Abstract
Anaplastic thyroid cancer (ATC) is a rare malignancy and has a very poor prognosis due to its aggressive behavior and resistance to treatment. No effective treatment modalities are currently available. Lenvatinib has shown encouraging results in the patients with radioiodine-refractory differentiated thyroid cancer (DTC); however, lenvatinib monotherapy has a relatively low efficacy against ATC. In this study, we assessed the antitumor effects of a combination of lenvatinib and microtubule inhibitor paclitaxel in ATC cells in vitro and in vivo. Our data showed that lenvatinib monotherapy was less effective than paclitaxel monotherapy in ATC cell lines and xenografts. The addition of lenvatinib to paclitaxel synergistically inhibited colony formation and tumor growth in nude mice, and induced G2/M phase cell cycle arrest and cell apoptosis as compared to lenvatinib or paclitaxel monotherapy. Taken together, this is the first study to suggest that lenvatinib/paclitaxel combination may be a promising candidate therapeutic strategy for ATC.
Keywords: Thyroid cancer, lenvatinib, paclitaxel, combined therapy, antitumor effects
Introduction
Anaplastic thyroid cancer (ATC) accounts for 1-2% of all thyroid cancers and has an annual incidence of 2 million per year [1,2]. ATC can arise de novo or from preexisting well-differentiated thyroid cancer (WDTC). Although ATC is relatively rare, it is one of the most aggressive and deadly human cancers due to its invasive growth behavior and a high propensity for distant metastasis [2]. ATC has a dismal prognosis. Median survival from the time of diagnosis is 4 to 12 months; 5-year survival rate is less than 10% [2,3]. In general, treatment modalities for ATC include surgery, chemotherapy, radiotherapy and multimodal therapy. Athough multimodality approaches have been shown to be superior to monotherapy in some patients [4,5], ATC still carries a very poor prognosis. Thus, improved therapeutic strategies against ATC should be sorely needed.
Lenvatinib, a multi-targeted tyrosine kinase inhibitor, selectively inhibits vascular endothelial growth factor receptors 1-3 (VEGFR1-3), fibroblast growth factor receptors 1-4 (FGFR1-4), platelet derived growth factor receptor-alpha (PDGFRα), mast/stem cell growth factor receptor (SCFR), RET and c-kit [6]. It was approved by the US Food and Drug Administration in February 2015 as the second agent for the treatment of radioiodine-refractory differentiated thyroid cancer (DTC) [7]. In a pivotal phase III study of 392 patients with radioiodine-refractory DTC, lenvatinib monotherapy was shown to improve progression-free survival and the response rate [8]. Although several lenvatinib-treated ATC patients in a phase II trial conducted in Japan obtained transient objective response, there were no RECIST (Response Evaluation Criteria in Solid Tumors) responses [9]. Overall, lenvatinib has shown relatively low efficacy in the patients with ATC when administered as a monotherapy.
A combination of kinase inhibitor pazopanib (targeting VEGFR) and paclitaxel was demonstrated to exhibit a synergistic antitumor effects against ATC in vitro and in vivo. Importantly, pilot anecdotal data from an ATC patient with lung metastasis treated with a combination of pazopanib and paclitaxel suggested marked and durable regression of metastatic disease (confirmed RECIST response) [10]. These data support the feasibility of clinical application of a combinatorial strategy for ATC treatment. Thus, in this study, we first attempted to test the antitumor activity of a combination of lenvatinib and paclitaxel against ATC through a series of in vitro and in vivo studies.
Materials and methods
ATC cell lines
ATC cell lines C643, 8305C and 8505C were provided by Dr. Haixia Guan (The First Affiliated Hospital of China Medical University, Shenyang, P. R. China) and Dr. Lei Ye (Ruijin Hospital, Shanghai, P. R. China), respectively. Cells were routinely cultured at 37°C in RPMI 1640 medium with 10% fetal bovine serum (FBS) (Invitrogen Technologies, Inc., CA). In some experiments, cells were treated with lenvatinib or/and paclitaxel at the indicated concentrations and times. Lenvatinib and paclitaxel were purchased from Selleck Chemicals, LLC (Houston, TX) and MedChem Express, LLC (Princeton, NJ), respectively. The drugs were dissolved in dimethylsulfoxide (DMSO), aliquoted and stored at -80°C until further use. The same volume of DMSO was used as the vehicle control.
Cell proliferation assay
Cells (2000/well) were seeded into 96-well plates and cultured with various concentrations of lenvatinib or paclitaxel for 48 h. Next, 3-(4,5-Dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to evaluate cell proliferation. IC50 values were calculated using the Reed-Muench method [11]. After drug treatment at the indicated time-points, 10 μL of 5 mg/mL MTT (Sigma, Saint Louis, MO) was added to the medium and incubated for 4 h; this was followed by addition of 150 μL of DMSO and further 15-min incubation. The plates were then read on a microplate reader using a test wavelength of 570 nm and a reference wavelength of 670 nm. Three triplicates were performed for each data point.
Colony formation assay
Colony formation assay was performed using monolayer culture. Cells (4000/well) were seeded into 12-well plates and cultured with the indicated concentrations of lenvatinib and paclitaxel, individually or in combination. The medium was refreshed every 2 days. After 14 days of culture, surviving colonies (≥50 cells per colony) were fixed with methanol and stained with 0.5% crystal violet, and the colonies were then counted. Each experiment was performed in triplicate.
Cell cycle analysis
8305C cells in the exponential growth phase were serum starved for 12 h. After co-culture with 5 µM lenvatinib and 500 nM paclitaxel, individually or in combination, for 48 h, cells were harvested, washed twice in PBS, and fixed in 70% ethanol on ice for at least 30 min. Cells were then stained with propidium iodide (PI) solution (50 μg/mL PI, 50 μg/mL RNase A, 0.1% Triton-X, 0.1 mM EDTA). Cell cycles were analyzed based on DNA content using a flow cytometer (BD Biosciences, NJ).
Apoptosis assay
C643 and 8505C cells were treated with the indicated concentrations of lenvatinib and paclitaxel, individually or in combination, for 72 h. Cells were then harvested, washed with PBS, and subjected to sequential staining with Annexin V-FITC/PI Detection Kit (Roche Applied Science, Penzberg, Germany) by flow cytometer according to the manufacturer’s protocol. Early apoptotic cells show Annexin V-FITC+/PI- staining patterns, whereas late apoptotic cells exhibit Annexin V-FITC+/PI+ staining patterns. They were collectively called apoptotic cells. Each experiment was performed in triplicate.
Xenograft tumor assay in nude mice
Female athymic nude mice were purchased from SLAC laboratory Animal Co., Ltd. (Shanghai, PR. China) and housed in a specific pathogen-free (SPF) environment. C643 cells (3×106) were injected subcutaneously into the flanks of mice at the age of 5 weeks. When tumors grew to ~5 mm in diameter, mice were grouped into four groups (six mice per group) and administered the following treatments: vehicle control, 5 mg/kg paclitaxel via intraperitoneal injection (twice per week), 5 mg/kg/day lenvatinib via oral route and a combination of these two drugs. Tumor volume was measured every 2 days during the course of the therapy, and was calculated by the formula (width)2 × length/2. After treatment for 12 days, tumors were harvested and weighted.
Tumor tissues were embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin (H&E). Cell proliferation ability was assessed by quantification of Ki-67 staining (percentage of positive cells). In brief, anti-human Ki-67 antibody (BD Pharmingen) was 1:150 diluted and immunostaining was done according to a standard protocol using DAB Substrate Kit (ZSGB-BIO). At least 1000 Ki-67-positive cells were scored by visual examination of 5 randomly selected fields (×200 magnification). In addition, to evaluate the effect of different treatments on animal hepatocytes, we performed H&E staining of liver sections and analyzed the serum levels of aspartate transaminase (AST) and alanine transaminase (ALT) by spectrophotometry, as previously described [12].
Results
Lenvatinib potentiates colony formation-inhibitory effect of paclitaxel in ATC cells
Given the encouraging clinical activity of lenvatinib in DTC, we tested its antitumor effects in ATC. We also evaluated the antitumor effects of paclitaxel in ATC cells. MTT assay was performed to test the dose-course of the effect of lenvatinib and paclitaxel on the proliferation in ATC cell lines C643, 8305C and 8505C. As shown in Figure 1A and 1B, lenvatinib and paclitaxel significantly inhibited the proliferation of ATC cells in a dose-dependent manner; the IC50 values ranged from 4.60 to 10.97 μM and from 1.99 to 9.97 nM, respectively. Next, we tested the effect of lenvatinib and paclitaxel, individually or in combination, on colony formation in these cells. As shown in Figure 2, paclitaxel at the indicated concentrations significantly inhibited colony forming ability in monolayer culture as compared to the control, whereas lenvatinib at the indicated concentrations had less effect on colony formation of ATC cells as compared to paclitaxel. As expected, a combination of these two drugs caused a synergistic inhibitory effect on colony formation in ATC cells as compared to lenvatinib or paclitaxel monotherapy.
Figure 1.
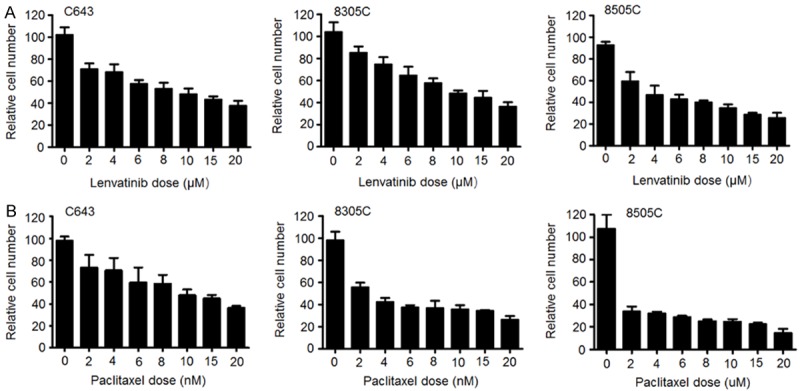
Inhibitory effect of lenvatinib and paclitaxel on the proliferation of ATC cells. Cells were treated with the indicated concentrations of lenvatinib (A) and paclitaxel (B) for 48 h, followed by MTT assay to evaluate cell proliferation. IC50 values of these two drugs for each cell line were calculated using the Reed-Muench method [11].
Figure 2.
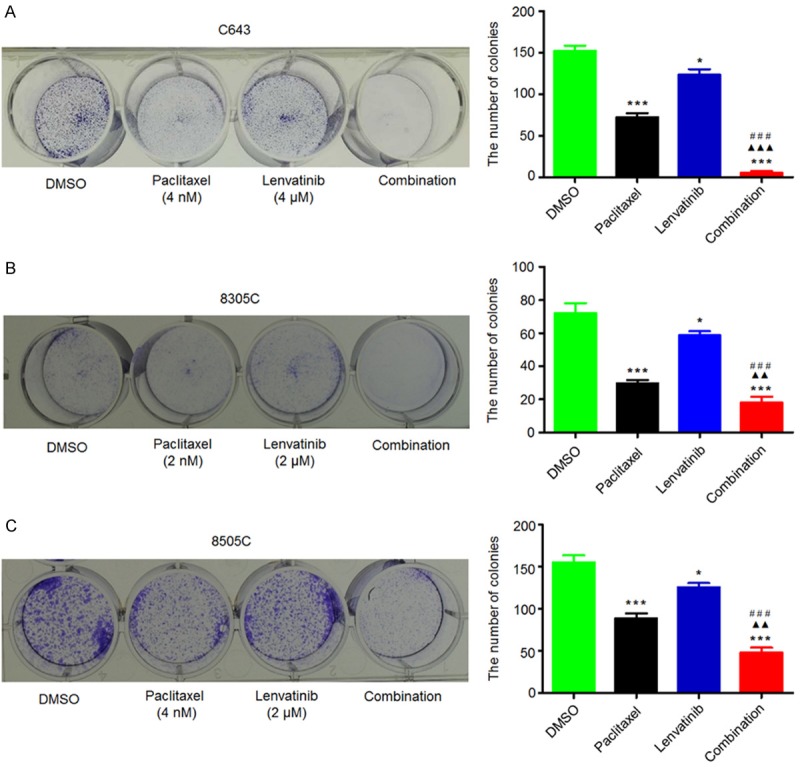
Synergistic inhibition of colony formation of ATC cells by lenvatinib and paclitaxel. Representative images of colony formation in C643 (A), 8305C (B) and 8505C (C) cells treated with vehicle control (DMSO) or lenvatinib and paclitaxel at the indicated concentrations, individually or in combination, are shown in left panel. Quantitative analysis of colony numbers is shown in right panel. Data are presented as mean ± SD of values from three different assays. Statistically significant differences are indicated: *, P < 0.05; ***, P < 0.001 for comparison with control; ▲▲, P < 0.01; ▲▲▲, P < 0.001 for comparison with paclitaxel monotherapy; ###, P < 0.001 for comparison with lenvatinib monotherapy.
Lenvatinib potentiates the cell cycle arrest and apoptotic effects of paclitaxel in ATC cells
To examine the possibility that lenvatinib may act in part by potentiating the cell cycle effects of paclitaxel, we performed fluorescence-activated cell sorting (FACS) analysis to evaluate the effect of lenvatinib and paclitaxel, individually or in combination, on cell cycle. As shown in Figure 3, both lenvatinib and paclitaxel monotherapies increased the proportion of 8305C cells in the G2/M phase of cell cycle; the addition of lenvatinib to paclitaxel synergistically increased the percentage of 8305C cells in G2/M phase as compared to lenvatinib or paclitaxel monotherapy. These findings suggest that lenvatinib/paclitaxel synergy may in part be attributable to the combined cell cycle effect of lenvatinib and paclitaxel.
Figure 3.
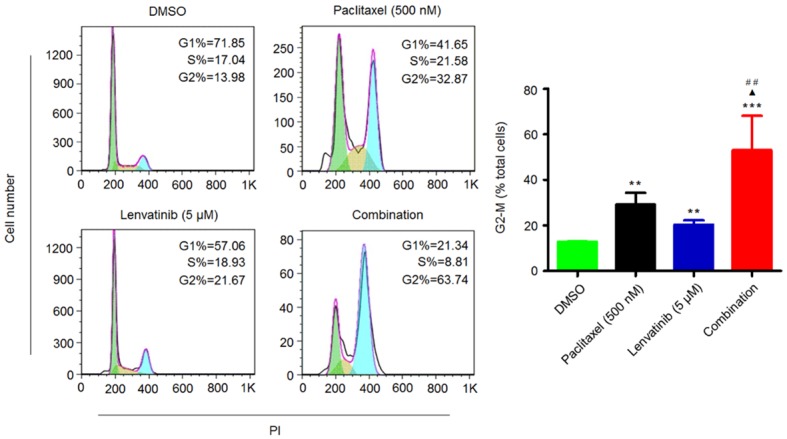
Synergistic induction of G2/M phase cell cycle arrest of 8305C cells by lenvatinib and paclitaxel. Cells were treated with vehicle control or lenvatinib and paclitaxel at the indicated concentrations, individually or in combination, for 48 h. DNA content was then measured by flow cytometry to determine cell cycle fractions. Representative flow cytometric histograms are shown in the left panel. The percentage of 8305C cells in the G2/M phase is indicated in the right panel. Statistically significant differences are indicated: **, P < 0.01; ***, P < 0.001 for comparison with control; ▲, P < 0.05 for comparison with paclitaxel monotherapy; ##, P < 0.01 for comparison with lenvatinib monotherapy.
Next, we also evaluated the effect of lenvatinib and paclitaxel, individually or in combination, on the apoptosis in ATC cells. As shown in Figure 4, C643 and 8505C cells treated with lenvatinib and paclitaxel, individually or in combination, at the indicated concentrations showed a dramatic increase in both early and late apoptosis in comparison with the control. Similar to the findings in Figure 3, a synergistic effect was expectedly found when these two cell lines were treated with a combination of lenvatinib and paclitaxel as compared to lenvatinib or paclitaxel monotherapy (Figure 4).
Figure 4.
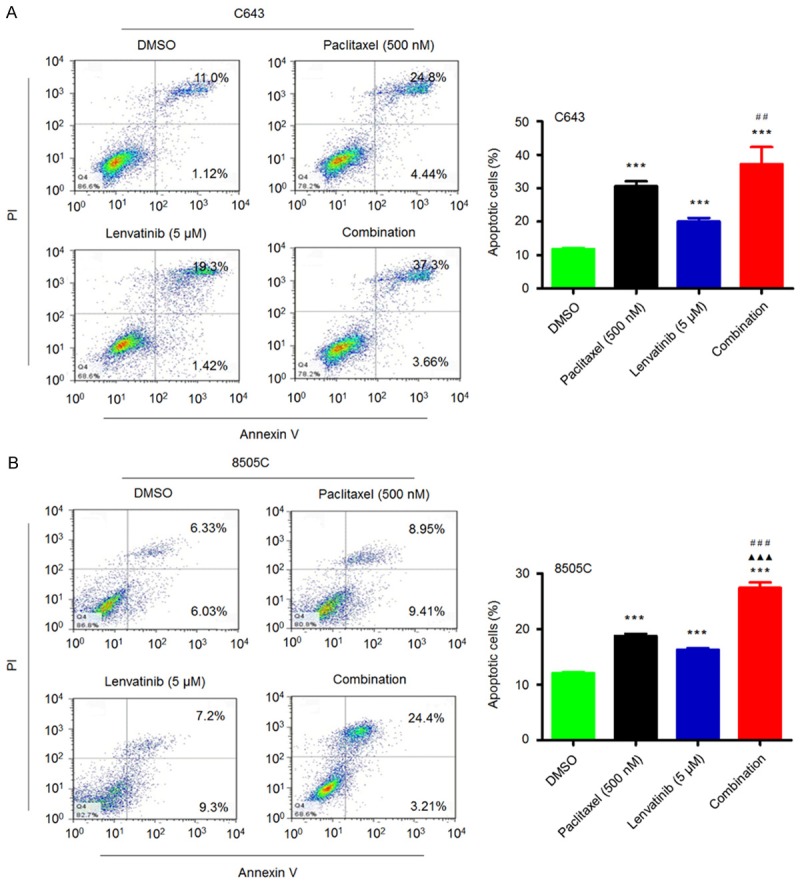
Synergistic induction of the apoptosis of ATC cells by lenvatinib and paclitaxel. C643 (A) and 8505C (B) cells were treated with vehicle control or lenvatinib and paclitaxel at the indicated concentrations, individually or in combination, for 72 h. The percentage of early apoptotic (bottom right quarter) and late apoptotic (top right) cells is presented in the figures (left panel). The data are presented as mean ± SD of values from three independent experiments in the right panel. Statistically significant differences are indicated: ***, P < 0.001 for comparison with control; ▲▲▲, P < 0.001 for comparison with paclitaxel monotherapy; ##, P < 0.01; ###, P < 0.001 for comparison with lenvatinib monotherapy.
Lenvatinib potentiates the antitumor effects of paclitaxel in ATC in vivo
To determine whether the observed antitumor effects of lenvatinib and paclitaxel in vitro can be seen in vivo, we established C643 tumor xenografts in nude mice and treated these mice with lenvatinib and paclitaxel, individually or in combination, at the indicated concentrations and time points. As shown in Figure 5A, the growth of C643 cell-derived xenograft tumors in the lenvatinib-treated and paclitaxel-treated groups was slower than that in control group. A synergistic effect was observed in the combined treatment group as compared to monotherapy group. Notably, body weight, an indicator of the health of the animals, did not show a significant difference among different treatment groups (data not shown). At the end of the experiment, the tumors were isolated and weighted. As shown in Figure 5B, the mean tumor weight in paclitaxel-treated mice was significantly lower than that in control mice. Tumor weights of lenvatinib-treated mice were also lower than those of control-treated mice; however, the difference was not statistically significant. Similarly, we also found a synergistic effect in mice treated with combined lenvatinib and paclitaxel as compared to mice treated with each drug alone (Figure 5B).
Figure 5.
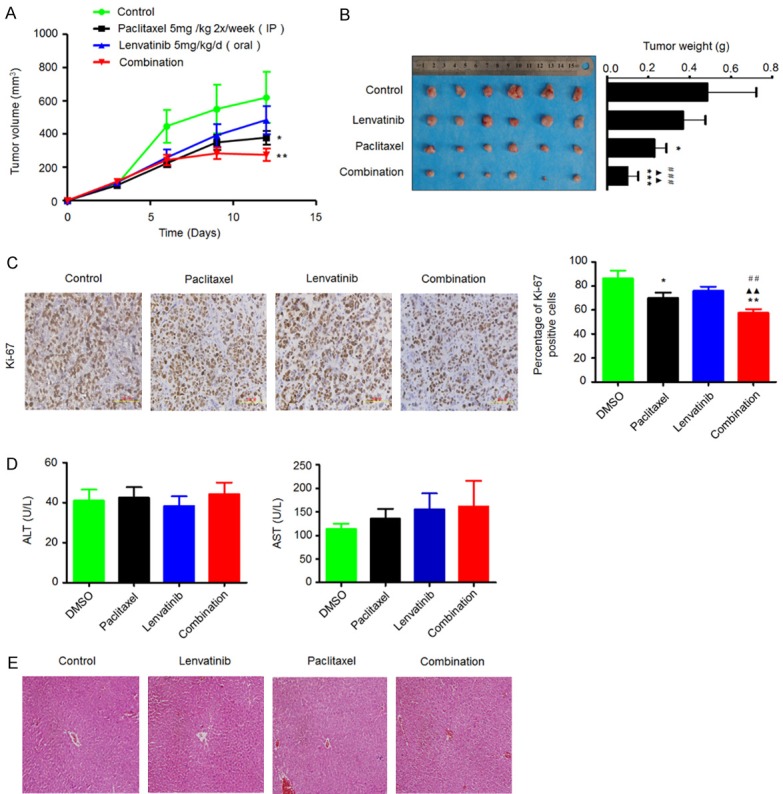
Synergistic inhibition of the growth of C643-derived xenograft tumors by lenvatinib and paclitaxel. Time course of tumor growth (A) and animal weight (B) was measured in each group at the indicated time points of various treatments. Data are presented as mean ± SD (n=6/group). Representative images of tumors from mice treated with vehicle control, lenvatinib and paclitaxel, individually or in combination are shown in the left panel. Bar graphs represents mean tumor weight from mice with the indicated treatments (right panel). Data are presented as mean ± SD (n=6/group). (C) Representative Ki-67 stained sections of xenograft tumors from different groups are shown in the left panel. Bar graphs represent mean ± SD of the numbers of Ki-67-positive cells from 5 microscopic fields in each group (right panel). (D) Serum levels of aspartate transaminase (AST) and alanine transaminase (ALT) were measured in the indicated mice by spectrophotometric methods. Data are presented as mean ± SD (n=6/group). (E) Representative H&E stained liver sections from the indicated mice. Statistically significant differences are indicated: *, P < 0.05; **, P < 0.01; ***, P < 0.001 for comparison with control; ▲▲, P < 0.01 for comparison with paclitaxel monotherapy; ##, P < 0.01; ###, P < 0.001 for comparison with lenvatinib monotherapy.
To quantitatively evaluate the proliferation index of tumors treated with lenvatinib and paclitaxel, individually or in combination, tumor sections were stained with the Ki-67 antibody. As shown in Figure 5C, the number of Ki-67-positive cells in paclitaxel-treated tumors was lower than that in control tumors, while lenvatinib monotherapy did not significantly affect the Ki-67 staining index as compared to the control. As expected, combined treatment caused a synergistic effect on tumor proliferation as compared to lenvatinib or paclitaxel monotherapy. Importantly, there was no significant difference with respect to serum levels of AST and ALT among three treatment groups (Figure 5D). In addition, histopathological evidences also showed that hepatic tissues exhibited normal large polygonal cells with prominent round nuclei and eosinophilic cytoplasm, and few spaced hepatic sinusoids arranged in-between the hepatic cords with fine arrangement of Kupffer cells in all experimental animals (Figure 5E). Although mild edema of liver cells was found in a few drug-treated mice, current treatment modalities did not cause any significant liver injury in mice. Collectively, our data suggest the safety and efficacy of a combination of lenvatinib and paclitaxel for ATC treatment.
Discussion
ATC is a rare form of undifferentiated cancer with a high mortality rate [2]. Complete surgical resection of the central compartment, combined with adjuvant therapy, is the main treatment option for ATC [13]. However, these coventional therapies have been shown not to be very effective in such patients. Fortunately, molecular characterization of ATC tumors in recent years has facilitated a better understanding of its molecular pathogenesis and exploration of new potential treatments for this disease [14].
In recent years, several biological agents have showed promise and may become potential therapies for ATC, despite the limited number of ATC patients in these studies. For example, in a phase I trial of combretastatin A-4 phosphate, one of three patients with ATC achieved complete remission and was alive at 30 months [15]. In another phase I study, intermittent high-dose gefitinib and docetaxel achieved a 4-month partial remission in one patient with ATC [16]. In addition, one of two ATC patients in a phase II trial of axitinib achieved a partial remission, while another patient had progressive disease [17]. These observations suggest that antitumor activity of new potential agents against ATC should be further investigated.
It is the fact that some small molecule inhibitors targeting tyrosine kinases, including pazopanib, vandetanib, cabozantinib, sorafenib and lenvatinib, have shown clinical benefit in advanced thyroid cancer, and some of them have been approved by the US FDA for treatment of thyroid cancer [18-22]. In a previous study, durable clinical responses were observed in about half of the patients with radioiodine-refractory DTC who received pazopanib, a multi-kinase inhibitor against VEGFR [23]. However, in a multiinstitutional phase II trial, although transient disease regression was observed in several ATC patients, there were no confirmed RECIST responses [24]. These results suggest that pazopanib monotherapy has minimal single-agent clinical activity in ATC. Of note, pazopanib was demonstrated to potentiate the antitumor effects of paclitaxel or other antimicrotubule inhibitors against ATC in vitro and in vivo [10]. Importantly, an ATC patient with lung metastases receiving a combined treatment of pazopanib and paclitaxel attained marked and durable regression of metastatic disease [10]. These encouraging findings suggest that the combination of small molecule tyrosine kinase inhibitors and antimicrotubule inhibitors such as paclitaxel may be a promising therapeutic strategy for ATC.
Lenvatinib, an oral multikinase inhibitor, was approved by the US FDA in February 2015 as the second agent for the treatment of radioiodine-refractory DTC [7]. Its antitumor activity has been demonstrated in variety of solid tumors including thyroid cancer [6]. Statistically significant improvement in progression free survival (PFS) has been reported from several phase I or II trials of lenvatinib in the patients with advanced radioiodine-refractory DTC [6,25]. In a phase II study conducted in Japan, 3 of 11 (27%) ATC patients receiving lenvatinib attained transient disease regression; however, there were no confirmed RECIST responses [9], suggesting that lenvatinib has relatively low effectiveness of single agent for ATC treatment. Given the largely disappointing outcomes of lenvatinib monotherapy in ATC, we thus attempted to investigate the feasibility of a combinatorial strategy for ATC treatment. In the present study, we combined lenvatinib with paclitaxel and determined their antitumor effects in ATC in vitro and in vivo. Our data showed that lenvatinib monotherapy was less effective than paclitaxel monotherapy in ATC cell lines and xenografts. More importantly, we found that lenvatinib potentiated the effect of paclitaxel against ATC in vitro and in vivo. The combined therapy synergistically inhibited colony formation and tumor growth in nude mice, and induced G2/M phase cell cycle arrest and cell apoptosis in ATC as compared to lenvatinib or paclitaxel monotherapy. These findings suggest that combinatorial therapies may be effective in the context of ATC. This is also supported by the latest clinical trials that the FDA has approved lenvatinib in combination with everolimus for second-line treatment of renal cell carcinoma following treatment with anti-angiogenic agent [7]. Moreover, several clinical trials are currently underway to investigate the antitumor activities of lenvatinib monotherapy or in combination with other agents in various human cancers [26].
In summary, our data demonstrate that lenvatinib enhances the antitumor effects of paclitaxel in ATC. Lenvatinib/paclitaxel synergy was observed not only in vitro but also in vivo. Collectively, these encouraging preliminary results suggest that the lenvatinib/paclitaxel combination may be a promising therapeutic approach for ATC.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81372217 and 81572627).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Are C, Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol. 2006;13:453–64. doi: 10.1245/ASO.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 3.Kebebew E, Greenspan FS, Clark OH, Woeber KA, McMillan A. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer. 2005;103:1330–5. doi: 10.1002/cncr.20936. [DOI] [PubMed] [Google Scholar]
- 4.Ito K, Hanamura T, Murayama K, Okada T, Watanabe T, Harada M, Ito T, Koyama H, Kanai T, Maeno K, Mochizuki Y, Amano J. Multimodality therapeutic outcomes in anaplastic thyroid carcinoma: improved survival in subgroups of patients with localized primary tumors. Head Neck. 2012;34:230–7. doi: 10.1002/hed.21721. [DOI] [PubMed] [Google Scholar]
- 5.Yau T, Lo CY, Epstein RJ, Lam AK, Wan KY, Lang BH. Treatment outcomes in anaplastic thyroid carcinoma: survival improvement in young patients with localized disease treated by combination of surgery and radiotherapy. Ann Surg Oncol. 2008;15:2500–5. doi: 10.1245/s10434-008-0005-0. [DOI] [PubMed] [Google Scholar]
- 6.Cabanillas ME, Habra MA. Lenvatinib: role in thyroid cancer and other solid tumors. Cancer Treat Rev. 2016;42:47–55. doi: 10.1016/j.ctrv.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Eisai, Inc. Lenvima [package insert] Woodcliff Lake, NJ: Eisai, Inc.; 2015. [Google Scholar]
- 8.Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372:621–30. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi S, Tahara M, Kiyota N, et al. Phase II study of lenvatinib (LEN), a multi-targeted tyrosine kinase inhibitor, in patients (PTS) with all histologic subtypes of advanced thyroid cancer (differentiated, medullary and anaplastic) [abstract] . Ann Oncol. 2014;25(Suppl 4) abstr 995PD. [Google Scholar]
- 10.Isham CR, Bossou AR, Negron V, Fisher KE, Kumar R, Marlow L, Lingle WL, Smallridge RC, Sherman EJ, Suman VJ, Copland JA, Bible KC. Pazopanib enhances paclitaxel-induced mitotic catastrophe in anaplastic thyroid cancer. Sci Transl Med. 2013;5:166ra3. doi: 10.1126/scitranslmed.3004358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welkos S, O’Brien A. Determination of median lethal and infectious doses in animal model systems. Methods Enzymol. 1994;235:29–39. doi: 10.1016/0076-6879(94)35128-7. [DOI] [PubMed] [Google Scholar]
- 12.Reitman S, Frankel S. A colourimetric method for the determination of Serum glutamic-oxaloacetic and glutamic-pyruvic transaminase. Am J Clin Pathol. 1957;28:56–61. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 13.Keutgen XM, Sadowski SM, Kebebew E. Management of anaplastic thyroid cancer. Gland Surg. 2015;4:44–51. doi: 10.3978/j.issn.2227-684X.2014.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP, Xu B, Schultz N, Berger MF, Sander C, Taylor BS, Ghossein R, Ganly I, Fagin JA. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016;126:1052–66. doi: 10.1172/JCI85271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dowlati A, Robertson K, Cooney M, Petros WP, Stratford M, Jesberger J, Rafie N, Overmoyer B, Makkar V, Stambler B, Taylor A, Waas J, Lewin JS, McCrae KR, Remick SC. A phase I pharmacokinetic and translational study of the novel vascular targeting agent combretastatin a-4 phosphate on a single-dose intravenous schedule in patients with advanced cancer. Cancer Res. 2002;62:3408–16. [PubMed] [Google Scholar]
- 16.Fury MG, Solit DB, Su YB, Rosen N, Sirotnak FM, Smith RP, Azzoli CG, Gomez JE, Miller VA, Kris MG, Pizzo BA, Henry R, Pfister DG, Rizvi NA. A phase I trial of intermittent high-dose gefitinib and fixed-dose docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2007;59:467–75. doi: 10.1007/s00280-006-0286-6. [DOI] [PubMed] [Google Scholar]
- 17.Cohen EE, Rosen LS, Vokes EE, Kies MS, Forastiere AA, Worden FP, Kane MA, Sherman E, Kim S, Bycott P, Tortorici M, Shalinsky DR, Liau KF, Cohen RB. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J. Clin. Oncol. 2008;26:4708–13. doi: 10.1200/JCO.2007.15.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CAPRELSA® (vandetanib) Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2014. Mar, [Google Scholar]
- 19.Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI, Smit JW, Chung J, Kappeler C, Peña C, Molnár I, Schlumberger MJ DECISION investigators. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319–28. doi: 10.1016/S0140-6736(14)60421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.COMETRIQ® (cabozantinib) [summary of product characteristics] Hampshire, UK: TMC Pharma Services, Ltd; 2014. Mar 26, [Google Scholar]
- 21.LENVIMATM (lenvatinib) [prescribing infomation] Woodcliff Lake, NJ: Eisai, Inc.; 2015. Feb, [Google Scholar]
- 22.Marotta V, Sciammarella C, Vitale M, Colao A, Faggiano A. The evolving field of kinase inhibitors in thyroid cancer. Crit Rev Oncol Hematol. 2015;93:60–73. doi: 10.1016/j.critrevonc.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME, Rubin J, Sideras K, Morris JC 3rd, McIver B, Burton JK, Webster KP, Bieber C, Traynor AM, Flynn PJ, Goh BC, Tang H, Ivy SP, Erlichman C Endocrine Malignancies Disease Oriented Group; Mayo Clinic Cancer Center; Mayo Phase 2 Consortium. Efficacy of pazopanib in progressive, radioiodinerefractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol. 2010;11:962–72. doi: 10.1016/S1470-2045(10)70203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bible KC, Suman VJ, Menefee ME, Smallridge RC, Molina JR, Maples WJ, Karlin NJ, Traynor AM, Kumar P, Goh BC, Lim WT, Bossou AR, Isham CR, Webster KP, Kukla AK, Bieber C, Burton JK, Harris P, Erlichman C Mayo Phase 2 Consortium; Mayo Clinic Endocrine Malignances Disease Oriented Group. A multiinstitutional phase 2 trial of pazopanib monotherapy in advanced anaplastic thyroid cancer. J Clin Endocrinol Metab. 2012;97:3179–84. doi: 10.1210/jc.2012-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hewett Y, Ghimire S, Farooqi B, Shah BK. Lenvatinib-A multikinase inhibitor for radioiodinerefractory differentiated thyroid cancer. J Oncol Pharm Pract. 2016 doi: 10.1177/1078155216680119. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26. https://clinicaltrials.gov/ct2/results?term=lenvatinib & Search=Search (accessed 24 May 2016)


