Abstract
Mutiple microRNAs are implicated in oral squamous cell carcinoma (OSCC), which is characterized by a high rate of proliferation and nodal metastasis. Data from the present study showed that miR-381-3p is significantly underexpressed in both OSCC tissues and cell lines. Overexpression of miR-381-3p led to marked suppression of proliferation and cell cycle progression of OSCC cells and promotion of apoptosis. Notably, fibroblast growth factor receptor 2 (FGFR2) was downregulated by miR-381-3p through direct interactions with its 3’ untranslated region. Knockdown of FGFR2 recapitulated the growth suppressive effect of miR-381-3p. Conversely, restoring FGFR2 expression attenuated miR-381-3p-induced effects in OSCC cells. Expression patterns of miR-381-3p and FGFR2 were inversely correlated in OSCC tissues. Our collective results provide novel evidence that miR-381-3p acts as a tumor suppressor in OSCC by directly targeting FGFR2, thereby presenting a promising therapeutic target.
Keywords: miR-381-3p, oral squamous cell carcinoma, FGFR2, proliferation
Introduction
Oral cancer is the most common head-and-neck cancer type. Oral squamous cell carcinoma (OSCC) accounts for ~90% total oral cancers, with more than 300,000 new cases diagnosed each year worldwide [1]. Despite considerable improvements in surgery and radiation therapy, the 5-year survival rate of OSCC has not improved significantly over the past two decades and its incidence continues to increase among young people and women [2]. Enhanced understanding of the molecular mechanisms underlying the development and progression of OSCC is therefore essential for identifying novel and effective therapeutic targets.
MicroRNAs (miRNAs or miR) are a class of small noncoding RNAs that can suppress post-transcriptional gene expression via binding to the 3’-untranslated region (UTR) of messenger RNAs (mRNAs), effectively repressing translation or inducing sequence-specific mRNA degradation [3]. As a newly identified family of gene regulators, miRNAs are involved in modulating numerous cellular processes, including proliferation, differentiation and apoptosis [4]. In particular, emerging evidence has revealed that miRNAs are abnormally expressed in various cancers and may act as either oncogenes or tumor suppressor genes [4]. miRNAs have been shown to play critical roles in oral cancer. For instance, miR-1271 is markedly decreased in OSCC and acts as tumor suppressor [5], miR-497 plays a key role in OSCC metastasis [6], and upregulation of miR-372 and -373 is associated with lymph node metastasis and poor prognosis of oral carcinomas [7]. These findings support the significant involvement of miRNAs in oral tumorigenesis and progression. Recent miRNA microarray data showed downregulation of miR-381-3p in OSCC, compared with adjacent normal tissues [8]. Aberrant expression of miR-381-3p has consistently been reported in several human cancer types, including lung cancer [9], hepatocellular carcinoma [10], colon cancer [11,12] and osteosarcoma [13]. However, the potential roles and mechanisms of action of miR-381-3p in human OSCC remain to be established.
In the current study, we showed that expression of miR-381-3p is suppressed in OSCC tissues and cell lines. Overexpression of miR-381-3p inhibited OSCC cell proliferation, induced cell cycle arrest and apoptosis, and reduced tumor growth in nude mice. Moreover, the oncogene, fibroblast growth factor receptor 2 (FGFR2), was identified as a direct and functional target of miR-381-3p in oral carcinogenesis.
Materials and methods
Patient specimens and cell culture
Surgical tumor specimens and adjacent tissue samples were obtained from 18 patients in the Second Affiliated Hospital of Nanchang University. None of the patients had received radiotherapy or chemotherapy before surgery. Samples were collected, immediately snap-frozen in liquid nitrogen, and stored at -80°C until further use. This study was approved by the Ethic and Research Committees of the Second Affiliated Hospital of Nanchang University and written informed consent obtained from all patients. Human tongue squamous cell carcinoma cell lines, SCC-9 and Tca-8113, were purchased from American Type Culture Collection (ATCC, Manassas, VA), and cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and 1% antibiotics. Human oral keratinocytes (HOK) were cultured in oral keratinocyte medium (ScienCell Research Laboratories, San Diego, Carlsbad, CA) according to the manufacturer’s instructions. All cells were maintained in a humidified chamber under 5% CO2 at 37°C.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from tissues and cell cultures using TRIzol reagent and quantified with Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA). miRNAs were isolated with the miRNeasy Mini Kit (Qiagen, Hilden, Germany). qRT-PCR was performed in an Applied Biosystems 7500 instrument (Applied Biosystems, Foster City, CA) using SYBR Premix Ex TaqTM (TaKaRa, Dalian, China) according to the manufacturer’s instructions. Expression of genes was evaluated based on the threshold cycle (Ct), and relative expression levels calculated using the 2-ΔΔCt method after normalization with reference to expression of GAPDH or U6.
Lentivirus transduction
Lentiviral miR-381-3p and FGFR2 expression constructs and the respective negative control lentiviruses were purchased from Hanbio (Shanghai, China). SCC-9 and Tca-8113 cell lines were infected with recombinant lentivirus transducing units in the presence of 5 mg/mL polybrene (Sigma-Aldrich, St Louis, MO) according to the manufacturer’s protocol.
Cell proliferation and colony formation
Cell viability was determined with the (3-4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. Infected SCC-9 and Tca-8113 cells were cultured in 96-well plates at a density of 3.0 × 103 cells per well and maintained for 1, 2, 3 and 4 days, respectively. MTT solution (20 μL) was added and incubated for 4 h at 37°C, followed by removal of culture medium and addition of 150 μL dimethyl sulfoxide. Absorbance at 490 nm was measured using a microplate spectrophotometer (Bio-Tek Instruments Inc., Winosski, VT). The results were determined as an average of three independent experiments. For the clonogenic assay, 500 cells were seeded into six-well plates and cultured for 12 days. After fixing in 1.0% crystal violet, visible colonies were counted.
Cell cycle and apoptosis analysis
Analysis of the cell cycle and apoptosis was conducted as described previously [14]. For cell cycle analysis, SCC-9 and Tca-8113 cells were harvested, washed and fixed in 75% ethanol at 4°C overnight. Fixed cells were washed with PBS and mixed with propidium iodide (PI, 50 mg/mL) for 30 min. Cell cycle profiles were analyzed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). For assessment of apoptosis, the Annexin V/PI Apoptosis Detection Kit (Beyotime, Jiangsu, China) was used following the manufacturer’s instructions. Apoptosis was analyzed with FACS.
Tumor xenograft model
All animal experiments were undertaken in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, with the approval of the Scientific Investigation Board of the Nanchang University. Healthy female BALB/c nude mice (4-5 weeks of age) were obtained from the Experimental Animal Center of Shanghai. SCC-9 cells (2.0 × 106) infected with miR-381-3p or control lentivirus were subcutaneously administered into flanks of BALB/c nude mice. Tumor sizes were monitored by measuring length (A) and width (B) with a slide caliper every 7 days, and the volume of implanted tumor calculated using the formula: V = (A × B2) × 0.5. After 5 weeks, mice were sacrificed, and tumors removed and weighed.
Plasmid construction and luciferase reporter assay
The wild-type 3’-UTR sequence of FGFR2 predicted to interact with miR-381-3p or a mutated sequence within the target site was synthesized and inserted into psiCHECK2 vector (Promega, Madison, WI). Wild-type or mutant luciferase reporter constructs were transfected into SCC-9 and Tca-8113 cells infected with miR-381-3p or control lentivirus. Forty-eight hours after transfection, firefly and renilla luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega), according to the manufacturer’s instructions.
Western blotting
Whole cell protein lysates of tissues and cells were prepared using RIPA Lysis and Extraction Buffer supplemented with protease inhibitors (Roche, Indianapolis, IN). Protein concentrations were determined with a Pierce BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL), and 30 μg protein loaded and separated on 10% sodium dodecyl sulfate polyacrylamide gels and transferred to polyvinylidene difluoride membrane (Millipore, Bedford, MA). Following transfer, membranes were blocked with 5% skimmed milk and incubated with the appropriate primary antibodies against FGFR2 and GAPDH (Proteintech Group, Wuhan, China). Antigen-antibody complexes on the membrane were detected with enhanced chemiluminescence reagent.
Statistical analysis
Data are expressed as means ± standard deviation (SD) of at least three independent experiments. SPSS 13.0 software was used for statistical analysis. Differences between two groups were compared using Student’s t-test. Spearman’s correlation was used to determine the association between miR-381-3p and FGFR2 expression. Differences were considered statistically significant at P < 0.05.
Results
miR-381-3p is significantly downregulated in OSCC tissues and cell lines
Initially, we examined miR-381-3p expression in 18 pairs of tongue OSCC specimens and matched adjacent normal specimens. qRT-PCR results showed that the average expression of miR-381-3p is significantly lower in cancer than normal specimens (Figure 1A). Consistently, miR-381-3p expression was decreased in the OSCC cell lines, SCC-9 and Tca-8113, relative to normal HOK (Figure 1B). Our results provide novel evidence of miR-381-3p downregulation in human OSCC tissues and cell lines.
Figure 1.
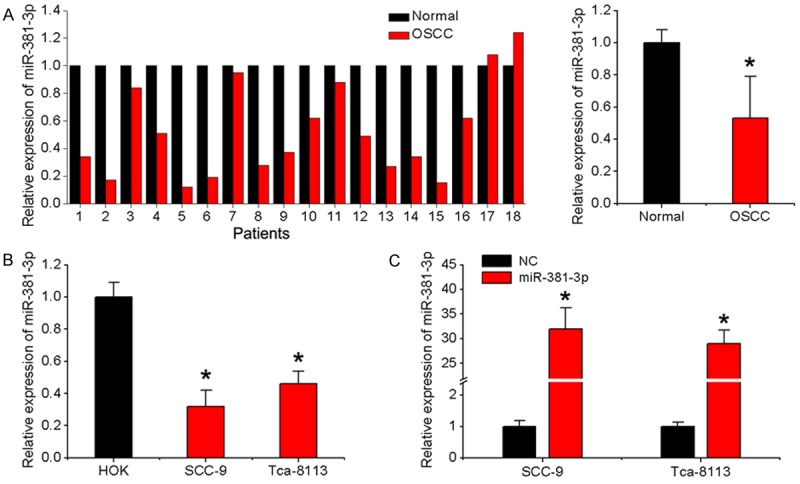
miR-381-3p expression in OSCC tissues and cell lines. A. Relative miR-381-3p expression in 18 pairs of OSCC specimens and matched adjacent normal specimens detected with qRT-PCR. U6 was used as an internal control. B. qRT-PCR analysis of miR-381-3p expression in OSCC cell lines (SCC-9 and Tca-8113) and HOK cells. C. SCC-9 and Tca-8113 cells were infected with miR-381-3p or negative control lentivirus, and qRT-PCR used to determine miR-381-3p expression. Data represent means ± SD of three independent experiments. *P < 0.05.
Overexpression of miR-381-3p inhibits OSCC cell proliferation and induces apoptosis
To determine whether miR-381-3p dysregulation modulates oral tumorigenesis, OSCC cell lines SCC-9 and Tca-8113 were transduced with recombinant lentivirus carrying the miR-381-3p gene and overexpression confirmed via qRT-PCR (Figure 1C). As evident from the MTT assay, miR-381-3p overexpression induced dramatic suppression of OSCC cell viability (Figure 2A). The inhibitory effect of miR-381-3p on OSCC cell proliferation was further confirmed using the colony formation assay. The number of colonies was significantly decreased in cells overexpressing miR-381-3p, compared to the negative control group (Figure 2B). The flow cytometry assay was further employed to measure cell cycle distribution and apoptosis. miR-381-3p-overexpressing cells displayed a significant increase in the proportion of cells at the G1/G0 phase and decrease in cells at the S phase (Figure 2C). In addition, apoptotic rate was significantly increased upon overexpression of miR-381-3p in the indicated cells (Figure 2D). Our data strongly suggest that miR-381-3p exerts growth inhibitory effects on OSCC cells and acts as a potential tumor suppressor.
Figure 2.
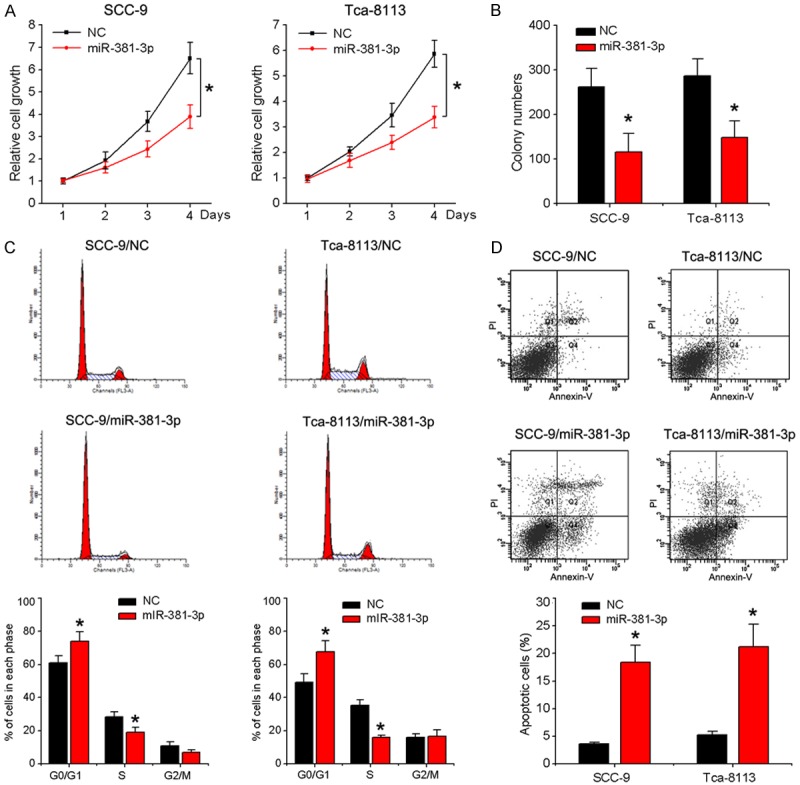
Effects of miR-381-3p on proliferation, cell cycle and apoptosis of OSCC cells. SCC-9 and Tca-8113 cells were infected with miR-381-3p or control lentivirus. A. Cell viability was detected with the MTT assay. B. Counting of colony numbers. C. Flow cytometry analysis of cell cycle progression. D. Apoptosis was measured via flow cytometry. Data represent means ± SD of three independent experiments. *P < 0.05.
FGFR2 is a direct target of miR-381-3p
To clarify the mechanism by which miR-381-3p exerts inhibitory effects on OSCC cells, we searched for target genes using two bioinformatic algorithms, TargetScan and Microrna.org. One putative target gene, FGFR2, widely reported to be involved in cancer cell proliferation and tumor growth, attracted our attention (Figure 3A). Accordingly, we examined FGFR2 expression at the transcriptional and translational levels in the infected OSCC cells via qRT-PCR and western blot, respectively. Overexpression of miR-381-3p significantly suppressed FGFR2 at both the mRNA and protein levels in SCC-9 and Tca-8113 cells (Figure 3B). To ascertain whether FGFR2 is regulated via direct binding of miR-381-3p to its 3’-UTR region, the FGFR2 3’-UTR fragment containing the miR-381-3p binding site and a corresponding mutant fragment were subcloned into the psiCHEK-2 vector and transfected into SCC-9 cells infected with miR-381-3p or control lentivirus (Figure 3A). Compared with the control group, luciferase activity was significantly suppressed in psiCHEK-2-FGFR2-3’-UTR-transfected but not psiCHEK-2-FGFR2-3’-UTR mutant-transfected cells (Figure 3C). To further establish the precise role of miR-381-3p in FGFR2 regulation, qRT-PCR analysis of FGFR2 expression in OSCC and normal tissues was performed. As shown in Figure 3D, expression of FGFR2 in OSCC tissues was lower than that in the normal tissue counterparts. In addition, FGFR2 and miR-381-3p levels were inversely correlated (Figure 3E). The results indicate that miR-381-3p binds directly to FGFR2 3’-UTR to repress its expression.
Figure 3.
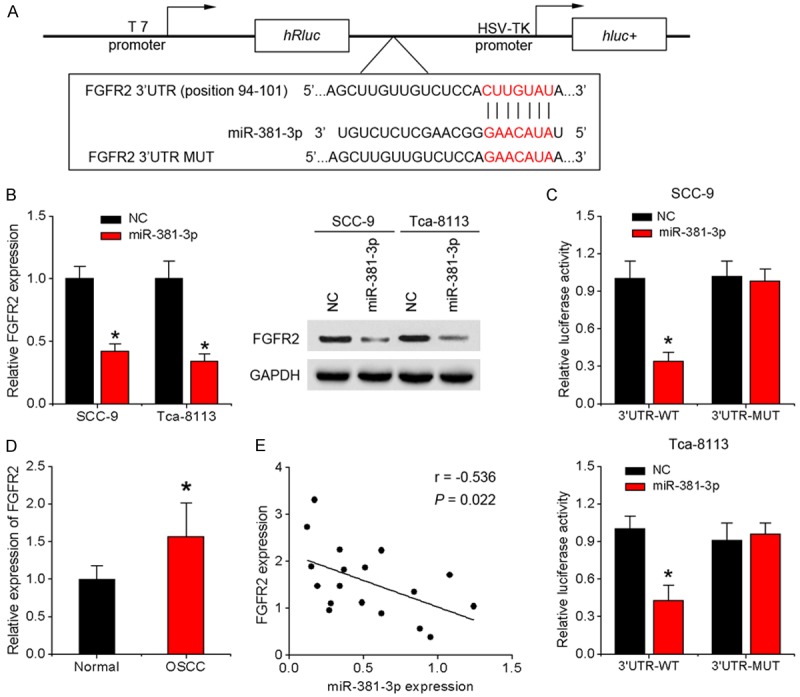
FGFR2 is a direct downstream target of miR-381-3p. A. A fragment of FGFR2 3’-UTR containing the wild-type (WT) miR-381-3p or mutant binding site was cloned downstream of the reporter gene vector. B. Levels of FGFR2 mRNA and protein were measured using qRT-PCR and western blot, respectively, in response to overexpression of miR-381-3p in SCC-9 and Tca-8113 cells. C. Luciferase activity of wild-type or mutant FGFR2 3’-UTR luciferase vectors in the presence of miR-381-3p. D. FGFR2 mRNA expression in 18 pairs of tongue OSCC specimens and matched adjacent normal specimens. E. Scatter plot showing the correlation between miR-381-3p and FGFR2 expression in OSCC tissues. Data represent means ± SD of three independent experiments. *P < 0.05.
Alterations in FGFR2 expression influence the effects of miR-381-3p on OSCC cells
Given that FGFR2 is closely associated with tumor cell proliferation [15,16], we eliminated FGFR2 in SCC-9 cells using specific siRNAs, with a view to further exploring its function (Figure 4A). Functional assays disclosed that knockdown of FGFR2 inhibits OSCC cell proliferation, induces G1 arrest, and increases apoptosis, resembling the inhibitory effects of miR-381-3p (Figure 4B). To further determine whether deregulation of FGFR2 is involved in suppression of cell proliferation by miR-381-3p, SCC-9 cells overexpressing miR-381-3p were infected with FGFR2 lentivirus encoding the full-length sequence without the 3’UTR region (Figure 4C). FGFR2 overexpression led to a marked increase in cell proliferation and rescued miR-381-3p-induced cell cycle arrest and apoptosis (Figure 4D), providing further evidence of its role as a downstream mediator of miR-381-3p.
Figure 4.
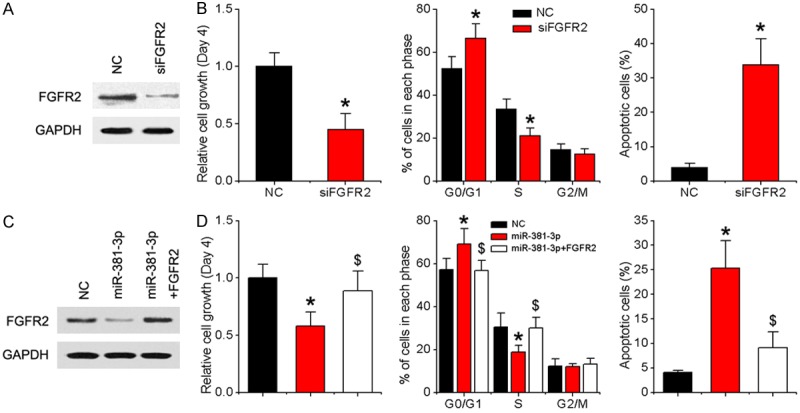
miR-381-3p suppresses OSCC growth by downregulating FGFR2. A. SCC-9 cells were transfected with FGFR2 siRNA or negative control. After 48 h, the FGFR2 protein level was examined via western blot analysis. B. MTT, cell cycle and apoptosis assays. C. SCC-9 cells were infected with NC, miR-381-3p lentivirus or miR-381-3p and FGFR2 lentivirus. After 48 h, FGFR2 protein levels were examined via western blot analysis. D. MTT, cell cycle and cell apoptosis assays. Data represent means ± SD of three independent experiments. *P < 0.05 vs. NC group, $P < 0.05 vs. miR-381-3p group.
miR-381-3p suppresses tumor growth of OSCC cells in nude mice
Finally, we investigated whether the in vitro findings are reproducible in a mouse xenograft model. SCC-9 cells overexpressing miR-381-3p or negative control were subcutaneously injected into nude mice. Consistent with in vitro observations, miR-381-3p significantly inhibited tumor growth in vivo (Figure 5A and 5B). The size of subcutaneous tumors derived from miR-381-3p overexpressing cells was significantly smaller than that of control cells (Figure 5A). A ~3-fold decrease in weight was observed in miR-381-3p-overexpressing tumors, compared to controls (Figure 5C). Additionally, miR-381-3p overexpression led to a significant decrease in the number of hyperproliferative Ki-67+ tumor cells (Figure 5D). qRT-PCR and western blot analyses of tumor tissues confirmed elevated miR-381-3p with reduced FGFR2 expression in miR-381-3p-overexpressing tumors (Figure 5E and 5F).
Figure 5.
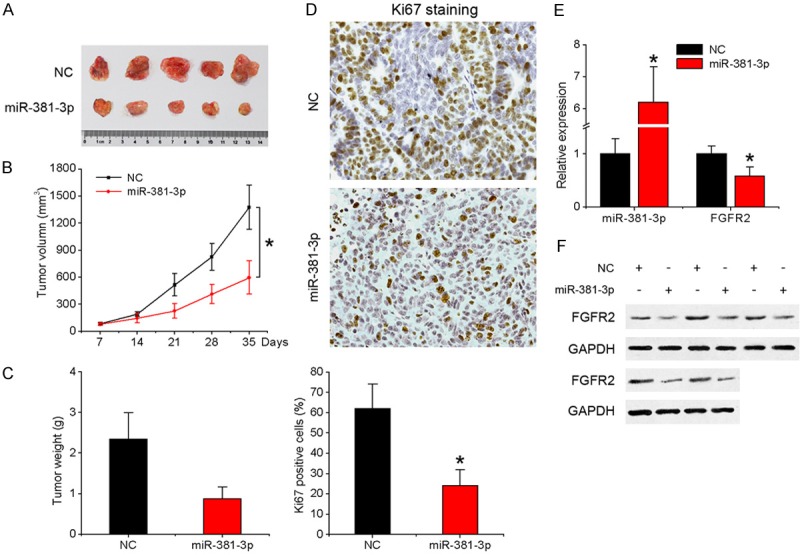
Overexpression of miR-381-3p inhibits OSCC tumorigenesis in vivo. Tumors were detected every 7 days after implantation of SCC-9 cells infected with miR-381-3p or control lentivirus. A. Representative images of the tumors formed. B. Tumor volume. C. Mean weight. D. Ki-67-stained sections of transplanted tumors (×400). E. qRT-PCR analysis of expression of miR-381-3p and FGFR2 in tumor tissues. F. Western blot analysis of FGFR2 expression in tumors. Data represent means ± SD of three independent experiments. *P < 0.05.
Discussion
Emerging research suggests that miRNAs play essential roles in progression of OSCC [17,18]. Previous miRNA microarray analyses showed that miR-381-3p is downregulated in OSCC tissues, compared with adjacent normal tissues [8]. However, since then, no further expression or functional data on miR-381-3p in OSCC have been reported. Here, we demonstrate that miR-381-3p functions as a tumor suppressor that negatively controls OSCC development. In our experiments, expression of miR-381-3p was significantly downregulated in OSCC tissues and cell lines. Functional studies revealed that overexpression of miR-381-3p induced inhibition of OSCC cell proliferation, cell cycle arrest and apoptosis and reduction of tumor growth in nude mice. Furthermore, miR-381-3p negatively regulated FGFR2 expression by directly targeting the 3’-UTR of its mRNA. Knockdown of FGFR2 recapitulated the growth suppressor function of miR-381-3p whereas restoring FGFR2 expression attenuated the effects of miR-381-3p in OSCC cells.
Downregulation of miR-381-3p is a frequent event in multiple cancer types [10-12,19], suggesting an important role in tumorigenesis and tumor progression. He et al. reported that miR-381 is significantly downregulated in CRC and correlated with distant metastasis and tumor, node, and metastasis stage, and miR-381 significantly inhibits CRC cell invasion, migration, and epithelial-mesenchymal transition through targeting Twist1 [12]. Xia and co-workers demonstrated that miR-381 expression is significantly decreased in EOC tissues and cell lines and its overexpression leads to marked inhibition of EOC cell proliferation, migration, and invasion via YY1 suppression [20]. The group of Li reported that low expression of miR-381 is a favorite prognostic factor and enhances chemosensitivity of osteosarcoma through the LRRC4-mediated mTOR pathway [19]. On the other hand, miR-381 functions as an “oncomir” in glioma progression, increasing both in vitro and in vivo proliferation through regulation of LRRC4 [21]. These controversial results suggest that the function of miR-381-3p is tumor-specific and highly dependent on its targets in different cancer cell types. Consistent with previous observations, we observed frequent downregulation of miR-381-3p in OSCC tissues and cell lines. Overexpression of miR-381-3p inhibited OSCC cell proliferation, induced cell cycle arrest and apoptosis and reduced tumor growth in nude mice, supporting the theory that deregulation of miR-381-3p plays a critical role in modulating oral carcinogenesis.
To better understand the tumor inhibitory effect of miR-381-3p, bioinformatics analysis was used and FGFR2 identified as a putative target of miR-381-3p. Using the 3’-UTR luciferase reporter assay, FGFR2 was further confirmed as a direct target of miR-381-3p. Increased miR-381-3p expression was accompanied by downregulation of FGFR2 in OSCC cells. The FGFR2 gene, located at human chromosome 10q26, is a member of FGFR family, which transduces FGF signals to cells [22]. Aberrant FGFR2 signaling activation has been shown to be involved in human carcinogenesis, and FGFR2 participates in several critical processes, such as cell proliferation, survival, migration and differentiation [23-26]. The crucial roles of FGFR2 in a variety of cancers support its potential utility as a therapeutic target. Several FGFR kinase inhibitors, including brivanib, dovitinib and SU-6668, effectively suppress tumor proliferation and are currently being tested for application in cancer therapy [27-29]. Data from the current study revealed downregulation of FGFR2 in OSCC tissues and a positive effect on OSCC cell proliferation based on RNA interference experiments. Moreover, FGFR2 overexpression rescued the growth suppressive effect of miR-381-3p. In clinical tissues, FGFR2 expression was inversely correlated with that of miR-381-3p. Our collective data strongly support a role of FGFR2 as a downstream mediator of miR-381-3p-dependent regulation of OSCC.
In summary, miR-381-3p is downregulated in OSCC and functions as an inhibitor of tumor growth through suppression of FGFR2. The newly discovered miR-381-3p/FGFR2 axis provides insights into the mechanisms underlying oral carcinogenesis and presents potential therapeutic targets for OSCC.
Disclosure of conflict of interest
None.
References
- 1.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–16. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Sano D, Myers JN. Metastasis of squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev. 2007;26:645–62. doi: 10.1007/s10555-007-9082-y. [DOI] [PubMed] [Google Scholar]
- 3.Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12:99–110. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- 4.Esquela-Kerscher A, Slack FJ. Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 5.Kong D, Zhang G, Ma H, Jiang G. miR-1271 inhibits OSCC cell growth and metastasis by targeting ALK. Neoplasma. 2015;62:559–66. doi: 10.4149/neo_2015_067. [DOI] [PubMed] [Google Scholar]
- 6.Hu J, Xu JF, Ge WL. MiR-497 enhances metastasis of oral squamous cell carcinoma through SMAD7 suppression. Am J Transl Res. 2016;8:3023–31. [PMC free article] [PubMed] [Google Scholar]
- 7.Tu HF, Chang KW, Cheng HW, Liu CJ. Upregulation of miR-372 and -373 associates with lymph node metastasis and poor prognosis of oral carcinomas. Laryngoscope. 2015;125:E365–70. doi: 10.1002/lary.25464. [DOI] [PubMed] [Google Scholar]
- 8.Shiah SG, Hsiao JR, Chang WM, Chen YW, Jin YT, Wong TY, Huang JS, Tsai ST, Hsu YM, Chou ST, Yen YC, Jiang SS, Shieh YS, Chang IS, Hsiao M, Chang JY. Downregulated miR329 and miR410 promote the proliferation and invasion of oral squamous cell carcinoma by targeting Wnt-7b. Cancer Res. 2014;74:7560–72. doi: 10.1158/0008-5472.CAN-14-0978. [DOI] [PubMed] [Google Scholar]
- 9.Rothschild SI, Tschan MP, Jaggi R, Fey MF, Gugger M, Gautschi O. MicroRNA-381 represses ID1 and is deregulated in lung adenocarcinoma. J Thorac Oncol. 2012;7:1069–77. doi: 10.1097/JTO.0b013e31824fe976. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q, Zhao S, Pang X, Chi B. MicroRNA-381 suppresses cell growth and invasion by targeting the liver receptor homolog-1 in hepatocellular carcinoma. Oncol Rep. 2016;35:1831–40. doi: 10.3892/or.2015.4491. [DOI] [PubMed] [Google Scholar]
- 11.Liang Y, Zhao Q, Fan L, Zhang Z, Tan B, Liu Y, Li Y. Down-regulation of MicroRNA-381 promotes cell proliferation and invasion in colon cancer through up-regulation of LRH-1. Biomed Pharmacother. 2015;75:137–41. doi: 10.1016/j.biopha.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 12.He X, Wei Y, Wang Y, Liu L, Wang W, Li N. MiR-381 functions as a tumor suppressor in colorectal cancer by targeting Twist1. Onco Targets Ther. 2016;9:1231–9. doi: 10.2147/OTT.S99228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Zhao C, Yu Z, Chen J, She X, Li P, Liu C, Zhang Y, Feng J, Fu H, Wang B, Kuang L, Li L, Lv G, Wu M. Low expression of miR-381 is a favorite prognosis factor and enhances the chemosensitivity of osteosarcoma. Oncotarget. 2016;7:68585–68596. doi: 10.18632/oncotarget.11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia LF, Wei SB, Gong K, Gan YH, Yu GY. Prognostic implications of micoRNA miR-195 expression in human tongue squamous cell carcinoma. PLoS One. 2013;8:e56634. doi: 10.1371/journal.pone.0056634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks AN, Kilgour E, Smith PD. Molecular pathways: fibroblast growth factor signaling: a new therapeutic opportunity in cancer. Clin Cancer Res. 2012;18:1855–62. doi: 10.1158/1078-0432.CCR-11-0699. [DOI] [PubMed] [Google Scholar]
- 16.Tokunaga R, Imamura Y, Nakamura K, Ishimoto T, Nakagawa S, Miyake K, Nakaji Y, Tsuda Y, Iwatsuki M, Baba Y, Sakamoto Y, Miyamoto Y, Saeki H, Yoshida N, Oki E, Watanabe M, Oda Y, Bass AJ, Maehara Y, Baba H. Fibroblast growth factor receptor 2 expression, but not its genetic amplification, is associated with tumor growth and worse survival in esophagogastric junction adenocarcinoma. Oncotarget. 2016;7:19748–61. doi: 10.18632/oncotarget.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu T, Liu K, Wu Y, Fan J, Chen J, Li C, Yang Q, Wang Z. MicroRNA-9 inhibits the proliferation of oral squamous cell carcinoma cells by suppressing expression of CXCR4 via the Wnt/beta-catenin signaling pathway. Oncogene. 2014;33:5017–27. doi: 10.1038/onc.2013.448. [DOI] [PubMed] [Google Scholar]
- 18.Jia LF, Huang YP, Zheng YF, Lyu MY, Wei SB, Meng Z, Gan YH. miR-29b suppresses proliferation, migration, and invasion of tongue squamous cell carcinoma through PTEN-AKT signaling pathway by targeting Sp1. Oral Oncol. 2014;50:1062–71. doi: 10.1016/j.oraloncology.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Zhao C, Yu Z, Chen J, She X, Li P, Liu C, Zhang Y, Feng J, Fu H, Wang B, Kuang L, Li L, Lv G, Wu M. Low expression of miR-381 is a favorite prognosis factor and enhances the chemosensitivity of osteosarcoma. Oncotarget. 2016;7:68585–96. doi: 10.18632/oncotarget.11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia B, Li H, Yang S, Liu T, Lou G. MiR-381 inhibits epithelial ovarian cancer malignancy via YY1 suppression. Tumour Biol. 2016;37:9157–67. doi: 10.1007/s13277-016-4805-8. [DOI] [PubMed] [Google Scholar]
- 21.Tang H, Liu X, Wang Z, She X, Zeng X, Deng M, Liao Q, Guo X, Wang R, Li X, Zeng F, Wu M, Li G. Interaction of hsa-miR-381 and glioma suppressor LRRC4 is involved in glioma growth. Brain Res. 2011;1390:21–32. doi: 10.1016/j.brainres.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 22.Katoh Y, Katoh M. FGFR2-related pathogenesis and FGFR2-targeted therapeutics (Review) Int J Mol Med. 2009;23:307–11. doi: 10.3892/ijmm_00000132. [DOI] [PubMed] [Google Scholar]
- 23.Zhao X, Zhou Y, Chen YU, Yu F. miR-494 inhibits ovarian cancer cell proliferation and promotes apoptosis by targeting FGFR2. Oncol Lett. 2016;11:4245–51. doi: 10.3892/ol.2016.4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsimafeyeu I, Khasanova A, Stepanova E, Gordiev M, Khochenkov D, Naumova A, Varlamov I, Snegovoy A, Demidov L. FGFR2 overexpression predicts survival outcome in patients with metastatic papillary renal cell carcinoma. Clin Transl Oncol. 2017;19:265–268. doi: 10.1007/s12094-016-1524-y. [DOI] [PubMed] [Google Scholar]
- 25.Wu XY, Xu H, Wu ZF, Chen C, Liu JY, Wu GN, Yao XQ, Liu FK, Li G, Shen L. Formononetin, a novel FGFR2 inhibitor, potently inhibits angiogenesis and tumor growth in preclinical models. Oncotarget. 2015;6:44563–78. doi: 10.18632/oncotarget.6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu DY, Guo QS, Li YL, Cui B, Guo J, Liu JX, Li P. Twist1 correlates with poor differentiation and progression in gastric adenocarcinoma via elevation of FGFR2 expression. World J Gastroenterol. 2014;20:18306–15. doi: 10.3748/wjg.v20.i48.18306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J, Song KD, Kim JH, Im GH, Yoon S, Namgung M, Hwang JH, Lee JH, Choi D. Characterization of brivanib therapy response in hepatocellular carcinoma xenografts using (1)H HR-MAS spectroscopy and histopathology. Mol Med Rep. 2013;8:1425–31. doi: 10.3892/mmr.2013.1690. [DOI] [PubMed] [Google Scholar]
- 28.Bhide RS, Lombardo LJ, Hunt JT, Cai ZW, Barrish JC, Galbraith S, Jeyaseelan R Sr, Mortillo S, Wautlet BS, Krishnan B, Kukral D, Malone H, Lewin AC, Henley BJ, Fargnoli J. The antiangiogenic activity in xenograft models of brivanib, a dual inhibitor of vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1 kinases. Mol Cancer Ther. 2010;9:369–78. doi: 10.1158/1535-7163.MCT-09-0472. [DOI] [PubMed] [Google Scholar]
- 29.Van TT, Hanibuchi M, Goto H, Kuramoto T, Yukishige S, Kakiuchi S, Sato S, Sakaguchi S, Dat le T, Nishioka Y, Akiyama S, Sone S. SU6668, a multiple tyrosine kinase inhibitor, inhibits progression of human malignant pleural mesothelioma in an orthotopic model. Respirology. 2012;17:984–90. doi: 10.1111/j.1440-1843.2012.02193.x. [DOI] [PubMed] [Google Scholar]


