Abstract
Objective
Prostaglandins (PGs) function in various reproductive processes, including luteolysis, maternal pregnancy recognition, conceptus development, and parturition. Our earlier study has shown that PG transporters ATP-binding cassette, subfamily C, member 4 (ABCC4) and solute carrier organic anion transporter family, member 2A1 (SLCO2A1) are expressed in the uterine endometrium in pigs. Since several other PG transporters such as ABCC1, ABCC9, SLCO4C1, and SLCO5A1 are known to be present in the uterine endometrium, this study investigated the expression of these PG transporters in the porcine uterine endometrium and placenta.
Methods
Uterine endometrial tissues were obtained from gilts on day (D) 12 and D15 of the estrous cycle and days 12, 15, 30, 60, 90, and 114 of pregnancy.
Results
ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNAs were expressed in the uterine endometrium, and levels of expression changed during the estrous cycle and pregnancy. Expression of ABCC1 and ABCC9 mRNAs was localized mainly to luminal and glandular epithelial cells in the uterine endometrium, and chorionic epithelial cells during pregnancy. Conceptuses during early pregnancy and chorioallantoic tissues from mid to late pregnancy also expressed these PG transporters. Estradiol-17β increased the expression of ABCC1 and SLCO5A1, but not ABCC9 and SLCO4C1 mRNAs and increasing doses of interleukin-1β induced the expression of ABCC9, SLCO4C1, and SLCO5A1 mRNAs in endometrial explant tissues.
Conclusion
These data showed that several PG transporters such as ABCC1, ABCC9, SLCO4C1, and SLCO5A1 were expressed at the maternal-conceptus interface, suggesting that these PG transporters may play an important role in the establishment and maintenance of pregnancy by regulating PG transport in the uterine endometrium and placenta in pigs.
Keywords: Pig, Uterus, Endometrium, Prostaglandin, Prostaglandin Transporters
INTRODUCTION
Prostaglandins (PGs) are derived from arachidonic acid, a polyunsaturated fatty acid in the cell membrane phospholipids, by sequential enzymatic actions of cyclooxygenase 1 and 2 and other intracellular enzymes [1]. PGs play key roles in many reproductive processes such as luteolysis, implantation, conceptus development and parturition in many mammalian species [2,3]. Among the various PGs, it has been shown that PGE2 and PGF2α are involved in conceptus implantation in pigs [4,5]. In pigs, the conceptuses undergo dramatic morphological changes from spherical to filamentous form and secrete hormones and cytokines during the implantation period [4]. Estrogen secreted by the elongating conceptus acts as a maternal recognition signal by changing the direction of luteolytic PGF2α secretion from the uterine blood vessel to the uterine lumen. Thus, PGF2α does not move to the ovary, but exerts luteolytic action on the corpus luteum (CL), so that CL can remain and secrete progesterone (P4) for maintenance of pregnancy [4]. It has been shown that PGE2 is produced by the implanting conceptuses and endometrium at the time of implantation [6,7]. PGE2 has a luteotrophic effect in the ovary [5].
Because PGs are lipid-derived hormones bearing negative charge, PGs cannot pass through the plasma membrane easily. Thus, PG transporters are required to mediate the transport of PGs through the plasma membrane [1]. In our earlier study, we have shown that PG transporters such as ATP-binding cassette, subfamily C, member 4 (ABCC4; also known as multidrug resistance protein 4 [MRP4]) and solute carrier organic anion transporter family, member 2A1 (SLCO2A1, also known as PG transporter [PGT] or organic anion-transporting polypeptide 2A1 [OATP2A1]), are expressed in the uterine endometrium during early pregnancy in pigs [8]. ABCC4 and SLCO2A1 mRNAs are expressed in a stage and cell type specific manner in the uterine endometrium during pregnancy, and their expression is increased by interleukin-1β (IL1B) during the implantation period [8]. In addition to ABCC4 and SLCO2A1, many other members of the ABCCs and SLCO family are known to be involved in PG transport through the cell membrane [9]. Among those, we selected, for the microarray and RNA-seq analyses, four PG transporters, ABCC1 (also known as MRP1), ABCC9 (also known as sulfonylurea receptor 2 [SUR2]), SLCO4C1 (also known as OATP4C1), and SLCO5A1 (also known as OATP5A1), because these have been shown to be differentially expressed in the uterine endometrium during early pregnancy [10,11].
ABCC1 expression has been shown in many species, and in human, ABCC1 mRNA is expressed in various tissues including placenta, liver, and kidney [12,13]. ABCC9 is a subunit of ATP-sensitive K+ channel and is expressed in many tissues including myometrium, cardiac and skeletal muscle [14]. SLCO4C1 and SLCO5A1 are also expressed in many tissues including kidney, liver, and brain [15,16]. In addition to transporting PG, these transporters are also involved in the transport of various molecules, including organic anions, amphipathic organic compounds, and drugs [17]. Although these transporters are known to mediate transport of various molecules including PGs in many tissues, expression and function of these PG transporters in the establishment and maintenance of pregnancy have not been determined.
During the implantation period in pigs, the elongating conceptuses produce estrogen and IL1B2. Estrogen acts as a signal for maternal recognition of pregnancy. These conceptus-derived factors induce expression of many endometrial genes [4]. Although it has been shown that expression of ABCC1 gene is induced by estrogen and progesterone in human trophoblast cells [18], regulatory mechanisms of the expression of PG transporter genes, ABCC1, ABCC9, SLCO4C1, and SLCO5A1 are not well understood.
Therefore, to understand the expression and regulation of PG transporters in porcine uterine endometrium during the estrous cycle and pregnancy, we evaluated i) expression of ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNAs in the uterine endometrium during the estrous cycle and pregnancy, in the conceptus of early pregnancy, and in chorioallantoic tissues during pregnancy; ii) localization of ABCC1 and ABCC9 mRNAs in the uterine endometrium; and iii) effects of steroid hormones, IL1B, and interferon gamma (IFNG) on ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNA expression in the endometrial tissues.
MATERIALS AND METHODS
Animals and tissue preparation
All experimental procedures involving animals were conducted in accordance with the Guide for Care and Use of Research Animals in Teaching and Research and approved by the Institutional Animal Care and Use Committee of Yonsei University. Sexually mature crossbred female gilts were assigned randomly to either cyclic or pregnant status. Gilts were observed for estrous behavior daily and artificially inseminated at the onset of estrus (day [D] 0) and 24 h later with fresh boar semen. The reproductive tracts of gilts were obtained immediately after they were slaughtered at a local slaughterhouse on either D 12 or D15 of the estrous cycle and on D12, D15, D30, D60, D90, or D114 of pregnancy (n = 3 to 6 gilts/d/status). Pregnancy was confirmed by the presence of apparently normal filamentous conceptuses in uterine flushings on D12 and D15 and presence of embryos and placenta in the later days of pregnancy. Chorioallantoic tissues were obtained from D30, D60, D90, and D114 of pregnancy (n = 3 to 4/d).
Endometrium, dissected free of myometrium, was collected from the middle portion of each uterine horn, snap-frozen in liquid nitrogen, and stored at −80°C prior to RNA extraction. For in situ hybridization, cross-sections of endometrium were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS; pH 7.4) for 24 h and then embedded in paraffin as previously described [8].
Total RNA extraction and cloning of porcine ABCC1, ABCC9, SLCO4C1, and SLCO5A1 cDNAs
Total RNA was extracted from endometrial, chorioallantoic, and conceptus tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s recommendations. The quantity of RNA was assessed spectrophotometrically, and integrity of RNA was validated by electrophoresis in 1% agarose gel.
Four micrograms each of total RNA from endometrial tissues and 1 μg each from conceptus tissues were treated with DNase I (Promega, Madison, WI, USA) and reverse transcribed using SuperScript II Reverse Transcriptase (Invitrogen, USA) to obtain cDNAs. The cDNA templates were then diluted 1:4 with nuclease-free water and amplified by polymerase chain reaction (PCR) using Taq polymerase (Takara Bio, Shiga, Japan), and specific primers based on porcine ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNA sequences. The PCR conditions, sequences of the primer pairs, and expected product sizes are listed in Table 1. The PCR products were separated on 2% agarose gel and visualized by ethidium bromide staining. The identity of each amplified PCR product was verified by sequence analysis after cloning into the pCRII vector (Invitrogen, USA).
Table 1.
Summary of PCR primer sequences and expected product sizes
| Primer | Sequence of forward (F) and reverse (R) primers (5′→3′) | Annealing temperature (°C) | Product size (bp) | No. of cycles | GenBank accession no. |
|---|---|---|---|---|---|
| For in situ hybridization | |||||
| ABCC1 | F: AGA CCT GGA CCT GGT TCT CC R: CGT CCA GAC CTC TTC CTC TG |
54 | 292 | 40 | XM_005662115.1 |
| ABCC9 | F: ACC TGT TGG CGA TAC CTC AC R: GAC AAG GCA GAG GAA AAT GC |
54 | 216 | 40 | XM_005655658.1 |
| For RT-PCR and/or real-time RT-PCR | |||||
| ABCC1 | F: AGT TTA TTC CCA CTT CAA CGA GAC R: TGC AGT GAG TAA GAC ACT GAG AGG |
60 | 267 | 40 | XM_005662115.1 |
| ABCC9 | F: AAG ATA TCA CCT GGA CAG CTA TGA G R: AGG TTC AGA TTC ATT TAC ATT GCT C |
60 | 274 | 40 | XM_005655658.1 |
| SLCO4C1 | F: TTT ACC AGG TAC AGA AGA AAT TCA AG R: ATT TAG GTA AAA ATG TGG CAA ATC C |
60 | 215 | 40 | XM_003480882.2 |
| SLCO5A1 | F: CTG TTC ATA GTG ACC TTC ATC ACA G R: TAA AAA TAA AGC CAA CGA ATT TGA G |
60 | 295 | 40 | XM_005663031.1 |
| RPL7 | F: AAG CCA AGC ACT ATC ACA AGG AAT ACA R: TGC AAC ACC TTT CTG ACC TTT GG |
60 | 177 | 40 | NM_001113217 |
PCR, polymerase chain reaction; RT-PCR, reverse transcription-PCR; ABCC1, ATP-binding cassette, subfamily C, member 1; ABCC9, ATP-binding cassette, subfamily C, member 9; SLCO4C1, solute carrier organic anion transporter family, member 4C1; SLCO5A1, solute carrier organic anion transporter family, member 5A1; RPL7, ribosomal protein L7.
Quantitative real-time reverse transcription-PCR
The levels of expression of ABCC1, ABCC9, SLCO4C1, and SLCO5A1 genes in endometrial and chorioallantoic tissues were analyzed by real-time RT-PCR using the Applied Biosystems StepOnePlus System (Applied Biosystems, Foster City, CA, USA) and SYBR Green method. cDNAs were synthesized from 4 μg each of the total RNA isolated from different uterine endometrial tissues. Newly synthesized cDNAs (total volume of 21 μL) were diluted 1:4 with sterile water and then used for PCR. The Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) was used for PCR reactions. The final reaction volume of 20 μL included 2 μL of cDNA, 10 μL of 2X Master mix, 2 μL of each primer, and 4 μL of double distilled water. PCR conditions and sequences of primer pairs are listed in Table 1. Results are reported as expression relative to that detected on D12 of the estrous cycle or that detected in control explant tissues after normalization of the transcript amount to the endogenous ribosomal protein L7 (RPL7) control by the 2−ΔΔCT method as previously described [19].
Non-radioactive in situ hybridization
Non-radioactive in situ hybridization was performed to determine the localization of ABCC1 and ABCC9 expression in the uterine endometrium as previously described with some modifications [20]. Sections (5 μm thick) were rehydrated by immersion in successive baths of xylene, 100% ethanol, 95% ethanol, diethylpyrocarbonate (DEPC) treated water, and DEPC treated PBS. Tissue sections were boiled in citrate buffer (pH 6.0) for 10 min. After washing in DEPC treated PBS, they were digested using 5 μg/mL proteinase K (Sigma, St. Louis, MO, USA) in Tris-ethylenediaminetetraacetic acid (EDTA) buffer (100 mM Tris-HCl, 50 mM EDTA, pH 7.5) buffer at 37°C. After post-fixation in 4% paraformaldehyde, tissue sections were incubated twice, for 15 min each, in PBS containing 0.1% active DEPC and equilibrated for 15 min in 5× sodium chloride-sodium citrate (SSC) [21]. The sections were pre-hybridized for 2 h at 68°C in the hybridization mix (50% formamide, 5× SSC, 500 μg/mL herring sperm DNA, 250 μg/mL yeast tRNA). Sense and antisense riboprobes for each gene were generated using partial cDNAs cloned into pCRII vectors by linearizing with appropriate restriction enzymes and labeling with digoxigenin (DIG)-UTP using a DIG RNA Labeling kit (Roche, Indianapolis, IN, USA). The probes were denatured for 5 min at 80°C and added to the hybridization mix. The hybridization reaction was carried out overnight at 68°C. Pre-hybridization and hybridization reactions were performed in a box saturated with 5× SSC-50% formamide solution to avoid evaporation, and no coverslips were used. After hybridization, sections were washed for 30 min in 2× SSC at room temperature, 1 h in 2× SSC at 65°C, and 1 h in 0.1× SSC at 65°C. Probes bound to the sections were detected immunologically, using sheep anti-DIG Fab fragments covalently coupled to alkaline phosphatase and nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate (toluidine salt) as the chromogenic substrate, according to the manufacturer’s protocol (Roche, USA).
Explant cultures
To determine the effect of steroid hormones, estradiol-17β (E2) and P4, and IL1B on the expression of ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNAs in the uterine endometrium, endometrial explant tissues obtained from gilts on D12 of the estrous cycle were cultured as previously described [8]. Endometrium on D12 of the estrous cycle was dissected from the myometrium and placed into warm, phenol red free Dulbecco’s modified Eagle’s medium/F-12 culture medium (DMEM/F-12; Sigma, USA) containing penicillin G (100 IU/mL) and streptomycin (0.1 mg/mL). The endometrium was minced with scalpel into small pieces (2 to 3 mm3), and aliquots of 500 mg were placed into T25 flasks with serum free modified DMEM/F-12 containing 10 μg/mL insulin (Sigma, USA), 10 ng/mL transferrin (Sigma, USA), and 10 ng/mL hydrocortisone (Sigma, USA). Endometrial explants were cultured immediately after mincing in the presence of ethanol (control), E2 (50 ng/mL; Sigma, USA), P4 (3 ng/mL; Sigma, USA), P4+E2, P4+E2+ICI182,780 (ICI; an estrogen receptor antagonist; 200 ng/mL; Tocris Bioscience, Ellisville, MO, USA), or P4+E2+RU486 (RU, a progesterone receptor antagonist; 30 ng/mL; Sigma, USA) for 24 h with shaking in an atmosphere of 5% CO2 in air at 37°C. To determine the effects of IL1B on expression of endometrial genes, explant tissues were treated with 0, 1, 10, or 100 ng/mL IL1B (Sigma, USA) in the presence of both E2 (50 ng/mL) and P4 (3 ng/mL) at 37°C for 24 h. Explant tissues were then collected and total RNA or protein was extracted for real-time RT-PCR and immunoblot analysis, respectively, to determine the expression levels of ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNAs. These experiments were conducted using endometrium on D12 of the estrous cycle, and treatments were performed in triplicate using tissues obtained from three gilts.
Statistical analysis
Data from real-time RT-PCR for ABCC1, ABCC9, SLCO4C1, and SLCO5A1 expression during the estrous cycle and pregnancy were subjected to least squares analysis of variance using the general linear models procedures of SAS (Cary, NC, USA). As sources of variation, the model included day, pregnancy status (cyclic or pregnant, D12 and D15 post-estrus), and their interactions, to evaluate steady-state levels of ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNAs, and effects of treatment and animal, to evaluate effects of steroid hormones on ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNAs. Preplanned orthogonal contrasts (control vs E2; control vs P4; E2 vs E2+P4; E2+P4 vs E2+P4+ICI; and E2+P4 vs E2+P4+RU) were used to test for effects of treatments in the explant cultures. Data from real-time RT-PCR performed to assess the effect of the day of pregnancy (D12, D15, D30, D60, D90, and D114) in the endometrium, effect of the day of pregnancy (D30, D60, D90, and D114) in chorioallantoic tissue, on the expression of ABCC1, ABCC9, SLCO4C1, and SLCO5A1, and data from IL1B dose-response studies were analyzed by least squares regression analysis. Data are presented as least squares means with SEM. A p-value less than 0.05 was considered significant, whereas a p-value of 0.05 to 0.10 was considered a trend toward significance.
RESULTS
Expression of ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNAs in the uterine endometrium during the estrous cycle and pregnancy in pigs
To determine the expression of mRNAs for PG transporters ABCC1, ABCC9, SLCO4C1, and SLCO5A1 in the porcine uterine endometrium during the estrous cycle and pregnancy, we performed real-time RT-PCR analysis (Figure 1). On D12 and D15 post-estrus, expression of ABCC1 mRNA was affected by pregnancy status (p<0.01) and day×status (p<0.05), but not by day (Figure 1A). Levels of ABCC9 and SLCO4C1 mRNAs were affected by pregnancy status (p<0.05), but not by day or day×status (Figure 1B, 1C). Levels of SLCO5A1 mRNA were affected by pregnancy status, day, and day×status (p<0.05) (Figure 1D). Expression of ABCC1 and SLCO5A1 mRNAs was greater on D12 of pregnancy than on D12 of the estrous cycle. Steady-state levels of ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNAs changed during pregnancy with the highest levels on D30 for ABCC1 and ABCC9, on D90 for SLCO4C1, and on D12 for SLCO5A1 (cubic effect of day for ABCC1, p<0.01; linear effect of day for ABCC9, p<0.05; cubic effect of day for SLCO4C1, p<0.05; linear effect of day for SLCO5A1, p = 0.788) (Figure 1A–1D).
Figure 1.
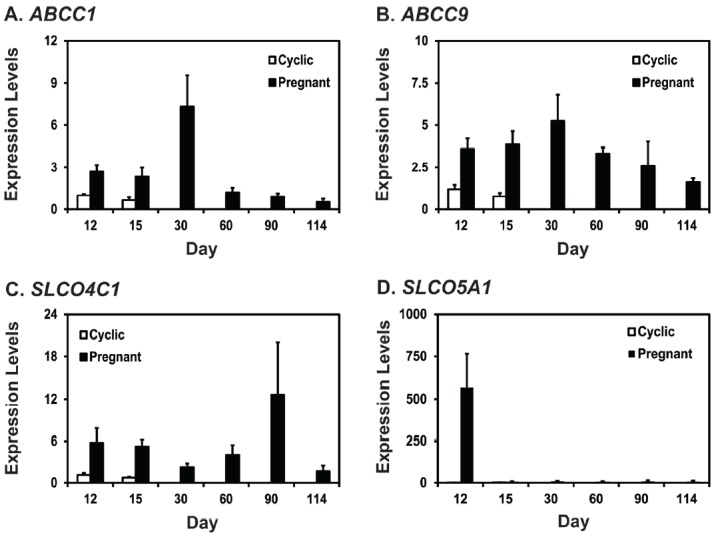
Expression of ABCC1 (A), ABCC9 (B), SLCO4C1 (C), and SLCO5A1 (D) mRNAs in porcine uterine endometria during the estrous cycle and pregnancy. Endometrial tissue samples from cyclic and pregnant gilts were analyzed by real-time RT-PCR and data are reported as expression relative to that detected on day (D) 12 of the estrous cycle after normalization of the transcript amount to the endogenous RPL7 control. Data are presented as least squares means with standard error. ABCC1, ATP-binding cassette, subfamily C, member 1; ABCC9, ATP-binding cassette, subfamily C, member 9; SLCO4C1, solute carrier organic anion transporter family, member 4C1; SLCO5A1, solute carrier organic anion transporter family, member 5A1; RT-PCR, reverse-transcription polymerase chain reaction; RPL7, ribosomal protein L7.
Expression of ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNAs in conceptuses during early pregnancy and in chorioallantoic tissues during late pregnancy
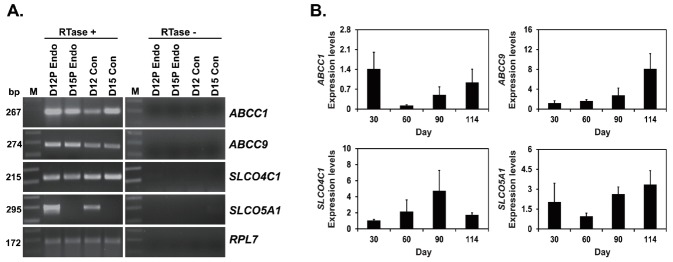
Next, to determine if ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNAs were expressed in conceptuses during early pregnancy, we performed RT-PCR using cDNAs from conceptuses from D12 and D15 of pregnancy. As shown in Figure 2A, ABCC1, ABCC9, and SLCO4C1 mRNAs were detected in conceptus on D12 of pregnancy, and SLCO5A1 mRNA was detected in conceptus on D12, but not on D15 of pregnancy. We also performed real-time RT-PCR to determine if levels of ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNAs change in chorioallantoic tissues during mid to late pregnancy. As shown in Figure 2B, levels of ABCC1 and ABCC9, but not SLCO4C1 and SLCO5A1 mRNAs changed in chorioallantoic tissues on D30, D60, D90, and D114 of pregnancy (quadratic effect of day for ABCC1, p = 0.075; linear effect of day for ABCC9, p<0.05).
Figure 2.
Expression of ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNAs by conceptuses from day 12 and day 15 of pregnancy and by chorioallantoic tissues during the later stages of pregnancy. (A) RT-PCR analysis of ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNAs in conceptuses on day (D) 12 and D15 of pregnancy was done using total RNA preparations. RPL7 was used as a positive control. RTase +/−, with (+) or without (−) reverse transcriptase; M, molecular weight marker; D12 Endo, endometrium on D12 of pregnancy; D12 Con, D12 conceptus; D15 Con, D15 conceptus. (B) Real-time RT-PCR analysis of the expression of ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNAs in chorioallantoic tissue samples on D30, D60, D90, and D114 of pregnancy. Data are reported as expression relative to that detected on D30 of pregnancy after normalization of the transcript amount to the endogenous RPL7 control, and data are presented as least squares means with standard errors. ABCC1, ATP-binding cassette, subfamily C, member 1; ABCC9, ATP-binding cassette, subfamily C, member 9; SLCO4C1, solute carrier organic anion transporter family, member 4C1; SLCO5A1, solute carrier organic anion transporter family, member 5A1; RT-PCR, reverse-transcription polymerase chain reaction; RPL7, ribosomal protein L7.
Localization of ABCC1 and ABCC9 mRNAs in the uterine endometrium during the estrous cycle and pregnancy in pigs
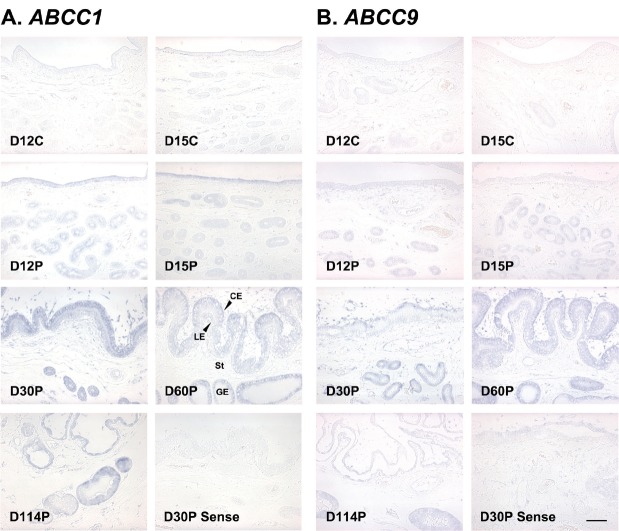
To determine the cell type(s) that express ABCC1 and ABCC9 mRNAs in the uterine endometrium, we performed in situ hybridization analysis. As shown in Figure 3, both ABCC1 and ABCC9 mRNAs were detected mainly in luminal (LE) and glandular epithelial (GE) cells in the endometrium, and also in chorionic epithelial (CE) and stromal cells in the chorioallantoic tissues (Figure 3A, 3B). Localization of SLCO4C1 and SLCO5A1 mRNAs could not be done, because the expression levels of SLCO4C1 and SLCO5A1 mRNAs in the tissues were too low and signal intensity was very weak.
Figure 3.
In situ hybridization analysis of ABCC1 (A) and ABCC9 (B) mRNAs in the uterine endometrium during the estrous cycle and pregnancy in pigs. Representative uterine sections from day 12 of pregnancy, hybridized with a digoxigenin-labeled sense ABCC1 or ABCC9 cRNA probe (sense) as a negative control, are shown. ABCC1, ATP-binding cassette, subfamily C, member 1; ABCC9, ATP-binding cassette, subfamily C, member 9; D, day; C, estrous cycle; P, pregnancy; LE, luminal epithelium; GE, glandular epithelium; St, stroma; CM, chorionic membrane. Bars = 100 μm.
Effects of steroid hormones, E2 and P4, and IL1B on ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNA expression in the uterine endometrium
Since the levels of ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNAs were significantly higher on D12 of pregnancy than those during the estrous cycle and E2 and IL1B from conceptus and/or P4 from corpus luteum (CL) regulate expression of many uterine genes in the endometrium during the peri-implantation period, we examined if E2, P4, and/or IL1B affect the expression of ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNAs in endometrial explant tissues from D12 of the estrous cycle (Figures 4, 5).
Figure 4.
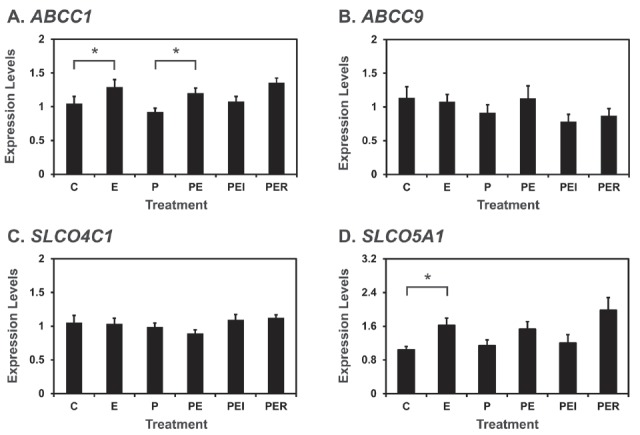
Effect of steroid hormones on the expression of ABCC1 (A), ABCC9 (B), SLCO4C1 (C), and SLCO5A1 (D) mRNAs in endometrial explant cultures. Endometrial explants from gilts on day 12 of the estrous cycle were cultured in DMEM/F-12 in the presence of control (C), E2 (E), P4 (P), E2+P4 (PE), E2+P4+ICI (I, an estrogen receptor antagonist) (PEI), or E2+P4+RU (R, a progesterone receptor antagonist) (PER) at 37°C for 24 h. All the experiments were done in triplicate with endometrium from three gilts for each treatment. Level of mRNA expression based on real-time RT-PCR analysis is relative to that for ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNAs in the control group of endometrial explants after normalization of transcript amounts to RPL7 mRNA. Data are presented as least squares means with standard errors. ABCC1, ATP-binding cassette, subfamily C, member 1; ABCC9, ATP-binding cassette, subfamily C, member 9; SLCO4C1, solute carrier organic anion transporter family, member 4C1; SLCO5A1, solute carrier organic anion transporter family, member 5A1; DMEM/F-12, Dulbecco’s modified Eagle medium/F-12; RT-PCR, reverse-transcription polymerase chain reaction. * p<0.05.
Figure 5.
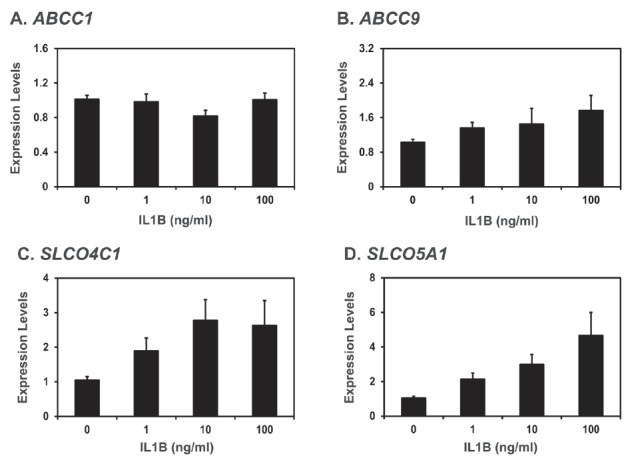
Effect of IL1B on ABCC1 (A), ABCC9 (B), SLCO4C1 (C), and SLCO5A1 (D) mRNA levels in endometrial explant cultures. Endometrial explants from gilts on day 12 of the estrous cycle were cultured in DMEM/F-12 with 0, 1, 10, 100 ng/mL IL1B in the presence of both E2 (50 ng/mL) and P4 (3 ng/mL), at 37°C for 24 h. All experiments were done in triplicate with endometrium from three gilts for each treatment. Level of mRNA expression determined by real-time RT-PCR analysis is relative to that for ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNAs in the control group of endometrial explants (0 ng/mL IL1B) after normalization of transcript amounts to RPL7 mRNA. Data are presented as least squares means with standard errors. IL1B, interleukin-1β; ABCC1, ATP-binding cassette, subfamily C, member 1; ABCC9, ATP-binding cassette, subfamily C, member 9; SLCO4C1, solute carrier organic anion transporter family, member 4C1; SLCO5A1, solute carrier organic anion transporter family, member 5A1; DMEM/F-12, Dulbecco’s modified Eagle medium/F-12; RT-PCR, reverse-transcription polymerase chain reaction; RPL7, ribosomal protein L7.
As shown in Figure 4, ABCC1 and SLCO5A1 mRNA levels were increased by E2 (control vs E2, p<0.05 for ABCC1 and SLCO5A1; P4 vs P4+E2, p<0.05 for ABCC1 and p = 0.064 for SLCO5A1). However, levels of ABCC9 and SLCO5C1 mRNAs were not affected by E2 and P4. As shown in Figure 5, IL1B did not affect the levels of ABCC1 and ABCC9 mRNAs, but increased the levels of SLCO4C1 and SLCO5A1 mRNAs in the endometrial tissues (quadratic effect of dose for SLCO4C1, p<0.05; linear effect of dose for SLCO5A1, p<0.01).
DISCUSSION
The significant findings of this study are: i) ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNAs are expressed in the uterine endometrium in pregnancy status and stage specific manner; ii) ABCC1 and ABCC9 mRNAs are localized mainly to LE, GE, and CE cells during mid to late pregnancy; iii) conceptus tissues on days 12 and 15 of pregnancy and chorioallantoic tissues from day 30 to term pregnancy express ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNAs; iv) E2 increases the expression of ABCC1 and SLCO5A1 mRNAs in the endometrial tissues; and v) IL1B stimulates the expression of SLCO4C1 and SLCO5A1 mRNAs in the endometrial tissues.
The expression patterns of ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNAs during pregnancy varied from one to the other. Levels of ABCC1 and ABCC9 mRNAs were highest on D30 of pregnancy; levels of SLCO4C1 mRNA were highest on D90 of pregnancy; and levels of SLCO5A1 mRNA were highest on D12 of pregnancy. Our previous study has shown that the patterns of ABCC4 and SLCO2A1 expression during pregnancy were biphasic with the highest levels on D12 and D90 of pregnancy [8]. These results suggest that different types of PG transporters function to transport PG in a stage specific manner during pregnancy.
In humans, it has been shown that ABCC1 and ABCC9 are expressed in the amniotic and chorionic membrane [22,23]. However, to our knowledge, expression of SLCO4C1 and SLCO5A1 in placental tissues has not been reported so far. Results of this study showed that conceptus tissues during early pregnancy and chorioallantoic tissues during mid to late pregnancy expressed ABCC1, ABCC9, SLCO4C1, and SLCO5A1 mRNAs, and levels of ABCC1 and ABCC9 mRNAs in chorioallantoic membrane changed during mid to late pregnancy. Interestingly, SLCO5A1 mRNA, which was expressed at the highest level in the uterine endometrium on D12 of pregnancy, was detectable in conceptus tissues on D12 of pregnancy, but not on D15 of pregnancy. Expression of ABCC4 and SLCO2A1 mRNA and protein has also been shown in conceptus tissues and chorioallaltoic tissues [8]. These observations indicate that conceptus tissues during early pregnancy and chorioallantoic tissues during late pregnancy express PG transporters dynamically, depending on the pregnancy stage.
Localization of ABCC1 and ABCC9 expression at the maternal-conceptus interface during pregnancy revealed that the expression of both ABCC1 and ABCC9 mRNAs were detected mainly in endometrial LE and GE cells and in CE and stromal cells in chorioallantoic tissues. ABCC1 and ABCC9 mRNAs were expressed by the same cell types, which express ABCC4 [8]. However, expression of ABCC1 and ABCC9 mRNAs was not detected in endothelial cells of endometrial blood vessels, which express SLCO2A1 [8]. In the porcine uterine endometrium, enzymes that synthesize PG, including PG-endoperoxide synthase 1 (PTGS1), PTGS2, PGE synthase (PTGES), and aldo-keto reductase family 1, member b1 (AKR1B1), are expressed in LE and GE cells in the endometrium and CE cells in chorioallantoic tissues during pregnancy [24,25], indicating that these cells may be the major cell types that produce PGs at the maternal-conceptus interface. Thus, PG transporters expressed by LE, GE, and CE cells may play an important role in trafficking PGs in these cell types during pregnancy. The cell types expressing SLCO4C1 and SLCO5A1 at the maternal-conceptus interface have not yet been determined.
Results of this study showed that levels of ABCC1 and SLCO2A1 mRNA were significantly higher on D12 of pregnancy than the estrous cycle and levels of ABCC9 and SLCO4C1 mRNA were higher during pregnancy than during the estrous cycle. In pigs, conceptus produces estrogen and IL1B2 during the implantation period [4]. The conceptus derived estrogen induces the expression of many uterine endometrial genes, including AKR1B1, fibroblast growth factor 7 (FGF7), interleukin 1 receptor accessory protein (IL1RAP), lysophosphatidic acid receptor 3 (LPAR3), secreted phosphoprotein 1 (SPP1), signal transducer and activator of transcription 1 (STAT1), and transient receptor potential vanilloid type 6 (TRPV6) [25–27]. The IL1B also increases the expression of many endometrial genes, including IL1B receptor genes IL1 receptor 1 (IL1R1) and IL1RAP, PG transporter genes ABCC4 and SLCO2A1, a calcium binding protein gene S100 calcium binding protein G (S100G), and a small lipid binding protein gene salivary lipocalin 1 (SAL1) [8,28]. Thus, we postulated that estrogen and/or IL1B from the conceptus might affect the expression of these PG transporters. Indeed, our explant culture study showed that E2 induced ABCC1 and SLCO5A1 mRNAs, and IL1B increased SLCO4C1 and SLCO5A1 mRNAs in endometrial tissues, indicating that conceptus factors play critical roles in PG synthesis and transport in the uterine endometrium.
Our previous study has also shown that IL1B, but not estrogen, increases the expression of ABCC4 and SLCO2A1 mRNAs, which show higher levels of endometrial expression on D12 of pregnancy than during the estrous cycle in pigs [8]. An especially interesting finding of this study is that mRNAs of PG transporters ABCC1 and SLCO5A1 were increased by estrogen. As mentioned above, estrogen did not increase the expression of ABCC4 and SLCO2A1 mRNAs in our previous study [8], but that observation has raised a question as to how PG movement is regulated in estrogen-induced pseudopregnant pigs. The length of estrous cycle is extended by up to more than 30 days after treatment with estrogen between days 11 and 15 of the estrous cycle [29], and levels of plasma PGF2α in utero-ovarian vein are low in pseudopregnant pigs [30]. Therefore, we postulated that any PG transporter(s) regulated by estrogen other than IL1B should be present and mediate PG movement into the uterine lumen in estrogen-induced pseudopregnancy. The results of the current study clearly showed that PG transporters regulated by estrogen were present in the uterine endometrium, suggesting that these transporters may be involved in the regulation of PG transport in estrogen-induced pseudopregnancy in pigs. Overall, these results indicate that both estrogen and IL1B2 of conceptus origin may differentially regulate the expression of PG transporters in the uterine endometrium, for the establishment and maintenance of pregnancy. Regulatory mechanism of the endometrial ABCC9 expression still needs further study.
In summary, this study showed that i) ABCC1, ABCC9, SLCO4C1, and SLCO5A1 genes are expressed in pregnancy stage and cell type specific manner in the uterine endometrium, ii) their expression during the implantation period is regulated by E2 or IL1B in the endometrial tissues, and iii) conceptuses during early pregnancy and chorioallantoic tissues during mid to late pregnancy also express ABCC1, ABCC9, SLCO4C1, and SLCO5A1. Our findings suggest that ABCC1, ABCC9, SLCO4C1, and SLCO5A1 play an important role in PG transport at the maternal-fetal interface for the establishment and maintenance of pregnancy in pigs.
ACKNOWLEDGMENTS
This study was supported by the Next Generation BioGreen 21 Program (#PJ01119103), Rural Development Administration, Republic of Korea.
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- 1.Schuster VL. Molecular mechanisms of prostaglandin transport. Annu Rev Physiol. 1998;60:221–42. doi: 10.1146/annurev.physiol.60.1.221. [DOI] [PubMed] [Google Scholar]
- 2.Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21:514–50. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- 3.Spencer TE, Bazer FW. Uterine and placental factors regulating conceptus growth in domestic animals. J Anim Sci. 2004;82(E-Suppl):E4–13. doi: 10.2527/2004.8213_supplE4x. [DOI] [PubMed] [Google Scholar]
- 4.Bazer FW, Johnson GA. Pig blastocyst-uterine interactions. Differentiation. 2014;87:52–65. doi: 10.1016/j.diff.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Ziecik AJ. Old, new and the newest concepts of inhibition of luteolysis during early pregnancy in pig. Domest Anim Endocrinol. 2002;23:265–75. doi: 10.1016/s0739-7240(02)00162-5. [DOI] [PubMed] [Google Scholar]
- 6.Geisert RD, Renegar RH, Thatcher WW, Roberts RM, Bazer FW. Establishment of pregnancy in the pig: I. Interrelationships between preimplantation development of the pig blastocyst and uterine endometrial secretions. Biol Reprod. 1982;27:925–39. doi: 10.1095/biolreprod27.4.925. [DOI] [PubMed] [Google Scholar]
- 7.Waclawik A. Novel insights into the mechanisms of pregnancy establishment: regulation of prostaglandin synthesis and signaling in the pig. Reproduction. 2011;142:389–99. doi: 10.1530/REP-11-0033. [DOI] [PubMed] [Google Scholar]
- 8.Seo H, Choi Y, Shim J, Yoo I, Ka H. Prostaglandin transporters ABCC4 and SLCO2A1 in the uterine endometrium and conceptus during pregnancy in pigs. Biol Reprod. 2014;90:100. doi: 10.1095/biolreprod.113.114934. [DOI] [PubMed] [Google Scholar]
- 9.Schuster VL. Prostaglandin transport. Prostaglandins Other Lipid Mediat. 2002;68–69:633–47. doi: 10.1016/s0090-6980(02)00061-8. [DOI] [PubMed] [Google Scholar]
- 10.Kim M, Seo H, Choi Y, et al. Analysis of stage-specific gene expression profiles in the uterine endometrium during pregnancy in pigs. PLoS One. 2015;10:e0143436. doi: 10.1371/journal.pone.0143436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samborski A, Graf A, Krebs S, et al. Transcriptome changes in the porcine endometrium during the preattachment phase. Biol Reprod. 2013;89:134. doi: 10.1095/biolreprod.113.112177. [DOI] [PubMed] [Google Scholar]
- 12.Cole SP. Multidrug resistance protein 1 (MRP1, ABCC1), a “multitasking” ATP-binding cassette (ABC) transporter. J Biol Chem. 2014;289:30880–8. doi: 10.1074/jbc.R114.609248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riches Z, Walia G, Berman JM, Wright TE, Collier AC. ATP-binding cassette proteins BCRP, MRP1 and P-gp expression and localization in the human umbilical cord. Xenobiotica. 2016;46:548–56. doi: 10.3109/00498254.2015.1091118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lovasz N, Ducza E, Gaspar R, Falkay G. Ontogeny of sulfonylurea-binding regulatory subunits of K(ATP) channels in the pregnant rat myometrium. Reproduction. 2011;142:175–81. doi: 10.1530/REP-10-0492. [DOI] [PubMed] [Google Scholar]
- 15.Kuo KL, Zhu H, McNamara PJ, Leggas M. Localization and functional characterization of the rat Oatp4c1 transporter in an in vitro cell system and rat tissues. PLoS One. 2012;7:e39641. doi: 10.1371/journal.pone.0039641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sebastian K, Detro-Dassen S, Rinis N, et al. Characterization of SLCO5A1/OATP5A1, a solute carrier transport protein with non-classical function. PLoS One. 2013;8:e83257. doi: 10.1371/journal.pone.0083257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447:653–65. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- 18.Evseenko DA, Paxton JW, Keelan JA. Independent regulation of apical and basolateral drug transporter expression and function in placental trophoblasts by cytokines, steroids, and growth factors. Drug Metab Dispos. 2007;35:595–601. doi: 10.1124/dmd.106.011478. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Olivier B, Walter W. A simplified in situ hybridization protocol using non-radioactively labeled probes to detect abundant and rare mrnas on tissue sections. Biochemica. 1998;1:10–6. [Google Scholar]
- 21.Olbrich HG, Michaelis H, Vandeplassche G, et al. Ultrastructural calcium distribution and myocardial calcium content in human idiopathic dilated cardiomyopathy. Cardiovasc Pathol. 1993;2:127–36. doi: 10.1016/1054-8807(93)90024-V. [DOI] [PubMed] [Google Scholar]
- 22.Aye IL, Paxton JW, Evseenko DA, Keelan JA. Expression, localisation and activity of ATP binding cassette (ABC) family of drug transporters in human amnion membranes. Placenta. 2007;28:868–77. doi: 10.1016/j.placenta.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Lybaert P, Hoofd C, Guldner D, et al. Detection of K(ATP) channels subunits in human term placental explants and evaluation of their implication in human placental lactogen (hPL) and human chorionic gonadotropin (hCG) release. Placenta. 2013;34:467–73. doi: 10.1016/j.placenta.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Blitek A, Waclawik A, Kaczmarek MM, et al. Expression of cyclooxygenase-1 and -2 in the porcine endometrium during the oestrous cycle and early pregnancy. Reprod Domest Anim. 2006;41:251–7. doi: 10.1111/j.1439-0531.2006.00646.x. [DOI] [PubMed] [Google Scholar]
- 25.Seo H, Choi Y, Shim J, Yoo I, Ka H. Comprehensive analysis of prostaglandin metabolic enzyme expression during pregnancy and the characterization of AKR1B1 as a prostaglandin F synthase at the maternal-conceptus interface in pigs. Biol Reprod. 2014;90:99. doi: 10.1095/biolreprod.113.114926. [DOI] [PubMed] [Google Scholar]
- 26.Johnson GA, Bazer FW, Burghardt RC, et al. Conceptus-uterus interactions in pigs: endometrial gene expression in response to estrogens and interferons from conceptuses. Soc Reprod Fertil Suppl. 2009;66:321–32. [PubMed] [Google Scholar]
- 27.Seo H, Kim M, Choi Y, Lee CK, Ka H. Analysis of lysophosphatidic acid (LPA) receptor and LPA-induced endometrial prostaglandin-endoperoxide synthase 2 expression in the porcine uterus. Endocrinology. 2008;149:6166–75. doi: 10.1210/en.2008-0354. [DOI] [PubMed] [Google Scholar]
- 28.Seo H, Choi Y, Shim J, Choi Y, Ka H. Regulatory mechanism for expression of IL1B receptors in the uterine endometrium and effects of IL1B on prostaglandin synthetic enzymes during the implantation period in pigs. Biol Reprod. 2012:87. doi: 10.1095/biolreprod.112.099051. [DOI] [PubMed] [Google Scholar]
- 29.Geisert RD, Zavy MT, Wettemann RP, Biggers BG. Length of pseudopregnancy and pattern of uterine protein release as influenced by time and duration of oestrogen administration in the pig. J Reprod Fertil. 1987;79:163–72. doi: 10.1530/jrf.0.0790163. [DOI] [PubMed] [Google Scholar]
- 30.Edgerton LA, Kaminski MA, Silvia WJ. Changes in uterine secretion of prostaglandin F2 alpha in response to oxytocin during the estrous cycle, early pregnancy, and estrogen-induced pseudopregnancy in swine. Biol Reprod. 1996;55:657–62. doi: 10.1095/biolreprod55.3.657. [DOI] [PubMed] [Google Scholar]