Abstract
Objective
The current study aimed to investigate the impact of high-fat diets composed of different animal and vegetable fat sources on serum metabolic health markers in Japanese quail, as well as the overall lipid content and fatty acid profiles of the edible bird tissues following significantly increased dietary lipid supplementation.
Methods
Fifty seven male quail were divided into six groups and fed either a standard diet or a diet enriched with one of five different fats (22% coconut oil, lard, palm oil, soybean oil, or sunflower oil) for 12 weeks. The birds were subjected to an oral glucose tolerance test following the feeding period, after which they were euthanized and blood, liver, breast, and thigh muscle samples collected. Total fat content and fatty acid profiles of the tissue samples, as well as serum uric acid, triglyceride, cholesterol, total protein, albumin, aspartate transaminase, and total bilirubin concentrations were assessed.
Results
High-fat diet feeding had no significant effects on the glucose tolerance of the birds. Dietary fatty acid profiles of the added fats were reflected in the lipid profiles of both the liver and breast and thigh muscle tissues, indicating successful transfer of dietary fatty acids to the edible bird tissues. The significantly increased level of lipid inclusion in the diets of the quail used in the present study was unsuccessful in increasing the overall lipid content of the edible bird tissues. Serum metabolic health markers in birds on the high-fat diets were not significantly different from those observed in birds on the standard diet.
Conclusion
Thus, despite the various high-fat diets modifying the fatty acid profile of the birds’ tissues, unlike in most mammals, the birds maintained a normal health status following consumption of the various high-fat diets.
Keywords: Metabolic Health Markers, High-fat Diets, Japanese Quail, Tissue Fatty Acid Profile
INTRODUCTION
The fatty acid profile of poultry meat is influenced by the fatty acid profile of the diet consumed by the birds during rearing, which in turn affects the metabolism and subsequent deposition of ingested lipids [1,2]. Vegetable oils and animal fats are often added to poultry diets as a concentrated source of energy resulting in improved growth performance and efficiency of feed utilization [3,4]. Most studies involving high-fat diet feeding in birds involve the domestic chicken and are focussed on improving meat quality by modification of the fatty acid profile of the edible bird tissues [1,5].
Dietary fat consumption in mammals results in metabolic disturbances, including alterations in glucose tolerance [6,7], dyslipidaemia [8], and hepatic steatosis [7,9], amongst others. No studies to our knowledge have focussed on the impact of high-fat poultry diets on the serum clinical biochemistry, as a measure of the overall health status of alternative poultry species. Research into high-fat diet induced metabolic disturbances in avian species, specifically alternative poultry species such as the Japanese quail, is somewhat limited despite the fact that it could have implications on production costs. The present study investigated the effects of different high-fat diets (composed of animal fat and vegetable oils with different fatty acid profiles) on glucose tolerance and serum uric acid, triglyceride, cholesterol, total protein, albumin, aspartate transaminase (AST), and total bilirubin concentrations as metabolic markers of health in male, Japanese quail. The fatty acid profiles and overall lipid content of the edible bird tissues (liver, breast, and thigh muscles) were also determined to confirm successful uptake of the dietary fats.
The current study made use of male Japanese quail in order to avoid the influence of the female ovarian cycle and related hormones on the results obtained and to eliminate the additional nutrient demands and metabolic costs associated with egg-laying [10]. Since the quail supplier was only able to determine the sex of the birds at four weeks of age, we were unable to start the experiment with the quails at a younger age. However, the rationale for starting the study with the quail at six weeks of age was to allow us to ensure that the total calories ingested by the birds, including the 22% of added dietary lipids, could be utilised for metabolic activities other than tissue growth. Since the quail in the present study were twelve weeks old following the first six weeks of the 12 week feeding period and male Japanese quail have been shown to reach approximately 98% of their mature body mass at twelve weeks of age [11], the quail were likely not in a critical linear growth phase.
MATERIALS AND METHODS
Ethical approval and statement of animal rights
The study was approved by the University of the Witwatersrand Animal Ethics Screening Committee (Ethics clearance number: 2013/02/05). All applicable international, national, and/or institutional guidelines for the care and use of animals were followed when carrying out the present research. All procedures performed in the present study were in accordance with the ethical standards of the University of the Witwatersrand Animal Ethics Screening Committee.
Animals and housing
Fifty seven, four-week old, male Japanese, jumbo quail (Coturnix coturnix japonica) (150.25 g±17.73 g), commercially sourced (SA Quailbreeders, Rockliff Farm, East London, South Africa) were used in the study. The birds were randomly divided into six groups (minimum of n = 8 birds in each group) and allocated to either a standard diet group which received commercial poultry feed (Epol, Centurion, South Africa) or to one of five high-fat diet groups that received the commercial poultry feed enriched with either coconut oil (Organic Cold-pressed Virgin coconut oil, Dis-Chem Pharmacies, Glen Austin, South Africa), lard (Norbert’s German butchery, Wilropark, Roodepoort, South Africa), palm oil (SupaCrisp, Super Olein Palm Oil, Felda Bridge Africa (PTY) Ltd, Johannesburg, South Africa), soyabean oil (d’lite, Willowton Group, Pietermaritzburg, South Africa) or sunflower oil (Sunfoil, Willowton Group, Pietermaritzburg, South Africa) at 22% of the mass of the feed (on a weight/weight basis; we did not take into account the different densities of the added dietary fats). The birds were fed their respective diets ad libitum, with free access to drinking water. All birds were housed individually in cages, with straw for bedding, in the animal unit of the Central Animal Service, University of the Witwatersrand. Lighting was restricted to 12 hours in each 24 hour period.
General experimental procedure
Following a two-week adaptation period, the experimental period commenced and continued for 12 weeks, during which the birds were fed their respective diets. Body mass was measured twice weekly and weight gain was calculated. After the 12 week feeding period, the birds were fasted for approximately 15 hours overnight and subjected to an oral glucose tolerance test (OGTT). Following the OGTT, the birds were re-fed and allowed a 72 h recovery period, after which they were euthanized using an anaesthetic overdose of Pentobarbital (Eutha-naze, Centaur Labs, South Africa) (200 mg/kg), administered via the wing vein. Blood samples (for serum measurements) were collected by cardiac puncture (using 20 G hypodermic needles) into serum separator, clot activator vacutainer tubes (Vacuette, Greiner Bio-One, Amphur Phanthong, Chonburi, Thailand) and gently inverted to ensure mixing. The bird’s livers were carefully excised and excess extraneous tissue was trimmed off. Samples of the liver tissue, from the same lobe of the liver each time, were then rinsed in saline solution, blotted dry, weighed and stored (−70°C) until further analysis. Relative liver mass (represented as a percentage of body mass) was calculated. Samples of breast and thigh muscle tissue were cut from each whole muscle, from each bird and stored (−70°C) until further analysis.
Specific experimental procedures
Oral glucose tolerance test
Following the overnight fast, the birds were subjected to an OGTT. Blood samples were collected via the wing vein and fasting blood glucose concentrations were measured using a glucometer (Ascensia Elite Blood glucose meter, Bayer Corporation, Mishawaka, IN, USA). Thereafter, a single dose of glucose solution (5 g/kg, 50% w/v glucose solution; Sigma-Aldrich, Seelze, Germany) was administered to the birds via orogastric intubation into the crop. Blood glucose concentrations (mmol/L) were then determined (using a glucometer) at fixed time intervals (15, 30, 60, 90, 120, and 180 minutes following glucose administration). Baseline glucose, peak glucose, glucose concentrations three hours following administration of the glucose load and area under the glucose curve (AUC) were then determined.
Serum metabolic/health profile analysis
The blood samples collected into the vacutainer tubes were centrifuged (Sorvall RT 6000 B, Du Pont, United kingdom) at 370×g and 22°C for 15 min. The serum was then collected and serum uric acid, triglyceride, cholesterol, total protein, albumin, AST, and total bilirubin concentrations were determined using an IDEXX Vetlab Analysis Machine (IDEXX Laboratories, Westbrook, ME, USA), according to manufacturer’s instructions.
Diet proximate analysis
The proximate content and energy analysis of the diets were performed at the Agricultural Research Council’s (ARC) Irene Analytical Services Laboratories, South Africa. Samples of each of the six diets were collected at the time of each feed preparation and frozen at −20°C. Upon completion of the feeding trial, the feed samples were pooled (per diet) and mixed. Samples were then sent to the ARC laboratory for analysis. Crude protein and fat content were determined as outlined by the Official Methods of Analysis of Analytical Chemists (2005; method numbers 954.01 and 920.39, respectively) [12]. Gross energy content (MJ/kg) of each diet was determined using an MC-1000 Modular Calorimeter (Energy Instrumentation, 135 Knoppieslaagte, Centurion, South Africa), according to manufacturer’s instructions.
Added dietary fats, liver, and muscle total fat content and fatty acid profile analysis
The total fat content (liver and muscle) and fatty acid profile (added dietary fats, liver, and muscle) analyses were also performed at the ARC, Irene Analytical Services Laboratories, South Africa. Tissue samples from all birds in a specific dietary group were pooled, freeze dried and then milled and analysed as composite, representative sample from each group. Total fat content was determined by the Soxhlet method as described by the Official Methods of Analysis of Analytical Chemists (2005; method number 920.39) [12]. For the fatty acid profile analyses, gas chromatography was used (HP6890 GC, Hewlett Packard, Bristol, UK) and nonadecanoic acid (C19:0) was used as the internal standard. Only the fatty acids that accounted for >1% of the total fatty acids in the respective tissue lipids are reported in the results tables.
Data analysis
All data are expressed as mean (standard deviation), unless otherwise stated. The data were analysed and plotted using Graphpad 5 Prism software (Graph-pad Software Inc, San Diego, CA, USA). A p-value of less than 0.05 was considered significant.
A one-way analysis of variance (ANOVA) with a Bonferroni post-hoc test was used to assess differences in initial and terminal body mass, dietary proximate components, OGTT parameters, serum parameters, and liver mass between birds in the various dietary groups after the 12-week feeding period. Bartlett’s test statistic for equal variance was used to define serum parameters with significantly different variance. The transformation used to correct for the unequal variance was the inverse function. A paired Student’s t-test was used to assess differences between initial and final body mass of the birds within each specific dietary group. Differences in blood glucose concentrations measured during the OGTT within a single dietary group were analysed using a repeated measures ANOVA with a Bonferroni post-hoc test. For the fatty acid profiles and total lipid content of the tissue samples data are presented as percentage of total fatty acids and percentage of tissue sample, respectively. Each assay was performed only once on the composite sample from each of the dietary groups.
RESULTS
Diet proximate analysis and fatty acid profile of added dietary fats
Table 1 shows the proximate content and energy analysis for the standard diet and the five high-fat diets. No significant differences (p>0.05, one-way ANOVA) in crude protein content were observed between the various diets. All diets enriched with fat had a significantly higher (p<0.0001, one-way ANOVA) fat content (ranging between 22.28%±0.70% and 26.13%±0.39% of total diet composition) compared to the standard diet (3.66%±0.08% of total diet composition). The high-fat diets composed of coconut oil, palm oil, soyabean oil, and sunflower oil had a significantly higher (p<0.0001, one-way ANOVA) energy content compared to that of the standard diet. The energy content of the high-fat diet composed of lard was not significantly different (p>0.05, one-way ANOVA) from that of the standard diet.
Table 1.
Proximate content of the standard and high-fat diets fed to male Japanese quail (Coturnix coturnix japonica) for 12 weeks1)
| Proximate component (%) | Diets | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| STD | CO | L | PO | SO | SuO | |
| Protein | 16.03 (0.51) | 11.66 (0.34) | 14.91 (2.64) | 14.61 (0.47) | 11.71 (0.15) | 11.90 (0.13) |
| Fat2) | 3.66 (0.08) | 23.28 (0.79)* | 22.44 (1.07)* | 24.39 (0.49)* | 26.13 (0.39)* | 22.28 (0.70)* |
| Energy (MJ/kg) | 15.85 (0.03) | 19.68 (0.28)** | 19.51 (1.03) | 20.87 (1.42)** | 20.02 (0.76)** | 20.45 (0.20)** |
STD, standard diet; CO, coconut oil; L, lard; PO, palm oil; SO, soyabean oil; SuO, sunflower oil.
Proximate component data presented as mean (standard deviation), n = 2 composite samples of each diet.
Fat was added at 22% on a weight/weight basis.
p<0.0001 when various dietary groups compared to STD (one-way analysis of variance);
p<0.05 when various dietary groups compared to STD (one-way analysis of variance).
Table 2 shows the results of the fatty acid profile analysis (fatty acids represented as a percentage of total fatty acids) for each of the added dietary fats used to formulate the five different high-fat diets. Coconut oil had the highest percentage of saturated fatty acids. Lard and palm oil had similar amounts of saturated, monounsaturated and polyunsaturated fatty acids. The highest percentage of polyunsaturated fatty acids was observed in the soyabean and sunflower oil. Soyabean oil had the lowest ratio of ω-6:ω-3 fatty acids and sunflower oil had the highest ratio. The lowest saturated to unsaturated fatty acid ratio was observed in the sunflower oil whereas coconut oil had the highest ratio.
Table 2.
Fatty acid profiles of dietary fats added to high-fat diets fed to male Japanese quail (Coturnix coturnix japonica) for 12 weeks1)
| Fatty acid | Added dietary fats | ||||
|---|---|---|---|---|---|
|
| |||||
| CO | L | PO | SO | SuO | |
| C8:0 (Caprylic acid) | 5.55 | nd | nd | nd | nd |
| C10:0 (Capric acid) | 6.21 | 0.11 | 0.02 | nd | nd |
| C14:0 (Myristic acid) | 19.47 | 1.52 | 1.07 | 0.08 | 0.06 |
| C16:0 (Palmitic acid) | 8.54 | 27.46 | 37.65 | 10.00 | 5.72 |
| C16:1 (Palmitoleic acid) | 0.01 | 1.77 | 0.20 | 0.08 | 0.06 |
| C18:0 (Stearic acid) | 3.14 | 17.54 | 3.85 | 5.04 | 5.44 |
| C18:1 ω-9 (Oleic acid) | 4.91 | 36.40 | 44.41 | 25.25 | 30.54 |
| C18:2 ω-6 (Linoleic acid) | 0.82 | 11.90 | 11.35 | 52.87 | 55.96 |
| C18:3 ω-3 (Alpha-linolenic acid) | nd | 0.60 | 0.23 | 4.88 | 0.09 |
| C22:0 (Behenic acid) | 0.03 | 0.01 | 0.06 | 0.59 | 1.08 |
| C13:0 (Tridecyclic acid) | 51.07 | nd | nd | nd | nd |
| Saturated FA | 94.16 | 47.69 | 43.56 | 16.58 | 13.12 |
| Monounsaturated FA | 4.97 | 38.90 | 44.76 | 25.57 | 30.79 |
| Polyunsaturated FA | 0.87 | 13.03 | 11.61 | 57.82 | 56.08 |
| Cis FA | 5.73 | 47.91 | 55.69 | 78.08 | 86.48 |
| ω-3 FA | 0.01 | 0.65 | 0.23 | 4.88 | 0.09 |
| ω-6 FA | 0.84 | 12.10 | 11.93 | 52.89 | 55.96 |
| ω-9 FA | 4.92 | 36.41 | 44.41 | 25.25 | 30.55 |
| ω6:ω3 | 59.93:1 | 18.67:1 | 52.56:1 | 10.85:1 | 643.26:1 |
| SFA:UFA | 16.12:1 | 0.92:1 | 0.77:1 | 0.20:1 | 0.15:1 |
CO, coconut oil; L, lard; PO, palm oil; SO, soyabean oil; SuO, sunflower oil; nd, not detected (below detectable threshold); FA, fatty acids; SFA, saturated fatty acids; UFA, unsaturated fatty acids.
Fatty acid data presesnted as a percentage of total fatty acids within the added dietary fats.
Body mass
The initial and final body masses (A), as well as the percentage body mass gains (B) of birds in the various dietary groups are shown in Figure 1. No significant differences in initial body mass (p>0.05, one-way ANOVA) were observed between birds in different dietary groups. Following the 12-week feeding period, birds in the soyabean and sunflower oil groups had a significantly higher (p<0.05, one-way ANOVA) final body mass (Figure 1A) and percentage body mass gain (Figure 1B) than those in the standard diet group. Birds in all five high-fat diet groups displayed a significantly higher (p<0.01, Student’s t-test) final body mass following the 12-week feeding period, compared to their initial body mass (before starting the feeding period) (Figure 1A). The final body mass of birds in the standard diet group however, was not significantly different (p>0.05, Student’s t-test) from their initial body mass.
Figure 1.
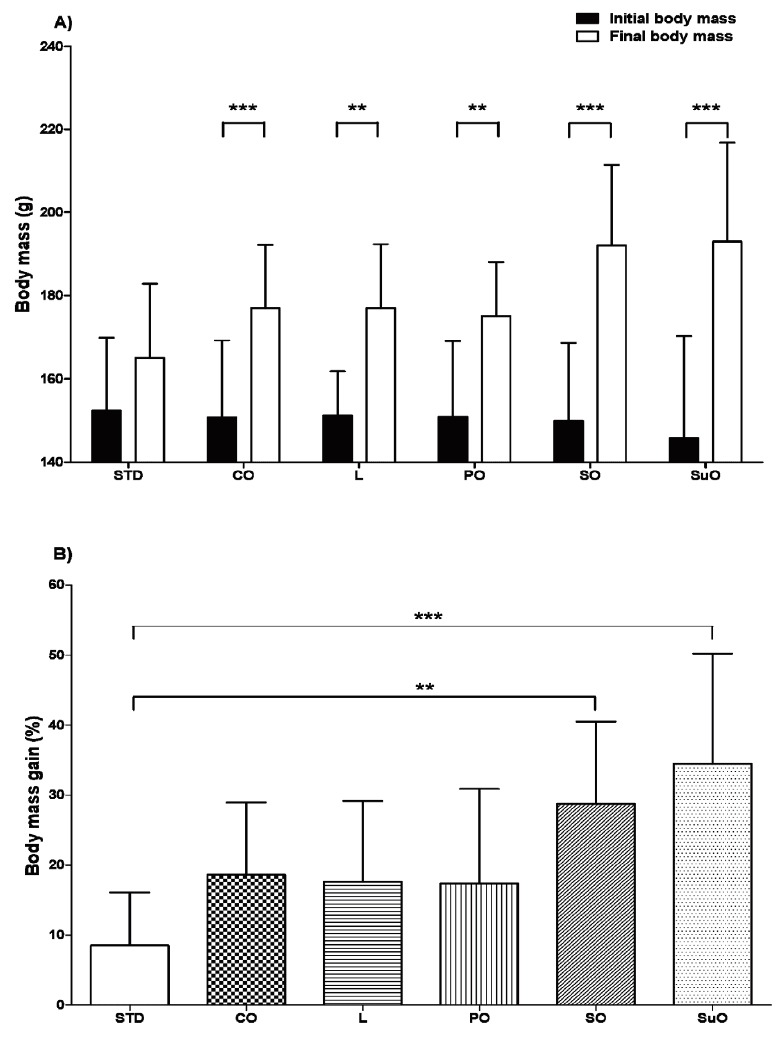
The initial (time: 0 weeks) and final (after 12 weeks of feeding) body masses (A) and percentage body mass gain (B) of male, Japanese quail (Coturnix coturnix japonica) fed either standard, commercial poultry feed (STD, standard diet) or one of five different high-fat diets. 57, four-week old, male Japanese quail were randomly assigned to 6 dietary groups and fed their respective diets for 12 weeks. Group 1 received standard, commercial poultry feed. Groups 2–6 received the standard, commercial poultry feed enriched with 22% added fat (on a weight/weight basis). The added fat was in the form of coconut oil (CO), lard (L), palm oil (PO), soyabean oil (SO), or sunflower oil (SuO). Data represented as means±standard deviation. n = 10 (soyabean oil and palm oil groups); n = 9 (sunflower oil and standard diet groups); n = 11 (coconut oil group); n = 8 (lard group). (A) ** p<0.01 when comparing initial versus final body mass within each individual dietary group. (B) ** p<0.01 and *** p<0.001 when comparing birds in the soyabean and sunflower oil groups, respectively, to the standard diet group.
Oral glucose tolerance test
The glucose tolerance curves (A) and AUC (B) obtained for birds from the various dietary groups, following 12 weeks of feeding, are shown in Figure 2. No significant differences (p>0.05, one-way ANOVA) in baseline blood glucose concentrations, peak blood glucose concentrations, blood glucose concentrations three hours following the glucose load, or in AUC were observed between birds from the different dietary groups. Blood glucose concentrations peaked 15 min following administration of the glucose load and were significantly higher (p<0.05, repeated measures ANOVA) than baseline blood glucose concentrations in all dietary groups. Following administration of the glucose load, blood glucose concentrations returned to normal in all high-fat diet groups at 60 min, unlike in the standard diet group, where blood glucose concentrations returned to normal at 120 min.
Figure 2.
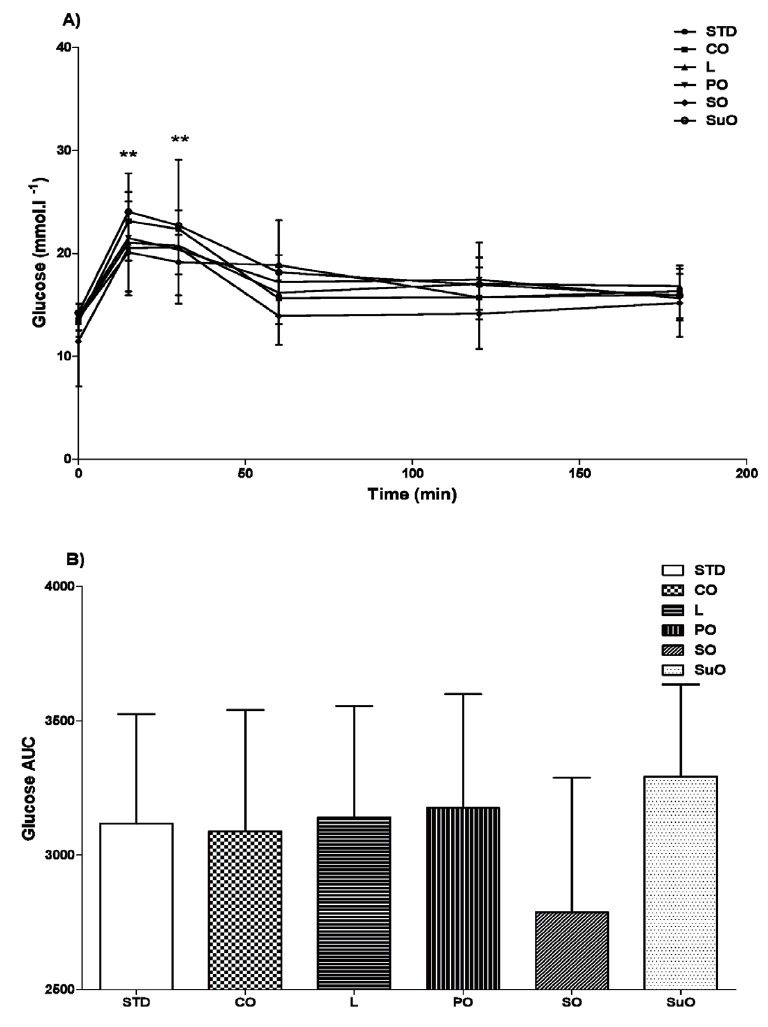
The glucose tolerance test curves (A) and area under the glucose curve (B) of male, Japanese quail (Coturnix coturnix japonica) fed either standard, commercial poultry feed (STD, standard diet) or one of five different high-fat diets. 57, four-week old, male Japanese quail were randomly assigned to 6 dietary groups and fed their respective diets for 12 weeks. Group 1 received standard, commercial poultry feed. Groups 2–6 received the standard, commercial poultry feed enriched with 22% added fat (on a weight/weight basis). The added fat was in the form of coconut oil (CO), lard (L), palm oil (PO), soyabean oil (SO) or sunflower oil (SuO). Data represented as means±standard deviation. n = 10 (soyabean oil and palm oil groups); n = 9 (sunflower oil and standard diet groups); n = 11 (coconut oil group); n = 8 (lard group). ** p<0.05 when comparing peak blood glucose concentrations, following an oral glucose load, to baseline blood glucose concentrations within each individual dietary group.
Serum metabolic markers of health
The serum parameters following the 12 week intervention are shown in Table 3. Serum triglyceride and cholesterol concentration data have been previously published in a corresponding manuscript, based on the same study performed by our lab [13]. No significant differences (p>0.05, one-way ANOVA) in serum uric acid, total protein, albumin, AST, total bilirubin and calcium were observed between the different dietary groups. Birds fed palm oil had significantly lower (p<0.05, one-way ANOVA) serum cholesterol concentrations compared to birds from the standard diet group. Birds from all five high-fat diet groups had significantly lower (p<0.05, one-way ANOVA) serum triglyceride concentrations compared to those in the standard diet group.
Table 3.
Serum parameters (metabolic/health markers) of Japanese quail (Coturnix coturnix japonica) following 12 weeks of either standard or high-fat diet feeding1)2),3)
| Serum parameters | Dietary groups | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| STD | CO | L | PO | SO | SuO | |
| Uric (mg/dL) | 7.3 (1.9) | 7.3 (3.1) | 5.0 (1.4) | 4.6 (1.9) | 6.1 (2.8) | 7.4 (2.9) |
| TPro (g/dL) | 3.1 (0.3) | 3.6 (0.8) | 3.1 (0.5) | 3.2 (0.7) | 3.7 (0.8) | 3.0 (0.4) |
| Alb (g/dL) | 0.6 (0.2) | 0.8 (0.4) | 0.7 (0.4) | 0.7 (0.3) | 1.0 (0.4) | 0.6 (0.2) |
| AST (U/L) | 608.5 (123.1) | 457.4 (160.8) | 494.0 (196.6) | 400.2 (101.3) | 521.0 (207.1) | 408.5 (138.2) |
| TBil (mg/dL) | 0.2 (0.1–0.6) | 0.2 (0.1–1.3) | 0.2 (0.1–0.3) | 0.1 (0.1–0.8) | 0.1 (0.1–0.6) | 0.1 (0.1–0.2) |
| Chol (mg/dL) | 206.0 (179.0–358.5) | 226.0 (201.5–235.5) | 195.0 (186.0–202.3) | 184.5 (156.0–205.0)* | 203.5 (178.0–218.0) | 207.0 (189.0–217.5) |
| Trig (mg/dL) | 238.4 (65.8) | 130.7 (48.0)# | 148.9 (64.7)* | 144.7 (54.7)** | 108.7 (38.0)*** | 120.1 (41.8)*** |
STD, standard diet; CO, coconut oil; L, lard; PO, palm oil; SO, soyabean oil; SuO, sunflower oil; Uric, uric acid; TPro, total protein; Alb, Albumin; AST, aspartate aminotransferase; Tbil, total bilirubin; Chol, cholesterol; Trig, triglycerides.
Cholesterol and triglyceride data published in a previous manuscript, based on the same birds in a study performed by our lab (Donaldson et al [13]).
n = 10 (soyabean oil, palm oil and coconut oil groups); n = 9 (sunflower oil and standard diet groups); n = 7 (lard group).
Data represented as mean (standard deviation) or median (interquartile range).
p<0.05,
p<0.01,
p<0.001 when comparing the various high-fat diet groups to the standard diet group.
Liver and muscle total fat content and fatty acid profile analysis
Liver
The absolute and relative liver mass and total liver lipid yield for birds from each dietary group are shown in Table 4. The dietary interventions did not significantly affect (p>0.05, one-way ANOVA) the absolute liver mass or percentage liver lipid yield. The relative liver mass of birds in the sunflower oil group was significantly lower (p<0.05, ANOVA) than that observed in the standard diet group, following twelve weeks of feeding. No significant differences (p>0.05, ANOVA) in relative liver mass were observed between the remaining dietary groups following twelve weeks of feeding. The highest percentage saturated, monounsaturated and polyunsaturated fatty acids was observed in the livers of birds receiving the coconut oil diet, standard diet and soyabean oil diet, respectively (Table 5). The lowest ratio of ω-6: ω-3 fatty acids was observed in the livers of birds receiving soyabean oil, while the birds receiving sunflower oil had the highest ratio of ω-6:ω-3 fatty acids. The saturated to unsaturated fatty acid ratio was lowest in the livers of the birds receiving soyabean oil, and highest in the livers of the birds receiving coconut oil.
Table 4.
Absolute and relative liver mass and total lipid yield of liver samples from male Japanese quail (Coturnix coturnix japonica) following 12 weeks of either standard or high-fat diet feeding1)
| Dietary groups | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| STD | CO | L | PO | SO | SuO | |
| Absolute liver mass (g) | 3.1 (0.67) | 2.93 (0.59) | 2.94 (0.40) | 3.02 (0.61) | 3.16 (0.88) | 2.51 (0.44) |
| Relative liver mass (%) | 1.87 (0.33) | 1.65 (0.28) | 1.68 (0.33) | 1.72 (0.31) | 1.68 (0.59) | 1.30 (0.24)* |
| Liver lipid yield (% of tissue) | 2.36 | 2.13 | 1.94 | 2.04 | 2.05 | 1.76 |
STD, standard; CO, coconut oil; L, lard; PO, palm oil; SO, soyabean oil; SuO, sunflower oil.
Liver mass data presented as mean (standard deviation). Liver lipid yield data presented as a percentage of total tissue sample.
p<0.05 when comparing the relative liver mass in the STD group to that of the SuO group.
Table 5.
Fatty acid profiles of the liver samples from male Japanese quail (Coturnix coturnix japonica) following 12 weeks of either standard or high-fat diet feeding1)
| Fatty acid | Dietary groups | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| STD | CO | L | PO | SO | SuO | |
| C12:0 (Lauric acid) | 0.10 | 6.33 | 0.14 | 0.11 | 0.08 | 0.09 |
| C14:0 (Myristic acid) | 0.58 | 5.61 | 0.72 | w0.38 | 0.25 | 0.25 |
| C16:0 (Palmitic acid) | 24.90 | 19.57 | 24.06 | 20.35 | 14.41 | 14.13 |
| C16:1 (Palmitoleic acid) | 4.04 | 1.63 | 1.19 | 0.68 | 0.40 | 0.41 |
| C18:0 (Stearic acid) | 13.97 | 17.66 | 16.64 | 16.60 | 18.60 | 20.05 |
| C18:1 ω-9 (Oleic acid) | 31.05 | 18.35 | 26.17 | 29.55 | 14.34 | 15.16 |
| C18:2 ω-6 (Linoleic acid) | 13.51 | 15.45 | 18.64 | 18.71 | 35.56 | 35.71 |
| C18:3 ω-3 (Alpha-linolenic acid) | 0.23 | 0.18 | 0.32 | 0.16 | 1.04 | 0.11 |
| C20:4 ω-6 (Arachidonic acid) | 6.86 | 7.83 | 6.65 | 9.42 | 8.33 | 10.48 |
| C23:0 (Tricosanoic acid) | 0.59 | 2.12 | 0.54 | 0.45 | 0.42 | 0.43 |
| C22:6 ω-3 (Docosahexaenoic acid) | 0.11 | 0.16 | 2.51 | 1.61 | 4.34 | 0.86 |
| Saturated FA | 41.13 | 52.57 | 42.80 | 38.36 | 34.29 | 35.65 |
| Monounsaturated FA | 35.78 | 21.04 | 27.88 | 30.56 | 15.22 | 15.74 |
| Polyunsaturated FA | 22.88 | 26.25 | 29.17 | 31.01 | 50.44 | 48.53 |
| Cis FA | 44.34 | 33.66 | 44.65 | 48.20 | 49.86 | 50.80 |
| ω-3 FA | 1.00 | 1.09 | 3.01 | 1.93 | 5.54 | 1.06 |
| ω-6 FA | 21.27 | 24.41 | 25.86 | 28.97 | 44.52 | 46.94 |
| DHA | 0.11 | 0.16 | 2.51 | 1.61 | 4.34 | 0.86 |
| ω-9 FA | 31.06 | 18.57 | 26.17 | 29.55 | 14.34 | 15.16 |
| ω6:ω3 | 21.27:1 | 22.39:1 | 8.59:1 | 15.01:1 | 8.04:1 | 44.28:1 |
| SFA:UFA | 0.70:1 | 1.11:1 | 0.75:1 | 0.62:1 | 0.52:1 | 0.55:1 |
STD, standard diet; CO, coconut oil; L, lard; PO, palm oil; SO, soyabean oil; SuO, sunflower oil; FA, fatty acids; DHA, docosahexaenoic acid; SFA, saturated fatty acids; UFA, unsaturated fatty acids.
Fatty acid data presented as a percentage of total fatty acids within composite tissue sample.
Breast and thigh muscles
The total lipid yield (as a percentage of total tissue sample) for the breast and thigh muscle samples from each dietary group are shown in Figure 3. The breast muscle of all birds from the high-fat diet groups had a higher percentage lipid yield compared to that of the standard diet group, with those from the coconut oil group being the highest and those from the palm oil group being the lowest. In the thigh muscles of the birds from the various dietary groups, only those from the palm oil and soyabean oil groups had a higher percentage lipid yield compared to the standard diet group. The remaining high-fat diet groups all had a lower percentage lipid yield compared to the standard diet group.
Figure 3.
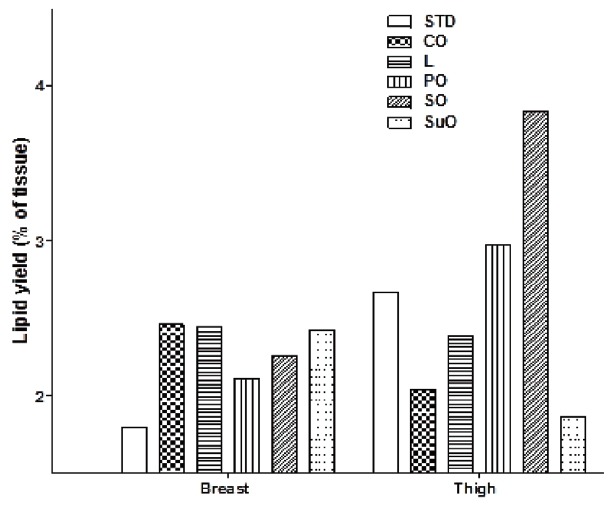
The percentage lipid yield of the breast and thigh muscle tissue of male, Japanese quail (Coturnix coturnix japonica) fed either standard, commercial poultry feed (STD, standard diet) or one of five different high-fat diets. 57, four-week old, male Japanese quail were randomly assigned to 6 dietary groups and fed their respective diets for 12 weeks. Group 1 received standard, commercial poultry feed. Groups 2–6 received the standard, commercial poultry feed enriched with 22% added fat (on a weight/weight basis). The added fat was in the form of coconut oil (CO), lard (L), palm oil (PO), soybean oil (SO), or sunflower oil (SuO). Data represented as means. n = 1 for all dietary groups [liver and muscle samples were collected from each bird, in each specific dietary group (minimum of n = 8 birds in each group) and analysed as a composite (representative sample) from each group].
Table 6 and Table 7 show the fatty acid profile (fatty acids represented as a percentage of total fatty acids) of the breast and thigh muscles, respectively, from birds from the various dietary groups. The highest percentage saturated fatty acids was observed in the birds receiving coconut oil, in both their breast and thigh muscles. The birds receiving palm oil had the highest percentage monounsaturated fatty acids in both their breast and thigh muscles and the highest percentage polyunsaturated fatty acids was observed in birds receiving sunflower oil, in both their breast and thigh muscles. The ratio of ω-6:ω-3 fatty acids was lowest in the breast and thigh muscles of birds receiving lard and highest in the breast and thigh muscles of the birds receiving sunflower oil. The saturated to unsaturated fatty acid ratio was lowest in the breast and thigh muscles of the birds receiving sunflower oil and highest in the breast and thigh muscles of the birds receiving coconut oil.
Table 6.
Fatty acid profiles of the breast muscle samples from male Japanese quail (Coturnix coturnix japonica) following 12 weeks of either standard or high-fat diet feeding1)
| Fatty acid | Dietary groups | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| STD | CO | L | PO | SO | SuO | |
| C12:0 (Lauric acid) | 0.06 | 14.84 | 0.29 | 0.10 | 0.13 | 0.12 |
| C14:0 (Myristic acid) | 0.67 | 10.69 | 0.94 | 0.57 | 0.49 | 0.46 |
| C14:1 (Myristoleic acid) | 0.11 | 1.02 | 0.04 | 0.05 | 0.04 | nd |
| C16:0 (Palmitic acid) | 20.61 | 16.46 | 22.54 | 18.25 | 17.92 | 14.79 |
| C16:1 (Palmitoleic acid) | 7.94 | 4.68 | 2.91 | 2.04 | 1.64 | 1.04 |
| C18:0 (Stearic acid) | 6.72 | 7.44 | 9.55 | 6.73 | 7.92 | 8.06 |
| C18:1 ω-9 (Oleic acid) | 41.89 | 25.24 | 39.39 | 49.34 | 31.46 | 30.58 |
| C18:2 ω-6 (Linoleic acid) | 17.61 | 13.82 | 17.99 | 17.54 | 35.04 | 41.90 |
| C18:3 ω-3 (Alpha-linolenic acid) | 0.44 | 0.33 | 0.45 | 0.24 | 1.37 | 0.25 |
| C20:4 ω-6 (Arachidonic acid) | 2.78 | 3.54 | 2.66 | 3.70 | 2.39 | 1.57 |
| Saturated FA | 28.39 | 50.29 | 34.26 | 25.97 | 27.04 | 23.93 |
| Monounsaturated FA | 50.28 | 31.26 | 43.16 | 51.80 | 31.73 | 30.54 |
| Polyunsaturated FA | 21.17 | 18.33 | 22.46 | 22.23 | 39.51 | 44.09 |
| Trans FA | 0.17 | 0.08 | 0.12 | 0.05 | 1.73 | 1.41 |
| Cis FA | 59.28 | 38.98 | 57.30 | 66.83 | 64.78 | 71.07 |
| ω-3 FA | 0.56 | 0.61 | 1.23 | 0.62 | 1.81 | 0.50 |
| ω-6 FA | 20.56 | 17.60 | 20.86 | 21.42 | 37.57 | 43.60 |
| ω-9 FA | 41.94 | 25.29 | 39.55 | 49.34 | 31.42 | 30.58 |
| ω6:ω3 | 37.00:1 | 28.87:1 | 16.97:1 | 34.77:1 | 20.71:1 | 87.92:1 |
| SFA:UFA | 0.40:1 | 1.01:1 | 0.52:1 | 0.35:1 | 0.38:1 | 0.32:1 |
STD, standard diet; CO, coconut oil; L, lard; PO, palm oil; SO, soyabean oil; SuO, sunflower oil; FA, fatty acids; SFA, saturated fatty acids; UFA, unsaturated fatty acids; nd, not detected (below detectable threshold).
Fatty acid data presented as a percentage of total fatty acids within composite tissue sample.
Table 7.
Fatty acid profiles of the thigh muscle samples from male Japanese quail (Coturnix coturnix japonica) following 12 weeks of either standard or high-fat diet feeding1)
| Fatty acid | Dietary groups | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| STD | CO | L | PO | SO | SuO | |
| C12:0 (Lauric acid) | 0.26 | 37.06 | 0.25 | 2.36 | 0.08 | 0.11 |
| C14:0 (Myristic acid) | 0.75 | 14.90 | 1.21 | 1.48 | 0.29 | 0.27 |
| C16:0 (Palmitic acid) | 19.51 | 11.13 | 26.61 | 18.08 | 10.97 | 9.20 |
| C16:1 (Palmitoleic acid) | 6.29 | 2.65 | 2.05 | 1.28 | 0.89 | 0.54 |
| C18:0 (Stearic acid) | 4.83 | 3.63 | 7.95 | 3.60 | 4.49 | 5.99 |
| C18:1 ω-9 (Oleic acid) | 40.79 | 16.57 | 39.62 | 52.22 | 29.24 | 29.89 |
| C18:2 ω-6 (Linoleic acid) | 25.02 | 10.64 | 19.00 | 19.19 | 50.21 | 51.93 |
| C18:3 ω-3 (Alpha-linolenic acid) | 0.64 | 0.25 | 0.50 | 0.24 | 2.06 | 0.21 |
| Saturated FA | 25.69 | 67.75 | 36.70 | 25.86 | 16.32 | 16.10 |
| Monounsaturated FA | 47.49 | 20.29 | 40.42 | 53.84 | 30.47 | 30.70 |
| Polyunsaturated FA | 26.67 | 11.86 | 21.00 | 20.27 | 53.16 | 53.21 |
| Trans FA | 0.15 | 0.05 | 1.88 | 0.03 | 0.08 | 0.05 |
| Cis FA | 65.66 | 27.16 | 56.70 | 71.38 | 79.43 | 81.77 |
| ω-3 FA | 0.75 | 0.34 | 0.88 | 0.34 | 2.22 | 0.27 |
| ω-6 FA | 25.88 | 15.88 | 20.00 | 19.90 | 50.84 | 52.83 |
| ω-9 FA | 40.79 | 16.57 | 39.62 | 52.22 | 29.27 | 29.89 |
| ω6:ω3 | 34.55:1 | 46.29:1 | 22.76:1 | 59.10:1 | 2.91:1 | 197.60:1 |
| SFA:UFA | 0.35:1 | 2.11:1 | 0.60:1 | 0.35:1 | 0.20:1 | 0.19:1 |
STD, standard diet; CO, coconut oil; L, lard; PO, palm oil; SO, soyabean oil; SuO, sunflower oil; FA, fatty acids; SFA, saturated fatty acids; UFA, unsaturated fatty acids.
Fatty acid data presented as a percentage of total fatty acids within composite tissue sample.
DISCUSSION
The present study investigated the effects of feeding male, Japanese quail high-fat diets formulated with coconut oil, lard, palm oil, soyabean oil, or sunflower oil (added dietary fat at 22% of the mass of the feed) on the overall lipid content and fatty acid profiles of the liver and breast and thigh muscle tissues, as well as on the body mass, glucose tolerance and various serum metabolic markers of health. The majority of studies involving high-fat diet feeding in poultry are focussed on improving the fatty acid profile of the animal products for human consumption, without investigating the health status of the birds during production. The present study is unique in the fact that these parameters were examined (in conjunction with the tissue fatty acid profile analyses) following the high-fat diet feeding, as a means of assessing any possible deleterious health-related consequences, which could ultimately affect poultry production costs.
Generally, the lipid profiles of the various high-fat diets used in the present study were mirrored in the fatty acid profiles of the bird tissues. Thus, the livers, breast and thigh muscles of birds fed coconut oil and those fed palm oil contained the highest percentage of saturated and monounsaturated fatty acids, respectively. Whilst the tissues of birds receiving soyabean and sunflower oil, contained the highest percentage of polyunsaturated fatty acids, which also mirrored the fatty acid profiles of these oils. This study is in agreement with previous studies in the Japanese quail which have also demonstrated that the fatty acid profile of the diet is to a large extent reflected in the bird tissues [14]. Thus, the enrichment of animal-derived food products (poultry included) with beneficial polyunsaturated fatty acids (specifically ω-3), through dietary modification, is a popular approach in substantially increasing the dietary intake of ω-3 polyunsaturated fatty acids in humans [15]. Fatty acids provided to humans through the enrichment of animal products (i.e. poultry meat) are more stable to the effects of processing than if the fats and oils were added to the food products during the manufacturing process [16].
Avian breast muscles are generally characterised by a lower lipid content compared to that of the thigh muscles. In the present study, birds fed the standard diet, as well as those fed diets formulated with palm oil and soybean oil displayed lower lipid content in their breast muscles compared to that in their thigh muscles. Genchev et al [17] observed a similar trend in both Faraon and White English breeds of Japanese quail, where both breeds displayed lower lipid content in the breast muscles (3.27% and 1.58%, respectively) compared to the thigh muscles (5.43% and 3.86%, respectively). The quail were fed standardised starter (0 to 17 days old) and finisher (18 to 31 days old) diets with slightly lower energy content and higher protein content [17] than the standard diet used in the present study. Vitula et al [18] also observed lower lipid content in breast muscles (130.64±50.41 g/kg) versus thigh muscles (299.60±69.46 g/kg) of Japanese quail. In the present study with dietary coconut oil, lard and sunflower oil, a different pattern of lipid deposition was observed. The mechanisms involved in the preferential deposition of dietary lipids either in the breast or thigh muscles of Japanese quail needs to be further investigated. To assess the effects of the high fat diets on the metabolic health of the quail, we deliberately used diets with a much higher fat content than that used by previous investigators. Interestingly, despite the high-fat content of the diets, the breast and thigh muscles of the Japanese quail from all high-fat diet groups in the present study, had an average (±SD) lipid yield of 2.34%± 0.15% and 2.62%±0.80%, respectively, which is comparable to previous studies in which the lipid yield of quail breast and thigh muscles ranged between 1.0% and 3.4%, in birds that were fed a much lower percentage of dietary fat [19].
The high-fat diet feeding in the present study significantly increased the body mass of the quail over the 12-week feeding period, however, no significant differences in percentage body mass gain or final body mass measurements were observed between birds in the various high-fat diet groups. When comparing percentage body mass gain between the high-fat diet groups to that of the standard diet group, only the birds receiving soyabean oil and those receiving sunflower oil had a significantly increased body mass gain compared to the standard diet group. It was noted however that the birds receiving the soyabean and sunflower oils had large amounts of feed/oil stuck to their feathers upon termination which could have accounted for the differences in percentage body mass gain observed. The dressed mass/carcass mass of the birds upon termination should be used in future studies, in order to allow for accurate comparison. In agreement with the results of the current study, Liu et al [20] also observed no significant differences in final body mass of Japanese quail fed diets containing 50 g/kg of soyabean oil, hydrogenated soyabean oil, chicken fat, or menhaden fish oil, for a period of 28 weeks. Previous studies in broilers have also yielded similar results and have shown that as long as the ratios of energy to protein within the formulated high-fat diets are balanced, the type of dietary fat has no influence on weight gain or final body mass [21], even though the fatty acid profiles of the diets are mirrored in the body tissues.
Majority of the high-fat diets in the present study also had no significant effect on the absolute and relative liver masses of the birds in the various dietary groups, however the relative liver mass of birds in the sunflower oil group was significantly lower than that observed in the standard diet group, following twelve weeks of feeding. The reduced relative liver mass observed in the sunflower oil group could be due to the significantly greater percentage body mass gain observed in these birds compared to that of the standard diet group, thus resulting in a significantly lower relative liver mass compared to that of the standard diet group. Al Daraji et al [22] observed significantly increased relative liver weights of Japanese quail fed diets supplemented with fish oil or flax seed oil (3% inclusion level) compared to those receiving diets supplemented with sunflower oil or corn oil (3% inclusion level). The differences in relative liver weights were accredited to the high levels of ω-3 fatty acids and low ω-6:ω-3 fatty acid ratios of the fish and flax seed oils [22], which has been shown to improve productive performance of the quails [14]. Even with the large variations in ω-6:ω-3 fatty acid ratios of the added dietary fats used in the present study, majority of the absolute and relative liver masses were not different between dietary groups. In contrast, previous studies observed a decrease in liver size with increasing levels of dietary fat in broiler chickens supplemented with varying levels of lard [23,24]. The decrease in liver size of the chickens receiving supplementary dietary fat was attributed to a decrease in hepatic lipase activity as a result of the increased proportion of fat calories compared to glucose calories available to the chickens [23,24].
High-fat diet feeding has also been associated with impaired glucose tolerance and insulin resistance, which results due to changes in the relationship between insulin sensitivity and pancreatic β-cell function [6]. Increased fasting blood glucose and insulin levels, as well as increased glucose and insulin area under the curve values, observed following an OGTT, are common signs of impaired glucose tolerance [6]. In the present study, none of the diagnostic signs of impaired glucose tolerance were observed, following consumption of the various high-fat diets for 12 weeks. The fatty acid profile of ingested fat is a powerful modulator of insulin action, with both fatty acid chain length and degree of saturation modifying insulin release [25]. Dietary intake of saturated fatty acids impairs insulin action, whereas insulin action is improved following the intake of ω-3 polyunsaturated fatty acids [25,26]. Studies in humans have shown that increased intake of saturated fatty acids is positively associated with degree of insulin resistance, whereas the intake of polyunsaturated fatty acids is associated with improved insulin sensitivity/resistance and glucose tolerance [27]. Our results are not in agreement with these studies as even with the large variations in fatty acids of the added dietary fats used in the present study, no significant differences were observed in any of the parameters of glucose tolerance assessed, between the different high-fat diet groups. However, blood glucose concentrations in all high-fat diet groups returned to normal levels a lot sooner than that observed in the standard diet group, following administration of the glucose load. Thus, the intake of additional polyunsaturated fatty acids (over and above that present in the standard diet), even in relatively small quantities in the high-fat diets which were composed predominantly of saturated fatty acids, seems to be related to improved insulin sensitivity and thus glucose clearance from the blood, which is in agreement with that observed by Lovejoy et al [27] in humans. Thus, the Japanese quail were able to tolerate the increased dietary fat of differing levels of saturated and unsaturated fatty acids, such that their glucose tolerance was unaffected.
The effects of the high-fat diet feeding on the overall health status (specifically kidney and liver function) of the birds were also evaluated by assessing serum parameters, including uric acid, as well as parameters which form part of routinely performed liver function tests such as total protein, albumin, AST, and total bilirubin. In birds, uric acid is the primary product formed from the metabolic catabolism of amino acids and purines [28]. Measurement of serum uric acid concentrations in birds is reflective of the intake of protein, the excretion of nitrogen, as well as antioxidant function within the bird [29]. Clinically, serum uric acid concentrations are used to assess avian renal function, with hyperuricemia (elevated serum uric acid levels) often being associated with renal disease [28]. In the present study, no significant differences in serum uric acid were observed following the high-fat diet feeding. Serum concentrations of albumin, total protein, AST, and total bilirubin are often measured, amongst others, as they reflect different functions of the liver, including the synthesis of proteins (serum albumin and total protein), the excretion of anions and formation of bile (serum bilirubin), as well as the actual integrity of the hepatocytes themselves (serum AST) [30]. No significant differences in the aforementioned serum parameters were observed between birds receiving the various diets. Thus, the high-fat diet feeding did not impair liver function of the Japanese quails.
Serum concentrations of triglycerides and cholesterol are assessed as a means of evaluating the lipidaemic status of humans and animals. The effects of various high-fat diets on serum lipid parameters in mammals and birds are somewhat contradictory. In general, high-fat diet feeding has been shown to result in significantly increased serum triglyceride and cholesterol concentrations in mammals and birds alike [31,32]. However, in the present study, the high-fat diet feeding resulted in significantly lower serum triglyceride concentrations in all high-fat diet groups compared to that observed in the standard diet group. As discussed in a previous, corresponding publication by our lab [13], the high-fat diet-induced reduction in serum triglycerides was attributed to a possible reduction in hepatic de novo fatty acid synthesis, as a large number of fatty acids were being supplied to the birds via the various high-fat diets. Peebles et al [33] also observed significantly lower serum triglyceride concentrations in broiler chickens fed a diet supplemented with 7% lard compared to that observed in control chickens, which was attributed to a metabolic over-compensation by the birds in response to the high-fat diet. In contrast, in a previous study by our lab [34], no significant differences in serum triglyceride levels were observed in Guinea fowl and Muscovy duck fed a high-fat diet composed of 20% palm oil and 2% lard, compared to birds receiving the standard diet. Thus, there seems to be some inter-avian species disparity in lipid metabolism, which needs to be further investigated. The lack of consistency between results from various studies involving the effects of high-fat diet feeding on serum cholesterol concentrations in birds also suggests some variation in lipid handling and cholesterol biosynthesis pathways between different avian species. In the present study, birds fed the high-fat diet supplemented with 22% palm oil displayed significantly lower serum cholesterol levels compared to birds receiving the other high-fat diets, as well as those receiving the standard diet. This is not in agreement with the previous study from our lab [34] in which significantly increased serum cholesterol concentrations were observed, in both Guinea fowl and Muscovy duck fed a high-fat diet composed of 20% palm oil and 2% lard, compared to birds receiving the standard diet. Monfaredi et al [35] also observed significantly increased serum cholesterol concentrations in broiler chickens, with increasing levels of dietary inclusion of soyabean oil and tallow.
From the data obtained in the present study, we were able to conclude that dietary supplementation of the various fats, including those high in saturated fats, was well-tolerated by the quail and did not have any negative influences on the growth performance of the birds. The fatty acid profile, ω-6:ω-3 polyunsaturated fatty acid ratios, as well as the proportions of saturated to unsaturated fatty acids of the liver, breast and thigh muscle tissues of the Japanese quail were reflective of the added dietary fats consumed by the quail during the study period. The significantly increased level of dietary lipid inclusion did not affect the overall lipid content deposited in the edible bird tissues. We also confirmed the notable differences in lipid content between the breast versus thigh muscles of the Japanese quail, which could have health consequences in terms of human consumption of the different cuts of poultry meat. More importantly, no adverse effects of the high-fat diet feeding, in terms of the health status of the birds, were observed.
ACKNOWLEDGMENTS
This work was supported by grants from the National Research Foundation, Oppenheimer Memorial Trust Fund and Health Sciences Faculty Research Committee of the University of the Witwatersrand. The authors would like to thank Busisani Lembede, Eliton Chivandi and Karmel Pillay for their technical assistance.
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- 1.Crespo N, Esteve-Garcia E. Dietary fatty acid profile modifies abdominal fat deposition in broiler chickens. Poult Sci. 2001;80:71–8. doi: 10.1093/ps/80.1.71. [DOI] [PubMed] [Google Scholar]
- 2.Jankowski J, Zdunczyk Z, Mikulski D, et al. Fatty acid profile, oxidative stability, and sensory properties of breast meat from turkeys fed diets with a different n-6/n-3 PUFA ratio. Eur J Lipid Sci Technol. 2012;114:1025–35. [Google Scholar]
- 3.Wu H, Gong LM, Guo L, Zhang LY, Li JT. Effects of the free fatty acid content in yellow grease on performance, carcass characteristics, and serum lipids in broilers. Poult Sci. 2011;90:1992–8. doi: 10.3382/ps.2010-01298. [DOI] [PubMed] [Google Scholar]
- 4.Zhang B, Haitao L, Zhao D, Guo Y, Barri A. Effect of fat type and lysophosphatidylcholine addition to broiler diets on performance, apparent digestibility of fatty acids, and apparent metabolizable energy content. Anim Feed Sci Technol. 2011;163:177–84. [Google Scholar]
- 5.Velasco S, Ortiz LT, Alzueta C, et al. Effect of inulin supplementation and dietary fat source on performance, blood serum metabolites, liver lipids, abdominal fat deposition, and tissue fatty acid composition in broiler chickens. Poult Sci. 2010;89:1651–62. doi: 10.3382/ps.2010-00687. [DOI] [PubMed] [Google Scholar]
- 6.Honors MA, Hargrave SL, Kinzig KP. Glucose tolerance in response to a high-fat diet is improved by a high-protein diet. Obesity. 2012;20:1859–65. doi: 10.1038/oby.2011.297. [DOI] [PubMed] [Google Scholar]
- 7.Fraulob JC, Ogg-Diamantino R, Fernandes-Santos C, Aguila MB, Mandarim-de-Lacerda CA. A mouse model of metabolic syndrome: Insulin resistance, fatty liver and non-alcoholic fatty pancreas disease (NAFPD) in C57BL/6 mice fed a high fat diet. J Clin Biochem Nutr. 2010;46:212–23. doi: 10.3164/jcbn.09-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan NI, Mahboob T. Antioxidant, hepatoprotective and antiatherogenic effects of curcumin on high fat diet induced dyslipidemia in rats. Pak J Nutr. 2014;13:537–45. [Google Scholar]
- 9.Gauthier MS, Favier R, Lavoie JM. Time course of the development of non-alcoholic hepatic steatosis in response to high-fat diet-induced obesity in rats. Br J Nutr. 2006;95:273–81. doi: 10.1079/bjn20051635. [DOI] [PubMed] [Google Scholar]
- 10.Williams TD. Mechanisms underlying the costs of egg production. BioScience. 2005;55:39–48. [Google Scholar]
- 11.Raji A, Alade N, Duwa H. Estimation of model parameters of the Japanese quail growth curve using Gompertz model. Arch Zootec. 2014;63:429–35. [Google Scholar]
- 12.AOAC. Official methods of analysis of AOAC International. 18th ed. Gaithersburg, MD: AOAC International; 2005. [Google Scholar]
- 13.Donaldson J, Pillay K, Madziva MT, Erlwanger KH. The effect of different high-fat diets on erythrocyte osmotic fragility, growth performance and serum lipid concentrations in male, Japanese quail (Coturnix coturnix japonica) J Anim Physiol Anim Nutr. 2015;99:281–9. doi: 10.1111/jpn.12250. [DOI] [PubMed] [Google Scholar]
- 14.Dalton MN. master’s thesis. Blacksburg VA: Virginia Polytechnic Institute; 2000. Effects of dietary fats on reproductive performance, egg quality, fatty acid composition of tissue and yolk and prostaglandin levels of embryonic tissues in Japanese quail (Coturnix coturnix japonica) [Google Scholar]
- 15.Zduńczyk Z, Jankowski J. Poultry meat as functional food: Modification of the fatty acid profile – A review. Ann Anim Sci. 2013;13:463–80. [Google Scholar]
- 16.Williams CM. Dietary fatty acids and human health. Ann Zootech. 2000;49:165–80. [Google Scholar]
- 17.Genchev G, Ribarski S, Afanasjev D, Blohin I. Fattening capacities and meat quality of Japanese quails of Faraon and White English breeds. J Cent Eur Agric. 2005;6:495–500. [Google Scholar]
- 18.Vitula F, Suchý P, Straková E, et al. Energy value of meat in selected species of feathered game. Acta Vet Brno. 2011;80:197–202. [Google Scholar]
- 19.Ribarski S, Genchev A. Effect of breed on meat quality in Japanese quails (Coturnix coturnix japonica) Trakia J Sci. 2013;11:181–8. [Google Scholar]
- 20.Liu D, Veit HP, Wilson JH, Denbow DM. Long-term supplementation of various dietary lipids alters bone mineral content, mechanical properties and histological characteristics of Japanese quail. Poult Sci. 2003;82:831–9. doi: 10.1093/ps/82.5.831. [DOI] [PubMed] [Google Scholar]
- 21.Sanz M, Flores A, Lopez-Bote CJ. The metabolic use of energy from dietary fat in broilers is affected by fatty acid saturation. Br Poult Sci. 2000;41:61–8. doi: 10.1080/00071660086411. [DOI] [PubMed] [Google Scholar]
- 22.Al Daraji HJ, Al Mashadani HA, Mirza HA, Al Hayani WK, Al Hassani A. Effect of feeds containing different fats on certain carcass parameters of Japanese quail. ARPN J Agric Biol Sci. 2011;6:6–11. [Google Scholar]
- 23.Latour MA, Peebles ED, Boyle CR, Brake JD. The effects of dietary fat on growth performance, carcass composition, and feed efficiency in the broiler chick. Poult Sci. 1994;73:1362–9. doi: 10.3382/ps.0731362. [DOI] [PubMed] [Google Scholar]
- 24.Peebles FD, Cheaney JD, Brake JD, Boyle CR, Latour MA. Effects of added dietary lard on body weight and serum glucose and low density lipoprotein cholesterol in randombred broiler chickens. Poult Sci. 1997;76:29–36. doi: 10.1093/ps/76.1.29. [DOI] [PubMed] [Google Scholar]
- 25.Newman RE, Bryden WL, Kirby AC, Storlien LH, Downing JA. Dietary n-3 and n-6 fatty acids alter avian glucose metabolism. Br Poult Sci. 2005;46:104–13. doi: 10.1080/00071660400023987. [DOI] [PubMed] [Google Scholar]
- 26.Riccardi G, Giacco R, Rivellese AA. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr. 2004;23:447–56. doi: 10.1016/j.clnu.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Lovejoy JC. The influence of dietary fat on insulin resistance. Curr Diab Rep. 2002;2:435–40. doi: 10.1007/s11892-002-0098-y. [DOI] [PubMed] [Google Scholar]
- 28.Kolmstetter CM, Ramsay EC. Effects of feeding on plasma uric acid and urea concentrations in Blackfooted Penguins (Spheniscus demersus) J Avian Med Surg. 2000;14:177–9. [Google Scholar]
- 29.Wright PA. Nitrogen excretion: three end products, many physiological roles. J Exp Biol. 1995;198:273–81. doi: 10.1242/jeb.198.2.273. [DOI] [PubMed] [Google Scholar]
- 30.Limdi JK, Hyde GM. Evaluation of abnormal liver function tests. Postgrad Med J. 2003;79:307–12. doi: 10.1136/pmj.79.932.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayala I, Castillo AM, Adánez G, et al. Hyperlipidemic chicken as a model of non-alcoholic steatohepatitis. Exp Biol Med. 2009;234:10–6. doi: 10.3181/0807-RM-219. [DOI] [PubMed] [Google Scholar]
- 32.Kim HJ, Kim JH, Noh S, et al. Metabolomic analysis of livers and serum from high-fat diet induced obese mice. J Proteome Res. 2011;10:722–31. doi: 10.1021/pr100892r. [DOI] [PubMed] [Google Scholar]
- 33.Peebles ED, Cheaney JD, Brake JD, et al. Effects of added lard fed to broiler chickens during the starter phase. 2. Serum lipids. Poult Sci. 1997;76:1648–54. doi: 10.1093/ps/76.12.1648. [DOI] [PubMed] [Google Scholar]
- 34.Donaldson J, Dangarembizi R, Mtetwa B, Madziva MT, Erlwanger KH. The progressive effects of a high-fat diet on erythrocyte osmotic fragility, growth performance and serum triglyceride and cholesterol levels in Guinea fowl (Numida meleagris) and Muscovy duck (Cairina moschata) J Anim Physiol Anim Nutr. 2014;98:867–74. doi: 10.1111/jpn.12149. [DOI] [PubMed] [Google Scholar]
- 35.Monfaredi A, Rezaei M, Sayyahzadeh H. Effect of supplemental fat in low energy diets on some blood parameters and carcass characteristics of broiler chicks. South Afr J Anim Sci. 2011;41:24–32. [Google Scholar]


