Abstract
Introduction
We and others have shown that the angiotensin type 2 (AT2) receptor agonist, compound 21 (C21), provides neuroprotection and enhances recovery in rodent stroke models yet the mechanism involved is not known. Moreover, C21 treatment is associated with an anti-inflammatory response. Here we tested the hypothesis that C21 mediates neuroprotection by upregulating the neuroprotective and anti-inflammatory cytokine interleukin (IL)-10.
Methods
Wistar rats were subjected to 3 h MCA suture occlusion and treated at reperfusion with C21 (0.03 mg/kg) ± IL-10 neutralizing antibody (0.1 mg/kg) both given i.p. Infarct size, behavioral outcomes, and molecular analysis were performed at 24 h post-injury. Primary rat neurons were used to test the direct neuroprotective effect of C21 in vitro.
Results
C21 treatment reduced infarct size, improved functional outcome and decreased the pro-inflammatory cytokine, tumor necrosis factor alpha (TNF-α) in the ischemic hemisphere compared to saline. Anti-IL-10 co-treatment blocked the C21 induced reduction in infarct size and inflammation, and the improvement in behavioral outcome. In vitro, C21 treatment increased neuron survival and reduced cell apoptosis after oxygen glucose deprivation (OGD) and OGD/reoxygenation. These effects were mediated through AT2R stimulation.
Conclusion
C21 provides direct neuroprotection as well as indirect protection through IL-10.
Keywords: Stroke, Angiotensin Type 2 Receptor, Compound 21, Neuroprotection, Interleukin 10
1. Introduction
Angiotensin type 2 (AT2R) receptor stimulation with the non-peptide agonist, compound 21 (C21), has been shown to provide neuroprotection and functional recovery after experimental ischemic stroke in rodents (Alhusban et al., 2015; Joseph et al., 2014; McCarthy et al., 2014; Min et al., 2014). C21 reduced infarct size after both permanent and temporary middle cerebral artery occlusion (MCAO). In addition, C21 reduced neuronal apoptosis and decreased mortality after stroke (Schwengel et al., 2016). Nevertheless, the mechanism underlying C21-mediated neuroprotection is still not known. In our hands, we reported an upregulation of the neuroprotective cytokine, interleukin (IL)-10 with C21 treatment at 24 h in the ischemic hemisphere after 3 h MCAO. Moreover, we reported an increase in the number of IL-10 positive cells at 7 days with a single dose of C21 after 90-min MCAO (Alhusban et al., 2015). Whether the increased IL-10 was causally related to the improved outcome remained unknown.
IL-10 is an anti-inflammatory cytokine that mediates its actions through activation of the JAK1-STAT3 (Janus Kinase 1 - Signal Transducer and Activator of Transcription 3) signaling pathway (Sabat et al., 2010). IL-10 has been shown to provide direct neuroprotection in vivo and in vitro. Administration of exogenous IL-10 centrally and systemically decreases the infarct size in rats after permanent focal ischemia (Spera et al., 1998), while IL-10 knockout mice show larger infarct volume following middle cerebral artery occlusion (Grilli et al., 2000). Moreover, post-ischemic IL-10 gene transfer attenuates brain infarction in rats subjected to focal and global ischemia (Ooboshi et al., 2005). Interestingly, neuroprotection by systemic immune cells such as regulatory T and B cells have also been shown to be mediated through IL-10 production (Bodhankar et al., 2013, 2014; Liesz et al., 2009; Liesz et al., 2013). In vitro, IL-10 protects murine cortical and cerebellar neurons from excitotoxic damage and oxygen glucose deprivation (OGD) by activating phosphatidylinositide 3-kinases (PI-3K) and signal transducer and activator of transcription 3 (STAT-3) pathways (Bachis et al., 2001; Grilli et al., 2000; Sharma et al., 2011).
Studies examining the protective role of AT2R stimulation using in vitro neuronal injury models are inconclusive. The peptide AT2R agonist, CGP-42112, but not C21, protected primary cortical neurons against glucose deprivation (Lee et al., 2012). In addition, Wu et al. showed neuroprotection with angiotensin receptor blockers (ARBs) but not CGP-42112 pretreatment against OGD/reoxygenation injury (Wu et al., 2010); however, these effects involved AT1R blockade and not indirect AT2R stimulation (Wang et al., 2014; Wu et al., 2010).
In this study, we aimed to determine the contribution of IL-10 to the neuroprotective effect of C21 using temporary MCAO in vivo. In addition, we tested the direct neuroprotective effect of C21 in vitro in an ischemia/reoxygenation injury model.
2. Materials and methods
Experiments were approved by the Care of Experimental Animal Committee of Augusta University/Institutional Animal Care and Use Committee (IACUC) of the Charlie Norwood Veterans Affairs Medical Center, Augusta, GA.
2.1. Middle cerebral artery occlusion and treatment
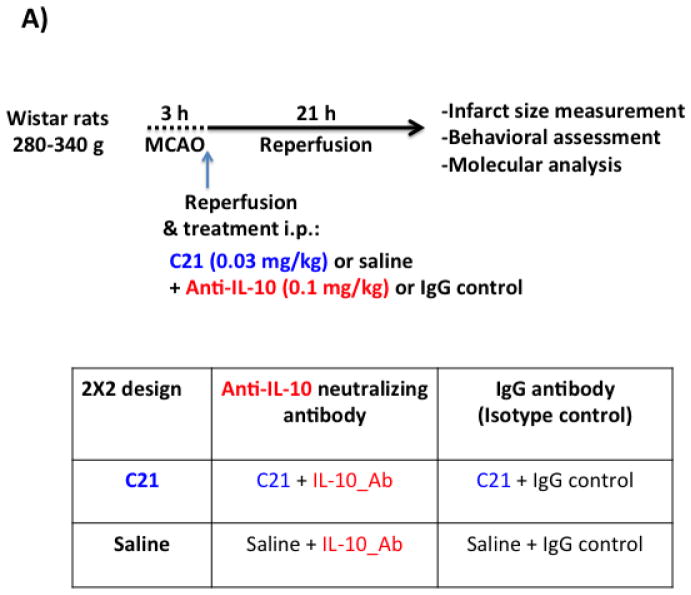
Adult male Wistar rats (280–340 g) were subjected to 3 h middle cerebral artery occlusion (MCAO) followed by reperfusion for 21 h to achieve ischemia/reperfusion injury in vivo as previously described (Alhusban et al., 2015). Animals were treated according to a 2X2 study design (Fig 1.A). C21 was administered intraperitoneal (i.p.) at a dose of 0.03 mg/kg, which has previously shown AT2R mediated neuroprotection in our hands using the same stroke model (Alhusban et al., 2015). Anti-IL-10 neutralizing antibody (Invitrogen) was given i.p. in a separate syringe (to avoid physical interaction) at a dose of (0.1 mg/kg) to block the effects of endogenous IL-10 (Cai et al., 2012). Immunoglobulin G (IgG) isotype control antibody was given to the rats that did not receive anti-IL-10. Animals were killed at 24 h and brains were collected. Behavioral outcome was assessed just before euthanasia. Sham animals were subjected to the same surgical procedure without actual MCAO occlusion.
Fig 1. IL-10 is involved in C21 mediated neuroprotection after ischemia/reperfusion injury in vivo.
A: Schematic diagram representing the study outline and treatment groups. Wistar rats were subjected to 3 h MCAO using the suture model followed by reperfusion for 21 h. Treatments were administered i.p. at reperfusion in separate syringes. Animals were assigned to one of four treatment groups in a 2X2 study design as outlined in the table.
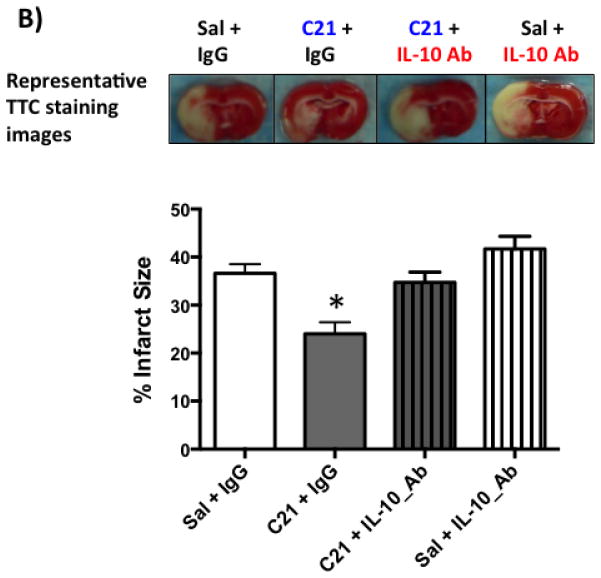
B: Representative TTC stained brain sections and quantification of infarct size from the four treatment groups. C21 treatment reduced infarct size at 24 h after 3 h MCAO. Co-treatment with anti-IL-10 neutralizing antibody abrogated the neuroprotective effect of C21 treatment. N=7–9 per group. *P<0.05 vs other groups (Two-way ANOVA).
2.2. Infarct size analysis
At 24 h, rats were killed after transcardial perfusion with ice cold PBS following ketamine/xylazine anesthesia. Brains were removed and sliced into seven 2 mm-thick coronal sections (A to G) and stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC) (Sigma Chemical Co., Missouri, USA). Areas of the infarct, ischemic, and non-ischemic hemispheres were measured using ImageJ software (NIH) in a blinded fashion and infarct size was calculated with edema correction using the following formula: 100 X (non-stroked – (stroked – infarct))/non-stroked.
2.3. Western blotting
Ischemic hemispheres of B, C, D, and E brain sections were homogenized using a hand homogenizer. Protein was quantified using Pierce BCA protein assay kit (Thermo Scientific), and 50 μg protein aliquots were run on SDS-PAGE as described previously (Guan et al., 2011), transferred to nitrocellulose membranes and probed with mouse anti-TNF-α (Abcam) and rabbit anti-cleaved caspase 3 (Cell Signaling) antibodies (Ishrat et al., 2015). Rabbit anti-GAPDH (Cell Signaling) and mouse anti-β-actin (Sigma) were used as loading controls depending on the molecular weight and species of the probed proteins. Membranes were further probed with peroxidase-conjugated goat anti-rabbit and anti-mouse secondary antibodies. Optical densities of bands were quantified using ImageJ software (NIH), divided by the loading control, and normalized to the control group.
2.4. Behavioral testing
Motor behavior assessment was conducted in a blinded fashion.
Bederson score
This test examines animal behavior in an open field. Rats were scored from 0–3. One point is given for each of the following: forelimb flexion when suspended by tail; decreased resistance to lateral push; and contralateral circling (Bederson et al., 1986).
Beam walk score
This test examines animal balance on a horizontal beam. Rats were placed on a beam for 1 min and scored from 0 to 6 as follows: balances on the beam with a steady posture = 0, grasps side of the beam = 1, hugs the beam with 1 limb falling = 2, hugs the beam with 2 limbs falling = 3, falls off the beam within 40 to 60 s = 4, falls off the beam within 20 to 40 s = 5; falls off the beam in less than 20 s = 6 (Watanabe et al., 2004).
2.5. Primary neurons isolation and culture
Primary rat cortical neurons were isolated from embryonic day 17 (E17) pregnant Wistar rat embryos as described (Dhandapani and Brann, 2003). Brain cortices were dissected and plated at 1 X 106 cells/ml on poly-D-lysine coated 24-well plates. Neurons were cultured in neurobasal medium (GIBCO) containing 2% B27 (GIBCO) and 0.5 mM L-glutamine, to select for neurons. Staining for Microtubule-associated protein 2 (MAP-2, a neuronal marker, Abcam, dilution 1/300) and anti-glial fibrillary acidic protein (GFAP, a marker for astrocytes, Sigma-Aldrich, dilution 1/300) showed that more than 90% of cells in our culture were neurons with less than 10% glia. Neurons were maintained in growth medium at 37 °C in a 95% air, and 5% CO2 humidified atmosphere. Experiments were conducted between days in vitro (DIV) 10–12, when neurons express mature neuronal markers as previously characterized (Dhandapani and Brann, 2003).
2.6. OGD/reoxygenation protocol
To mimic in vivo ischemia/reperfusion conditions, cells were subjected to OGD for 3, 6 and 9 h followed by 6 h of reoxygenation. Durations of OGD and reoxygenation were based on our preliminary experiments. For OGD, cells were switched to glucose free DMEM (GIBCO) medium and incubated in a hypoxia chamber (ProOx C21, Biospherix, NY) (94% N2, 5% CO2, and <1% O2 at 37 °C). Reoxygenation was achieved by replacing the DMEM with the growth medium and further incubation under 95% air, 5% CO2 at 37 °C. In one experiment, cells were treated with increasing concentrations of C21 from start of OGD. In other experiments, cells were treated at reoxygenation with C21 ± PD123319 (AT2R blocker, 1 μM) or anti-IL-10 neutralizing antibody (1 μg/ml). For Western blotting, medium was removed and cells were washed twice with ice cold PBS then collected in RIPA lysis buffer.
2.7. Cell viability (MTT assay)
The assay is based on the ability of living cells to reduce 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT, Sigma) into insoluble formazan (Dhandapani and Brann, 2003). At the end of the experiment, MTT was added to each well to a final concentration of 0.5 mg/ml for 4 h at 37°C. The medium was removed, and DMSO was added to dissolve the formazan. After 5 min incubation, absorbance was measured at 540 nm using a microplate reader (Synergy HT, BioTek, VT).
2.8. Cytotoxicity detection (LDH assay)
Cell death was determined by measuring Lactate dehydrogenase (LDH) activity in the media (culture supernatant) using a cytotoxicity detection kit (Roche, IN) according to the manufacturer’s protocol.
2.9. Statistical analyses
Statistical tests were carried out using GraphPad Prism software. Since a 2X2 study design was conducted for the in vivo experiments, a two-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons was used. For in vitro experiments, one-way ANOVA or t-tests were used. P value <0.05 was considered significant. Data is presented as mean ± S.E.M. In vitro experiments were repeated at least twice.
3. Results
3.1. IL-10 is involved in C21 mediated neuroprotection in vivo
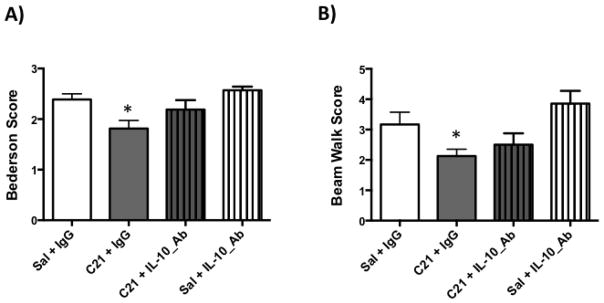
We have previously shown that C21 provides neuroprotection and IL-10 upregulation after stroke (Alhusban et al., 2015). To examine the involvement of IL-10 in C21 mediated neuroprotection, Wistar rats were subjected to experimental conditions outlined in (Fig 1.A) as described in the methods section. As we have previously reported, C21 treated rats showed a reduction in infarct size compared to saline treatment. IL-10 neutralization abrogated the neuroprotective effect of C21 supporting the involvement of IL-10 in C21 mediated neuroprotection in vivo (Fig 1.B). Moreover, C21 treatment improved Bederson and beam walk scores at 24 h. IL-10 neutralization partially blocked this behavioral recovery (Fig 2.A, 2.B).
Fig 2. IL-10 neutralization does not prevent the C21 mediated functional outcome improvement.
A & B: C21 treatment improved Bederson and beam walk scores compared to saline control group, while IL-10 neutralization partially blocked this functional improvement. N=7–9 per group. *P<0.05 vs Sal+IgG and Sal+IL-10_Ab (Two-way ANOVA).
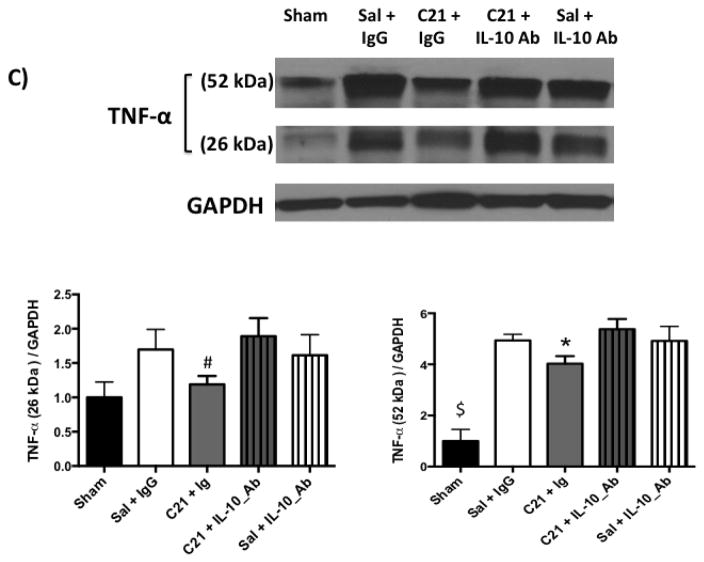
C: Measuring the pro-inflammatory cytokine, TNF-α, using Western blotting showed a band at 52 kDa corresponding to the homotrimeric secreted protein and a band at 26 kDa corresponding to the membrane bound form. Both bands showed similar trend. Stroke lead to an increase in the pro-inflammatory cytokine, TNF-α, in the ischemic hemisphere compared to the sham group. C21 ablated the increase in TNF-α in ischemic hemisphere compared to saline and this was reversed by Anti-IL-10. *P<0.05 vs Sal+IgG and C21+IL-10_Ab. #P<0.05 vs C21+IL-10_Ab group. $P<0.05 vs other groups. n=5–7 per group
IL-10 has been shown to exert its anti-inflammatory action through inhibiting transcription of specific pro-inflammatory genes including tumor necrosis factor alpha (TNF-α) (Murray, 2005). Stroke increased TNF-α in the ischemic hemisphere compared to sham animals. C21 treatment decreased TNF-α compared to saline treated group and co-administration of IL-10 neutralizing antibody blocked this effect (Fig 2.C).
3.2. C21 provides direct neuroprotection in vitro
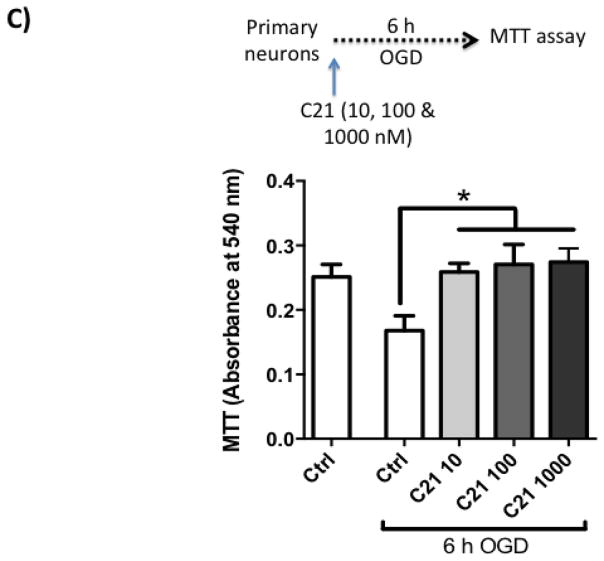
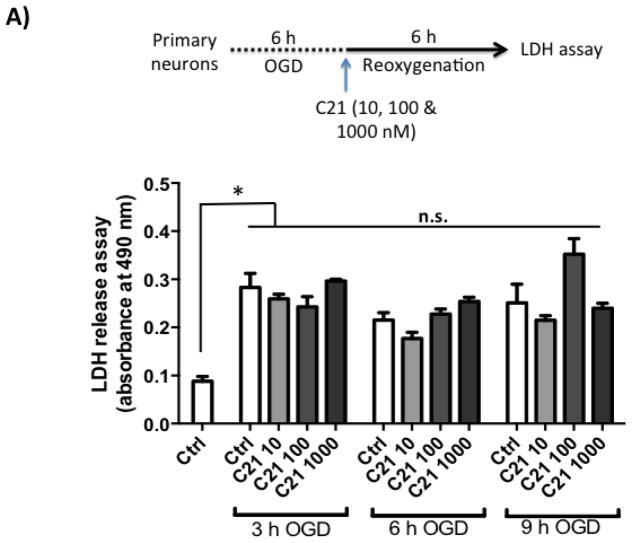
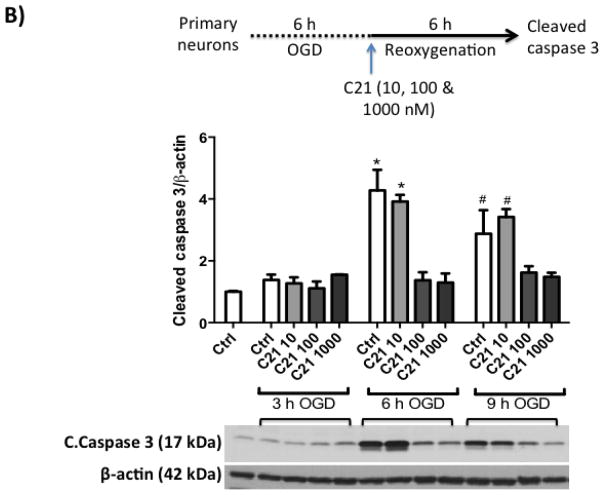
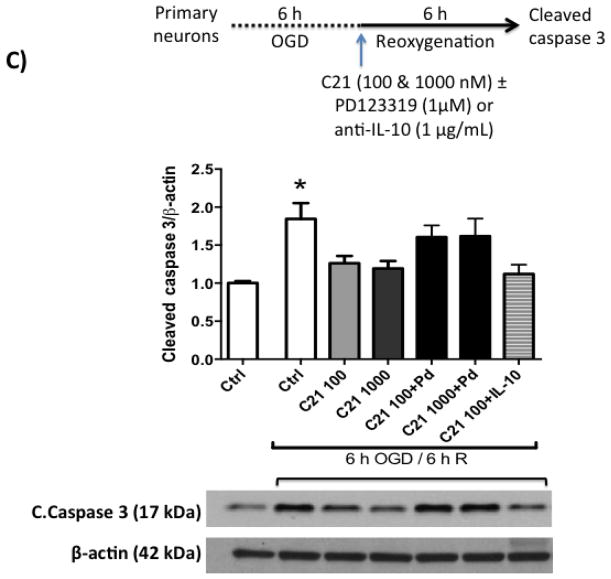
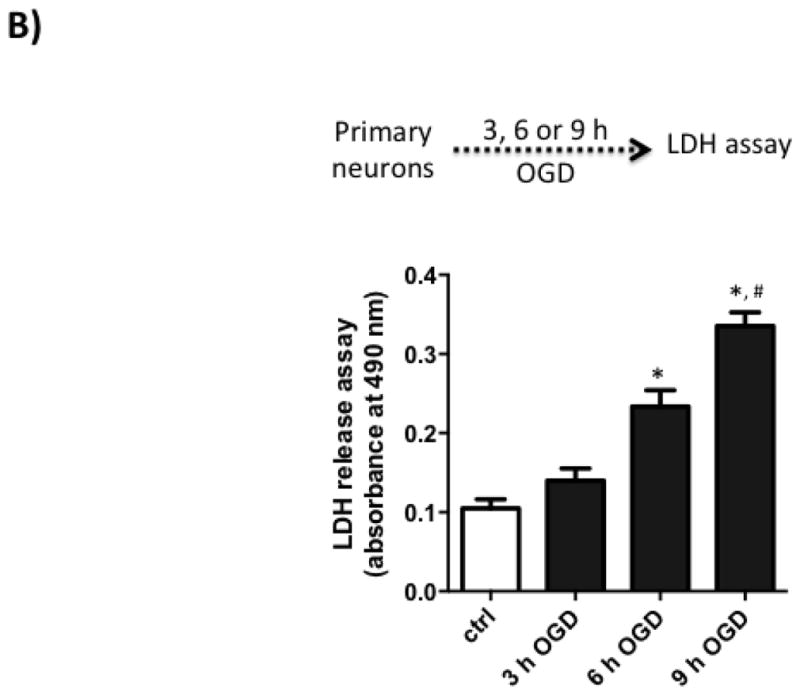
Next, we wanted to test if C21 has a direct neuroprotective effect in vitro. For this, we used primary rat cortical neurons (Fig 3.A). We subjected the neuron culture to 3, 6 and 9 h of OGD, which resulted in time dependent increased cell death as measured by LDH assay (Fig 3.B). 6 h OGD time point provided optimal cell death (>2-fold increase in LDH release). Treating neurons at the beginning of 6 h OGD with C21 increased cell viability in the different concentrations examined (10, 100, and 1000 nM) as measured by MTT assay (Fig 3.C). To mimic our in vivo ischemia/reperfusion injury model, neurons were subjected to 3, 6, or 9 h OGD followed by 6 h of reoxygenation, which resulted in >2-fold increase in LDH release. C21 treatment at reoxygenation was not able to reduce the LDH release (Fig 4.A) but significantly reduced cleaved caspase 3 at 100 and 1000 nM concentrations (Fig 4.B). This anti-apoptotic effect of C21 was blocked with the AT2R antagonist, PD123319, but not anti-IL-10 neutralizing antibody (Fig 4.C). Together, these results suggest that C21 exerts a direct neuroprotective effect in vitro that is independent of IL-10.
Fig 3. C21 provides neuroprotection against OGD.
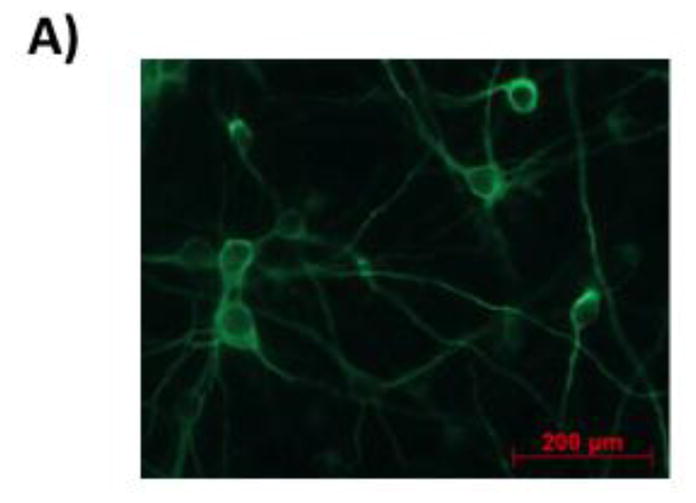
A: Immunostaining of the primary neurons culture for the neuronal marker MAP2 at day 10 in vitro (DIV 10).
B: Time dependent increased cell death with OGD. Lactate dehydrogenase (LDH) release into the supernatant increased in a time dependent manner after 3, 6, and 9 h of OGD. N=6. One-way ANOVA: *P<0.05 vs control and 3 h OGD, #P<0.05 vs 6 h OGD
C: C21 in different concentrations increased cell survival after 6 h OGD as measured by MTT assay. N=3–4. *P<0.05 vs OGD control
Fig 4. C21 provides neuroprotection against OGD/reperfusion.
A: Neurons were subjected to 3, 6 and 9 h of OGD followed by 6 h of reoxygenation which lead to >2 fold increase in LDH assay. C21 did not affect LDH release into the supernatant. N=3. *P<0.05 vs Normoxic control.
B: C21 in 100 and 1000 nM concentrations decreased cleaved caspase 3 as measured by western blotting in cell lysate. N=3. *P<0.05 vs respective 100, 1000 nM groups and normoxic control. #P<0.05 vs respective 100, 1000 nM groups and normoxic control.
C: C21 anti-apoptotic effect is mediated through AT2R stimulation. C21 anti-apoptotic effect was blocked by PD123319 but not anti-IL-10 neutralizing antibody. N=3. *P<0.05 vs normoxic control, 100 nM, 100 nM, and 100nM+IL-10_Ab.
4. Discussion
Our data prove a causal role of IL-10 in the neuroprotective and anti-inflammatory effects of C21 after temporary MCAO in vivo. In addition, the current results show direct neuroprotection with C21 treatment after OGD/reoxygenation injury in vitro.
Recent reports have pointed out the involvement of IL-10 in C21-mediated beneficial effects in renal inflammation, and myocardial ischemia models through AT2R stimulation (Curato et al., 2010; Dhande et al., 2013; Namsolleck et al., 2013). C21 has been shown to provide renoprotection by directly increasing the levels of IL-10 in the kidney via nitric oxide (NO) signaling (Dhande et al., 2013). Similarly, C21 exerts anti-inflammatory response in lipopolysaccharide-activated THP-1 macrophages via increased IL-10 production (Dhande et al., 2015). Furthermore, C21 protects against myocardial ischemia through increasing IL-10 production by CD8(+)AT2R(+) T cells (Curato et al., 2010). We have previously reported C21 mediated IL-10 upregulation, which correlated with neuroprotection after experimental stroke (Alhusban et al., 2015). In the current study, we aimed to determine whether IL-10 upregulation was causally related to the neuroprotective effect of C21. IL-10 neutralization blocked the C21-mediated neuroprotection and anti-inflammatory effect after ischemia/reperfusion injury in vivo. Other agents have also been shown to mediate IL-10 upregulation and neuroprotection. For example, the endogenous peptides, melanocortins have been shown to induce IL-10 production and reduce tissue damage after cerebral ischemia and traumatic brain injury (Bitto et al., 2012; Spaccapelo et al., 2013).
C21 could be increasing IL-10 production from different cellular sources. C21 has poor penetration into the brain through the intact blood brain barrier (BBB) (Shraim et al., 2011). However, BBB is disrupted in our in vivo ischemia/reperfusion injury model of 3 h temporary MCAO as previously characterized (Fagan et al., 2006; Kozak et al., 2008). Therefore, it is possible that C21 can penetrate to the ischemic brain tissue and stimulate IL-10 production by resident brain cells. We have previously shown that IL-10 co-localizes with neurons after ischemic stroke (Fouda et al., 2013). Moreover, C21 is speculated to stimulate an IL-10 producing, anti-inflammatory microglia phenotype (McCarthy et al., 2013). C21 may also increase IL-10 production from systemic immune cells. Anti-IL-10 neutralizing antibody was given in this study via the i.p. route and thus it can neutralize the IL-10 produced by resident brain cells as well as systemic immune cells. Many publications have shown that IL-10 is involved in the systemic immune cells neuroprotective actions, mainly regulatory B- and T-lymphocytes (Bodhankar et al., 2013, 2014; Liesz et al., 2009; Liesz et al., 2013). Recent studies have identified immunomodulatory AT2R+ T cells that exert protection against myocardial infarction through IL-10 (Skorska et al., 2015). In accordance, preliminary studies from our lab show increased regulatory T-cells in rat blood and brain after stroke with C21 treatment (data not shown). Cellular sources of C21 mediated IL-10 upregulation post-stroke are to be examined in future studies.
In our study, IL-10 blockade did not appear to completely reverse the improvement in functional outcome at the 24 h time point examined, suggesting that other mediators could be involved in C21 mediated functional recovery. One of the potential mediators is brain derived neurotrophic factor (BDNF). We have previously published that BDNF is involved in the proangiogneic effect of C21. In addition, knocking down of BDNF abrogated the behavioral recovery mediated through AT1R blockade with candesartan treatment (Fouda et al., 2016). Long-term studies are warranted to examine the mechanism of C21-induced functional recovery after stroke.
While Lee et al. showed no neuroprotection with C21 treatment in vitro (Lee et al., 2012), we have seen a direct neuroprotective effect with C21 treatment on rat primary neurons. This was manifested by increased cell survival and reduction in apoptosis. Interestingly, C21 provided neuroprotection under both OGD and OGD/reoxygenation. The discrepancy between our results and the previous report could be due to the different in vitro stroke model used. While Lee et al. used only glucose deprivation, we used a more clinically relevant model combining both oxygen and glucose deprivation in addition to the reoxygenation insult. The mechanism of this direct neuroprotection is still to be elucidated. Interestingly, a recent report described AT2R expression in neurons mitochondria. Treatment of neurons with oxidative stress inducers increased AT2R expression, which decreased mitochondrial respiration (Valenzuela et al., 2016). This suggests a neuroprotective effect of AT2R through regulation of mitochondrial respiration.
In conclusion, our results show that IL-10 is involved in C21 neuroprotective action in vivo. In addition C21 elicited direct neuroprotection on primary neurons in vitro.
Acknowledgments
This study was supported by Veterans Affairs (VA) Merit Review (BX000891), RO1-NS063965 and R21-NS088016 to SCF. AE is a Research Career Scientist at the Charlie Norwood Veterans Affairs Medical Center in Augusta, Georgia. This work was supported in part by VA Merit Award (BX000347), VA Research Career Scientist Award, and NIH award (R01NS083559) to AE.
The authors thank Vicore Pharma (Göteborg, Sweden) for the supply of C21 and AstraZeneca for the supply of candesartan.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- Alhusban A, Fouda AY, Bindu P, Ishrat T, Soliman S, Fagan SC. Compound 21 is pro-angiogenic in the brain and results in sustained recovery after ischemic stroke. Journal of hypertension. 2015;33:170–180. doi: 10.1097/HJH.0000000000000364. [DOI] [PubMed] [Google Scholar]
- Bachis A, Colangelo AM, Vicini S, Doe PP, De Bernardi MA, Brooker G, Mocchetti I. Interleukin-10 prevents glutamate-mediated cerebellar granule cell death by blocking caspase-3-like activity. The Journal of neuroscience. 2001;21:3104–3112. doi: 10.1523/JNEUROSCI.21-09-03104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke; a journal of cerebral circulation. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Bitto A, Polito F, Irrera N, Calo M, Spaccapelo L, Marini HR, Giuliani D, Ottani A, Rinaldi M, Minutoli L, Guarini S, Squadrito F, Altavilla D. Protective effects of melanocortins on short-term changes in a rat model of traumatic brain injury*. Critical care medicine. 2012;40:945–951. doi: 10.1097/CCM.0b013e318236efde. [DOI] [PubMed] [Google Scholar]
- Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H. IL-10-producing B-cells limit CNS inflammation and infarct volume in experimental stroke. Metabolic brain disease. 2013;28:375–386. doi: 10.1007/s11011-013-9413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H. Treatment of experimental stroke with IL-10-producing B-cells reduces infarct size and peripheral and CNS inflammation in wild-type B-cell-sufficient mice. Metabolic brain disease. 2014;29:59–73. doi: 10.1007/s11011-013-9474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai ZP, Parajuli N, Zheng X, Becker L. Remote ischemic preconditioning confers late protection against myocardial ischemia-reperfusion injury in mice by upregulating interleukin-10. Basic research in cardiology. 2012;107:277. doi: 10.1007/s00395-012-0277-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curato C, Slavic S, Dong J, Skorska A, Altarche-Xifro W, Miteva K, Kaschina E, Thiel A, Imboden H, Wang J, Steckelings U, Steinhoff G, Unger T, Li J. Identification of noncytotoxic and IL-10-producing CD8+AT2R+ T cell population in response to ischemic heart injury. Journal of immunology (Baltimore, Md: 1950) 2010;185:6286–6293. doi: 10.4049/jimmunol.0903681. [DOI] [PubMed] [Google Scholar]
- Dhandapani K, Brann D. Neuroprotective effects of estrogen and tamoxifen in vitro: a facilitative role for glia? Endocrine. 2003;21:59–66. doi: 10.1385/endo:21:1:59. [DOI] [PubMed] [Google Scholar]
- Dhande I, Ali Q, Hussain T. Proximal tubule angiotensin AT2 receptors mediate an anti-inflammatory response via interleukin-10: role in renoprotection in obese rats. Hypertension. 2013;61:1218–1226. doi: 10.1161/HYPERTENSIONAHA.111.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhande I, Ma W, Hussain T. Angiotensin AT2 receptor stimulation is anti-inflammatory in lipopolysaccharide-activated THP-1 macrophages via increased interleukin-10 production. Hypertension research : official journal of the Japanese Society of Hypertension. 2015;38:21–29. doi: 10.1038/hr.2014.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan SC, Kozak A, Hill WD, Pollock DM, Xu L, Johnson MH, Ergul A, Hess DC. Hypertension after experimental cerebral ischemia: candesartan provides neurovascular protection. Journal of hypertension. 2006;24:535–539. doi: 10.1097/01.hjh.0000209990.41304.43. [DOI] [PubMed] [Google Scholar]
- Fouda A, Kozak A, Alhusban A, Switzer J, Fagan S. Anti-inflammatory IL-10 is upregulated in both hemispheres after experimental ischemic stroke: Hypertension blunts the response. Experimental & translational stroke medicine. 2013;5:12. doi: 10.1186/2040-7378-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouda AY, Alhusban A, Ishrat T, Pillai B, Eldahshan W, Waller JL, Ergul A, Fagan SC. Brain-Derived Neurotrophic Factor Knockdown Blocks the Angiogenic and Protective Effects of Angiotensin Modulation After Experimental Stroke. Molecular neurobiology. 2016 doi: 10.1007/s12035-015-9675-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli M, Barbieri I, Basudev H, Brusa R, Casati C, Lozza G, Ongini E. Interleukin-10 modulates neuronal threshold of vulnerability to ischaemic damage. European journal of neuroscience. 2000;12:2265–2272. doi: 10.1046/j.1460-9568.2000.00090.x. [DOI] [PubMed] [Google Scholar]
- Guan W, Somanath PR, Kozak A, Goc A, El-Remessy AB, Ergul A, Johnson MH, Alhusban A, Soliman S, Fagan SC. Vascular protection by angiotensin receptor antagonism involves differential VEGF expression in both hemispheres after experimental stroke. PloS one. 2011;6:e24551. doi: 10.1371/journal.pone.0024551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishrat T, Mohamed IN, Pillai B, Soliman S, Fouda AY, Ergul A, El-Remessy AB, Fagan SC. Thioredoxin-interacting protein: a novel target for neuroprotection in experimental thromboembolic stroke in mice. Molecular neurobiology. 2015;51:766–778. doi: 10.1007/s12035-014-8766-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JP, Mecca AP, Regenhardt RW, Bennion DM, Rodriguez V, Desland F, Patel NA, Pioquinto DJ, Unger T, Katovich MJ, Steckelings UM, Sumners C. The angiotensin type 2 receptor agonist Compound 21 elicits cerebroprotection in endothelin-1 induced ischemic stroke. Neuropharmacology. 2014;81c:134–141. doi: 10.1016/j.neuropharm.2014.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak W, Kozak A, Johnson MH, Elewa HF, Fagan SC. Vascular protection with candesartan after experimental acute stroke in hypertensive rats: a dose-response study. The Journal of pharmacology and experimental therapeutics. 2008;326:773–782. doi: 10.1124/jpet.108.139618. [DOI] [PubMed] [Google Scholar]
- Lee S, Brait VH, Arumugam TV, Evans MA, Kim HA, Widdop RE, Drummond GR, Sobey CG, Jones ES. Neuroprotective effect of an angiotensin receptor type 2 agonist following cerebral ischemia in vitro and in vivo. Experimental & translational stroke medicine. 2012;4:16. doi: 10.1186/2040-7378-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nature medicine. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- Liesz A, Zhou W, Na SY, Hammerling GJ, Garbi N, Karcher S, Mracsko E, Backs J, Rivest S, Veltkamp R. Boosting regulatory T cells limits neuroinflammation in permanent cortical stroke. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:17350–17362. doi: 10.1523/JNEUROSCI.4901-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy CA, Vinh A, Miller AA, Hallberg A, Alterman M, Callaway JK, Widdop RE. Direct angiotensin AT2 receptor stimulation using a novel AT2 receptor agonist, compound 21, evokes neuroprotection in conscious hypertensive rats. PloS one. 2014;9:e95762. doi: 10.1371/journal.pone.0095762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy CA, Widdop RE, Deliyanti D, Wilkinson-Berka JL. Brain and retinal microglia in health and disease: an unrecognized target of the renin-angiotensin system. Clinical and experimental pharmacology & physiology. 2013;40:571–579. doi: 10.1111/1440-1681.12099. [DOI] [PubMed] [Google Scholar]
- Min LJ, Mogi M, Tsukuda K, Jing F, Ohshima K, Nakaoka H, Kan-No H, Wang XL, Chisaka T, Bai HY, Iwanami J, Horiuchi M. Direct Stimulation of Angiotensin II Type 2 Receptor Initiated After Stroke Ameliorates Ischemic Brain Damage. American journal of hypertension. 2014 doi: 10.1093/ajh/hpu015. [DOI] [PubMed] [Google Scholar]
- Murray PJ. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proc Natl Acad Sci U S A. 2005;102:8686–8691. doi: 10.1073/pnas.0500419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namsolleck P, Boato F, Schwengel K, Paulis L, Matho KS, Geurts N, Thone-Reineke C, Lucht K, Seidel K, Hallberg A, Dahlof B, Unger T, Hendrix S, Steckelings UM. AT2-receptor stimulation enhances axonal plasticity after spinal cord injury by upregulating BDNF expression. Neurobiology of disease. 2013;51:177–191. doi: 10.1016/j.nbd.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Ooboshi H, Ibayashi S, Shichita T, Kumai Y, Takada J, Ago T, Arakawa S, Sugimori H, Kamouchi M, Kitazono T, Iida M. Postischemic gene transfer of interleukin-10 protects against both focal and global brain ischemia. Circulation. 2005;111:913–919. doi: 10.1161/01.CIR.0000155622.68580.DC. [DOI] [PubMed] [Google Scholar]
- Sabat R, Grutz G, Warszawska K, Kirsch S, Witte E, Wolk K, Geginat J. Biology of interleukin-10. Cytokine & growth factor reviews. 2010;21:331–344. doi: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Schwengel K, Namsolleck P, Lucht K, Clausen BH, Lambertsen KL, Valero-Esquitino V, Thone-Reineke C, Muller S, Widdop RE, Denton KM, Horiuchi M, Iwai M, Boato F, Dahlof B, Hallberg A, Unger T, Steckelings UM. Angiotensin AT2-receptor stimulation improves survival and neurological outcome after experimental stroke in mice. Journal of molecular medicine (Berlin, Germany) 2016;94:957–966. doi: 10.1007/s00109-016-1406-3. [DOI] [PubMed] [Google Scholar]
- Sharma S, Yang B, Xi X, Grotta JC, Aronowski J, Savitz SI. IL-10 directly protects cortical neurons by activating PI-3 kinase and STAT-3 pathways. Brain research. 2011;1373:189–194. doi: 10.1016/j.brainres.2010.11.096. [DOI] [PubMed] [Google Scholar]
- Shraim N, Mertens B, Clinckers R, Sarre S, Michotte Y, Van Eeckhaut A. Microbore liquid chromatography with UV detection to study the in vivo passage of compound 21, a non-peptidergic AT(2) receptor agonist, to the striatum in rats. Journal of neuroscience methods. 2011;202:137–142. doi: 10.1016/j.jneumeth.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Skorska A, von Haehling S, Ludwig M, Lux CA, Gaebel R, Kleiner G, Klopsch C, Dong J, Curato C, Altarche-Xifro W, Slavic S, Unger T, Steinhoff G, Li J, David R. The CD4(+) AT2R(+) T cell subpopulation improves post-infarction remodelling and restores cardiac function. Journal of cellular and molecular medicine. 2015;19:1975–1985. doi: 10.1111/jcmm.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaccapelo L, Galantucci M, Neri L, Contri M, Pizzala R, D’Amico R, Ottani A, Sandrini M, Zaffe D, Giuliani D, Guarini S. Up-regulation of the canonical Wnt-3A and Sonic hedgehog signaling underlies melanocortin-induced neurogenesis after cerebral ischemia. European journal of pharmacology. 2013;707:78–86. doi: 10.1016/j.ejphar.2013.03.030. [DOI] [PubMed] [Google Scholar]
- Spera PA, Ellison JA, Feuerstein GZ, Barone FC. IL-10 reduces rat brain injury following focal stroke. Neuroscience letters. 1998;251:189–192. doi: 10.1016/s0304-3940(98)00537-0. [DOI] [PubMed] [Google Scholar]
- Valenzuela R, Costa-Besada MA, Iglesias-Gonzalez J, Perez-Costas E, Villar-Cheda B, Garrido-Gil P, Melendez-Ferro M, Soto-Otero R, Lanciego JL, Henrion D, Franco R, Labandeira-Garcia JL. Mitochondrial angiotensin receptors in dopaminergic neurons. Role in cell protection and aging-related vulnerability to neurodegeneration. Cell death & disease. 2016;7:e2427. doi: 10.1038/cddis.2016.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Pang T, Hafko R, Benicky J, Sanchez-Lemus E, Saavedra JM. Telmisartan ameliorates glutamate-induced neurotoxicity: roles of AT(1) receptor blockade and PPARgamma activation. Neuropharmacology. 2014;79:249–261. doi: 10.1016/j.neuropharm.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Okuda Y, Nonoguchi N, Zhao MZ, Kajimoto Y, Furutama D, Yukawa H, Shibata MA, Otsuki Y, Kuroiwa T, Miyatake S. Postischemic intraventricular administration of FGF-2 expressing adenoviral vectors improves neurologic outcome and reduces infarct volume after transient focal cerebral ischemia in rats. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2004;24:1205–1213. doi: 10.1097/01.WCB.0000136525.75839.41. [DOI] [PubMed] [Google Scholar]
- Wu X, Kihara T, Hongo H, Akaike A, Niidome T, Sugimoto H. Angiotensin receptor type 1 antagonists protect against neuronal injury induced by oxygen-glucose depletion. British journal of pharmacology. 2010;161:33–50. doi: 10.1111/j.1476-5381.2010.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]