Abstract
Background:
Beta-sitosterol (BS) is a compound discovered to be present in numerous plants. A number of interesting biomedical properties have been attributed to BS, including immuno-modulating and anti-inflammatory activities. Therefore, the aim of this report was to evaluate its anti-inflammatory capacity by applying various rodent experimental tests.
Methods:
To carry out the objective of the study we applied the methods indicated here. Two of the adopted methods were based on the passive reverse Arthus reaction: the rat paw edema test and the rat pleurisy assay. We also applied two methods related with the non-specific acute inflammation: the mouse ear edema test, and the mouse mieloperoxidase activity assay.
Results:
The results obtained in all tests established a significant anti-inflammatory potential of BS. In the rat paw edema test we found an inhibitory effect which goes from 50-70%; in the rat pleurisy assay our findings with respect to the volume of pleural exuded showed a reduction of 46%, as well as a 20% low amount of neutrophils in comparison with the level of the control group. In the mouse ear edema test we found a mean inflammatory inhibition of 75%, and with respect to mieloproxidase activity the results showed a significant inhibition induced by the three doses of BS.
Conclusions:
In the present study we determined a potent anti-inflammatory capacity of BS in specific and non-specific types of acute inflammation in rodents.
Keywords: Beta-sitosterol, anti-inflammatory assays, mouse, at
Introduction
Inflammation is a relevant physiological event which is needed for the maintenance of tissue homeostasis; it is a reaction of an organism to cell and tissue damage caused by a variety of agents, as well as to autoimmune events (Perez et al., 2014). During this process, microcirculatory events occur, including vascular permeability, changes in the movement, recruitment and accumulation of leukocytes, besides the release of inflammatory mediators (Medzhitov, 2010). Resolution is necessary to restore the original morphology and function of the affected tissue. This is an active process coordinated by a number of intracellular and extracellular molecules. In the process, a reduction of proinflammatory mediators occur, in addition to the release of pro-resolving mediators which prevents migration of leukocytes and increases their apoptosis (Serhan and Savill, 2005; Serhan et al., 2007). These events start the resolution process and are required to restore tissue homeostasis. When inflammation is unresolved, or excessive initial inflammation is produced, a variety of chronic inflammatory diseases can be developed affecting a number of organs; for example, organs that may be affected are those which belong to the respiratory and digestive systems. Likewise, inflammatory diseases developed can also originate rheumatoid arthritis, a systemic, autoimmune disease characterized by chronic inflammation mainly affecting the joints, where it can produce a progressive degree of deformity and functional disability related with progressive cartilage destruction, as well as with damage in tendons, ligaments and bones. Moreover, the disease can affect other organs, such as eyes, lungs, pleura, heart, skin, and blood vessels (McIlness and Schett, 2011; Gaffo et al., 2006).
Pharmacologic treatments are mainly designed to resolve, deter or improve the quality of life affected by inflammatory diseases. A number of drugs have been designed and applied depending on the affected organ, the status of the disease and the characteristics of the involved patient; however, the therapy can be expensive, and its effect may be symptomatic, non-permanent, and could cause collateral damage. For example, non-steroidal anti-inflammatory drugs can have adverse effects on bone tissue by modulating the proliferation, differentiation, adhesion, and migration of osteoblasts (Garcia-Martinez et al., 2015); some of the mentioned drugs, as well as aspirin, have been associated with upper gastrointestinal tract injury, including bleeding and ulcers (Goldstein, 2004; Goldstein and Crier, 2015), and they confer an increased risk for thrombotic and congestive heart failure (Farkouh et al., 2007). Furthermore, another risk factor to be considered is the concurrent use of anti-inflammatory drugs with other medications such as anticoagulants, corticosteroids, serotonin reuptake inhibitors or antihypertensive agents (Goldstein and Cryer, 2015; Kalafutova et al., 2014). This complexity makes the search for new compounds worthwhile, with efficacy both to reduce the pathophysiological mechanisms leading to the disease, and to lessen few if any, toxic side effects. Plant extracts or plant-derived compounds are under investigation in order to accomplish these purposes. In these efforts, a number of screening tests can be used in rodents, including the immune complex-induced reversed passive Arthus reaction (Bailey and Sturm, 1983; Szalai et al., 2000).
Beta-sitosterol (BS) is a vegetable-derived compound found in plants such as rice, wheat, corn, nut, peanut, and particularly in cat’s claw (Uncaria tomentosa), where it has been suggested to be involved in the curative properties attributed to the plant on inflammation, viral damage, ulcer, cancer development, as well as in the enhancement of the immune system (Heitzman et al., 2005; Ling and Jones, 1995). BS is a chemical structurally related to cholesterol, but more slowly absorbed into the intestinal tract, interfering with the cholesterol absorption and preventing its rise in serum (Tapiero et al., 2003). Besides, BS is suggested to modulate the immune function, inflammation, and pain levels by controlling the production of inflammatory cytokines (Awad and Fink, 2000). Based on the abovementioned information, we extended studies in the present report on the capacity of BS as an anti-inflammatory agent by applying various tests in rodents.
Material and methods
Chemicals and animals
BS, ibuprofen, prednisone, chicken egg albumin, indomethacin, 12-O-tetradecanoylphorbol-13-acetate (TPA), tetramethyl benzidine (TMB), hexadecyltrimethyl ammonium bromide, acetone, sodium acetate, hydrogen peroxide, as well as ethylic ether and NaCl were purchased from Sigma Chemicals (St. Louis, MO, USA). Ovalbumin antiserum (rabbit polyclonal to ovalbumin) was obtained from Abcam (Mexico City).
For the assay, we used eight-week-old male Wistar rats with a mean weight of 250 g and mouse (CD1) with a mean weight of 25 g. The animals were obtained from the National Institute of Rehabilitation and maintained in metallic cages, at 23° C, in a 12 h dark-light cycle, and permitted to freely consume food (Rodent Lab Chow 5001, Purina) and water. The experimental procedure was approved by the Committee of Ethics and Biosecurity of the National Rehabilitation Institute.
Inductions of rat paw edema
For this assay, we followed the reverse passive Arthus reaction (Pflum and Graeme, 1979) with some modifications. Initially, the substance corresponding to each experimental group was administered, followed by the induction of the antigen-antibody reaction to each rat. We used 30 rats organized in 6 groups with 5 individuals each: a negative control group that was intra-gastrically (IG) administered with mineral oil; three other groups that were IG administered with BS (50, 100 and 200 mg/kg), previously diluted in mineral oil; and finally, two more groups intraperitoneally (IP) administered with known anti-inflammatory drugs, in which one group was injected 200 mg/kg of ibuprofen (a non-steroidal anti-inflammatory agent), and the other group was injected with 10 mg/kg of prednisone (a steroidal anti-inflammatory agent). All animals were immediately injected with 0.1 ml of anti-ovalbumin antibody (diluted 1:3) in the hindquarter of the plantar area of the right leg, and in the contralateral leg; rats were injected with 0.1 ml of 0.9 % NaCl. In a subsequent step, all animals were intravenously (IV) injected with a suspension of egg albumin (25 mg/kg).
The volume of the swollen leg was determined with a digital pletysmometer 3 h after the ovoalbumin injection. The paw edema value was established by determining the difference between the volume obtained in the treated leg and in the control (left leg). For data calculation, the volume of the swollen leg was considered to represent 100% inflammation.
Pleurisy assay in rats
This assay was also based on the reverse passive Arthus reaction (Pflum and Graeme, 1979) with some modifications. In the first instance, we injected 0.2 ml anti-ovalbumin antibody (diluted 1:10) to the pleural cavity of rats in order to induce the immunocomplex; 20 min later all animals were IV injected with 25 mg/kg of ovalbumin. Six groups with 5 rats each were used for the experiment. Two positive control groups were included in the study: one IP administered ibuprofen (200 mg/kg), and another IP injected 10 mg/kg of prednisone; besides these groups, three more groups were IG treated with BS diluted in mineral oil (50, 100, and 200 mg/kg, respectively); finally, we included a negative control group IG administered with mineral oil. Six hours after the pleural inoculation, rats were sacrificed by cervical dislocation; then, the exuded volume of the pleural cavity was quantified and centrifuged at 3000g for 5 min. The sediment was placed on a slide, fixed with methanol and stained with Giemsa for 10 min. Then, a differential counting of neutrophiles and lymphocytes was made.
Mouse ear edema assay
For this test, we followed the guidelines described by Young and De Joung (1989) to measure inflammation using TPA. All substances were dissolved in acetone. Five groups with five individuals each were topically administered with TPA (2.5 μg) as phlogistic agent, in both the internal and external surface of the right ear of each mouse. Also, 20 μl of acetone were applied to the left ear of each mouse. One hour later, BS (0.5, 1.0 and 1.5 mg) was topically applied to the right ear of the individuals of three groups. Besides, we also included a group administered indomethacin (0.5 mg/ear) as positive control. Four hours after the chemical administration, mice were cervically dislocated, and a section (7mm diameter) of the central portion of both ears was obtained and weighed.
The value of the edema was calculated by the difference between the weight of the treated right ear and the non-treated left ear. The inhibitory effect of the edema (in percentage) was calculated in comparison with the edema detected in the TPA treated group.
Inhibition of mieloperoxidase (MPO) activity
For this assay, we followed the method described by Suzuki et al. (1983) modified by Young and De Joung (1989) for use in a plate reader. MPO activity was measured from the ears of mice treated with TPA, BS, and indomethacin. These ears belonged to individuals from the previously described mouse ear edema assay. They had been weighted and kept frozen at - 20 °C until use. At this time, ears were homogenized and centrifuged at 11 200g at 4 °C for 20 min. After that, 30 μl of the supernatant were added to the reaction mixture which consisted of phosphate buffer (50 mmol/l, pH 5.4), TMB (1.6 mmol/l), 0.5% hexadecyltrimethyl ammonium bromide, and H2O2 (1 mmol/l). The reaction was carried out for 3 min at 37 °C and then it was stopped by adding sodium acetate 1.46 M. Absorbances of the samples were read at 630 nm. The enzyme inhibition (expressed in percentage) corresponded to the absorbance difference between the treated groups with respect to the TPA treated group.
Statistics
The statistical analysis of data obtained from the different anti-inflammatory assays was performed with an ANOVA followed by the Student t test. For this purpose, we used the SPSS 11.0.1 computer program (Statistical software, Inc., Chicago, IL, USA).
Results
The results obtained in the rat paw edema test are shown in Figure 1. The three tested doses of BS were found to induce a significant, dose dependent inhibition of the edema in comparison with the result found in the control group. The inhibition was 51, 63, and 70% with 50, 100, and 200 mg/kg of BS, respectively. Interestingly, the high dose of BS was 17% more effective than ibuprofen and 11% more effective than prednisone.
Figure 1.
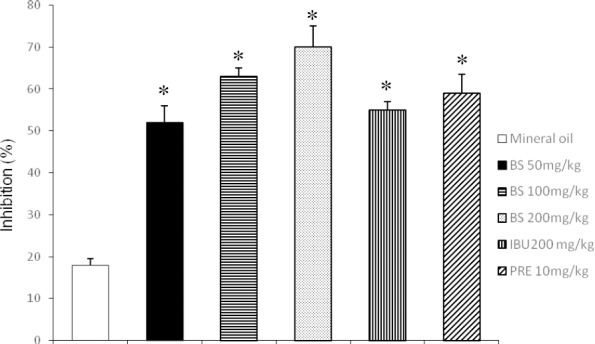
Anti-inflammatory effect of beta-sitosterol (BS) in the rat paw edema test
IBU = Ibuprofen; PRE = Prednisone. Each bar represents the mean ± SEM of five animals per group. *Statistically significant difference with respect to the result of mineral oil; ANOVA and Student t tests, p < 0.05
In the pleurisy assay, the effect of BS on the exudative pleural effusion induced with the antigen/antibody reaction corresponded to 2.1, 1.8, and 2.2 ml with the low, intermediate and high tested doses (Figure 2). This result represents a mean reduction of 46% with respect to the amount of the control (3.8 ml), and a higher reduction with respect to the level induced by ibuprofen (31%) and prednisone (36%).
Figure 2.
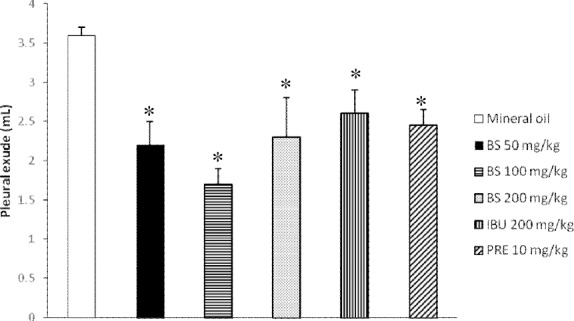
Anti-inflammatory effect of beta-sitosterol (BS); Determination of rat pleural effusion
IBU = Ibuprofen; PRE = Prednisone. Each bar represents the mean ± SEM of five animals per group. *Statistically significant difference with respect to the result of mineral oil; ANOVA and the Student t tests, p≤0.05
In regard to the amount of neutrophils and lymphocytes determined in the pleural cavity of rats, the results are presented in Figure 3. In this study, the percentage of neutrophils induced by BS corresponded to 60, 57 and 59 % with 50, 100, and 200 mg/kg of the chemical, respectively (Figure 3A); these values were about 20% lower than those observed in the negative group, as well as more than 6% of the observed values in the prednisone and ibuprofen treated mice. In regard to lymphocytes, results determined with BS after the antigen/antibody reaction showed a duplication of the value obtained in the negative control group, and only about 4% lower than the one observed in rats administered ibuprofen or prednisone (Figure 3B).
Figure 3.
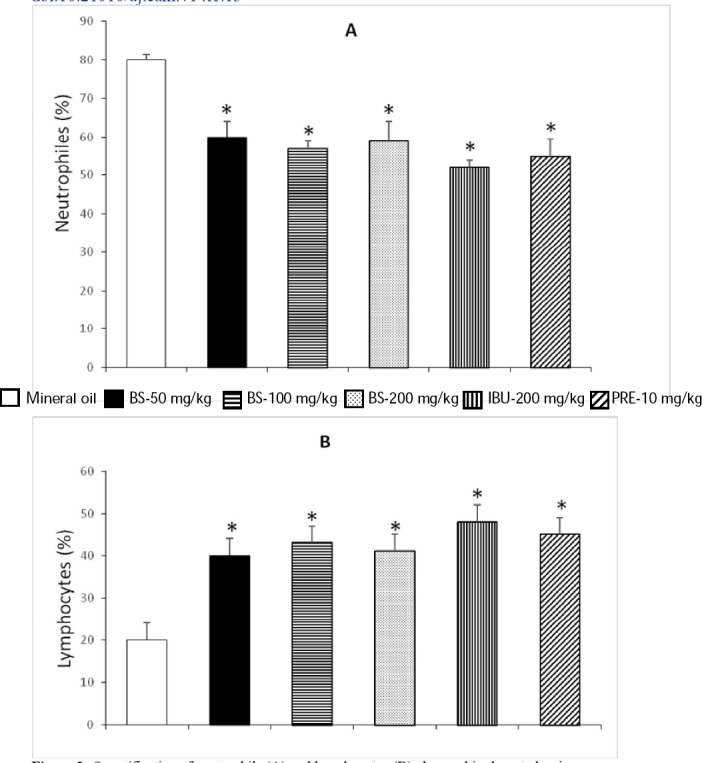
Quantification of neutrophils (A) and lymphocytes (B) observed in the rat pleurisy assay
IBU = Ibuprofen; PRE = Prednisone. Each bar represents the mean ± SEM of five animals per group. *Statistically significant difference with respect to the result obtained for mineral oil; ANOVA and Student t tests, p<0.05
Table 1 shows results concerning the mouse auricular edema test. In this assay we determined a dose-dependent inhibition induced by BS, with a mean effect of 75%, which is slightly higher than the effect produced by indomethacin; however, with 1.5 mg/ear of BS the difference reached 14%.
Table 1.
Anti-inflammatory effect of beta-sitosterol (BS) in the mouse ear edema test
| Treatment | Dose (mg/ear) | Edema (mg) | Inhibition % |
|---|---|---|---|
| TPA | 0.0025 | 19.7+0.60 | -- |
| IND | 0.5 | 5.9+0.72 | 70.3* |
| BS | 0.5 | 6.7+0.69 | 66.0* |
| BS | 1.0 | 5.4+1.5 | 74.2* |
| BS | 1.5 | 3.1+0.93 | 84.1* |
IND=indomethacin. The obtained results represent the mean ± EEM of 5 mice per group.
Statistically significant difference with respect to 12-O-tetradecanoylphorbol-13-acetate (TPA) treated animals. ANOVA and Student t tests, p≤0.05
Finally, in regard to the myeloperoxidase assay, we found a significant inhibitory effect produced with the tested doses of BS, the high dose of BS (1.5 mg/ear) being slightly better than the inhibition observed with indomethacin (Table 2).
Table 2.
Anti-inflammatory effect of beta-sitosterol (BS); Determination of mieloperoxidase activity
| Treatment | Dose (mg/ear) | Inhibition % |
|---|---|---|
| TPA | 0.0025 | -- |
| IND | 0.5 | 95.36* |
| BS | 0.5 | 56.39* |
| BS | 1.0 | 79.20* |
| BS | 1.5 | 98.90* |
IND=indomethacin. The inhibiton percentage was calculated according to the equation %I=DAc-DAp x 100/DAc; where DAc is the arithmetic mean of the control absorbance, and DAp the arithmetic mean of the tested agent absorbance. The obtained results represent the mean ± EEM of 5 mice per group.
Statistically significant difference with respect to the value obtained in 12-O-tetradecanoylphorbol-13-acetate (TPA) treated animals; ANOVA and Student t tests, p≤0.05
Discussion
Inflammation is a process that can represent a prelude for the development of the final disease, which is the case of a chronic inflammatory state in the digestive apparatus that may conclude with gastrointestinal cancer (Susuki et al., 2005); however, it could also correspond to the main clinical symptom that characterizes a number of well-established pathologies, for example, the systemic inflammatory response syndrome (Fry, 2012). Therefore, due to its high public health relevance, investigations are constantly in progress to find new drugs with better characteristics than those on the market. In these efforts, the identification of plants with anti-inflammatory properties has produced valuable knowledge. Plant species with this property have been found in a variety of families, including Rubiaceae, Moraceae, Gentianaceae, Compositae, Myrtaceae, Arecaceae, and Boraginaceae, among others (He et al., 2014; Huang et al., 2013; Jamkhande et al., 2013; Dwevedi et al., 2015; Uddin et al., 2014; Anyanwu et al., 2015; Piscoya et al., 2001; Naressi et al., 2012). Authors in this research field have generally proposed that medicinal effects of plant extracts were due to synergism among the identified compounds, which include flavonoids, tannins, acethophenone derivatives, and sterols, among others. Moreover, due to this variety of useful chemicals, medicinal plants may usually act on various diseases, complicating then the correlation of a compound’s effect with a specific illness. Therefore, another route of investigation is the determination of pharmacological effects induced by specific compounds.
Phytosterols are plant derived steroid alcohols that are part of the cell membrane and they have structural resemblance to cholesterol, which enables them to displace low density lipoprotein cholesterol in the intestine (Choudary and Trans, 2011). Three phytosterols predominate in the human herbal nutrition: BS, campesterol, and stigmasterol (Saeidnia et al., 2014).
In the present report, we evaluated the effect of BS by using two types of acute inflammatory reactions, one of them corresponded to an immune reaction (reversed passive Arthus reaction in the rat) and the other to a non-specific reaction (mouse ear edema assay). Our results showed that BS had a prominent anti-inflammatory potential in both types of inflammation. In regard to the Arthus reaction, BS inhibited the rat paw edema induced by the reaction antigen/antibody from 50-70%. Moreover, the compound also decreased 46% the volume of the pleural fluid induced by the same reaction; besides, an increase of neutrophils by BS was also observed in the same assay. These results are concordant with both the inhibition of edema and the infiltration of inflammatory cells.
The reversed passive Arthus reaction is an immunologically induced inflammatory response characterized by immune complex deposition, complement fixation, polymorphonuclear leukocyte infiltration and tissue damage (Pflum and Graeme, 1979). The anti-inflammatory activity of BS inhibited the reversed passive Arthus reaction probably because of the inhibition of the mediators that give rise to the full Arthurs lesion; in this sense, other authors have provoked ovoalbumin-induced asthmatic mice, and found that BS mitigated the inflammation by eosinophil infiltration and mucus hypersecretion by goblet hyperplasia, and that various inflammatory biomarkers also decreased in the lung tissue and brochoalveolar lavage fluid (Yuk et al., 2007; Han et al., 2014; Liz et al., 2013; Mahajan and Metha, 2011; Loizou et al., 2010).
Furthermore, our results also showed that BS diminished the edema produced by TPA, as well as the mieloperoxidase activity, results which were related to a non-specific inflammatory reaction. In this respect, studies regarding the anti-inflammatory effect of BS have been published since the early 80s, where authors showed such an effect employing carrageenan-induced edema, cotton pellet implantation and Brewer’s yeast induced pyrexia in rats (Gupta et al., 1980). From that time onward a number of anti-inflammatory, antypiretic, antinociceptive, and immunomodulating activities have also been reported (Bouic, 2002; Saeidnia et al., 2014).
However, in vitro and in vivo experimental studies on the BS anti-inflammatory potential had been limited until recently, and they are now being increasingly advanced by the relevance that is being demonstrated for the studied compound. This type of research has shown a number of actions produced by BS: it was able to inhibit both vascular adhesion and intracellular adhesion molecule 1 expression in TNF-αlpha-stimulated human aortic endothelial cells; in 2,4-dinitrofluorobenzene-induced mouse dermatitis, it was able to significantly reduce the clinical severity by inhibiting the infiltration of inflammatory cells, and the levels of IgE, interleukin-4, and histamine in the serum of treated mice; also, in the mouse carrageenan-induced inflammation air pouch model, it was determined that the chemical promoted a time- and dose-dependent increase of the calcium uptake in activated neutrophils, and inhibited myeloperoxidase, adenosine deaminase, interleukin-1β and the tumor necrosis factor. The mentioned effects, as well as the findings of the present report, support the suggested beneficial effects of BS for a number of diseases, which include benign prostatic hyperplasia, colon and breast cancer, atherosclerosis, and gastrointestinal ulceration (Wilt et al., 1999; Choudary and Trans, 2011; Hewing and Fisher, 2012; Tovey, 2015).
Conclusion
The present study demonstrated the prominent anti-inflammatory potential of BS in two types of acute inflammatory reactions: one was an immune reaction expressed by the reversed passive Arthus reaction in the rat, and the other was a non-specific reaction shown in the mouse ear edema assay. Its effect was at least similar to the selected reference anti-inflammatory compounds.
Acknowledgments
The authors wish to thank Dr. Rebeca E. Franco y Bourland, Head of the Biochemistry Service, for her support.
References
- 1.Anyanwu GO, Ur-Rehman N, Onyeneke CE, Rauf K. Medicinal plants of the genus Anthocleista-A review of their ethnobotany, phytochemistry and pharmacology. J Etnopharmacol. 2015;175:648–667. doi: 10.1016/j.jep.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 2.Awad AB, Fink CS. Phytosterols as anticancer dietary components: evidence and mechanism of action. J Nutr. 2000;130:2127–2130. doi: 10.1093/jn/130.9.2127. [DOI] [PubMed] [Google Scholar]
- 3.Bailey PJ, Sturm A. Immune complexes and inflammation. A study of the activity of anti-inflammatory drugs in the reverse passive Arthus reaction in the rat. Biochem Pharmacol. 1983;32:475–481. doi: 10.1016/0006-2952(83)90526-9. [DOI] [PubMed] [Google Scholar]
- 4.Bouic PJ. Sterols and sterolins: new drugs for the immune system? Drug Discov Today. 2002;7:775–778. doi: 10.1016/s1359-6446(02)02343-7. [DOI] [PubMed] [Google Scholar]
- 5.Choudhary SP, Trans LS. Phytosterols: perspectives in human nutrition and clinical therapy. Curr Med Chem. 2011;18:4557–4567. doi: 10.2174/092986711797287593. [DOI] [PubMed] [Google Scholar]
- 6.Dwevedi A, Sharma K, Sharma YK. Cadamba: A miraculus tree having enormous pharmacological implications. Pharmacogn Rev. 2015;9:107–113. doi: 10.4103/0973-7847.162110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farkouh ME, Greenberg JD, Jeger RV, Ramanathan K, Verheugt FW, Chesebro JH, Kishner H, Hochman JS, Lay CL, Ruland S, Mellein B, Matchaba PT, Fuster V, Abramson SB. Cardiovascular outcomes in high risk patients with osteoarthritis treated with ibuprofen, naproxen or lumiracoxib. Ann Rheum Dis. 2007;66:764–770. doi: 10.1136/ard.2006.066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fry DE. Sepsis, systemic inflammatory response, and multiple organ dysfunction: the mystery continues. Am Surg. 2012;78:1–8. [PubMed] [Google Scholar]
- 9.Gaffo A, Saag KG, Curtis JR. Treatment of rheumatoid arthritis. Am J Health Syst Pharm. 2006;63:2451–2465. doi: 10.2146/ajhp050514. [DOI] [PubMed] [Google Scholar]
- 10.García-Martínez O, De Luna Bertos E, Ramos-Torrecillas J, Manzano-Moreno FJ, Ruiz C. Repercussions of NSAIDS drugs on bone tissue: the osteoblast. Life Sci. 2015;123:72–77. doi: 10.1016/j.lfs.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein JL. Challenges in managing NSAID-associated gastrointestinal tract injury. Digestion. 2004;691(Suppl):25–33. doi: 10.1159/000076554. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein JL, Cryer B. Gastrointestinal injury associated with NSAID use: a case study and review of risk factors and preventative strategies. Drug Healthc Patient Saf. 2015;7:31–41. doi: 10.2147/DHPS.S71976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta MB, Nath R, Shrivastava N, Shanker K, Kishor K, Bhargava KP. Anti-inflammatory and antipyretic activities of beta-sitosterol. Planta Med. 1980;39:157–163. doi: 10.1055/s-2008-1074919. [DOI] [PubMed] [Google Scholar]
- 14.Han NR, Kim HM, Jeong HJ. The ß-sitosterol attenuates atopic dermatitis-like skin lesions through down-regulation of TSLP. Exp Biol Med. 2014;239:454–464. doi: 10.1177/1535370213520111. [DOI] [PubMed] [Google Scholar]
- 15.He C, Li W, Zhang JJ, Qu SS, Li JJ, Wang LY. Determination of ß-sitosterol and total sterols content and antioxidant activity of oil in acai (Euterpe oleracea) Zhongguo Zhong Yao Za Zhi. 2014;39:4620–4624. [PubMed] [Google Scholar]
- 16.Heitzman ME, Neto CC, Winiarz E, Vaisberg AJ, Hammond GB. Ethnobotany, phytochemistry and pharmacology of Uncaria (Rubiaceae) Phytochemistry. 2005;66:5–29. doi: 10.1016/j.phytochem.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 17.Hewing B, Fisher EA. Preclinical mouse models and methods for the discovery of the causes and treatments of atherosclerosis. Expert Opin Drug Discov. 2012;7:207–216. doi: 10.1517/17460441.2012.660143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang GJ, Deng JS, Huang SS, Wang SY, Chang YS, Kuo YH. Bioassay guided isolation and identification of anti-inflammatory active compound from the root of Ficus formosana. J Agric Food Chem. 2013;61:11008–11015. doi: 10.1021/jf4033766. [DOI] [PubMed] [Google Scholar]
- 19.Jamkhande PG, Barde SR, Patwekar SL, Tidke PS. Plant profile, phytochemistry and pharmacology of Cordia dichotoma (Indian cherry): a review. Asian Pac J Trop Biomed. 2013;3:1009–1016. doi: 10.1016/S2221-1691(13)60194-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalafutova S, Juraskova B, Vlcek J. The impact of combinations of non-steroidal anti-inflammatory drugs and anti-hypertensive agents on blood pressure. Adv Clin Exp Med. 2014;23:993–1000. doi: 10.17219/acem/37357. [DOI] [PubMed] [Google Scholar]
- 21.Ling WH, Jones PJ. Dietary phytosterols: a review of metabolism, benefits and side effects. Life Sci. 1995;57:195–206. doi: 10.1016/0024-3205(95)00263-6. [DOI] [PubMed] [Google Scholar]
- 22.Liz R, Zanatta L, dos Reis GO, Horst H, Pizzolatti MG, Silva FR, Fröde TS. Acute effect of ß-sitosterol on calcium uptake mediates anti-inflammatory effect in murine activated neutrophils. J Pharm Pharmacol. 2013;65:115–122. doi: 10.1111/j.2042-7158.2012.01568.x. [DOI] [PubMed] [Google Scholar]
- 23.Loizou S, Lekakis I, Chrousos GP, Moutsatsou P. Beta-sistosterol exhibits anti-inflammatory activity in human aortic endothelial cells. Mol Nutr Food Res. 2010;54:551–558. doi: 10.1002/mnfr.200900012. [DOI] [PubMed] [Google Scholar]
- 24.Mahajan SG, Mehta AA. Suppression of ovalbumin-induced Th2-driven airway inflammation by ß-sitosterol in a guinea pig model of asthma. Eur J Pharmacol. 2011;650:458–464. doi: 10.1016/j.ejphar.2010.09.075. [DOI] [PubMed] [Google Scholar]
- 25.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 26.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Naressi MA, Ribeiro MA, Bersani-Amado CA, Zamuner ML, Costa WF, Tanaka CM, Sarragiotto MH. Chemical composition, anti-inflammatory, molluscidal and free-radical scavenging activities of the leaves of Ficus radicans ‘Variegata’ (Moraceae) Nat Prod Res. 2012;26:323–330. doi: 10.1080/14786411003754223. [DOI] [PubMed] [Google Scholar]
- 28.Perez DA, Vago JP, Athaide RM, Reis AC, Teixeira MM, Sousa LP, Pinho V. Switching off key signaling survival molecules to switch on the resolution of inflammation. Mediators Inflamm. 20142014:829851. doi: 10.1155/2014/829851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pflum LR, Graeme ML. The Arthus reaction in rats, a possible test for anti-inflammatory and antirheumatic drugs. Agents Action. 1979;9:184–189. doi: 10.1007/BF02024732. [DOI] [PubMed] [Google Scholar]
- 30.Piscoya J, Rodriguez Z, Bustamante SA, Okuhama NN, Miller MJ, Sandoval M. Efficacy and safety of freeze-dried cat’s claw in osteoarthritis of the knee: mechanism of action of the species Uncaria guianensis. Inflamm Res. 2001;50:442–448. doi: 10.1007/PL00000268. [DOI] [PubMed] [Google Scholar]
- 31.Saeidnia SIA, Gohari AR, Abdollahi M. The story of beta-sitosterol-A review. European J of Med Plants. 2014;4:590–609. [Google Scholar]
- 32.Serhan CN, Brain CD, Buckley CD, Gilroy DW, Haslett C, O’Neill LA, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:225–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 34.Susuki K, Ota H, Sasagawa S, Sakatani T, Fujikura T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem. 1983;132:345–352. doi: 10.1016/0003-2697(83)90019-2. [DOI] [PubMed] [Google Scholar]
- 35.Susuki R, Kohno H, Sugie S, Tanaka T. Dose-dependent promoting effect of dextran sodium sulfate on mouse colon carcinogenesis initiated with azoxymethane. Histol Histophathol. 2005;20:483–492. doi: 10.14670/HH-20.483. [DOI] [PubMed] [Google Scholar]
- 36.Szalai AJ, Digerness SB, Agrawal A, Kearney JF, Bucy RP, Niwas S, Kilpatrick JM, Babu YS, Volanakis JE. The Arthus reaction in rodents: species-specific requirement of complement. J Inmunol. 2000;164:463–468. doi: 10.4049/jimmunol.164.1.463. [DOI] [PubMed] [Google Scholar]
- 37.Tapiero H, Townsend DM, Tew KD. Phytosterols in the prevention of human pathologies. Biomed Pharmacother. 2003;57:321–325. doi: 10.1016/s0753-3322(03)00104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tovey FI. Role of dietary phospholipids and phytosterols in protection against peptic ulceration as shown by experiments on rats. World J Gastroenterol. 2015;21:1377–1384. doi: 10.3748/wjg.v21.i5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uddin G, Rauf A, Siddiqui BS, Muhammad N, Khan A, Shah SU. Anti-nociceptive, anti-inflammatory and sedative activities of the extracts and chemical constituents of Diospyros lotus. L Phytomedicine. 2014;21:954–959. doi: 10.1016/j.phymed.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Wilt TJ, MacDonald R, Ishani A. beta-sitosterol for the treatment of benign prostatic hyperplasia: a systematic review. BJU Int. 1999;83:976–983. doi: 10.1046/j.1464-410x.1999.00026.x. [DOI] [PubMed] [Google Scholar]
- 41.Young JM, De Joung LM. Cutaneous models of inflammation for evaluation of topical and systemic pharmacological agents. In: Spector S, Back N, editors. Modern methods in pharmacology. Pharmacological methods in the control of inflammation Alan R Liss. New York: 1989. pp. 215–231. [Google Scholar]
- 42.Yuk JE, Woo JS, Yun CY, Lee JS, Kim JH, Song GY, Yang EJ, Hur IK, Kim IS. Effects of lactose-beta-sitosterol and beta-sitosterol on ovalbumin-induced lung inflammation in actively sensitized mice. Int Immunopharmacol. 2007;7:1517–1527. doi: 10.1016/j.intimp.2007.07.026. [DOI] [PubMed] [Google Scholar]


