Abstract
Background:
Kundmannia anatolica Hub.-Mor. is an endemic specie of Apiaceae diversified in Turkey. Several parts of the plant may contain essential oils in different quantity which can be influenced by environmental factors, mainly altitude. The aim of this study was to test whether there is any altitude effect on volatile chemical constituents of essential oil obtained from the fruits of K. anatolica growing spontaneously in different altitudes of Lakes Region in Turkey.
Materials and Methods:
K. anatolica was collected in 2015 at different altitudes (400, 820, 1002 and 1560 m) of Lakes Region Turkey. The fruits of the plants were distilled for 3 h using a Clevenger type apparatus according to the British Pharmacopiea (1980). Essential oils of the fruits were collected using hydro distillation method and analyzed by GC-MS/FID.
Results:
Essential oil contents of fruits increased by corresponding increase in altitude level. Predominant compounds were a-Pinene (27.87-61.94%) and β-Pinene (24.92-36.46%) of the total oil of K. anatolica. Other important compounds were α-Thujene (2.66-8.15%), l-Limonene (1.83-8.23%), α-Phellandrene (1.85-5.01%) and these compounds were higher in low altitudes.
Conclusion:
Altitude change affected the terpenoid biosynthesis and oxygenated monoterpenes generally and were greatest when low; while sesquiterpene constituents were greatest at high altitudes. The influence of altitude seems to be an important factor for yielding the chemical profile of K. anatolica essential oils. Thus, the location of the plant must be taken into account depending on the intended use.
Keywords: Altitude, essential oil composition, Kundmannia anatolica Hub.-Mor
Introduction
Apiaceae (Umbelliferae) is one of the largest families of flowering plants with more than 3500 species spread across 455 genera (Arbizu et al., 2014). In Turkey, it is represented by 101 genera belonging to 485 species included in 511 taxa and endemism rate of the family is about 37.3% with 181 species (Özhatay et al., 2009; Baser and Kırımer, 2014). Lake District of Turkey located on the boundary between Mediterranean and Iran-Turan geographic regions is very rich in the number of plant species, particularly in Apiaceae family. It was reported that more than 70 taxa included in the Apiaceae family has been found in the floristic studies conducted in the region (Davis, 1988; Sağıroğlu and Duman, 2006; Yıldırımlı and Selvi, 2006; Pimenov and Leonov, 2014). Members of the family Apiaceae are usually comprised of aromatic plants having a distinctive flavour attributed to high concentration of volatile terpenic compounds (Kılıc, 2014a, b).
Kundmannia is a typical genus of the Apiaceae and are well represented in Spain, France, Italy, Turkey, Syria and North Africa. The genus Kundmannia consists of five species; K. anatolica, K. syriaca, K. insulana, K. pastinacifolia and K. sicula (Djarri et al., 2008). The genus Kundmannia is represented by K. anatolica and K. syriaca in Turkey with 100% endemism (Özhatay et al., 2009). Kundmannia anatolica Hub.-Mor. is located at elevations between 550-650 m in Southeast Anatolia region of Turkey (Davis et al., 1988; Çinbilgel and Gökçeoğlu, 2010). However, our recent field observations in the region showed the species spread at elevations between 400-1560 m in Lakes Region of Turkey in C3 Antalya, Isparta and Burdur (Karadogan et al., 2016). Similar to the other taxa of the genus Kundmannia, K. anatolica produces essential oils, which can be extracted from different parts of the plant, including the fruit, root and flower as well as stem and leafs, with significant diversity in yield and composition. In general, the relative composition of essential oils varies remarkably with geographical position, climate conditions and several other factors (Arruda et al., 2012). There are many studies related to the essential oils in Apiaceae species. To our best knowledge, there is no study performed on the chemical composition of fruit from K. anatolica, but essential oil compounds and antimicrobial activity of aerial parts of the specie has been recently reported by Paksoy et al. (2016). Some studies on the essential oil composition of fruit and aerial parts from K. sicula growing in Algeria and Tunisia have been carried out (Djarri et al., 2008; Abderrahim et al., 2013; Faidi et al., 2014).
However, this study was aimed at testing possible altitudinal effect on volatile chemical constituents of essential oil obtained from the fruits of K. anatolica growing spontaneously in different altitudes of Lakes Region in Turkey. This is the first report on the composition of essential oils from K. anatolica fruits.
Materials and Methods
Plant Material
K. anatolica was collected in 2015 at different altitudes of Lakes Region Turkey (Table 1). All samples were collected at full flowering stage for species identification and fruit maturing stage for essential oil analyses. Plant materials were identified by Dr. Hasan ÖZÇELİK of the Department of Biology at Süleyman Demirel University, Isparta according to “Flora of Turkey” (Davis et al., 1988) and voucher specimens (63.36.1.1) and deposited in the Herbarium GUL, at Suleyman Demirel University, Turkey. As a description of K. anatolica: Erect, branched, glabrous perennial with terete very finely ridged stems, up to 100 cm. Basal leaves ovate-triangular, 10-30 χ 5-10 cm, 1-2-pinnate; ultimate segments ovate, pinnatifid or irregularly toothed, 2-3 χ 1.5-3 cm, petiole 8-15 cm. Upper cauline leaves 1-pinnate with linear-oblong to linear segments 30 χ 1.5-2 mm and broad sheathing petioles. Umbel rays 20-30, subequal, 1.5-3.5 cm. Bracts up to 12, narrow oblong-setaceous, 2-5 mm long. Umbellules 20-25 flowered; pedicels 1.5-4 mm. Bracteoles 3-6, linear, 1-5 mm. Petals yellow. Fruit oblong-cylindrical, 3.5-1.5 χ 1-1.5 mm, not attenuate towards apex, glabrous, ridges of mericarps broadly filiform, vittae numerous, irregularly arranged. Stylopodium conical.
Table 1.
Location of the analyzed populations of K.anatolica Hub.-Mor.
| Localities | Habitat | Latitude | Longitude | Altitude (m) | |
|---|---|---|---|---|---|
| Sutfuler District - Qandir Village | (A) | Macchie and red pine forest | 37°45’ 58"7 | 30°94’87"5 | 400 |
| Altinyayla District | (B) | Macchie and red pine forest | 37°06’ 09"6 | 29°53’31"3 | 820 |
| Atabey District | (C) | Pastures and open forests. | 37°94’ 62"2 | 30°63’60"8 | 1002 |
| Egirdir District-Barla Village | (D) | Pine forest opens | 38°08’ 92"7 | 30°78’97"7 | 1560 |
Essential oil analysis
Seeds of the plants were distilled for 3 h using a Clevenger type apparatus according to the British Pharmacopiea (1980). The oil obtained was stored at -4°C in a sealed vial until chemical analyses.
Gas chromatography-mass spectrometry
GC-MS analysis was performed on QP5050 GC-MS equipped with FID detector. The GC was equipped with CP-Wax 52 CB capillary column (50 m x 0.32 mm; film thickness = 0.25 μm) and helium was used carrier gas with flow rate of 1 mL/min. The GC oven was heated from 60°C to 230°C at a rate of 3°C/min, the final temperature was then maintained during 20 min. The injector was maintained at a temperature of 250°C. Injection volume 0.1 mL of 1% solution prepared in n-hexane; split ratio 20:1. The mass spectrometer was operating in EI mode at 70 eV with mass scan range of 40- 450 amu. Identification of constituents was done on the basis of RI (determined with reference to homologous series of n-alkanes C8-C25, under identical experimental condition), MS library search (NIST 08MS Library (Version 2.0 f) and Wiley MS 9th edition), and by comparison with MS literature data (Adams, 2007). The relative amounts of individual components were calculated based on GC peak area (FID response) without using correction factor.
Data analyses
The data was statistically analyzed using a completely randomized design (CRD) using SPSS (19.0) software. Means of the main constituents of the essential oils were compared by Duncan’s multiple range test at %5 of significance level.
Results and Discussion
Fresh fruits of K. anatolica afforded yellowish oils, with yields (mean of four replicates) 0.7% (A), 0.9% (B), 1.0% (C) and 1.2% (D) (v/w), respectively. The essential oil components of K. anatolica growing at different localities are given in Table 2. In the results of GC/FID; 38, 46, 31 and 35 components were identified and accounted for 98.89%, 98.51%, 97.17% and 98.28% of the total essential oils for A, B, C and D localities, respectively.
Table 2.
Percentage composition of the essential oil of the fruits of K.anatolica Hub.-Mor.
| No | RI | Chemical Component | % | |||
|---|---|---|---|---|---|---|
| A | B | C | D | |||
| 1 | 899 | n-Nonane | - | 0.05 | - | - |
| 2 | 925 | Tricyclene | 0.24 | 0.25 | - | 0.07 |
| 3 | 929 | α-Thuj ene | 8,15 | 8,02 | 2,66 | 3,46 |
| 4 | 938 | α-Pinene | 31,91 | 27,87 | 61,94 | 59,05 |
| 5 | 955 | Camphene | 1,61 | 1,87 | 1,02 | 1,16 |
| 6 | 959 | Verbenone | - | 0,04 | - | - |
| 7 | 949 | α-Phellandrene | 5,01 | 4,7 | 1,85 | 2,08 |
| 8 | 970 | β-Pinene | 36,46 | 33,07 | 24,92 | 26,98 |
| 9 | 980 | 2-Amylfuran | - | 0,07 | - | - |
| 10 | 986 | β-Myrcene | 1,17 | 2,41 | 0,2 | 0,26 |
| 11 | 993 | Heptanol<2,6-dimethyl-2-> | - | - | 0,06 | 0,05 |
| 12 | 1005 | l-Phellandrene | 0,23 | 0,24 | 0,06 | 0,06 |
| 13 | 1007 | α-Terpinene | 0,06 | 0,07 | - | - |
| 14 | 1011 | β-Cymene | 0,16 | 0,49 | 0,04 | 0,04 |
| 15 | 1025 | l-Limonene | 7,31 | 8,23 | 1,83 | 2,35 |
| 16 | 1033 | cis-Ocimene | 0.06 | 0.1 | - | 0.02 |
| 17 | 1041 | 1,5-Heptadiene | 0.16 | 0.07 | 0.11 | 0.07 |
| 18 | 1055 | γ-Terpinene | 0.16 | 0.82 | 0.04 | 0.05 |
| 19 | 1062 | Octilin | - | 0.5 | 0.4 | 0.08 |
| 20 | 1082 | trans-2-caren-4-ol | - | 0.09 | - | - |
| 21 | 1088 | α-Terpinolene | 0.12 | 0.25 | - | - |
| 22 | 1115 | α-Campholenealdehyde | 0.24 | 0.26 | 0.05 | 0.05 |
| 23 | 1142 | trans-Pinocarveole | 0.25 | 0.28 | 0.06 | - |
| 24 | 1149 | Verbenol | 0.36 | 0.37 | - | - |
| 25 | 1164 | βinocarvone | 0.13 | 0.16 | - | - |
| 26 | 1175 | β-Mentha-1,5-dien-8-ol | 0.05 | 0.07 | - | - |
| 27 | 1186 | α-Terpineol | 0.19 | 0.27 | - | 0.04 |
| 28 | 1192 | Myrtenal | 0.1 | 0.14 | - | - |
| 29 | 1195 | Linalylpropionate | 0.13 | 0.22 | - | 0.04 |
| 30 | 1198 | β-Allylanisole | - | 0.14 | - | - |
| 31 | 1200 | trans-Myrtenol | 0.2 | 0.18 | - | - |
| 32 | 1207 | 3-Tetradecene, (Z) | 0.12 | 0.14 | 0.25 | 0.2 |
| 33 | 1213 | n-Octylacetate | - | - | 0.28 | - |
| 34 | 1217 | trans-Carveol | 0.05 | 0.07 | - | - |
| 35 | 1221 | trans-3-Caren -2-ol | 0.05 | 0.06 | - | - |
| 36 | 1236 | d-Carvone | - | 0.4 | 0.01 | - |
| 37 | 1242 | Thymolmethyleether | 0.03 | 0.17 | 0.09 | 0.05 |
| 38 | 1261 | (E)-Caryophyllene | - | - | 0.06 | - |
| 39 | 1265 | α-Muurolene | 0.07 | 3.77 | 0.07 | 0.06 |
| 40 | 1278 | Endobornylacetate | 0.13 | 0.2 | - | 0.04 |
| 41 | 1322 | Methylgeranate | 0.26 | 0.42 | 0.1 | 0.22 |
| 42 | 1441 | α-Humulene | 0.1 | 0.54 | - | 0.11 |
| 43 | 1457 | Limoneneoxide | 0.24 | 0.13 | 0.08 | 0.09 |
| 44 | 1464 | Octylbutyrate | - | 0.5 | 0.06 | - |
| 45 | 1485 | Germacrene-D | - | 0.1 | 0.11 | 0.03 |
| 46 | 1501 | α-Farnesene | 0.07 | - | - | 0.51 |
| 47 | 1504 | β-Bisabolene | 1.66 | 0.21 | 0.16 | 0.26 |
| 48 | 1516 | Myristicin | 1.47 | - | 0.09 | 0.13 |
| 49 | 1520 | δ-Cadinene | - | 0.26 | - | 0.06 |
| 50 | 1524 | 2-Methyldodecan- 1-ol | - | - | - | 0.05 |
| 51 | 1532 | (-)-a-Santalal | - | - | 0.05 | 0.07 |
| 52 | 1539 | α-cadinene | - | - | - | 0.07 |
| 53 | 1584 | 3-Hexadecene, (Z) | 0.11 | 0.12 | 0.27 | 0.25 |
| 54 | 1623 | γ-epoxy-elemene | - | - | 0.04 | - |
| 55 | 1786 | 9-Octadecene, (E) | 0.07 | 0.12 | 0.21 | 0.17 |
| Total identified | 98.89 | 98.51 | 97.17 | 98.28 | ||
| Monoterpene hydrocarbons | 92.65 | δ8.44 | 94.56 | 95.58 | ||
| Oxygenated monoterpenes | 2.15 | 3.08 | 0.67 | 0.45 | ||
| Sesquiterpene hydrocarbons | 1.96 | 4.89 | 0.42 | 1.11 | ||
| βhenolic compounds | 1.5 | 0.17 | 0.18 | 0.22 | ||
| Fatty acid methyl esters, Alcohols, Alkenes and Alkanes | 0.3 | 0.88 | 0.79 | 0.67 | ||
| Others | 0.63 | 1.93 | 1.34 | 0.92 | ||
| Total | 31 | 38 | 46 | 35 | ||
Note: The percentages are based on GC peak areas. RI, retention index
The essential oil consists mainly of monoterpene hydrocarbons (88.44-95.58%) followed by sesquiterpene hydrocarbons (0.42-4.89%) and oxygenated monoterpenes (0.45-3.08%). Moreover, levels of phenolic compounds in A essential oil were also detected in a higher percentage (1.5%) than other localities (0.17-0.22%). Essential oils from A and B localities contain higher oxygenated monoterpenes (2.15% for A oil and 3.08% for B oil) and sesquiterpene hydrocarbons (1.96% A oil and 4.89% for B oil). The predominant compounds were a-Pinene accounting for 27.87% (B) to 61.94% (C) and and β-Pinene 24.92% (C) to 36.46 % (A) of the total oil of K. anatolica. Other major compounds were α-Thujene (2.66 to 8.15%), l-Limonene (1.83 to 8.23%), α-Phellandrene (1.85 to 5.01%) and camphene (1.02 to 1.87%). The results indicated that there were significant differences between the contents of main components: a-Pinene (p<0.01), β-Pinene (p≤0.05), a-Phellandrene (p<0.05), a-Thujene (p<0.01) and l-Limonene (p<0.01) in oils (Table 3).
Table 3.
The main components of essential oil of K. anatolica Hub.-Mor. from four altitudes
| Component | A | B | C | D | Anova |
|---|---|---|---|---|---|
| α-Thujene | 8,15±0,30 a | 8,02±0,37 a | 2,66±0,22 c | 3,46±0,27 b | ** |
| α-Pinene | 31,91±3,28 b | 27,87±3,25 b | 61,94±2,47 a | 59,05±1,10 a | ** |
| α-Phellandrene | 5,01±1,37 a | 4,7±1,10 a | 1,85±0,26 b | 2,08±0,31 b | * |
| β-Pinene | 36,46±1,98 a | 33,07±2,09 a | 24,92±2,54 b | 26,98±1,14 b | * |
| l-Limonene | 7,31±1,32 a | 8,23±1,40 a | 1,83±0,21 b | 2,35±0,51 b | ** |
Values of major compounds are given as means ± SD.
at p<0.05;
: significant at p<0.01.
Among the monoterpenic hydrocarbons a-Pinene predominates in the oil from C (61.94%) and D (59.05%) and it is also present at high concentrations in A oils (31.91%) and B oils (27.87%). On the other hand, essential oils form A and B have higher concentration of β-Pinene (36.46%, 33.07%, respectively) than C and D (24.92%, 26.98%, respectively) (Table 2). Essential oils from the populations of A and B have a high level of a-Thujene (8.15%, 8.02%), l-Limonene (7.31%, 8.23%) and a-Phellandrene (5.01%, 4.7%), which are about two to three-fold as high compared with the same class of compounds identified in the oil from C and D. Concerning the content of sesquiterpene hydrocarbons, the proportion of the compounds changes drastically. In fact, a-Muurolene content was higher in B (3.77%) than other localities (0.07%) although all localitie contain similar compounds (Table 2).
It is interestingly noticeable that some of the compounds, such as a-Thujene, a-Phellandrene, β-Pinene, β-Myrcene, l-Limonene and γ-Terpinene decreased in quantity with altitudinal increase, while constituents like a-Pinene increase in quantity with a decrease in altitude. Interestingly, several sesquiterpene constituents like Heptanol <2,6-dimethyl-2->, (-)-a-Santalal and a-cadinene, which were observed at high altitude, were not observed in low altitude. In contrast, some oxygenated monoterpenes such as Verbenol, Pinocarvone, p-Mentha-1,5-dien-8-ol, Myrtenal, trans-Myrtenol, trans-Carveol and trans-3-Caren -2-ol were only detected at low altitudes (Table 2).
To our knowledge, there are few reports on essential oil compounds of Kundmannia species (Djarri et al., 2008; Faidi et al., 2014; Paksoy et al., 2016). Essential oil compositions of K. sicula from Algerian were analyzed previously by Djarri et al (2008) and those from Tunisian by Faidi et al (2014). Essential oil compositions and antimicrobial activity of K. syriaca and K. anatolica from Turkey have been reported for the first time by Paksoy et al (2016). However, Paksoy et al (2016) identified the essential oil constituents from the aerial parts of K. anatolica and reported that spathulenol (17.4%), curdione (4.6%), apritone (4.4%) and germacrene-d (4.5%) were the major components. Faidi et al (2014) reported that the major compounds of essential oils from the inflorescences with mature seeds of K. Sicula growing in Tunisia were identified as isocurcumenol (10.1%), hexadecanoic acid (10.9%), spathulenol (3.4%), 10-epi-geudesmol (.5%), a-cubebene (6.1%) and trans-dihydro occidentalol (6.6%).
Although spathulenol was the major compound in K. anatolica, K. syriaca and K. sicula collected from different localities, it was not detected in essential oils of seed samples of K. anatolica in the present study. Taking into account previous studies and comparing present results with those previously reported in relation to the analysis of K. anatolica essential oils from Turkey; we noticed a clear variation in major components present in essential oils from different plant parts and populations of K. anatolica. Thus, spathulenol detected as the main constituent in the essential oils derived from aerial parts of the species growing in Turkey, whereas a-Pinene and β-Pinene considered as one of the prominent chemical constituents of the seed essential oils in our study. Additionally, it was found that differences between major compounds of Kundmannia species when compared to each other. These variations can be explained by the differences of mainly part of plant utilized, genotype, the plant ecotype, geographic origins or adaptive process to some particular and local ecological conditions (Chalchat et al., 1995; Djarri et al., 2008; Faidi et al., 2014). The origin of changes should be sought mainly in the differences in the nature of soil on the one hand and solar radiation on the other. Both factors involve the activation or inactivation of certain enzymatic groups, leading to the predominance of a particular biosynthetic pathway (Arruda et al., 2012).
Figure 1.
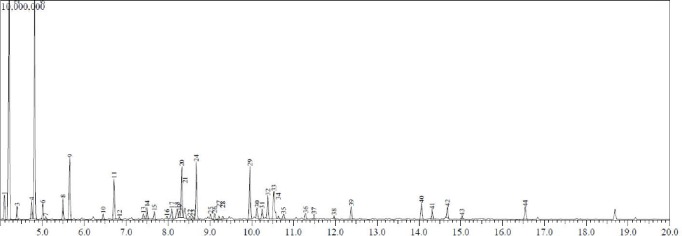
Gas Chromatogram with mass spectral data for location A essential oil components. On the chromatogram x-axis is retention time (RT) in minutes and on the y-axis is abundance in arbitrary units.
Figure 2.

Gas Chromatogram with mass spectral data for location B essential oil components. On the chromatogram x-axis is retention time (RT) in minutes and on the y-axis is abundance in arbitrary units.
Figure 3.
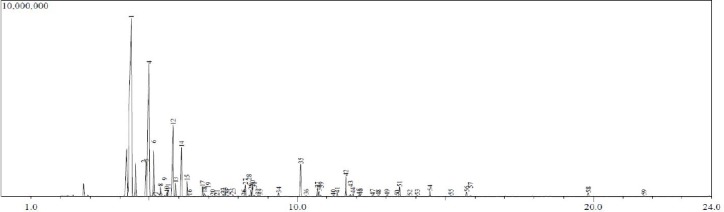
Gas Chromatogram with mass spectral data for location C essential oil components. On the chromatogram x-axis is retention time (RT) in minutes and on the y-axis is abundance in arbitrary units.
Figure 4.
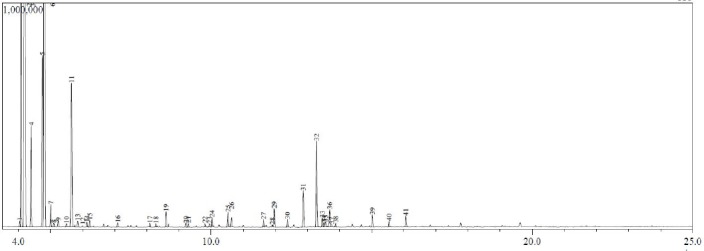
Gas Chromatogram with mass spectral data for location D essential oil components. On the chromatogram x-axis is retention time (RT) in minutes and on the y-axis is abundance in arbitrary units.
Altitudinal changes sea level is an important factor influencing terrestrial ecosystems. Thus, significant change in altitude level can bring about corresponding changes in temperature, relative humid, wind speed, available water, and radiation rate. As a result, changes in environmental conditions will alter many eco-physiological reactions in plant bodies. Secondary metabolites of K. anatolica are strongly influenced by edaphic factors, climate and geologic formation. The study area is located in transition area of Mediterranean climate and Mediterranean-Steppe climate. Climatic characteristics can show significant variations at short distances due to topographic structure. Sample areas at high altitudes located in Mediterranean-Steppe climate have calcareous soil textures with semi dry cool and semi mountainous humid climates. Low altitudes sample areas are located in Mediterranean climate with in general red and decalcified soils and very humid climates (Fakir et al., 2015). Hence, it is expected that changing ecological niches will alter the essential oil compositions and its components, as seen in the present study especially in the case of the constituents like: a-Pinene, β-Pinene, a-Phellandrene, a-Thujene and l-Limonene.
Conclusion
Essential oils of fruits from K. anatolica collected in Lakes Region of Turkey contain high percentage monoterpene-rich volatile fractions, a-Pinene and β-Pinene. These compounds used as base compound in many areas including pharmaceuticals, foods and medicine. On the other hand, altitude affected the terpenoid biosynthesis and oxygenated monoterpenes generally and were greater at low altitude, while sesquiterpene constituents were greater at high altitudes. In conclusion, the influence of altitude seems to be an important factor for improved yield in chemical profile of K. anatolica essential oils, and thus the location of the plant must be taken into account depending on the intended use. The results of the study revealed that this specie might be evaluated for its rich content of a-Pinene and β-Pinene by different industries.
Acknowledgements
The authors would like to thank to Dr. Hasan ÖZÇELİK from Süleyman Demirel University Biology Department for identifying the plants. This work is funded by TUBİTAK (Turkey Bilimsel ve Teknolojik Araştırma Kurumu, grant number: 113O284).
References
- 1.Abderrahim O, Martin G.J, Abdelaziz A. Botanical identification and ethno-medicinal uses of some underground part of medicinal plants collected and traded in Marrakech region. J. Med. Plants Res. 2013;7:2165–2169. [Google Scholar]
- 2.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. IL, USA: Allured Publications: Carol Stream; 1995. [Google Scholar]
- 3.Arbizu C, Ruess H, Senalik D, Simon P.W, Spooner D.M. Phylogenomics of the carrot genus (Daucus, Apiaceae) Am. J. Bot. 2014;101:1666–1685. doi: 10.3732/ajb.1400106. [DOI] [PubMed] [Google Scholar]
- 4.Arruda M, Viana H, Rainha N.R, Rosa J.S, Nogueira J.M.F, Barreto M.C. Antiacetylcholinesterase and antioxidant activity of essential oils from Hedychium gardnerianum Sheppard ex Ker-Gawl. Molecules. 2012;17:3082–3092. doi: 10.3390/molecules17033082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Başer K.H.C, Kırımer N. Essential Oil of Anatolian Apiaceae. Nat. Vol. Essent. Oils. 2014;1(1):1–50. [Google Scholar]
- 6.British Pharmacopoeia. HM Stationary Office, London 1980;II [Google Scholar]
- 7.Chalchat J.C, Garry R.P, Muhayimana A. Essential oil of Tagetes minuta from Rwanda and France: chemical composition according to harvesting location, growth stage and part of plant extracted. Journal of Essential Oil Research. 1995;7:375–386. [Google Scholar]
- 8.Çinbilgel İ, Gökçeoğlu M. Flora of Altınbeşik Cavern National Park (İbradı-Akseki, Antalya/Turkey) Biological Diversity and Conservation. 2010;3(3):85–110. [Google Scholar]
- 9.Davis P.H, Mill R.R, Tan K. Flora of Turkey and the East Aegean Islands. Suppl. Vol. 10. Edinburgh: 1988. [Google Scholar]
- 10.Djarri L, Medjroubi K, Akkal S, Elomri A, Seguin E, Groult M.L, Vérité P. Variability of two essential oils of Kundmannia sicula (L.) DC. a traditional Algerian medicinal plant. Molecules. 2008;13:812–817. doi: 10.3390/molecules13040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faidi K, El Mokni R, Joshi R.K, Hammami S, M’Henni M.F, Mighri Z. Comparative study on the chemical constituents of essential oils from different organs of the Sicilian Kundmannia (Kundmannia sicula L.) DC. (Apiaceae) growing spontaneously in Tunisia. Nat. Prod. Res. 2014;28:1680–1684. doi: 10.1080/14786419.2014.935942. [DOI] [PubMed] [Google Scholar]
- 12.Fakir H, Karatepe Y, Gürlevik N. Soil Characteristics of Some Endemic Geophytes growing around Lake District, Turkey. Research Journal of Biotechnology. 2015;10(12):51–58. [Google Scholar]
- 13.Karadogan T, Şanlı A, Özçelik H, Baydar H. Determination of the Species of the Umbelliferae Family and their Essential Oil Contents in Isparta and Burdur Province Located in Lakes Region (TUBİTAK project report) 2015 [Google Scholar]
- 14.Kılıc Ö. Essential oil composition and potential usefulness of two Bupleurum L. species from Turkey. Turk J Agric. Nat. Sci. 2014a;1:445–449. [Google Scholar]
- 15.Kılıc Ö. Essential oils composition of two endemic Umbelliferae herbs growing wild in Turkey. J Agric. Sci. Technol. A. 2014b;4:435–442. [Google Scholar]
- 16.Özhatay N, Akalın E, Özhatay E, Ünlü S. Rare and endemic taxa of Apiaceae in Turkey and their conservation significance. J. Fac. Pharm. 2009:40. [Google Scholar]
- 17.Paksoy M.Y, Diraz E, Digrak M, Tutar E, Karaman S. Essential oil composition and antimicrobial activity of two endemic Kundmannia SCOP. species from Turkey. Industrial Crops and Products. 2016;79:39–46. [Google Scholar]
- 18.Pimenov M.G, Leonov M.V. The Asian Umbelliferae biodiversity database (ASIUM) with particular reference to South-West Asian taxa. Turk J Bot. 2004;28:139–145. [Google Scholar]
- 19.Sağıroğlu M, Duman H. Ferula parva Freyn & Bornm. (Apiaceae) A contribution to an Enigmatic Species from Turkey. Turk. J. Bot. 2006;30:399–404. [Google Scholar]
- 20.Yıldırımlı Ş, Selvi B. A new genus Dumaniana Yıldırımlı&Selvi and a new species D. gelendostensis Yıldırımlı&Selvi (Apiaceae) from Isparta, Southwest Anatolia, Turkey and new combinations. Ot Sistematik Botanik Dergisi. 2006;13:1–8. [Google Scholar]


