Abstract
Background:
Gastric cancer is a serious health issue caused by H. pylori and claims more lives in developing and undeveloped countries. Hence, the need for a natural drug with several pharmacological activities with no adverse effect are highly recommended. The target of this study was to verify the anti-H. pyloric efficacy of mangiferin (MF) on H. pylori-infected AGS cells.
Materials and methods:
AGS cells were co-cultured with H. pylori and incubated with increased concentration of MF (10, 20, 50 and 100 μg/mL) or amoxicillin (AMX) and DMSO (control) group to assess its anti-H. pyloric effect by checking inhibitory zone, bacterial drug sensitivity test (MIC and MBC), adhesion and invasive property and various inflammatory markers.
Results:
Co-culturing of H. pylori-infected AGS cells with MF (100 μg) considerably increased (p<0.05) the inhibitory zone as well as substantially lowered (p<0.05) in the levels of MBC and MIC with decreased adhesion and invasive property in a dose-dependent manner and thus endorsing its anti H. pyloric activity and are almost equivalent to antibiotic AMX. Meanwhile, inflammatory markers such as NF-κΒ subunit p65, interleukins-1β, IL-8, and TNF-α were also markedly suppressed (p<0.01) on treatment with MF. In addition, the protein expression of inflammatory enzymes like COX-2 and iNOS were notably downregulated (p<0.05) in AGS cells incubated with MF.
Conclusion:
We, concluded that MF treatment with H. pylori-infected AGS cells significantly suppressed the adhesion and invasion process as well as deactivated NF-p65 thereby blocking inflammatory response and thus lower the incidence of gastric carcinoma.
Keywords: Gastric cancer, mangiferin, AGS cells, H. pylori, amoxicillin, anti-inflammatory
Introduction
Helicobacter pylori (H. pylori) is a helical shaped gram negative carcinogenic bacteria, commonly colonized in upper gastrointestinal (GI) tract, especially in the stomach. It has been estimated that 50 % of global population are infected with H. pylori due to its highly contagious nature (Yong et al., 2015). The pathogenesis of H. pylori infection is extremely complex owing to its multi-virulence factors like Cytotoxin-associated gene A (CagA), Cag pathogenicity island (Cag PAI), lipopolysaccharide (LPS), urease and Vacuolating cytotoxin A (VacA). Nevertheless, sustained inflammation, immunomodulation and oxidative stress (in the host) are the major reason for H. pylori-related diseases like peptic ulceration, gastritis, gastric mucosa-associated lymphoid tissue lymphoma and gastric cancer (Lawal et al., 2014; Sgouras et al., 2015). Gastric cancer is a serious problem caused by H. pylori since it claims more lives in developing and undeveloped countries attributing to poor sanitation facility. Hence, World Health Organization (WHO) categorize H. pylori as a group I human carcinogen. Moreover, several epidemiological studies reported that H. pylori are the major risk factor for gastric cancer (de Martel et al., 2013; Guggenheim and Shah, 2013).
Attachment of H. pylori with the host cell (epithelial cells) is the pivotal step in the initiation of H. pylori infection process (Geethangili et al., 2010). Once H. pylori attached to cell, it releases various virulence factors especially CagA, LPS and Vag A, which trigger the inflammatory process by elevating infiltration of subepithelial lamina by macrophages and neutrophils, are the putative contributor for a reactive oxygen species (ROS) generation (Zaidi et al., 2015; Naito and Yoshikawa, 2002). ROS activate the oxidant-sensitive transcription factor like nuclear factor kappa-B (NF-κΒ). Which in turn stimulate the expression of the inflammatory genes which codes for inflammatory enzymes like cyclooxygenase-2 (COX-2) and inducible oxide synthase (iNOS) as well as pro-inflammatory cytokines such as interleukins (IL-1β, IL-8) and tumor necrosis factor (TNF-α) (Sokolova et al., 2013; Polk and Peek, 2010; Handa et al., 2010). At present several antibiotics in the form of triple or tetra regimen are available in markets such as amoxicillin, clarithromycin, and metronidazole with one proton pump inhibitor (PPI) for treating gastric ulcer or gastritis caused by H. pylori infection, but they showed less inhibitory activity due to drug resistance as well as leads to numerous adverse events (Schinor et al., 2007). Hence, a natural medicinal plant with anti- H. pylori property with no adverse effect are badly required (Wang, 2014).
Mangiferin (MF) is a polyphenol (xanthonoid) commonly found in the bark, fruits, and leaves of Mangiferin indica L an in the roots of Salacia chinensis. MF (1,3,6,7-tertrahydroxy-C2-β-D-glucoside) is a C-glucosyl-xanthone with condensed aromatic ring and glucose moiety (Lv et al., 2016). MFt a broad spectrum of pharmaceutical activities such as antioxidant, anti-inflammation, anti-microbial, anti-invasive, anti-tumor (Wauthoz et al., 2007; Singh et al., 2012; Anand et al., 2015). Enormous studies had indicated that MF might act as an anti-carcinogen in various cancer cell line model attributing to its antioxidant, anti-inflammatory, anti-proliferative (cytotoxic), anti-adhesive, and pro-apoptotic effect (Rajendran et al., 2015; du Plessis-Stoman et al., 2011). Moreover, the gastroprotective activity of mangiferin was reported in an animal model (Carvalho et al., 2007). Therefore, we speculate that MF might exhibit anti-H. pyloric effect and might act as an anti-gastric carcinogenic agent to confirm it, we investigated the chemotherapeutic effect of MF by assessing antioxidant, anti-inflammatory and anti-proliferative activity on H. pylori-induced AGS cell model.
Materials and Methods
Chemicals
Mangiferin (98.0%), trypsin, ethylene-diamine-tetraacetic acid (EDTA), glutamine, cell lysis buffer, urease reagent, isopropanol, dimethyl sulfoxide (DMSO), and formalin were purchased from Sigma-Aldrich (MO, USA). F12 Hans medium, fetal bovine serum (FBS), penicillin-streptomycin, amoxicillin (AMX), phosphate buffered saline (PBS) were bought from Thermo Fisher Scientific Gibco (NY, USA).
H. pylori and Cell Culture
H. pylori (CagA+/VacA+ strain) was obtained from American Type Culture Collection (ATCC; MD, USA) and cultured as reported previously by Rao et al., (2012). Human gastric adenocarcinoma AGS cells were also procured from ATCC and cultured using F12 K (hams media) nutrition mixture medium with 10% fetal bovine sera, 1% penicillin-streptomycin, 1% glutamine at 37°C in 5% CO2. AGS cells were sub-cultured with 0.25% trypsin/0.53 EDTA (trypsinization) and seeded in 96-well cell culture plates.
Determination of antibacterial activity (zone of inhibition)
The anti-bacterial (anti-H. pylori) activity of MF was assessed by disk agar diffusion method by the method of Castillo-Juarez and others, (2007). Shortly, 100 μL of H. pylori cell suspension (1>×108 colony forming units/mL) was smeared onto nutrient agar plates. The sterile paper disks of 6 mm were saturated with different concentration (10, 20, 50 and 100 μg) of MF (based on the preliminary analysis; data not shown) dissolved in DMSO and impregnated on the agar surface (plate). DMSO alone was used as positive control (only H. pylori; HP group), and antibiotic amoxicillin (AMX, 10 μg/ml) was used as a standard for comparison. After 48 h of incubation at 37°C under the microaerophilic conditions, the zones of inhibition were measured and expressed as mm.
Evaluation of bacterial drug sensitivity (dilution test)
H. pylori suspension (1 x 108 CFU/mL) was combined with 10 μL of MF (various concentration) cultured in anaerobic conditions with 10% FBS at 37°C for 24 h (test suspension). Then, 100 μL of test suspension was mixed with DMSO reagent (different dilution rate and the turbidity) and were measured by a microplate reader at the absorbance of 600 nm for the minimum inhibitory concentration (MIC). Similarly, 100 μL of test suspension was added with an equal volume of urease reagent which consists of 3 mM PBS, 7 μg of phenol and 2% urea and incubated for 4 h at 37°C and measured by a microplate reader at the absorbance of 560 nm for the minimum bactericidal concentration (MBC).
Assessing adhesion and invasion activity
The adhesion or invasion activity of MF was determined as elaborated previously (Rao et al., 2012). In brief, AGS cells (5 χ 105/mL) were cultured in 24 well plates, and 500 μL of medium was added to each well and left for overnight at 37°C. Then, H. pylori suspension (10 μL) and various concentration of MF (10, 20, 50, and 100 μg) or AMX (10 μg) were added and left overnight at 37°C in 5% CO2 incubator. Then the non-adherent bacteria were washed off, using PBS. Finally, to the adhered cell (H. Pylori) urease reagent (500 μL) were added and quantified (using microplate reader at 560 nm). AGS cells infected only with H. pylori (Positive control) without any addition of MF or AMX and utilized to establish 100% of adhesion or invasion. Both the adhesion or invasion activity were expressed as the percentage of relative inhibition of H. pylori invasion or adhesion as compared to positive control.
Inflammatory markers
AGS cell suspension (1 χ 105/mL) were seeded in a petri dish (10 cm) and co-cultured with increased concentrations of MF for 48 h. Then, the cells were isolated and lysed using lytic buffer solution and centrifuged at 600 xg for 15 min at 4°C. Thus, prepared cell suspension was used for evaluating various inflammatory markers. AGS cell suspension was treated with Nuclear/Cytosolic Fractionation Kit (Cell Biolabs Inc, SD, USA) to separate cytosolic and nuclear fraction. Separated nuclear cell fraction was used to determine the NF-κΒ free p65 subunit using an ActivELISA kit (Imgenex, CA, USA). Also, the levels of pro-inflammatory cytokines such as IL-1β and IL-8 as well as TNF-α were quantified by ELISA method using commercial kits from Thermo Fisher Scientific (MA, USA) based on manufacturer’s protocol.
Western Immunoblot
The AGS cell suspension was prepared as previously indicated in inflammatory marker section. From the cell suspension, the total protein extract (for COX-2 and iNOS protein expression) were prepared and quantified using BCA protein assay kit (Sigma-Aldrich, MO, USA). An equal volume of protein (50 μg) was transferred to each well of 8% sodium dodecyl sulfate-polyacrylamide gel and then electrotransferred onto a polyvinylidene difluoride (PVDF) membrane.
Then, the membrane was blocked with TBS (Tris-buffered saline) solution which contains 0.3 % of Tween 20, 5 % of non-fat (skimmed) milk and 150 mM of sodium chloride. Followed by a wash with PBS and the membrane were probed with primary antibody-like anti-COX-2 and anti-iNOS polyclonal antibody (1:1000; Abcam, Cambridge, UK) or monoclonal β-actin (1:400; Santa Cruz Biotechnology, CA, USA) at 4°C for 10 h. The unbound antibodies were washed with TBS. Secondary antibody was incubated with horseradish peroxidase (HRP) linked antibody (1:500; Santa Cruz Biotechnology, CA, USA) for 1h at 37°C and washed again with TBS to remove unbound secondary antibody. β-actin will serve as a loading control for comparison with test samples. The membrane with the conjugated antibodies was detected by the enhanced chemiluminescence (Pierce; IL, USA) system, and the protein expression in the form of bands were measured using Bio-Rad image analyzer.
Statistical analysis
All the experiments were carried out in triplicate and expressed as mean ± standard deviation (SD). The difference between the MF treated groups or AMX group, and control group or HP group were analyzed using Student t-test by Statistical Package for the Social Sciences software (SPSS; Ver 23) from IBM Corporation (NY, USA). p value less than 0.05 was deemed as statistically significant.
Results and Discussion
Based on the recent survey and review (Rajendran et al., 2015), we would confirm that this is the first study conducted to explore the anti-H. pyloric activity of MF on AGS cell line. The disk agar diffusion method was employed to investigate the anti-H. pyloric activity of MF (with different concentration) on H. pylori-infected AGS cells. Figure. 1 depict the inhibitory zone (in diameter; mm) of different concentration of MF against H. pylori in AGS cells. MF 10 showed the least inhibitory zone size with 4 mm, whereas MF 100 showed better inhibitory zone size with 14 mm on compared with other MF concentrations. However, AMX exhibit highest inhibitory zone size with 15 mm. Nevertheless, no significant difference was noted between MF 100 and AMX in the inhibitory zone size. Thus, this disk agar diffusion technique inferred that MF significantly inhibited (p<0.05) the H. pylori in a dose-dependent manner and thereby indicating it’s anti- H. pylori activity. Previously, Singh and his coworkers, (2009) proved that mangiferin can exert antibacterial activity against different bacterial strain, but the exact mechanism behind the antibacterial activity is still obscure.
Figure 1.
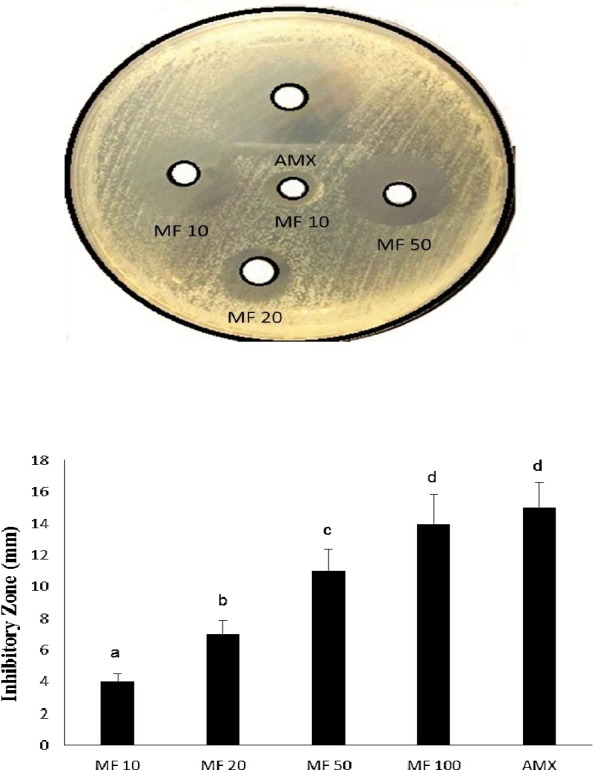
Inhibitory activity of MF on H. pylori-infected AGS cells. Values are expressed as the means ± SD. Data with different letters were significantly different (p<0.05).
Similar, to the previous parameters MF 100 (58% and 64%) presented greatest (p<0.05) anti-adhesive and anti-invasive effect. However, AMX (79% and 82%) showed the best anti-adhesive and anti-invasive effect than MF. Singh et al., (2012) also pointed out that mangiferin exerted antibacterial activity against 7 different bacterial strain and hypothesized that it would be due to higher anti-adhesive and anti-invasive activity.
Enormous studies had pointed out that inflammation is one the key event for the initiation and progression of gastric cancer related to H. pylori infection (Zaidi et al., 2015; Naito and Yoshikawa, 2002). IL-1β, IL-8, and TNF-α is a crucial proinflammatory cytokine that triggers inflammatory response during H. pylori infection. The H. pylori infected epithelial cells produce IL-8, which initiates the migration of neutrophils and thereby stimulating other pro-inflammatory cytokines (IL-1β and TNF-α) and ROS, which are responsible for the mucosal damage and leads to gastritis and eventually results in gastric cancer (Huang et al., 2014).
The bacterial drug sensitivity test (MIC and MBC) were used to determine the bactericidal and bacteriostatic value of any drug. MBC value is defined as the lowest concentration of sample that completely destroy the H. pylori (bactericidal). Also, MIC is the lowest concentration of sample that prevents the growth of H. pylori (bacteriostatic). Thus, finding both MIC and MBC is a crucial parameter for determining the effective dose for suppressing H. pylori infection (new drug) and thereby lower the risk of gastric cancer (Andrews, 2001). Hence, both MIC and MBC were assessed to check the effective dose for MF to exert its anti- H. pyloric activity. MF 100 displayed lowest MIC and MBC with 600 μg/mL, in comparison with other doses. AMX showed least MIC and MBC level with 400 μg/mL (Table 1). Similarly, Stoilova et al., (2005) also indicated that mangiferin exerts antibacterial activity with almost similar MBC value of standard antibiotics AMX. We did a cytotoxicity study (MTT assay), which showed that AGS cells proliferation were slightly reduced upon treatment with different concentration of MF (but no significant changes) and thus indicating that MF may trigger apoptosis or necrosis process in AGS cell but in the lower level (Data not shown).
Table 1.
Effect of MF on Bacterial drug sensitivity test (MIC and MBC) on H. pylori-infected AGS cells
| Groups | MIC (µg/mL) | MBC µg/mL) |
|---|---|---|
| MF 10 | 2500a | 2800a |
| MF 20 | 2100a | 2500a |
| MF 50 | 1500b | 1700b |
| MF 100 | 600c | 600c |
| AMX | 400c | 400c |
Values are expressed as the means ± SD. Data with different letters were significantly different (p=0.05).
As mentioned earlier, attachment of H. pylori to AGS cells (host epithelial cell) is a crucial step in the initiation of infection. The attached H. pylori then tend to form a colony and effectively deliver the virulent factors like CagA into AGS cells and leads to various pathological events like excessive ROS generation as well as an initiate inflammatory cascade (Geethangili et al., 2010; Zaidi et al., 2015). Hence, adhesion and subsequent invasion process of H. pylori have to be evaluated to examine the anti-gastric carcinoma property of any drug (since H. pylori adhesion and invasion may lead to gastric cancer). The urease reagent was used to assess the percentage of relative inhibition of H. pylori invasion or adhesion to AGS cells. The effect of MF on adhesion and invasive property on H. pylori-infected AGS cells were shown in figure 2.
Figure 2.
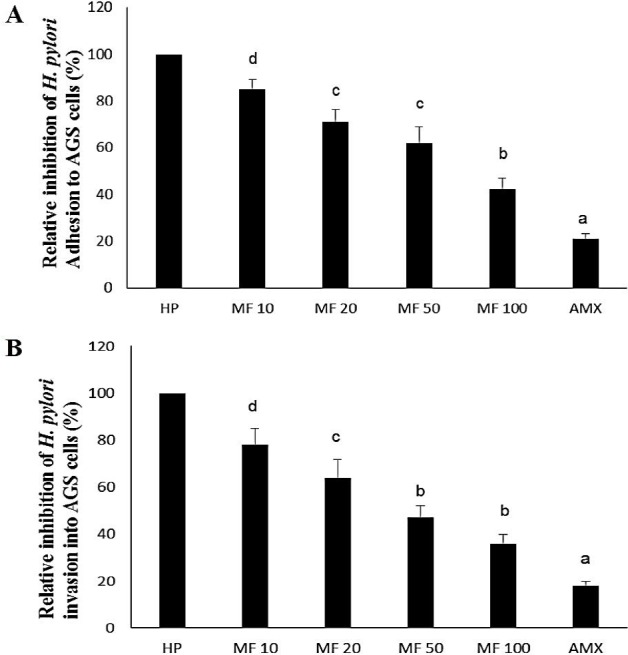
Effect of MF on adhesion (A) and invasive (B) property on H. pylori-infected AGS cells. Values are expressed as the means ± SD. Data with different letters were significantly different (p<0.05).
Table 2 represent the effect of MF on various inflammatory markers on H. pylori-infected AGS cells by ELISA method. A substantial inclination in the levels of a nuclear fraction of the NF-p65 subunit, IL-1β, IL-8, and TNF-α were observed in AGS cell seeded with H. pylori group (HP group). As brief in the previous section, attachment of H. pylori to AGS cells, release CagA an important virulent factor (immunomodulatory), which in turn activate NF-κΒ (dimer p50 and p65 subunits).
Table 2.
Effect of MF on inflammatory markers on H. pylori-infected AGS cells
| NF-p65 | IL-10 | IL-8 | TNF-α | |
|---|---|---|---|---|
| Groups | (pg/mL) | (pg/mL) | (ng/mL) | (pg/mL) |
| Control | 31.34±3.32 | 17.01±1.43 | 1.78±0.16 | 27.45±2.09 |
| HP | 108.53±10.87a** | 78.22±9.22a** | 11.56±1.35a** | 98.83±1.12a** |
| MF 10 | 93.12±7.44b* | 71.63±8.13b* | 9.97±1.04b* | 86.27±10.37b* |
| MF 20 | 79.57±8.40b** | 58.65±6.53 b** | 7.25±0.85b** | 71.54±7.01b** |
| MF 50 | 56.38±7.27b** | 42.46±3.56b** | 5.94±0.95b** | 57.35±6.05b** |
| MF 100 | 42.70±5.64b** | 30.60±3.77b** | 3.22±0.48b** | 40.63±6.32b** |
| AMX | 28.45±3.32b** | 21.63±4.53b** | 2.03±0.28b** | 29.46±4.10b** |
Values are expressed as the means ± SD. Statistical significance (p value):
p<0.01,
p<0.05
(a) compared with the control (DMSO) group, (b) compared with the HP group. These activate NF-p50, and p65 subunits translocate into nucleus from cytosol to transcribe several downstream inflammatory factor genes like IL-1β, IL-8, and TNF-α (Sokolova et al., 2013; Polk and Peek, 2010; Yong et al. 2015). Hence, the levels of NF-p65, IL-1β, IL-8, and TNF-α were significantly elevated (p<0.01) in H. pylori-infected AGS cells than control (non-infected AGS cells). Moreover, CagA can also stimulates reactive oxygen species (ROS) and thus activate NF-κB and contributes to inflammatory response (Zaidi et al., 2015; Naito and Yoshikawa, 2002). Meanwhile, H. pylori-infected AGS cells treated with different concentrations of MF exert a significant reduction (p<0.01) in the levels of the NF-p65 subunit, IL-1β, IL-8 and TNF-α in comparison with HP
Values are expressed as the means ± SD. Statistical significance (p value): **p<0.01, *p<0.05 (a) compared with the control (DMSO) group, (b) compared with the HP group. These activate NF-p50, and p65 subunits translocate into nucleus from cytosol to transcribe several downstream inflammatory factor genes like IL-1β, IL-8, and TNF-α (Sokolova et al., 2013; Polk and Peek, 2010; Yong et al. 2015). Hence, the levels of NF-p65, IL-1β, IL-8, and TNF-α were significantly elevated (p<0.01) in H. pylori-infected AGS cells than control (non-infected AGS cells). Moreover, CagA can also stimulates reactive oxygen species (ROS) and thus activate NF-κΒ and contributes to inflammatory response (Zaidi et al., 2015; Naito and Yoshikawa, 2002). Meanwhile, H. pylori-infected AGS cells treated with different concentrations of MF exert a significant reduction (p<0.01) in the levels of the NF-p65 subunit, IL-1β, IL-8 and TNF-α in comparison with HP group. It probably due to antioxidant and anti-inflammatory activity of MF would contribute to the inhibition of NF-p65 translocation from cytosol to nucleus (Lv et al., 2016). Also, Garrido-Suarez and his colleagues (2010), demonstrated that mangiferin could effectively suppress the NF-κΒ activation and thus downregulated the transcription of various pro-inflammatory cytokines.
Furthermore, the protein expression of inflammatory enzymes such as iNOS and COX-2 were evaluated using SDS-PAGE technique. Figure 3 portrait the protein expression pattern of iNOS and COX-2 on H. pylori -infected AGS cells. The levels of iNOS and COX-2 were exponentially upregulated (p<0.01) on AGS cell infected with H. pylori (HP group) on an equivalent with the control group (non-infected AGS cells). It might be due to activation of NF-κΒ, which subsequently upregulate iNOS and COX-2 expression. However, H. pylori-infected AGS cells co-cultured with increased concentration of MF, greatly downregulated (p<0.01) the protein expression levels of iNOS and COX-2. Thus, MF demonstrating it anti-inflammatory activity it would be due to suppression of excessive ROS generation and NF-κΒ activation. Our studies are in corroboration with the results of Beltran et al., (2004), who also demonstrated that mangiferin treatment markedly downregulated the expression of iNOS and COX-2 in vascular smooth muscle cells as well as in rat model. Also, Shin and others (2008), indicated that mangiferin could substantially downregulate iNOS and COX-2 expression in LPS induced RAW-264.7 cells.
Figure 3.
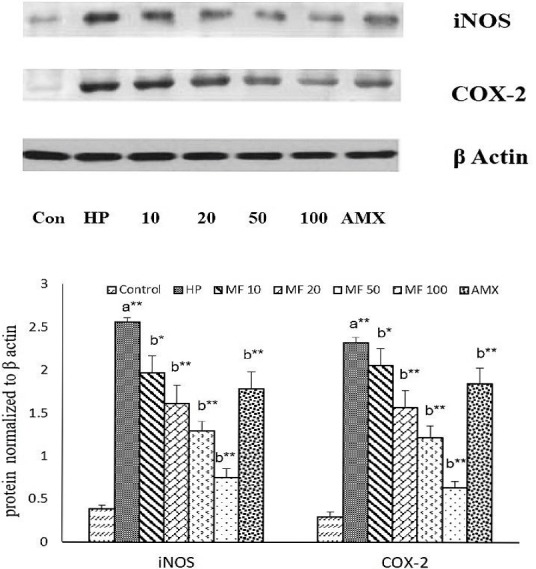
Effect of MF on protein expression of iNOS and COX-2 on H. pylori-infected AGS cells. Values are expressed as the means ± SD. Statistical significance (p value): **p<0.01, *p<0.05 (a) compared with the control (DMSO) group, (b) compared with the HP group.
From the above statement, it is clear that activation of NF-κΒ and subsequent pro-inflammatory markers are the major contributors to H. pylori-related diseases like gastritis, peptic ulceration, and gastric cancer. Therefore, MF attributing to its anti-inflammatory activity and antioxidative activity can notably inhibit NF-κΒ activation in H-pylori infected AGS cells and thus used for treating H. pylori-related diseases especially gastric cancer. Current experiment has few limitations such as omitting cytotoxicity parameters especially pertaining to apoptotic markers (TUNNEL) and cell cycle phases alterations by MF and well as metastasis (MMPs) and virulent factor gene or protein expression. Exclusion of those parameters would hamper the quality of our study. In addition, the precise mechanism for the anti-H. pyloric activity has to be explored by checking several signaling molecules related to inflammation would strengthen our experiment.
Conclusion
The present finding demonstrated that MF (100 μg) treatment with H. pylori-infected AGS cells significantly suppressed the adhesion and invasion process with increased inhibitory zone and less MIC and MBC levels (due to anti- H. pyloric activity). Moreover, suppression of NF-μ and thereby blocking other pro-inflammatory cytokines and inflammatory genes would considerably halt the inflammatory response (anti-inflammatory activity) and thereby lower the risk of gastric cancer. Also, preclinical and are required to endorse our present implications and can be considered as a chemotherapeutic agent against gastric cancer. In subsequent studies, the precise mechanism for anti- H. pylori activity of MF has to be explored.
Acknowledgement
The current experiment was funded by The Affiliated Hospital of Qingdao University Medical College. We greatly acknowledge Dr. Chung, for helping in statistical analysis.
References
- 1.Anand G, Ravinanthan M, Basaviah R, Shetty A. V. In vitro antimicrobial and cytotoxic effects of Anacardium occidentale and Mangifera indica in oral care. J. Pharm. Bioall. Sci. 2015;7(1):69–74. doi: 10.4103/0975-7406.148780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews J. M. Determination of minimum inhibitory concentrations. Journal Antimicrob. Chemo. 2001;48:5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 3.Beltrán A. E, Alvarez Y, Xavier F. E, Hernanz R, Rodriguez J, Núñez A. J, Alonso M. J, Salaices M. Vascular effects of the Mangifera indica L. extract (Vimang) Eur J. Pharmacol. 2004;499(3):297–305. doi: 10.1016/j.ejphar.2004.07.073. [DOI] [PubMed] [Google Scholar]
- 4.Carvalho A. C, Guedes M. M, de Souza A. L, Trevisan M. T, Lima A. F, Santos F. A, Rao V. S. Gastroprotective effect of mangiferin, a xanthonoid from Mangifera indica, against gastric injury induced by ethanol and indomethacin in rodents. Planta Medica. 2007;73(13):1372–1376. doi: 10.1055/s-2007-990231. [DOI] [PubMed] [Google Scholar]
- 5.Castillo-Juárez I, Rivero-Cruz F, Celis H, Romero I. Anti-Helicobacter pylori activity of anacardic acids from Amphipterygium adstringens. J Ethnopharmacol. 2007;114(1):72–77. doi: 10.1016/j.jep.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 6.de Martel C, Forman D, Plummer M. Gastric cancer: epidemiology and risk factors. Gastroenterol. Clin. North Am. 2013;42(2):219–240. doi: 10.1016/j.gtc.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 7.du Plessis-Stoman D, du Preez J. G. H, Van de Venter M. Combination treatment with oxaliplatin and mangiferin causes increased apoptosis and downregulation of NFrfS in cancer cell lines. Afr. J. Tradit. Complement. Altern. Med. 2011;8(2):177–184. doi: 10.4314/ajtcam.v8i2.63206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garrido-Suárez B. B, Garrido G, Delgado R, Bosch F, Rabí M. D. C. A Mangifera indica L. extract could be used to treat neuropathic pain and implication of mangiferin. Molecules. 2010;15(12):9035–9045. doi: 10.3390/molecules15129035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geethangili M, Fang S. H, Lai C. H, Rao Y. K, Lien H. M, Tzeng Y. M. Inhibitory effect of Antrodia camphorata constituents on the Helicobacter pylori-associated gastric inflammation. Food Chem. 2010;119(1):149–153. [Google Scholar]
- 10.Guggenheim D. E, Shah M. A. Gastric cancer epidemiology and risk factors. J. Surg. Oncol. 2013;107(3):230–236. doi: 10.1002/jso.23262. [DOI] [PubMed] [Google Scholar]
- 11.Handa O, Naito Y, Yoshikawa T. Helicobacter pylori: a ROS-inducing bacterial species in the stomach. Inflamm. Res. 2010;59(12):997–1003. doi: 10.1007/s00011-010-0245-x. [DOI] [PubMed] [Google Scholar]
- 12.Huang H. L, Ko C. H, Yan Y. Y, Wang C. K. Antiadhesion and anti-inflammation effects of noni (Morinda citrifolia) fruit extracts on AGS cells during Helicobacter pylori infection. J. Agric. Food Chem. 2014;62(11):2374–2383. doi: 10.1021/jf405199w. [DOI] [PubMed] [Google Scholar]
- 13.Lawal T. O, Olorunnipa T. A, Adeniyi B. A. Susceptibility testing and bactericidal activities of Theobroma cacao Linn. (cocoa) on Helicobacter pylori in an in vitro study. J. Herb. Med. 2014;4(4):201–207. [Google Scholar]
- 14.Lv X. L, Wang Y, Hu B, Kang L, Xin J, Zhang G. Y. Neurotherapeutic effect of mangiferin against hypoxic-ischemic encephalopathy in neonatal rats. Afr. J. Tradit. Complement. Altern. Med. 2016;13(2):229–236. [Google Scholar]
- 15.Naito Y, Yoshikawa T. Molecular and cellular mechanisms involved in Helicobacter pylori-induced inflammation and oxidative stress 1, 2. Free. Rad. Biol. Med. 2002;33(3):323–336. doi: 10.1016/s0891-5849(02)00868-7. [DOI] [PubMed] [Google Scholar]
- 16.Polk D. B, Peek R. M. Helicobacter pylori gastric cancer and beyond. Nat. Rev. Cancer. 2010;10(6):403–414. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajendran P, Rengarajan T, Nandakumar N, Divya H, Nishigaki I. Mangiferin in cancer chemoprevention and treatment: pharmacokinetics and molecular targets. J. Recept. Sig. Transd. 2015;35(1):76–84. doi: 10.3109/10799893.2014.931431. [DOI] [PubMed] [Google Scholar]
- 18.Rao Y. K, Lien H. M, Lin Y. H, Hsu Y. M, Yeh C. T, Chen C. C, Lai C. H, Tzeng Y. M. Antibacterial activities of Anisomeles indica constituents and their inhibition effect on Helicobacter pylori -induced inflammation in human gastric epithelial cells. Food Chem. 2012;132(2):780–787. [Google Scholar]
- 19.Schinor E. C, Salvador M. J, Ito I. Y, Dias D. A. Evaluation of the antimicrobial activity of crude extracts and isolated constituents from Chresta scapigera. Braz. J. Microbiol. 2007;38(1):145–149. [Google Scholar]
- 20.Sgouras D. N, Trang T. T. H, Yamaoka Y. Pathogenesis of Helicobacter pylori Infection. Helicobacter. 2015;20(S1):8–16. doi: 10.1111/hel.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin J. S, Kim D. H, Lee K. T. Articles: Mangiferin isolated from the rhizome of Anemarrhena asphodeloides inhibits the LPS-induced nitric oxide and prostagladin E2 via the NF-κΒinactivation in inflammatory macrophages. Nat. Prod. Sci. 2008;14(3):206–213. [Google Scholar]
- 22.Singh S. K, Kumar Y, Kumar S. S, Sharma V. K, Dua K, Samad A. Antimicrobial evaluation of mangiferin analogues. Ind. J. Pharm. Sci. 2009;71(3):328–331. doi: 10.4103/0250-474X.56023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh S. K, Tiwari R. M, Sinha S. K, Danta C. C, Prasad S. K. Antimicrobial evaluation of mangiferin and its synthesized analogues. Asian. Pac. J. Trop. Biomed. 2012;2(2):S884–S887. [Google Scholar]
- 24.Sokolova O, Borgmann M, Rieke C, Schweitzer K, Rothkötter H. J, Naumann M. Helicobacter pylori induces type 4 secretion system-dependent, but CagA-independent activation of M3s and NF-rfS/RelA at early time points. International J. Med. Microbiol. 2013;303(8):548–552. doi: 10.1016/j.ijmm.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Stoilova I, Gargova S, Stoyanova A, Ho I. Antimicrobial and antioxidant activity of the polyphenol mangiferin. Herba Polonica. 2005;51:1–2. [Google Scholar]
- 26.Wang Y. C. Medicinal plant activity on Helicobacter pylori related diseases. World. J. Gastroenterol. 2014;20(30):10368–10382. doi: 10.3748/wjg.v20.i30.10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wauthoz N, Balde A, Balde E. S, Van Damme M, Duez P. Ethnopharmacology of Mangifera indica L. bark and pharmacological studies of its main C-glucosylxanthone, mangiferin. Int. J. Biomed. Pharma. Sci. 2007;1(2):112–119. [Google Scholar]
- 28.Yong X, Tang B, Li B. S, Xie R, Hu C. J, Luo G, Qin Y, Dong H, Yang S. M. Helicobacter pylori virulence factor CagA promotes tumorigenesis of gastric cancer via multiple signaling pathways. Cell Comm. Sig. 2015;13(1) doi: 10.1186/s12964-015-0111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaidi S. F, Muhammad J. S, Usmanghani K, Sugiyama T. Pharmacological ins and outs of medicinal plants against Helicobacter pylori: A review. Pak. J. Pharm. Sci. 2015;28(3):1171–1176. [PubMed] [Google Scholar]


