Abstract
Background:
Starch-degrading amylase enzyme is important in biotechnological applications as food, fermentation, textile, paper and pharmaceutical purposes. The aim of current study to isolate alkaline thermostable α-amylase bacteria and then study the composition of medium and culture conditions to optimize cells growth and a-amylase production.
Materials and Methods:
Thermophilic amylase producing bacterium was isolated from local hot water-springs in Gazan city Saudi Arabia.
Results:
Phylogenetic analysis of 16 S rRNA sequence for the strain revealed that the strain have the same sequence of Bacillus subtilis. Maximum amylase production was observed, when B. subtilis cultured in medium containing starch at concentration 0.5%, and 10 g/L peptones as nitrogen source at pH 8.5 in when it was incubated for 48 h at 45°C.
Conclusion:
An amylase-producing bacterium were isolated from hot-spring water and was identified as B. subtilis. Amylase produced from B.subtilis had optimum temperature 45°C and pH 8.5 in shaking media.
Keywords: Thermophilic bacteria, Bacillus subtilis, α-amylase
Introduction
Amylases (E.C. 3.2.1.1.) are enzymes that degraded the internal α-1,4-O--glycosidic bonds in the starch to give diverse products including mono sugar glucose and oligo-sugar maltose. α - Amylases are obtained from various origins like plant, animal, bacterial, actinomycetes and fungal (Zambare, 2011). On the other hand, the microbial as fungal and bacterial amylases, are used for the industrial purposes. The advantages of microbial amylases are low cost, consistency, space, less production time and the modification and optimization process are very easy (Burhan et al., 2003). Fungi belonging to Aspergillus genus and thermophilic fungal such as Talaromyces emersonii, Thermomonospora fusca and Thermomyces lanuginosus have been mostly employed for a-amylase production (Bunni et al., 1989 and Jensen, Olsen, 1992). Thermophilic amylases enzyme was produced by Thermostable Actinomycetes such as Thermomonospora and Thermoactinomyces and some type of Bacillus sp. (Ben Massoud et al., 1999). Prakash& Jaiswal, (2009), Raul et al., (2014) and Saxena et al. (2007) referred that a commercial production of thermostable and alkaline α-amylases were produced by mesophile Bacillus sp. as Bacillus subtilis, Bacillus stearothermophilus, Bacillus licheniformis, and Bacillus amyloliquefaciens which have large applications. Setyorini et al. (2006) reported that good yield of alkaline and thermotolerant amylases were produced by Bacillus spp., Bacillus licheniformis, and Bacillus halodurans. The utilization of thermostable amylases in the industrial processes have many advantages of include reduce the contamination risk and temperature control cost, increasing substrates solubility, a decreasing viscosity which increases mixing and pumping (Lin et al., 1998). The highly thermophilic amylolytic enzymes that are active at (90°C) would directly effective for industrial processes. On the other hand, the production thermophilic a- amylase will need new technique for culturing of thermophilic bacteria (Leveque et al., 2000). The composition, concentration of media and cultural conditions must be optimized to give highly product of bacterial growth and amylase (Srivastava and Baruah, 1986, Bezbaruah et al., 1994). Generally, amylolytic enzymes that starch-degrading enzyme have wide roles in biotechnological industries as food, fermentation, textile, paper, pharmaceutical and sugar industries (Lin et al., 1997, Pandey et al., 2000).
The present study aimed to isolate alkaline thermostable α-amylase bacteria then studying the composition of medium and culture conditions to optimize bacterial growth and a-amylase production
Materials and Methods
Chemicals
The media components include yeast or beef extract, starch and peptone were analytical grade obtained from Sigma Co. (St. Louis, Mo).
Microorganism
Isolation the bacterial strain
Tested water samples were collected from Al-Ain Alhara (or Al- Khawaba), a hot spring located at Gazan, KSA. Samples were collected in sterile glass vessel that keep the temperature of the water samples constant. Fifty ml Mineral Starch Medium(MSM) was inoculated with 5 ml of water sample, then incubated at 45°C with shaking for 48 h.
An inoculum was transferred on Mineral Starch agar Medium (MSMA) agar media, incubated at 45°C for 48 h.
Media
Mineral Starch Medium (MSM) (g/l)
K2HPO4,0.5 g; KH2PO4,1g; NH4Cl, 1g; MgSO4.7H2O, 0.2g; Starch, 5g.
pH was adjusted to pH 8.0. 20 g/L agar was added in solid medium (MSMA).
Maintenance medium
The Bacillus subtilis was maintained on the medium containing the following composition (g/l): peptone, 10.0 g; beef extract, 10.0 g; NaCl, 5.0 g; starch, 10.0 g and agar 20 g. The slants were inoculated with B. subtilis and incubated at 45°C for 48 h. The slants were stored at 0-4°C in refrigerators.
Inoculum medium
The inoculum medium used for the growth of B.subtilis containing the following component (g/l) : beef extract, 5.0 g; peptone, 5.0 g; NaCl, 0.5 g; yeast extract, 5.0 g and starch, 1.0 g. The pH was adjusted to 7.0. The flask 250 ml containing 50 ml was inoculated with a slant of B. subtilis and incubated at 45°C on a rotary shaker (120 rpm) for 24h. Basal medium
The basal medium for α-amylase production contained (g/l): peptone, 10.0 g; yeast extract, 0.2 g; soluble starch, 10.0 g; NaCl, 2.0 g; MgSO4.7H2O, 0.5 g; KH2PO4, 3.0 g and CaCl2, 0.5 g. The pH was adjusted to 8.0. Fifty ml of basal medium were inoculated at 10% (v/v) level and incubated at 45°C on a rotary shaker for 48h. The enzyme activity was determined at different intervals times.
Optimizing the conditions for high yield of α-amylase by B. subtilis
Maximum yield of the enzyme was obtained by studying the impact of media composition and its conditions. Different types of carbon sources as starch, glycogen, maltose, glucose, xylose or glycerol was used as solo carbon source at concentration 10 g/L.
Optimum starch and nitrogen concentrations
Different concentrations of starch (0- 20 g/L) and nitrogen sources (3 g/L) were added to the basal medium. This nitrogen sources used (peptone, yeast extract, beef extract, malt extract, yeast+ peptone(1.5+1.5g), beef extract +peptone, NaNO3,(NH4)2SO4, peptone+NaNO3, peptone+(NH4)2SO4).
Effect of pH and temperature
The basal medium was adjusted at pH range (5.5 - 9.0) and at different temperature rang (25- 70°C).
The optimum time for α -amylase production
Fermentation medium with some modification 10 g/L starch as carbon source, Peptone (3 g/L), pH was adjusted to 8.5. to study the optimum time for α -amylase production, two flasks were taken every 24h from 0 - 7 days for determination of the growth of cells, final pH, and α-amylase activity.
Analytical Methods
Estimation of starch degradation
To monitor the rate of digestion of potato starch by B. subtilis, 1% potato starch granules was added to nutrient agar. The starch medium was inoculated with B. subtilis, the plates were incubated for 24 h at 45°C, these plates were flooded with Gram’s iodine solution. A clearing zones around the bacterial colonies were indicated the amylolytic activity.
Biochemical identification of bacterial strain
Several biochemical tests were done according Holt et al., (1994).
Phylogenetic analysis
Analysis of genes sequences data of bacteria was done by phylogenetic method using with reference sequences homology from the NCBI database using MEGA 4 programme (Tamura et al., 2007). Phylogenetic trees were constructed by distance matrix-based cluster algorithms viz. unweighted pair group method with averages unweighted pair group method using arithmetic averages (UPGMA), neighbour-joining, maximum likelihood, and maximum-parsimony analysis as described elsewhere (Rai et al., 2010). The trees were rooted using Escherichia coli strain K 12 MG1655 (accession no. U00096) as out group. The stability of trees obtained from above cluster analysis was assessed by using BOOTSTRAP program in sets of 1000 re-samplings (MEGA 4).
Growth estimation
Cells growth were measured spectrophotometrically at 600 nm. The blank was fermentation medium without inoculation. Absorbency converted to dry weight by using a standard curve.
Protein determination
Protein was assayed according to the method of Bradford (1976). The bovine albumin was used as standard.
Enzyme assay
The amount of reducing sugars released during starch hydrolysis by dinitrosalicylic acid (DNS) indicate the amylase activity according to (Bernfeld 1955). One unit of α-amylase is defined as the amount of enzyme releasing one mM reducing sugar equivalent of glucose per minute under the assay conditions.
Results
The initial screening of B.subtilis strain revealed zones of hydrolysis on starch agar plates with a distinct halo around the growth (Figure 1). The clearance around the culture colonies grown in starch agar media indicated that the starch has been hydrolyzed by the amylase produced by bacteria, whereas purple zone was the results of starch and iodine reaction.
Figure 1.

Clear Zone indicate hydrolysis of starch by B. subtilis grown on starch agar media after incubation at 50 °C for 24 h.
Molecular characterization of these strains was achieved by DNA isolation (CTAB method, according to Doyle and Doyle, 1990) then the 16S r DNA was analyzed. Further these amplified 16S rDNA sequences of the bacterial strains was blasted using online tool (MEGA 4). The taxonomical identification was achieved by the phylogenetic tree construction and the comparison of this bacterial strain sequences with other homologous bacterial sequences.
The isolated strain was spore forming, Gram-positive, rod-shaped and aerobic. The growth temperature was in the range of 30-60 C and several biochemical tests as catalase reaction-positive, oxidase reaction positive, nitrate oxidase positive, idols negative and maltose negative in addition to phylogenetic tree based on different species of Bacillus was constructed using neighbor joining method (Figure 2), indicated that strain is closely related with Bacillus subtilis strain.
Figure 2.
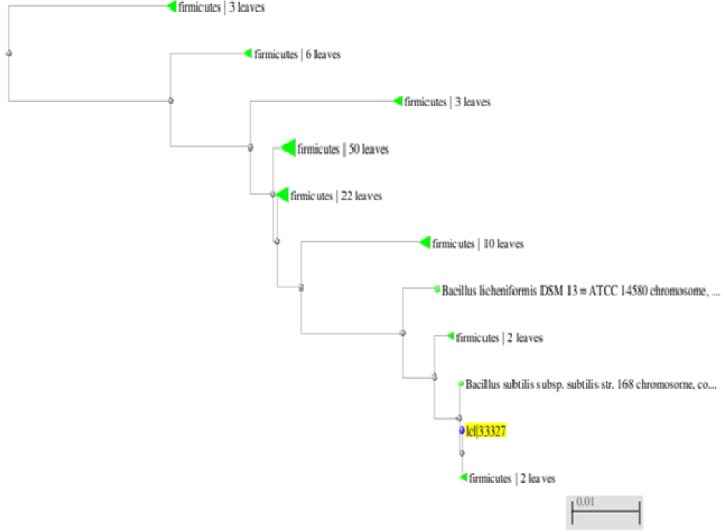
Identification of B. subtilis by using Phylogenetic tree method indicated that strain was closely related with Bacillus subtilis strain.
Influence of carbon source on production of α-amylase, as shown in (Figure 3) B. subtilis was able to grow in basal liquid medium supplied with different carbon sources as glycogen, starch, maltose, glucose, xylose or glycerol. Highest enzyme activity (10 U/ml) and cell growth were obtained at starch then maltose gave (08 U/ml).
Figure 3.
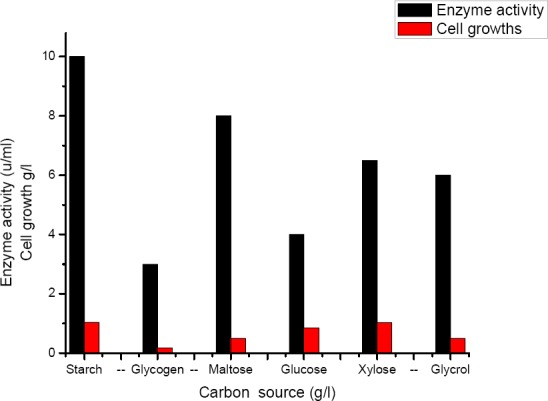
Effect of different carbon source (starch, glycogen, maltose, glucose, xylose and glycerol) on the activity of α-amylase produced by B. subtilis strain (U/ml)
For determination of the optimum concentration of starch was performed using various starch concentrations between (0- 20g/L). Optimum starch concentration (Figure 4) was found at the concentration of 0.5% where α-amylase activity was maximum (16 U/ml). On the other hand, the cells growth were increased with the increasing starch concentrations reaching its maximum at 2.17 g/l at 20 g/l(Figure 4).
Figure 4.
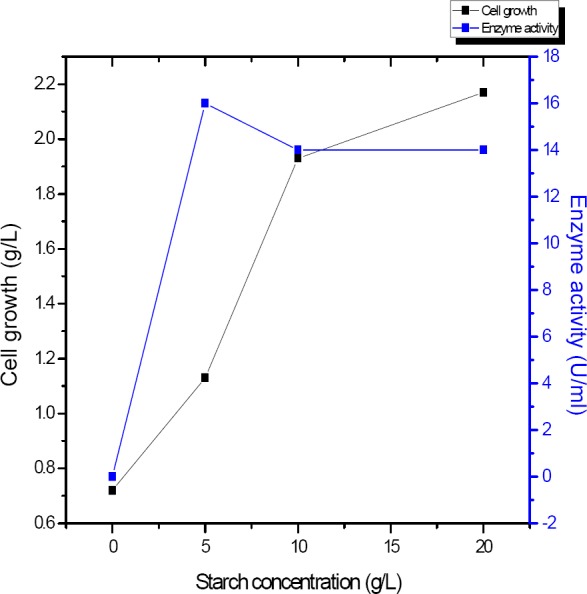
Effect of different concentration of starch (g/L) on the amount of α-amylase produced by B. subtilis strain (U/ml)
To investigate the influence of nitrogen sources as mentioned in methodology section in α-amylase production by B. subtilis. As shown in (Figure 5) the maximum bacterial growth (3.5 g/l) were observed at peptone when conjunct with ammonium sulphate. Peptone showed the highest enzyme activity (18 U/ml), while sodium nitrate alone or conjugated with peptone increased the enzyme activity slightly compared with peptone alone.
Figure 5.
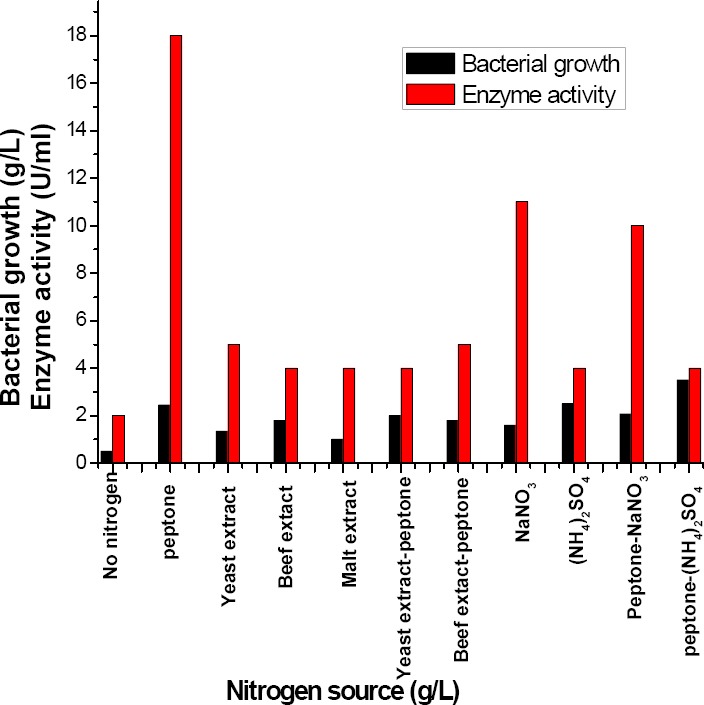
Influence of different nitrogen sources (g/L) on the amount of α-amylase produced by B. subtilis strain (U/ml)
This experiment was done to explore the effects of different peptone concentrations (0-10 g/l) in production of α-amylase by B. subtilis.
Figure 6 showed that, bacterial growth was increased with the increasing peptone concentrations at 10 g/l maximum bacterial growth were 1.96 g/l. The best peptone concentration for maximum α-amylase activity was 5-10 g/l.
Figure 6.
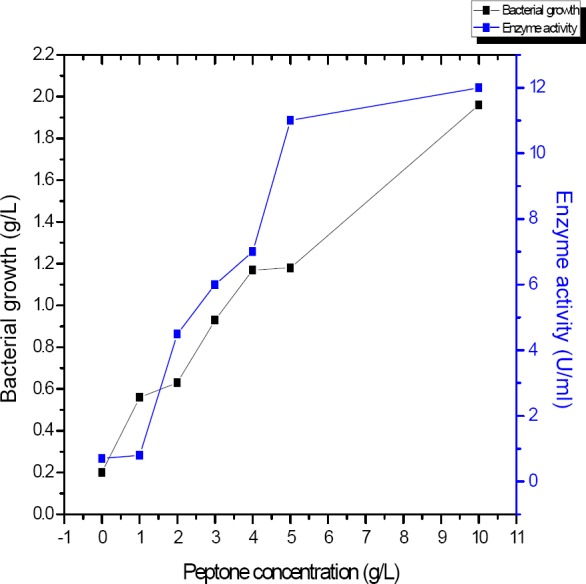
Influence of different concentrations of peptone on the amount of α-amylase produced by B. subtilis strain (U/ml)
To investigate the effect of initial pH on the production of α-amylase by B. subtilis. Different initial pH ranged from (5.5- 9.0) were studied. The initial pH of fermentation medium pH was adjusted by 1N HCl or1N NaOH.
Final pH was changed to the values ranging from 6.1 to 8.3, this change may be attributed to the metabolic activities of B. subtilis during the fermentation process. As shown in (Figure 7) the cell growth was maximum (1.00g/l) at initial and final pH of 9.0 Maximum enzyme(18 U/ml) was produced by B.subtilis at pH 8.5.
Figure 7.
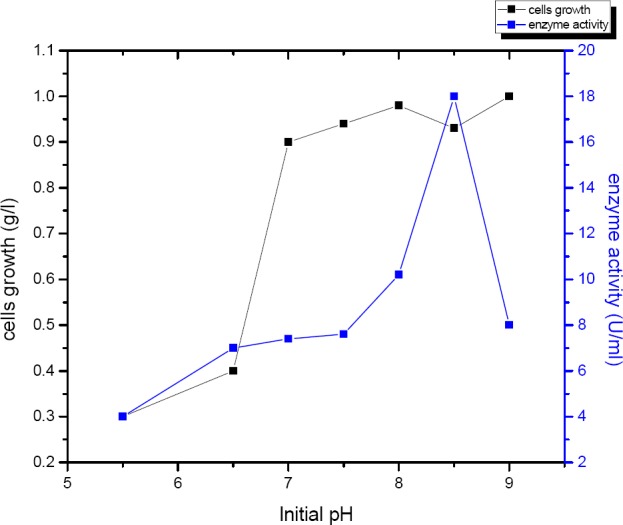
Effect of pH range (1-9) on the amount of α-amylase produced by B. subtilis strain (U/ml).
Different temperatures (25°C- 70°C) were tested for detecting the optimum temperature of enzyme activity. In (Fig. 8) enzyme activity revealed that bacteria yielded maximum α-amylase(20 U/ml) and bacterial growth (1.3 g/l) at 45°C.
Figure 8.
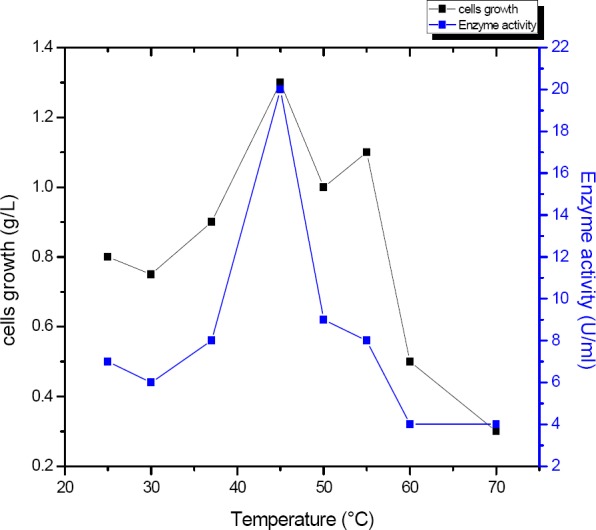
Impact of different temperature on the amount of α-amylase produced by B. subtilis strain (U/ml).
The maximum growth measured at 48h then the growth declined to reach the minimum level at 96, 120 and 144 h (Figure 9). Maximum enzyme activity about (25 U/ml) was produced after 48 h, then further extension of fermentation period the enzyme yield was shown a gradual decrease in enzyme activity.
Figure 9.
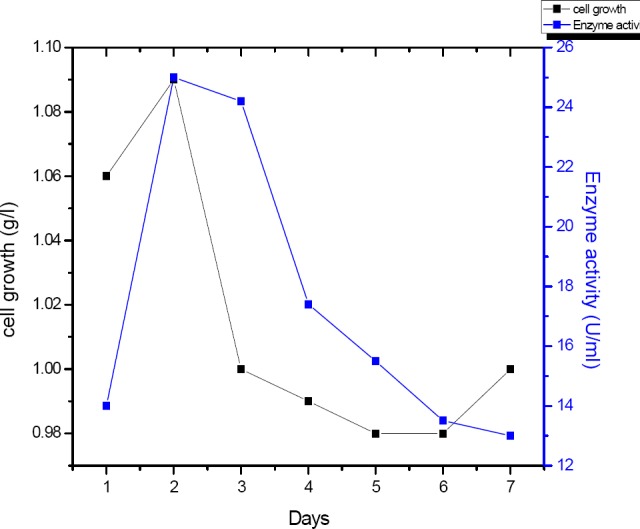
Impact of different incubation periods (days) on the amount of α-amylase produced by B. subtilis strain (U/ml).
Discussion
The initial screening of B. subtilis strain revealed zones of hydrolysis on starch agar plates. This clear zone indicated that B. subtilis a-amylase can be hydrolyzed starch.
Phylogenetic tree for different species of Bacillus was indicated that strain was closely related with B. subtilis strain. Our results in agree with Konsoula and Liakopoulou-Kyriakides, (2007), who were produce amylase from B. subtilis.
Starch was the best carbon source of α-amylase production by B. subtilis. These results were in agreement with El-Banna et al., (2007) results who found that presence starch enhanced, α-amylase production by B. subtilis ; whereas other carbon tested have no effect on α-amylase synthesis. (Božić, et al., 2011 & Roy et al., 2012) found α-amylase production by different bacterial strains were increased addition starch to medium.
Optimum starch concentration was found at the concentration of 0.5% where α-amylase activity was maximum (16 U/ml). A similar result was observed by Božić, et al., (2011) who found that 0.5 % starch was suitable concentration for α-amylase production by B. subtilis IP 5832. In contrast, Reyed, (2007) & Fatoni, A and Zusfahair,(2012) reported that α-amylase was maximum at concentrations 5% and 10% of starch respectively.
Peptone gave the highest enzyme activity (18 U/ml), while sodium nitrate and peptone conjunction with sodium nitrate increased the enzyme activity slightly. Similar results were found by El-Banna et al., (2007) who reported that peptone was best nitrogen source for amylase production (2.7μml-1) from B. subtilis. In addition to that, Oziengbe and Onilude (2012) found that peptone gave the highest α-amylase activity when was added to the production medium.
The bacterial growth were increased with increasing peptone concentrations till 10 g/L the bacterial growth reach maximum 1.96 g/l (Fig. 6). The best peptone concentration for maximum α-amylase activity were 5-10 g/L. These results were in accordance with the results of Ramachandran et al. (2004) who suggested that peptone (1%) gave an α-amylase activity in solid - state medium using coconut oil cakes as substrate. Fooladji and Sajjadian (2010) found that the addition of peptone (1 %) to mineral medium, improved growth and α-amylase activity.
Maximum enzyme production by B. subtilis was 18 U/ml at pH 8.5. Anupama & Jayaraman (2011) reported similar results that, at pH 8 maximum α-amylase was produced. On the other hand, Utong et al., (2006) described that at pH 6-9 range Bacillus sphericus was produced α-amylase. In contrast Roy et al., (2011) who reported that the optimum enzyme production from Bacillus subtilis strain AS-S01a was observed at pH 6.
The enzyme activity revealed that the bacteria yielded maximum α-amylase (20 U/ml) and bacterial growth (1.3 g/l) production at 45°C. Asad et al (2011) reported the same results that maximum amylase production from Bacillus WA21 was observed at 45°C. In addition to that, Roy et al, (2011) reported that alkaline α-amylase was produced by Bacillus subtilis strain AS-S01 at optimum temperature 45-55°C, this enzyme active over all tested temperatures.
Maximum cell growth and enzyme activity (25 U/ml) observed after 48 h measured. This result is in accordance with the results, Hassan and AbdKarim (2012) who found that, the highest enzyme activity of Bacillus subtilis were 39.9 U/g after 48 h of incubation.
Conclusion
An amylase-producing bacterium was isolated from hot-spring water identified as B subtilis. Amylase produced from B. subtilis had optimum temperature 45°C and pH 8.5 in shaking media. Optimum substrate concentration was achieved at 5 g/L starch and peptone at concentration of 10g/l was more suitable as nitrogen source for optimum enzyme activity (25 U/ml).
Acknowledgements
This research was funded by King Abdulaziz City of Science and Technology Saudi Arabia, grant no (79-34-T-A).
References
- 1.Anupama A, Jayaraman G. Detergent stable, halotolerant alpha-amylase from Bacillus Aquimaris VITP4 exhibits reversible unfolding. International Journal of Applid Biology and Pharmaceutical Technology. 2011;2(2):366–376. [Google Scholar]
- 2.Asad W, Asif M, Rasool AS. Extracellular enzyme production by indigenous thermophilic bacteria : partial purification and characterization of alpha-amylase by Bacillus sp. WA21. Pak J. Bot. 2011;43(2):1045–1052. [Google Scholar]
- 3.Ben Massoud E, Ben Ali M, Elluch N, Bejar S. Purification and properties of maltyoheptose and maltohexose forming amylase produced by Bacillus subtilis. Enzyme and Microbial Technology. 1999;34:662–666. [Google Scholar]
- 4.Bezbaruah RL, Gogoi BK, Pilla KR. Optimization of alkaline amylase Production by thermophilic Bacillus stearothermophilus AN002. J. Basic Microbiol. 1994;34:139. [Google Scholar]
- 5.Božić N, Ruiz J, Lóoez-Santin J, Vujčić Z. Optimization of the growth and alpha-amylase production of Bacillus subtilis IP 5832 in shake flask and laboratory fermenter batch cultures. Journal of the Serbian Chemical Society. 2011;76(7):965–972. [Google Scholar]
- 6.Bunni L, McHale L, McHale A.P. Production, isolation and partial characterization of an amylase system produced by Talaromyces emersonii CBS 814.70. Enzyme Microb. Technol. 1989;11:370–375. [Google Scholar]
- 7.Burhan A, Nisa U, Gokhan C, Omer C, Ashabil A, Osman G. Enzymatic properties of a novel thermostable, thermophilic, alkaline and chelator resistant amylase from an alkaliphilic Bacillus sp. isolate ANT-6. Process. Biochem. 2003;38:1397–1403. [Google Scholar]
- 8.Doyle JJ, Doyle J.L. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- 9.El-Banna TE, Abd-Aziz AA, Abou-Dobara MI, Ibrahim RI. Production and immobilization of alpha-amylase from Bacillus subtilis. Pakistan Journal of Biological Sciences. 2007;10(12):2039–2047. doi: 10.3923/pjbs.2007.2039.2047. [DOI] [PubMed] [Google Scholar]
- 10.Fatoni A, Zusfahair Thermophilic amylase from Thermus sp. isolation and its potential application for bioethanol production. Songklanakarin Journal of Science and Technology. 2012;34(5):525–531. [Google Scholar]
- 11.Fooladi j, Sajjaduan A. Screening the thermophilic and hyperthermophilic bacterial population of three Iranian hot-springs to detect the thermostable alpha-amylase producing strain. Iranian Journal of Microbiology. 2010;2(1):49–53. [PMC free article] [PubMed] [Google Scholar]
- 12.Hassan H, Abd Karim K. Utilization of agricultural by-products for alpha-amylase production under solid state fermentation by Bacillus subtilis. Engineering Journal. 2012;16(5):177–186. [Google Scholar]
- 13.Holt J. G, Krieg N. R, Sneath P. H. A, Staley J. T, Williams S. T. Bergey’s Manual of Determinative Bacteriology. 9th edn. Baltimore: Williams & Wilkins; 1994. [Google Scholar]
- 14.Jensen B, Olsen J. Physicochemical properties of a purified alpha amylase from the thermophilic fungus Thermomyces lanuginosus. Enzyme Microb. Technol. 1992;14:112–116. [Google Scholar]
- 15.Konsoula Z, Liakopoulou-Kyriakides M. Co-production of alpha-amylase and beta-galactosidase by Bacillus subtilis in complex organic substrates. Bioresource Technology. 2007;98:150–157. doi: 10.1016/j.biortech.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Leveque E, Janacek S, Haye B, Belarbi A. Thermophilic archeal amylolytic enzymes. Enzyme and Microbial Technology. 2000;26:3–14. [Google Scholar]
- 17.Lin LL, Chyau CC, Hsu WH. Production and properties of a raw starch-degrading amylase from the thermophilic and alkaliphilic Bacillus sp. TS-23. Biotechnology and Applied Biochemistry. 1998;28:61–68. [PubMed] [Google Scholar]
- 18.Lin LL, Hsu WH, Chu WS. A gene encoding for α-amylase from thermophillic Bacillus sp strain TS-23 and its expression in Escherichia coli. J. Appl Microbiol. 1997;82:325–334. doi: 10.1046/j.1365-2672.1997.00364.x. [DOI] [PubMed] [Google Scholar]
- 19.Oziengbe E.O, Onilude AA. Production of a thermostable A-amylase and its assay using Bacillus Licheniformis isolated from excavated land sites in Ibadan Nigeria. Bayero Journal of Pure and Applied Science. 2012;5(1):132–138. [Google Scholar]
- 20.Pandey A, Nigam P, Soccol CR, Soccol VT, Singh D, Mohan R. Advances in microbial amylases. Biotechnol Appl Biochem. 2000;31:35–152. doi: 10.1042/ba19990073. [DOI] [PubMed] [Google Scholar]
- 21.Prakash O, Jaiswal N. alpha-Amylase: An Ideal Representative of Thermostable Enzymes. Appl Biochem Biotechnol. 2009;160:2401–2414. doi: 10.1007/s12010-009-8735-4. [DOI] [PubMed] [Google Scholar]
- 22.Ramachandran S, Patel AK, Nampoothiri KM, Francis F, Nagy V, Szakacs G, et al. Coconut oil cake -A potential raw material for the production of a-amylase. Bioresource Technology. 2004;93(2):169–174. doi: 10.1016/j.biortech.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Rai SK, Roy JK, Mukherjee AK. Characterization of a detergent-stable alkaline protease from a novel thermophilic strain Paenibacillus tezpurensis sp. Appl. Microbiol. Biotechnol. 2010;85:1437–1450. doi: 10.1007/s00253-009-2145-y. [DOI] [PubMed] [Google Scholar]
- 24.Raul D, Biswas T, Mukhopadhyay S, Kumar SD, Gupta S. Production and Partial Purification of Alpha Amylase from Bacillus subtilis (MTCC 121) Using Solid State Fermentation. Biochemistry Research International. 2014:568141. doi: 10.1155/2014/568141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reyed RM. Biosynthesis and properties of extracellular amylase by encapsulation Bifidobatrium bifidum in batch culture. Australian Journal of Basic and Applied Sciences. 2007;1(1):7–14. [Google Scholar]
- 26.Roy JK, Rai SK, Mukherjee AK. Characterization and application of a detergent-stable alkaline alpha-amylase from Bacillus subtilis strain AS-S01a. International Journal of Biological Macromolecules. 2012;50(1):219–229. doi: 10.1016/j.ijbiomac.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Saxena R, Dutt K, Agarwal L, Nayyar P. A highly thermostable and alkaline amylase from a Bacillus sp. Bioresource Technology. 2007;98:260–265. doi: 10.1016/j.biortech.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Setyorini E, Takenaka S, Murakami S, Aoki K. Purification and characterization of two novel halotolerant extracellular proteases from Bacillus subtilis strain FP-133. Biosci Biotechnol Biochem. 2006;70:433–40. doi: 10.1271/bbb.70.433. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava RAK, Baruah JN. Culture Conditions for Production of Thermostable Amylase by Bacillus stearothermophilus. Appl. Environ. Microbiol. 1986;52:179–184. doi: 10.1128/aem.52.1.179-184.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamura A, Shimizu Y.K, Tanaka T, Kuroda K, Arakawa Y, Takahashi K, Mishiro S, Shimizu K, Moriyama M. Persistent infection of hepatitis E virus transmitted by blood transfusion in a patient with T-cell lymphoma. Hepatol. Res. 2007;37:113–120. doi: 10.1111/j.1872-034X.2007.00024.x. [DOI] [PubMed] [Google Scholar]
- 31.Utong J F, Al-Quadan Akel H. Effects of various growth conditions on the production of extracellular amylase from thermotolerant Bacillus sp. isolated from hot spring in Jordan. Journal of Biological Science. 2006;6(3):621–625. [Google Scholar]
- 32.Zambare V. Optimization of amylase production from Bacillus sp. using statistics based experimental design. Emir. J Food Agric. 2011;23(1):37–47. [Google Scholar]


