Abstract
Background:
Crocodile oil and its products are used as ointments for burns and scalds in traditional medicines. A new ointment formulation - crocodile oil burn ointment (COBO) was developed to provide more efficient wound healing activity. The purpose of the study was to evaluate the burn healing efficacy of this new formulation by employing deep second-degree burns in a Wistar rat model. The analgesic and anti-inflammatory activities of COBO were also studied to provide some evidences for its further use.
Materials and methods:
The wound healing potential of this formulation was evaluated by employing a deep second-degree burn rat model and the efficiency was comparatively assessed against a reference ointment - (1% wt/wt) silver sulfadiazine (SSD). After 28 days, the animals were euthanized and the wounds were removed for transversal and longitudinal histological studies. Acetic acid-induced writhing in mice was used to evaluate the analgesic activity and its anti-inflammatory activity was observed in xylene -induced edema in mice.
Results:
COBO enhanced the burn wound healing (20.5±1.3 d) as indicated by significant decrease in wound closure time compared with the burn control (25.0±2.16 d) (P<0.01). Hair follicles played an importance role in the physiological functions of the skin, and their growth in the wound could be revealed for the skin regeneration situation. Histological results showed that the hair follicles were well-distributed in the post-burn skin of COBO treatment group, and the amounts of total, active, primary and secondary hair follicles in post-burn 28-day skin of COBO treatment groups were more than those in burn control and SSD groups. On the other hand, the analgesic and anti-inflammatory activity of COBO were much better than those of control group, while they were very close to those of moist exposed burn ointment (MEBO).
Conclusions:
COBO accelerated wound closure, reduced inflammation, and had analgesic effects compared with SSD in deep second degree rat burn model. These findings suggest that COBO would be a potential therapy for treating human burns.
Abbreviations: COBO, crocodile oil burn ointment; SSD, silver sulfadiazine; MEBO, moist exposed burn ointment; TCM, traditional Chinese medicine; CHM, Chinese herbal medicine; GC-MS, gas chromatography-mass spectrometry.
Keywords: Crocodile oil burn ointment, Chinese herbal medicine, Burn healing, Hair follicles, Antinociceptive, Anti-inflammatory
Introduction
Burns are one of the most widespread injuries in accidents and still remain a global public health problem (Bell et al., 1993, Li et al., 2012). The burn injury may lead to complications such as long-term disability, prolonged hospitalization, loss of body extremities and even death (Upadhyay et al., 2009). Wound repair is the process that follows injury to the skin, which is initiated by an inflammatory response and the cells below the dermis begin to increase collagen production. Later, the epithelial tissue is regenerated (Wilgus, 2008). Although many advances have been made in our understanding and care of burn injuries, it is still a challenge to heal the burn injury for modern medicine (Li et al., 2012).
In the world there are already many topical therapies available for the burn injuries, such as, 1% silver sulfadiazine (SSD), moist exposed burn ointment (MEBO), mafenide acetate 10% cream, and povidone iodine (Hemmila et al., 2010, Jewo et al., 2009, Vloemans et al., 2003). But there were gradually some problems found in the treatments, for example, disturbing evidence has appeared that SSD could stimulate the inflammatory and cause the injury to the wounds against the healings (Li, 2011). And it was found that MEBO treatment was not always dependable, as some wounds got worse in the healing (Huang et al., 2004). Hence, it is still necessary and important to develop new drugs for the burn injuries.
Wound healing process is promoted efficiently by the use of traditional remedies, which are mainly based on plant and animal sources (De Oliveira et al., 2010, Li et al., 2012, Süntar et al., 2011, Sun et al., 2015, Tumen et al., 2011). Traditional Chinese medicines (TCM) could provide an important and effective source for the discovery of novel drugs, while side effects of topical TCM compounds are relatively rare (Li et al., 2011). In addition, further research has demonstrated the efficacy and pharmacological activities of TCM (Fan et al., 2010, Li et al., 2011, Xutian et al., 2009, Zhang et al., 2013).
Crocodiles belong to the general term of animals of Crocodyliae, Reptilia (Buchanan, 2009). Crocodylus siamensis is one of the species of freshwater crocodile that was originally distributed throughout Southeast Asia (Kang et al., 2012), which is now grown at a large scale in Thailand and the Guangdong province, China. Crocodile oil is extracted from the fatty tissues of crocodile; it contains high concentrations of saturated and unsaturated fatty acids. Crocodile oil was recorded to be very effective in the treatment of ailments, ranging from skin conditions to cancer, and has been used for centuries by traditional practitioners (Buthelezi et al., 2012). In Mexico, the oil is used for illnesses such as asthma, emphysema, and influenza. In Africa, crocodile oil is used for skin rashes and to promote wound healing (Buthelezi et al., 2012, De Villiers and Ledwaba, 2003, Lindsey, 1999). Crocodile oil and its products are used as ointments for burns and scalds in the traditional medicines, such as traditional Chinese and Southeast Asia medicine (Li et al., 2012, Tang, 2007). The study has found that crocodile oil could enhance cutaneous burn wound healing and reduce scar formation in rats (Li et al., 2012).
The aim of the study was to develop a more efficient drug which could not only have the advantage of crocodile oil on the burn wound healing, but also have significant antinociceptive and anti-inflammatory activities. Crocodile oil burn ointment (COBO) is a topical Chinese herbal medicine (CHM) compound made from one natural mineral, crocodile oil and the extraction of five herbal medicines. The formula and preparation method of COBO have been licensed by the national patent office of the Peoples’ Republic of China. Our previous experiment has shown good results for the treatment of burn injuries (Chen et al., 2011). The aim of the present study was to evaluate the wound healing potential of this new formulation by using the animal models.
Materials and methods
Preparation of crocodile oil burn ointment (COBO)
Crocodile oil was extracted from the fats using a previously described method, and contained high concentrations of palmitic acid, oleic acid, and linoleic acid (Li et al., 2012). COBO was made according to a TCM formula. Crocodile oil was formulated with the Chinese herbal extraction. The herbal components used were: Arnebia euchroma I.M. Johnst., root, dried; Astragalus membranaceu Moench, root, dried; Savia miltiorrhiza Bunge, root, dried; Sanguisorba officinalis L., root, dried; and Borneolum syntheticum (Chan et al., 2012). The compounds were purchased from the First Affiliated Hospital and botanically identified by a qualified botanist. Sesame oil and ethanol were used as extraction agents, and albolene served as a base.
The preparation of COBO was adopted as a low-temperature vacuum extraction, which could make the water-soluble and liposoluble constitutes release in the extraction solution. The entire process is carried out under a bacteria-free environment. At first, the herbal components (Arnebia euchroma I.M. Johnst., 20 g; Astragalus membranaceu Moench, 10 g; Savia miltiorrhiza Bunge, 10 g; Sanguisorba officinalis L.,10 g; and Borneolum syntheticum, 20 g, respectively) were extracted with 500 mL sesame oil and 500 mL ethanol in a rotary evaporator at 40-50 °C for 4~6 h, and the evaporated ethanol was returned to the evaporator each hour. Then the extraction was mixed until there was no ethanol, and was centrifuged with the speed (3000 rpm). Albolene was preheated in a liquid form, and was blended evenly with the filtered herbal extraction and crocodile oil in a proportion of 2: 1: 1. COBO was stored in sterile containers and placed in a clean environment. COBO was identified by Gas chromatography-mass spectrometry (GC-MS), and it contained borneol (C10H18O) at least 6% in total weight. The formula and preparation method of COBO have been licensed for national invention patent of the People’s Republic of China. The application no. is ZL 201010505834.X.
Biological activity tests
Animals
Male albino Wistar rats (200±20 g) and male Kuming mice (20±2 g) were purchased from SLRC Laboratory Animal Co., Ltd. Shanghai, China. The animals were kept on 12-hour-light/12-hour-dark cycle under the condition of a constant temperature of 21-22 °C and 60-65% humidity. And they were maintained on standard pellet diet and water ad libitum throughout the experiments. The experimental procedures were performed in accordance with the guidelines for the humane treatment of animals set by the Laboratory Animal Center.
Burn experiment
Burn injury
This study was performed in the Laboratory Animal Center. The rats were acclimated to the laboratory for 1 week prior to beginning the study and had free access to water and food at all times. The dorsal hair was shaved and depilated with 10% sodium sulfide solution 24 h before burn wound experiment, 70% alcohol was employed to sterilize the dorsal area. A deep second-degree burn wound of a surface diameter of 2.5 cm was inflicted using a 40-g glass full with water. The glass was heated in 100 °C boiling water for 10 min, and then applied perpendicularly to the shaved area with gravity alone without pressure on one side of the back for a period of 10 s (Lee et al., 2011, Li et al., 2012). Two burns were created on the dorsum of each animal except the sham group (Figure 1).
Figure 1.
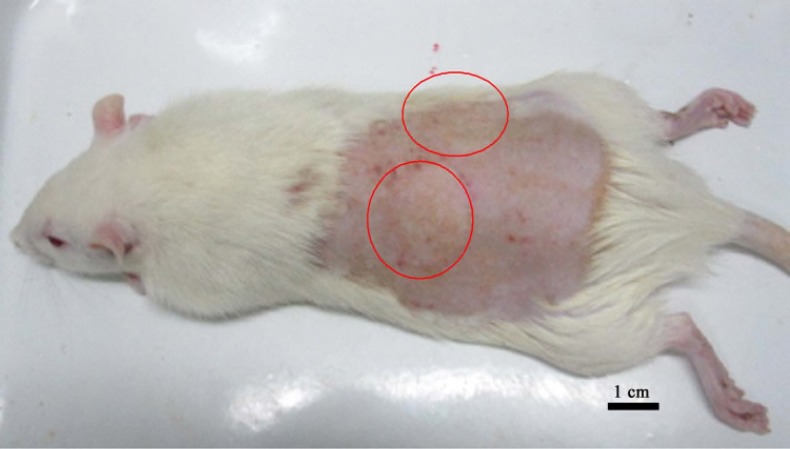
A deep second-degree burn wound of a surface (2.5 cm in diameter) was created; two burns were created on the dorsum of the animal except normal group (Sham group). Scale bar: 1.0 cm.
Experimental protocols
Twenty-four SPF-class rats were assigned equally into four groups using a random number table, and then caged individually after the burn experiment. The four groups were set as follows: sham group, burn control group treated with saline solution (12 burns for six rats, served as negative control), and silver sulfadiazine-treated group (SSD; 12 burns for six rats, served as positive control), and COBO-treated group (COBO; 12 burns for six rats,). Silver sulfadiazine cream (1% wt/wt) was purchased from the first affiliated hospital. And it was used as standard care. 0.3 g SSD or COBO per wound was applied slowly on the wound area, extending slightly outside the wound area to ensure inclusion of the wound edges. Treatments were repeated twice daily for 28 days. The first application was done directly after the wound injuring.
Optical photographs were taken from the wound area, using a SAMSUNG camera at an equal distance from the wound and right angle to the wound surface at 3, 7, 10, 14, 21, and 28 days. Wound contraction, wound closure time and amount of the edema, exudation and the firm of the wound surface were observed. The wound surface areas were calculated by tracing their contours using a transparent paper to evaluate wound contraction. (Feng et al., 2010). The percentage of wound contraction (%) = (wound size day 0- wound size day X)/wound size day 0x100%. At day 28 the experiment was terminated and the animals were euthanized by CO2 inhalation. The granulation tissues were excised for histological examination. The excisional skin biopsies were fixed in 4% neutral buffered formaldehyde solution for 24 h, processed and blocked with paraffin. The samples were evaluated in transversal and longitudinal planes.
To determine the alternations of skin structure in the 28 days’ post-burn skin, cross-sections through the longitudinal aspect of the scarred areas were made. The samples sectioned into 5 μm and stained with hematoxylin and eosin (HE). The samples were evaluated by the histological changes: the extent of re-epithelization, the maturation and organization of the epidermal squamous cells, the degree of granulation tissue formation, the boundary between the epidermal and the dermis, the structure in the hair follicles.
Embedded skin samples were sectioned in transverse plane at 8 μm made by microtome and stained by a special tetrachrome stain sacpic (Nixon, 1993). The active follicle was stained in red, and the amounts of total follicle or active follicle, primary follicle, and secondary follicle were assessed respectively. All measurements were evaluated by taking average number of follicles in eight high power fields (20xobjective), midway in the post-burn skins.
Acetic-acid-induced writhing response in mice
Forty mice were randomly and equally divided into five groups, as follows: saline solution group (negative control, 0.3 g/20 g), MEBO group (positive control, 0.3 g/20 g), and COBO groups (low, middle and high doses group for 0.05 g/20 g, 0.3/20 g and 0.6 g/20 g, respectively).
The test was carried out using the described technique previously (Zhou et al., 2008). Mice were injected intraperitoneally with 0.1 ml 10 g body weight of 0.6 % acetic acid in saline solution 0.5 h after the topical administration of tested drugs in the abdomens. The frequency of writhing occurring was recorded 20 min after the injection of acetic acid, and the time of first writhing was also evaluated. At the end of the experiment, the animals were euthanized by CO2 inhalation
Xylene-induced ear swelling in mice
The mice were randomly divided into five groups, consisting eight mice per group. The doses (0.05 g/20 g, 0.3 g/20 g and 0.6 g/20 g) of COBO groups were tested in the experiment. MEBO was served as positive control and saline solution as negative control; their doses were both 0.3 g/20 g.
The test was carried out using the described technique previously (Kou et al., 2005). In the beginning of the experiment, each animal received 50 μl of xylene on the anterior and posterior surfaces of the right ear lobe. The left ear was considered as control. Then 30 minutes later, the drugs were applied in the right ear. Two hour later, the animals were sacrificed by cervical dislocation and both ears were sampled. Circular sections were taken, using a cork borer with a diameter of 8 mm, and weighed. The degree of ear swelling was calculated based on the weight of left ear without applying xylene. At the end of the experiment, the animals were euthanized by CO2 inhalation.
Statistical analysis
Data are expressed as mean±SE and statistical significance between experimental and control values were analyzed by ANOVA followed by Dunnett’s test using Graph Pad Prism 2.01 (Graph Pad Software Inc.). A P-value < 0.05 was considered statistically significant. All morphometric parameters were done with Image Analyzer (Olympus Microscope BX61) by using image analyzing computer program (Image-Pro Plus 6.2).
Results
Effect of crocodile oil burn ointment (COBO) on the burn healing
The burn healing time in the COBO group (20.5±1.3 d) was shorter than in the burn control group (25.0±2.16 d) and SSD group (22.67±1.53 d) (Figure 2). Optical photographs were taken from the wound area at 3, 7 10, 14, 21, 28 days. In the first 3 days, there were brown scabs appearing in the burn wounds, which were not measurable different among the groups (Figure 3a, 3g, and 3m). On the 7th day, the scabs in the COBO group were gradually shedding, as in the other two groups there were not obviously changing (Figure 3b, 3h, and 3n). On the 10th day, we observed the significant improvement in the wound closure in COBO group (Figure 3c), comparing to the burn control group and SSD group (Figure 3i and 3o). After 14 days of treatment, the burn wounds in COBO group were smaller than those in the SSD groups (Figure 3d, 3j, and 3p). On the 28th day, the surface of the skin in COBO group (Figure 3r) was smoother and the color was almost close to the normal skin. The hair was well-distributed in the skin of COBO group, while there was not hair distribution partially in the burn control group (Figure 3f).
Figure 2.
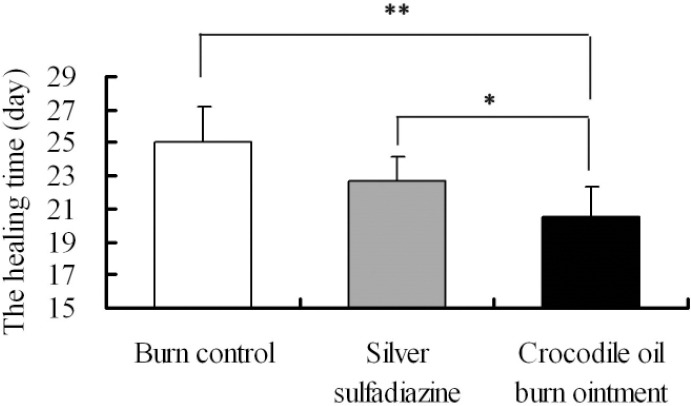
The healing time on Wistar rat dorsal wound. Values are mean± SE of twelve burns, Values in brackets represent statistic differences: * P < 0.05; ** P < 0.01.
Figure 3.
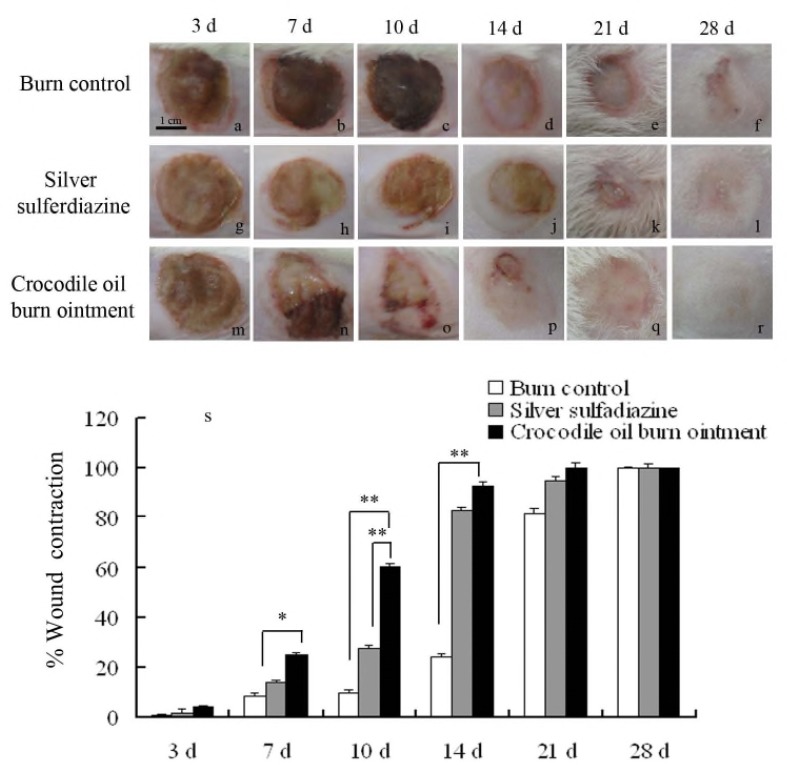
Wound contraction in different groups. Rat dorsal burn wound area photograph on the 3rd, 7th, 10th, 14th, 21st, 28th day after application of different treatments: burn control group (a-f), silver sulfadiazine group (g-l) and crocodile oil burn ointment group (m-r). Photographs were taken from a representative animal of each group. Scale bar: 1 cm. The percentage of wound contraction in different groups on the 3rd, 7th, 10th, 14th, 21st, 28th day (s): Burn control group, Silver sulfadiazine group, and Crocodile oil burn ointment group. Values are mean ± SE of twelve burns. * P < 0.05; ** P <0.01.
Effect of crocodile oil burn ointment (COBO) on the skin structure
At day 28 the experiment was terminated and the wound area was removed from the surviving animals for histological evaluation. Samples were cross-sectioned through the longitudinal aspect and stained with hematoxylin and eosin (HE). The result was showed in Figure 4. In the sham group, the epidermis was thin, the skin structure and collagen deposition were well organized and distributed (Figure 4a). In the burn control and silver sulfadiazine groups, the epidermis was much thicker than those in the normal group and the COBO group, few hair follicles structures existed, and collagen deposition was unevenly distributed (Figure 4b, 4c). In COBO group, there was full thickness re-epithelialization, where the epidermis was thin and well formed, and the hair follicle structure was well organized with sebaceous glands and sweat glands. The collagen deposition in the 28-days-postburn skin of COBO group was evenly distributed, compared to the other groups (Figure 4d).
Figure 4.
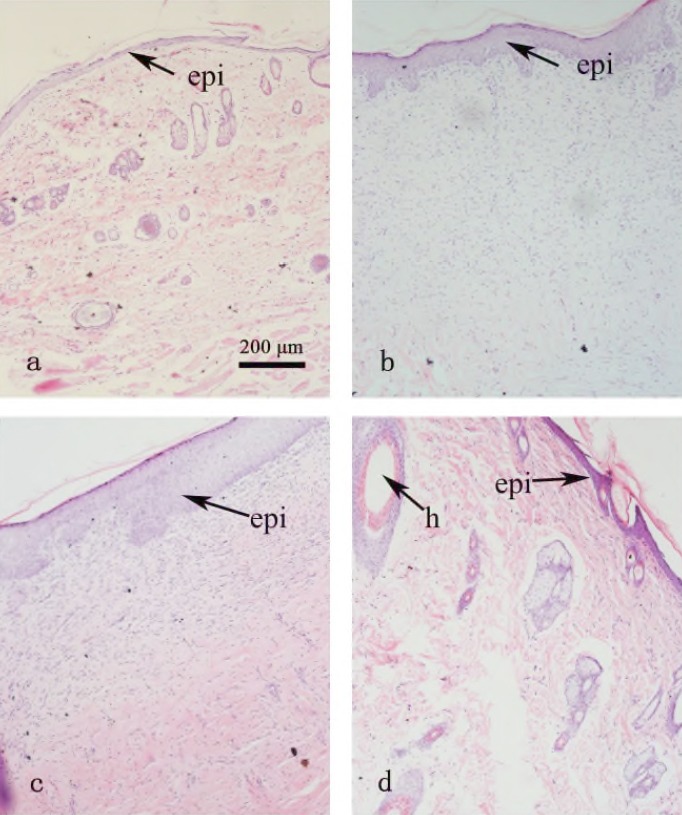
Hematoxylin and eosin (HE) staining in the post-burn 28 day rat dorsum biopsies. Cross-sections through the longitudinal aspect of the scarred areas were made. (a) Normal group, (b) Burn control group; (c) Silver sulfadiazine group; (d) Crocodile oil burn ointment group. epi, epidermis; h, hair follicle; Scale bar 200 μm; Original magnification x10.
Effect of crocodile oil burn ointment (COBO) on the activities and distribution of hair follicles in the post-burn skin
The distribution of the hair follicles was reflected the skin restructure in the burn wound healing. To further investigate the effect of COBO on the activities and distribution of hair follicles in the post-burn skin, samples were transverse-sectioned and stained with a special tetrachrome stain Sacpic. The result is shown in Figure 5. The histological examination on the 28th day showed that the primary and secondary follicles of the sham groups (Figure 5A, 5a) were well-organized and distributed, and they were both at high activities in follicle growth cycling (red staining). In burn control group (Figure 5B, 5b) and SSD group (Figure 5C, 5c), the hair follicles were distributed sparsely and unevenly, and their structures were disorganized; there were few primary and secondary follicles with little activities. In the COBO group (Figure 5D, 5d), most area was covered with hair, and the activities of the follicles were higher than the other groups.
Figure 5.
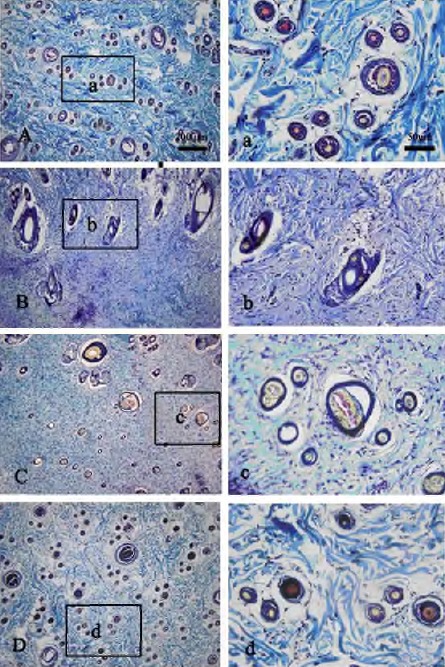
Sacpic staining in the post-burn 28 day rat dorsum biopsies. Embedded skin samples were sectioned in transverse plane. (A, a) Normal group; (B, b) Burn control group; (C, c) Silver sulfadiazine group; (D, d) Crocodile oil burn ointment group. Pictures (A - D) were taken at original magnification x10, Scale bar: 200 μm; Pictures (a - d) were taken at original magnification x40, Scale bar: 50 μm.
Quantitative analysis showed that on the 28th day the amounts of total and active follicles in the skin of the COBO group were close to those of the normal group, and were 75% and 67% more than those in the SSD group respectively (Figure 6a). While the amounts of primary and secondary follicle were about 75% and 50% more than those in the burn control group respectively (Figure 6b, 6c).
Figure 6.
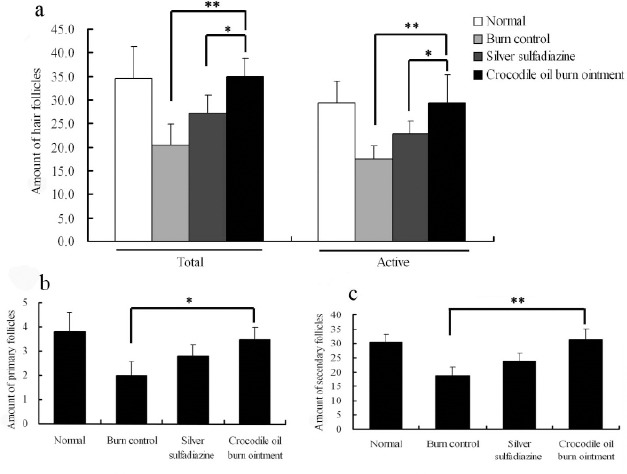
Assessment of the activity and distribution of hair follicles in the post-burn 28 day rat dorsum biopsies. Data were quantified from the samples which were stained with Sacpic. The active follicle was stained in red. The amounts of total follicle or active follicle (a), primary follicle (b), and secondary follicle (c) were measured respectively. All measurements were evaluated by taking average number of follicles in eight high power fields (20xobjective), midway in the post-burn skins. Values are mean ± SE of twelve burns. * P < 0.05; ** P < 0.01.
Antinociceptive activity of crocodile oil burn ointment (COBO)
Acetic acid-induced writhing was used to evaluate the analgesic activity of COBO; the results presented in Table 1 showed that COBO dose-dependently restrained the writhing response induced by intraperitoneal injection of acetic acid in mice with the inhibition rates of 24.6%, 41.3% and 48.6%. And COBO prolonged the time of first writhing with a higher inhibition rate of 84.4% at the dose of 0.3 g/20g in comparison to MEBO with an inhibition rate of 78.1%.
Table 1.
Effect of crocodile oil burn ointment (COBO) on acetic-acid-induced writhing response in mice
| Groups (N=8) | Dose (g/20g) | Writhing count | Inhibition (%) | The time of first writhing/min | Extension (%) |
|---|---|---|---|---|---|
| Control(salinesolution) | 0.3 | 62.3±14.8 | - | 3.2±0.5 | - |
| MEBO | 0.3 | 33.1±4.9** | 46.9 | 5.7±0.8** | 78.1 |
| COBO | 0.05 | 47.0±8.1* | 24.6 | 4.6±0.6* | 43.8 |
| COBO | 0.3 | 36.6±6.1** | 41.3 | 5.9±0.5** | 84.4 |
| COBO | 0.6 | 32.0±2.9** | 48.6 | 6.2±0.3** | 93.8 |
Each value represents the mean±SE of 8 mice. P values vs. control by F-test.
P < 0.05.
P < 0.01.
Anti-inflammatory activity of crocodile oil burn ointment (COBO)
The results presented in Table 2 showed that COBO suppressed xylene-induced ear swelling in mice with a dose-effect activity. At a dose of 0.3 g/20 g, the inhibition of xylene-induced ear swelling of COBO was not significantly different with that of MEBO.
Table 2.
Effect of crocodile oil burn ointment (COBO) on xylene-induced ear swelling in mice
| Groups(N=8) | Dose (g/20 g) | Ear swelling (%) | Inhibition (%) |
|---|---|---|---|
| Control(saline solution) | 0.3 | 3.1±0.8 | - |
| MEBO | 0.3 | 1.6±0.1** | 48.4 |
| COBO | 0.05 | 2.0±0.3* | 34.2 |
| COBO | 0.3 | 1.7±0.4** | 45.2 |
| COBO | 0.6 | 1.5±0.4** | 50.2 |
Each value represents the mean±SE of 8 mice. P values vs. control by F-test.
P < 0.05.
P < 0.01.
Discussion
The burn wound healing is a complex course divided into several phases, including inflammation, re-epithelization, and tissues remodeling (Süntar et al., 2011). In the present study, the phases were also monitored and observed for evaluating the wound healing. COBO was showed to be efficient for the burn healing, and significantly reduced the recovery time. COBO was formulated according to the topical CHM, and CHM and its extracts are commonly used topically in the clinic and show unique efficacy, especially for burns, pressure ulcers, cervical erosion, herpes zoster and chronic ulcers (Li et al., 2011). In the theory of Chinese herbalism, the interactions among the herbs which may produce synergistic effects were emphasized (Fan et al., 2010). The five herbal components in the COBO formula had their pharmacological actions as follows: Arnebia euchroma I.M. Johnst., a heat-clearing drug, has the effects of sterilization, heat-clearing and detoxification. Savia miltior rhiza Bunge can activate blood circulation on the wound surface. Sanguisorba officinalis L. can be slightly cold for clearing heat and sour for astringency. Astragalus membranaceu Moench has immunostimulant activity relieving edema. And Borneolum syntheticum can increase the penetration of other agents through the skin. Besides, crocodile oil from Crocodylus siamensis has the wound healing activity, and reduced scar formation in rats (Li et al., 2012). Hence, COBO is a complex herbal medicine whose main effects on the burn wound healing might be due to sterilization, anti-inflammation, promoting blood circulation and promoting granulation. It was supposed that COBO might inhibit the expressions of pro-inflammatory cytokines, and reduce swelling. Meanwhile, COBO might promote the expressions and secretions of growth factors in the wounds, accelating cell proliferation and new granulation formation to enhance the wound healing.
The repair of wounds is one of the most complex biological processes that occur during human life. And the ability to respond to injury and to repair tissue is a fundamental property of all multicellular organisms (Singer and Clark, 1999). The stage of new tissue formation occurs about 2-10 days after injury and is characterized by cellular proliferation and migration of different cell types (Gurtner et al., 2008), Myofibroblasts and re-epithelialization together mediate the contraction of the wound (Lin et al., 1995). The macroscopic results showed that the re-epithelialization of the wounds in the COBO group was accelerated in the first 10 days after burn injury, compared to the control group; wound closure was also faster on the COBO group. Hence, COBO could enhance the proliferation of myofibroblasts and re-epithelialization to recovery the wounds.
The skin performs many physiological functions in the body, such as, protection, sensation, heat regulation, control of evaporation, absorption, water resistance, and so on (Madison, 2003, Proksch et al., 2008, Stucker et al., 2002). The hair follicle is a fascinating miniorgan with a complex structure, which plays an importance role in the physiological functions of the skin (Muntener et al., 2011). Wound healing is a highly regulated process that is thought to be mediated in part by stem cells (Ito et al., 2005, Levy et al., 2005), while hair follicles are the niche of various stem cell populations and are a major source of cells responsible for regeneration of the hair, sebaceous glands and epidermis (Joachimiak et al., 2012). A skin wound leaves the stumps of hair follicles intact, and then a large contribution to the healed epidermis derives from these hair follicle remnants (Martin, 1997). So their growth in the wound could be revealed for the skin regeneration situation. In the present study, the hair follicles were well-distributed in the post-burn skin of COBO treatment, and the amounts of total, primary and secondary hair follicles in post-burn 28 day, skin of COBO treatment groups were more than those in burn control groups and SSD groups. These data were indicated that the skins were well of regeneration in the COBO treatment. COBO might stimulate the stem cell of hair follicle, and enhance the proliferation of the skin cells to heal the wounds.
A partial thickness skin wound leaves the roots of the hair follicles intact, which contributes to the healing of the epidermis. Thus the growth of hair follicles is a reflection of skin regeneration(Rochat et al., 1994), In the present study viable hair follicles were well distributed in the skin of COBO treated burns indicating improved skin regeneration compared with the other groups. Red staining of the connective tissue sheaths of the hair follicles on Sacpic staining is further proof that these follicles are actively dividing and differentiating
Burn injury triggers a series of physiological events at the wound site including inflammation and pain followed by new tissue formation over the course of the next several days (Feng et al., 2010, Martin and Leibovich, 2005). The antinociceptive and anti-inflammatory effects of COBO were investigated by acetic acid-induced writhing and xylene-induced ear swelling tests, respectively. In the present studies, the analgesic and ant-inflammatory activity of COBO were better than those of control group, while they were very close to that of MEBO. The active components of COBO, such as corodile oil and Borneolum syntheticum, could effiently defend against the inflammation. Such results suggest that COBO had remarkable analgesic and ant-inflammatory activities, which would promote the wound healing.
Conclusion
In summary, COBO could accelerate burn wound healing in a deep second-degree burn rat model and promote skin regeneration and the growth of hair follicles. And COBO also has analgesic and ant-inflammatory activities. These results suggest COBO would be a useful ointment for burn wound healing. Nevertheless, the repaired mechanism and clinical research of COBO needs to be further investigated
Competing interests
The authors have no conflicts of interest.
Acknowledgements
The present investigation was supported by the Science and Technology Foundation of Xiamen (No. 3502Z20133009), Guangzhou TuoLong Bio-technology Co., Ltd, GuangZhou (XDHT2013183A), and the National Science Foundation for Fostering Talents in Basic Research of the National Natural Science Foundation of China (Grant No.J1310027).
References
- 1.Bell G.D, Powell K.U, Burridge S.M, Harrison G, Rameh B, Weil J, Gant P.W, Jones P.H, Trowell J.E. Reinfection or recrudescence after apparently successful eradication of Helicobacter pylori infection: implications for treatment of patients with duodenal ulcer disease. QJM-An International Journal of Medicine. 1993;86:375–382. [PubMed] [Google Scholar]
- 2.Buchanan L.A. Kambara taraina sp. nov. (Crocodylia, Crocodyloidea), a new Eocene mekosuchine from Queensland, Australia, and a revision of the genus. Journal of Vertebrate Paleontology. 2009;29:473–486. [Google Scholar]
- 3.Buthelezi S, Southway C, Govinden U, Bodenstein J, du Toit K. An investigation of the antimicrobial and anti-inflammatory activities of crocodile oil. Journal of Ethnopharmacology. 2012;143:325–330. doi: 10.1016/j.jep.2012.06.040. [DOI] [PubMed] [Google Scholar]
- 4.Chan K, Shaw D, Simmonds M.S, Leon C.J, Xu Q, Lu A, Sutherland I, Ignatova S, Zhu Y.P, Verpoorte R, Williamson E.M, Duez P. Good practice in reviewing and publishing studies on herbal medicine, with special emphasis on traditional Chinese medicine and Chinese materia medica. Journal of Ethnopharmacology. 2012;140:469–475. doi: 10.1016/j.jep.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 5.Chen Q.X, Xiong Y.X, Li H.L, Hu Y.H, Chen L.P, Kang J.H, Zhang X.S. Crocodile oil burn ointment (COBO) and its preparation method. China Patent No. ZL 201010505834. 2011 [Google Scholar]
- 6.De Oliveira M.L, Nunes-Pinheiro D.C, Tome A.R, Mota E.F, Lima-Verde I.A, Pinheiro F.G, Campello C.C, de Morais S.M. In vivo topical anti-inflammatory and wound healing activities of the fixed oil of Caryocar coriaceum Wittm. seeds. Journal of Ethnopharmacology. 2010;129:214–219. doi: 10.1016/j.jep.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 7.De Villiers F.P, Ledwaba M.J. Traditional healers and paediatric care. S Afr Med J. 2003;93:664–665. [PubMed] [Google Scholar]
- 8.Fan A.Y, Lao L, Zhang R.X, Zhou A.N, Berman B.M. Preclinical safety evaluation of the aqueous acetone extract of Chinese herbal formula Modified Huo Luo Xiao Ling Dan. Zhong Xi Yi Jie He Xue Bao. 2010;8:438–447. doi: 10.3736/jcim20100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng X, Cheng G, Chen S.Y, Yang H, Huang W. Evaluation of the burn healing properties of oil extraction from housefly larva in mice. Journal of Ethnopharmacology. 2010;130:586–592. doi: 10.1016/j.jep.2010.05.044. [DOI] [PubMed] [Google Scholar]
- 10.Gurtner G.C, Werner S, Barrandon Y, Longaker M.T. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 11.Hemmila M.R, Mattar A, Taddonio M.A, Arbabi S, Hamouda T, Ward P.A, Wang S.C, Baker J.R., Jr Topical nanoemulsion therapy reduces bacterial wound infection and inflammation afterburn injury. Surgery. 2010;148:499–509. doi: 10.1016/j.surg.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang X.H, Rong X.Z, Du J. Beware of burn wound treated with MEBO. Academic Journal of Guangzhou Medical College. 2004;32:85–86. [Google Scholar]
- 13.Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris R.J, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair butnotto homeostasis of the epidermis. Nature Medicine. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 14.Jewo P.I, Fadeyibi I.O, Babalola O.S, Saalu L.C, Benebo A.S, Izegbu M.C, Ashiru O.A. A comparative study of the wound healing properties of moist exposed burnointment(MEBO) and silver sulphadiazine. Annals of Burns and Fire Disasters. 2009;22:79–82. [PMC free article] [PubMed] [Google Scholar]
- 15.Joachimiak R, Bajek A, Drewa T. Hair follicle as a novel source of stem cells. Postępy Higieny i Medycyny Doświadczalnej. 2012;66:181–186. doi: 10.5604/17322693.991445. [DOI] [PubMed] [Google Scholar]
- 16.Kang J.H, Zhang W.Q, Song W, Shen D.Y, Li S.S, Tian L, Shi Y, Liang G, Xiong Y.X, Chen Q.X. Apoptosis mechanismof human cholangiocarcinoma cells induced by bile extract from crocodile. Appl Biochem Biotechnol. 2012;166:942–951. doi: 10.1007/s12010-011-9482-x. [DOI] [PubMed] [Google Scholar]
- 17.Kou J, Ni Y, Li N, Wang J, Liu L, Jiang Z.H. Analgesic and anti-inflammatory activities of total extract and individual fractions of Chinese medicinal ants Polyrhachis lamellidens. Biological & Pharmaceutical Bulletin. 2005;28:176–180. doi: 10.1248/bpb.28.176. [DOI] [PubMed] [Google Scholar]
- 18.Lee J.A, Jeong H.J, Park H.J, Jeon S, Hong S.U. Acupuncture accelerates wound healing inburn-injured mice. Burns. 2011;37:117–125. doi: 10.1016/j.burns.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Levy V, Lindon C, Harfe B.D, Morgan B.A. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Developmental Cell. 2005;9:855–861. doi: 10.1016/j.devcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Li H.L, Chen L.P, Hu Y.H, Qin Y, Liang G, Xiong Y.X, Chen Q.X. Crocodile OilEnhances Cutaneous BurnWound Healing and Reduces Scar Formation in Rats. Academic Emergency Medicine. 2012;19:265–273. doi: 10.1111/j.1553-2712.2012.01300.x. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Zhao J, Liu J, Xiang F, Lu D, Liu B, Xu J, Zhang H, Zhang Q, Li X, Yu R, Chen M, Wang X, Wang Y, Chen B. Prospective randomized controlled study of a Chinese herbal medicine compound Tangzu Yuyang Ointment for chronic diabetic foot ulcers: a preliminary report. Journal of Ethnopharmacology. 2011;133:543–550. doi: 10.1016/j.jep.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 22.Li Y. Clinical observation of high dose Ambroxol on preventing and curing newborn respiratory distress syndrome. China Medical Herald. 2011;8:83–85. [Google Scholar]
- 23.Lin R.Y, Sullivan K.M, Argenta P.A, Meuli M, Lorenz H.P, Adzick N.S. Exogenous transforming growth factor-beta amplifies its own expression and induces scar formation in a model of human fetal skin repair. Annals of Surgery. 1995;222:146–154. doi: 10.1097/00000658-199508000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsey K.L, Jager A.K, Raidoo D.M, VanStaden J. Screening of plants used by Southern African traditional healers in the treatment of dysmenorrhoea for prostaglandin-synthesis inhibitors and uterine relaxing activity. Journal of Ethnopharmacology. 1999;64:9–14. doi: 10.1016/s0378-8741(98)00097-x. [DOI] [PubMed] [Google Scholar]
- 25.Madison K.C. Barrier function of the skin: “la raison d’etre” of the epidermis. Journal of Investigative Dermatology. 2003;121:231–241. doi: 10.1046/j.1523-1747.2003.12359.x. [DOI] [PubMed] [Google Scholar]
- 26.Martin P. Wound healing--aiming for perfectskin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 27.Martin P, Leibovich S.J. Inflammatorycells during wound repair: the good, thebadandthe ugly. Trends in Cell Biology. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Muntener T, Doherr M.G, Guscetti F, Suter M.M, Welle M.M. The canine haircycle - a guide forthe assessmentofmorphological and immunohistochemical criteria. Veterinary Dermatology. 2011;22:383–395. doi: 10.1111/j.1365-3164.2011.00963.x. [DOI] [PubMed] [Google Scholar]
- 29.Nixon A.J. A method for determining the activity state of hair follicles. Biotechnic & Histochemistry. 1993;68:316–325. doi: 10.3109/10520299309105637. [DOI] [PubMed] [Google Scholar]
- 30.Proksch E, Brandner J.M, Jensen J.M. The skin: an indispensable barrier. Experimental Dermatology. 2008;17:1063–1072. doi: 10.1111/j.1600-0625.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 31.Rochat A, Kobayashi K, Barrandon Y. Location of stem cells of human hair follicles by clonal analysis. Cell. 1994;76:1063–1073. doi: 10.1016/0092-8674(94)90383-2. [DOI] [PubMed] [Google Scholar]
- 32.Süntar I, Akkol E.K, Keleş H, Oktem A, Başer K.H.C, Yeşilada E. A novel wound healing ointment: A formulation of Hypericum perforatum oil and sage and oregano essential oils based on traditional Turkish knowledge. Journal of Ethnopharmacology. 2011;134:89–96. doi: 10.1016/j.jep.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 33.Singer A.J, Clark R.A. Cutaneous woundhealing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 34.Stucker M, Struk A, Altmeyer P, Herde M, Baumgartl H, Lubbers D.W. The cutaneous uptake of atmospheric oxygen contributes significantly to the oxygen supply of human dermis and epidermis. Journal of Physiology. 2002;538:985–994. doi: 10.1113/jphysiol.2001.013067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Z, Yue J, Zhang Q. Ionic Components of Wound Current at Mouse Skin Incisional Wounds. European Journal of BioMedical Research. 2015;1:3. [Google Scholar]
- 36.Tang L, Qin M.Z. Themedicinal researchand development prospects of crocodile. World Health Digest(in Chinese) 2007;4(10):66–68. [Google Scholar]
- 37.Tumen I, Akkol E.K, Suntar I, Keles H. Wound repair and anti-inflammatory potential of essential oils from cones of Pinaceae: preclinical experimental research in animal models. Journal of Ethnopharmacology. 2011;137:1215–1220. doi: 10.1016/j.jep.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 38.Upadhyay N.K, Kumar R, Mandotra S.K, Meena R.N, Siddiqui M.S, Sawhney R.C, Gupta A. Safety and healing efficacy of Sea buckthorn (Hippophae rhamnoides L.) seed oil on burn wounds in rats. Food and Chemical Toxicology. 2009;47:1146–1153. doi: 10.1016/j.fct.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Vloemans A.F, Soesman A.M, Suijker M, Kreis R.W, Middelkoop E. A randomised clinical trial comparing a hydrocolloid-derived dressing and glycerol preserved allograft skin in the management of partial thickness burns. Burns. 2003;29:702–710. doi: 10.1016/s0305-4179(03)00161-x. [DOI] [PubMed] [Google Scholar]
- 40.Wilgus T.A. Immune cells in the healing skin wound: influential players at each stage of repair. Pharmacological Research. 2008;58:112–116. doi: 10.1016/j.phrs.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 41.Xutian S, Zhang J, Louise W. New exploration and understanding of traditional Chinese medicine. The American Journal of Chinese Medicine. 2009;37:411–426. doi: 10.1142/S0192415X09006941. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Q.H, Sun Z.R, Yue J.H, Ren X, Qiu L.B, Lv X.L, Du W. Traditional Chinese medicine for pressure ulcer: a meta-analysis. International Wound Journal. 2013;10:221–231. doi: 10.1111/j.1742-481X.2012.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou M, Wang H, Suolangjiba Kou J, Yu B. Antinociceptive and anti-inflammatory activities of Aquilaria sinensis (Lour.) Gilg. Leaves extract. Journal of Ethnopharmacology. 2008;117:345–350. doi: 10.1016/j.jep.2008.02.005. [DOI] [PubMed] [Google Scholar]


