Abstract
Aim
To investigate whether the impact of dose escalation in our patient population represented an improvement in local control without increasing treatment related toxicity.
Materials and methods
A cohort of consecutive patients with colorectal liver metastases treated with stereotactic body radiation therapy (SBRT) between December 2002 and December 2013 were eligible for this study. Inclusion criteria were a Karnofsky performance status ≥80% and, according to the multidisciplinary tumor board, ineligibility for surgery or radiofrequency ablation. Exclusion criteria were a lesion size >6 cm, more than 3 metastases, and treatment delivered with other fractionation scheme than 3 times 12.5 Gy or 16.75 Gy prescribed at the 65–67% isodose. To analyze local control, CT or MRI scans were acquired during follow-up. Toxicity was scored using the Common Toxicity Criteria Adverse Events v4.0.
Results
A total of 40 patients with 55 colorectal liver metastases were included in this study. We delivered 37.5 Gy to 32 lesions, and 50.25 Gy to 23 lesions. Median follow-up was 26 and 25 months for these two groups. Local control at 2 and 3 years was 74 and 66% in the low dose group while 90 and 81% was reached in the high dose group. No significant difference in local control between the two dose fractionation schemes could be found. Grade 3 toxicity was limited and was not increased in the high dose group.
Conclusions
SBRT for colorectal liver metastases offers a high chance of local control at long term. High irradiation doses may contribute to enhance this effect without increasing toxicity.
Keywords: Colorectal, Metastases, Liver, Stereotactic body radiation therapy
1. Background
Liver metastases develop in up to 70% of patients with colorectal cancer. Resection is the ‘golden standard’ treatment with reported median survival of 44 months and 34–40% of patients being alive at 5 years.1 Because most of the patients are not eligible for surgery, other nonsurgical ablation techniques are used, with radiofrequency ablation (RFA) being the most widely applied treatment modality. Several factors have been described to impact the success rate of RFA. A tumor size >3 cm has been identified as a predictor of a higher relapse rate.2 The location of the tumors within the liver is also an important factor; in particular tumors adjacent to large hepatic vessels present a unique problem due to the cooling effect provided by the blood flowing through them.2 Location near the portal vein pedicles is also associated with increased complications because RFA in this area can cause injury to the main bile duct resulting in biliary stricture.2 Retrospective RFA series for colorectal liver metastases have shown site recurrence rates of 9–42% for percutaneous RFA and 5–14% for open RFA with median survival of 36 months.3, 4 For patients not eligible for RFA, stereotactic body radiation therapy (SBRT) offers the possibility to deliver potent biological doses to limited volumes of the liver in a few fractions. High local control rates at 2 years of 74–91% and median survival of 34 months have been reported after SBRT for colorectal liver metastases.5, 6
A few studies have assessed the role of dose escalation on the clinical outcomes after SBRT for liver metastases. In 2006 Wulf et al. found a significant improvement in 2 year local control (82 vs. 58%) with 12–12.5 Gy in 3 fractions or 26 Gy in 1 fraction vs. 30 Gy in 3 fractions.7 No severe toxicity was observed. Later on, in a multi-institutional phase I/II study, Rusthoven et al. evaluated the efficacy and tolerability of high dose SBRT.8 The dose was safely escalated from 36 till 60 Gy delivered in 3 fractions with 2 year local control rate of 92%. Only one patient experienced grade III (soft tissue) toxicity. Rule et al. studied three dose-escalation cohorts and showed a significant difference in local control between 60 Gy in 5 fractions vs. 30 Gy in 3 fractions.9 No patient experienced grade III or higher toxicity. Regardless of the above mentioned results, Vautravers-Dewas and colleagues did not find a significant difference in local control between 40 Gy in 4 fractions and 45 Gy in 3 for their cohort treated with SBRT.10
In 2010, our group reported a 2-year local control of 74% for patients with colorectal liver metastases treated mainly with 37.5 Gy in 3 fractions.5 Later on, and based on published data, the dose was escalated to 50.25 Gy also delivered in 3 fractions. This retrospective study investigated whether the increase in dose represented an improvement in local control without raising the treatment associated toxicity in our patient population.
2. Materials and methods
2.1. Design
This study was designed as a retrospective, observational, and single institution. It was performed in accordance with the code of ethics of the Helsinki declaration and approved by the Ethical Committee of Erasmus Medical Center (MEC-2015-029).
2.2. Population
All consecutive patients treated in our department between December 2002 and December 2013 were considered candidates for this study. Patients should fulfill the following criteria: diagnose of colorectal liver metastases, not eligible for surgery or radiofrequency ablation (RFA) according to the multidisciplinary tumor board, and a Karnofsky performance score of at least 80%. If extrahepatic disease was present, it had to be limited and potentially treatable with local therapies. Exclusion criteria for this study included: a tumor diameter >6 cm, more than 3 metastases per patient, and dose fractionation scheme other than 3 times 12.5 Gy or 16.75 Gy delivered at the 65–67% isodose.
2.3. Endpoints
Primary endpoints of this study were the assessment of local control and toxicity. Local control was defined as no in field progression during follow-up on CT or MR imaging. Toxicity was scored with the Common Toxicity Criteria (CTC) of the National Cancer Institute v 4.0. Secondary endpoint was overall survival. Factors related to local control were also investigated, including age, gender, and size and number of metastases.
2.4. Treatment preparation and delivery
Between 2002 and 2011 patients were positioned in a stereotactic body frame (Elekta Oncology Systems, Stockholm, Sweden) with abdominal compression to reduce respiratory tumor motion for planning and treatment purposes. Three computed tomography (CT) scans per patient were acquired; one in the arterial phase and one in the venous phase for tumor definition, and one large-volume non-enhanced scan for dose planning. Details about this procedure have been reported earlier.5, 11, 12, 13 From 2011, only one large contrast enhanced planning CT in the venous phase was acquired. The tumor delineations have always been reviewed by an experienced radiologist. The boundary of the metastasis was considered the border or contrast enhancement.
Since 2005, we have been implanting fiducial markers in the vicinity of the tumor to assess the respiratory motion of the area where the tumor is located. Initially the motion was measured with video fluoroscopy registrations and later on with a reconstruction of 4DCT registrations.14, 15
No margin between gross target volume and clinical target volume was used. Planning target volume (PTV) margins were initially based on the Karolinska experience.16, 17 With the introduction of fiducial markers, margins were individualized based on an in-house developed margin recipe that was used to calculate required PTV margin for each patient individually. The margin recipe takes as input the treatment technique (e.g. tracking or non-tracking), the amplitude of the respiratory-induced motion, and the distance between the center-off-mass of the marker configuration and the center of the tumor.15 For conventional linac based treatments the personalized margins that were applied clinically ranged between 8–10 mm superior–inferior, and 5–7 mm left–right and anterior–posterior. For CyberKnife treatments the personalized margins ranged between 5 and 7 mm in all directions. The dose was prescribed at the periphery of the PTV. Planning dose volume constraints for PTV and organs at risk have been already published. Over time the liver constraint was modified based on available new data.18
Until 2012 treatment was delivered with a conventional linac and using a daily CT (not in room) and MV-or KV-imaging for daily setup correction. Later on, the treatment was delivered with CyberKnife (Accuray, Sunnyvale, CA, USA), guided by an incorporated real time fiducial tracking system using KV imaging (Synchrony).
2.5. Follow-up
Patients had a CT or MRI scan at 1 month after irradiation and every 3 months thereafter during the first two years. After this period, imaging was performed every 6 months. Liver function examinations were also performed including bilirubin, albumin aspartate-and alanine amino transferases (AST and ALT), gamma glutamyl tranferase (GGT), and alkaline phosphatase (AF).
2.6. Statistics
Descriptive statistics including mean, median, standard deviation and range were calculated for each investigated parameter. For categorical data, percentages per category were calculated. To assess local control and survival, Kaplan–Meier analyses were performed. We also estimated the cumulative incidence for local control with death as competing risk.19 The one-way ANOVA test was carried out to evaluate differences on tumor diameter between the low and the high dose group. The Cox regression model was used to identify variables associated with local control.
All data were analyzed with SPSS version 20 (Statistical Package for the Social Sciences, Chicago IL) and the statistical program R version 2.13 (http://www.R-project.org).
Statistical significance was considered p ≤ 0.05.
3. Results
3.1. Population
Between December 2002 and December 2013 we treated 40 patients with 55 colorectal liver metastases that fulfilled the inclusion and exclusion criteria. Gender distribution was 31 male and 9 female. Median age was 70 years. A total of 32 lesions with a median diameter of 2.7 cm (0.7–6.2) were treated with 37.5 Gy, and 23 lesions with a median diameter of 2.2 cm (0.9–4.2) were treated with 50.25 Gy. No significant difference in diameter between the two groups was found (p = 0.3). Median follow-up (imaging) was 26 months (1.5–80) for the low dose group and 25 months (4.6–64) for the high dose group. Further details are presented in Table 1.
Table 1.
Demographics.
| Patients | 40 |
| Gender ratio (M:F) | 31:9 |
| Median (range) age (years) | 70 (35–86) |
| Patients with 1 metastasis | 27 |
| Patients with 2 metastases | 11 |
| Patients with 3 metastases | 2 |
| Metastases | 55 |
| Median (range) diameter (cm) | 2.5 (0.7–6.2) |
| Site (Couinaud segments) | |
| I | 5 |
| II | 0 |
| III | 3 |
| IV | 7 |
| V | 3 |
| VI | 5 |
| VII | 12 |
| VIII | 20 |
| Treatment with 3 × 12.5 Gy | 32 |
| Treatment with 3 × 16.75 Gy | 23 |
| Conventional linac vs. CyberKnife | 40:15 |
3.2. Local control
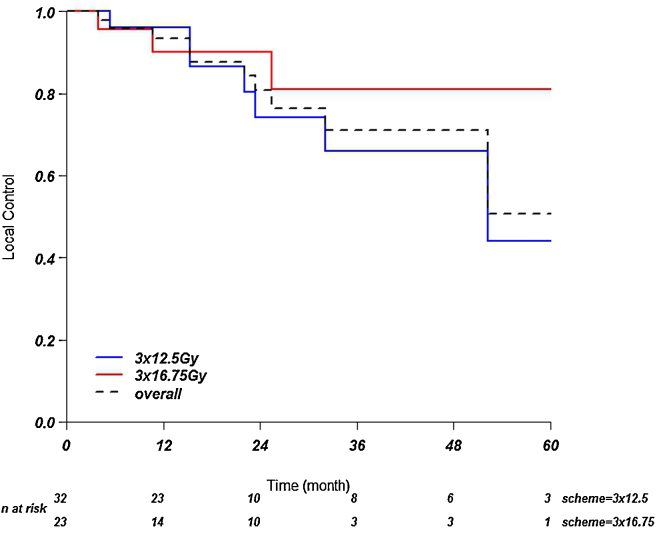
For the 37.5 Gy group the local control at 1, 2, and 3 years was 96, 74, and 66%, respectively. For the 50.25 Gy group, local control at 1, 2, 3 years was 90, 90 and 81%. See Fig. 1. No significant difference could be found between the two dose fractionation schemes (p = 0.44).
Fig. 1.
Local control. Kaplan–Meier curves showing local control over time.
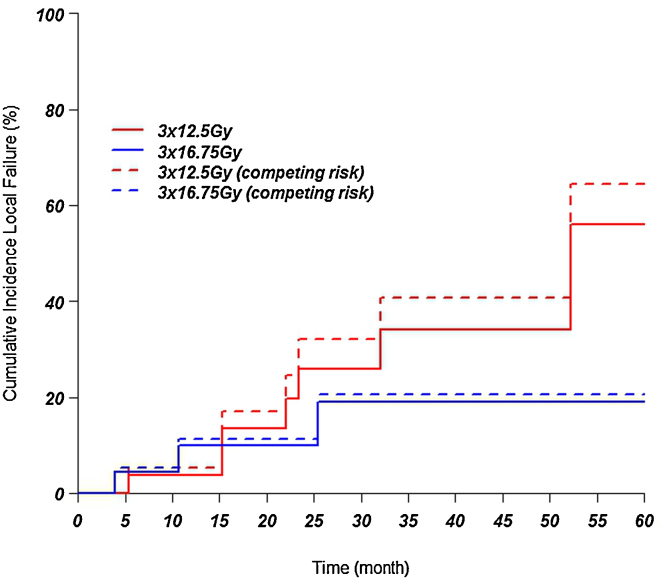
When analysing the local relapse incidence with the competing risk method, the 1, 2 and 3 years in the low dose group was 4, 18, and 23%. For the high dose group the values at 1, 2, and 3 years were 9, 9, and 16%. There was no significant difference between the two groups (p = 0.41). See Fig. 2.
Fig. 2.
Incidence of local failure after applying the Kaplan–Meier test and the competing risk method.
Univariate analysis showed that tumor diameter (>30 mm) and number of lesions had a significant impact on local control. In multivariate analysis only the number of lesions was significantly associated with local control (Table 2).
Table 2.
Factors influencing local control. Univariate and multivariate analysis.
| Factor | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| p value | HR 95% CI |
p value | HR 95% CI |
|
| Dose fractionation scheme | 0.44 | 0.59 (0.15–2.3) |
– | – |
| Diameter | 0.16 | 1.04 (0.98–1.1) |
0.13 | 1.04 (0.99–1.1) |
| Number of lesions | 0.05 | 2.5 (1.0–6.0) |
0.04 | 2.6 (1.05–6.2) |
| Age | 0.62 | 1.01 (0.95–1.08) |
– | – |
| Gender | 0.91 | 0.88 (0.11–7.0) |
– | – |
HR: Hazard ratio, CI: Confidence interval, p: Probability value (significance).
3.3. Survival
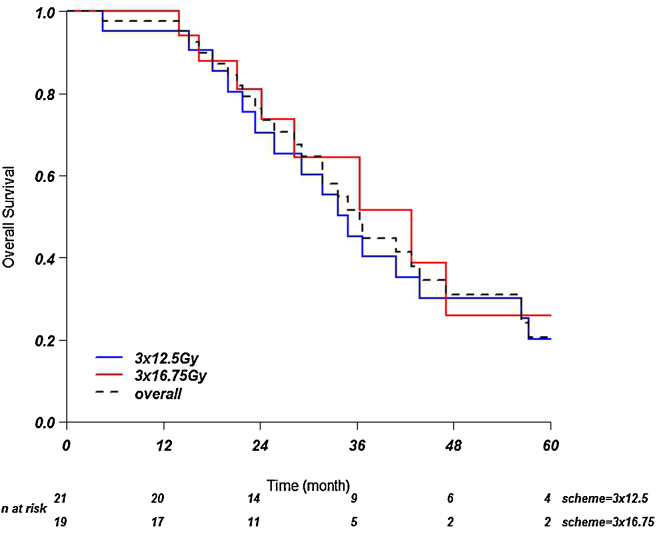
Overall survival at 1, 2 and 3 years in the group of patients treated with 37.5 Gy was 95, 69 and 48%, respectively. In the group treated with 50.25 Gy it was 94, 81 and 65%. Median overall survival in the low dose group was 35 months and in the high dose group was 43 months. The difference between the two groups was not significant (p = 0.46) (Fig. 3).
Fig. 3.
Overall survival. Kaplan–Meier curves showing overall survival over time.
3.4. Toxicity
We had no liver function investigations of five patients after treatment. Two patients were followed in other hospitals and three were referred to other specialists just after SBRT. In one patient we did not perform any liver function investigation before treatment.
Grade ≤2 hepatic toxicity was observed directly after SBRT in all patients except one.
Two cases of grade 3 asymptomatic elevation of gamma GGT were found in the 37.5 Gy group. One case of grade 3 GGT and aspartate transaminase elevation was detected in the 50.25 Gy group.
One patient treated in the low dose group experienced asthenia grade 3 with spontaneous recuperation two months after treatment. Another patient developed grade 2 fat atrophy and skin induration after SBRT for three subcapsular metastases. As described in a previous paper, one patient developed a portal hypertension syndrome with esophageal varices and one episode of melena after two SBRT treatments.5, 13 A case of a biliary tree dilatation was observed in a patient treated for a centrally located liver metastases with 50.25 Gy.
No case of grade 4 or 5 toxicity was observed.
4. Discussion
This study corroborates a high chance of local control after SBRT for colorectal liver metastases not eligible for other local treatments, including surgery and RFA. Local control rates at 2 years were 74% after the delivery of 37.5 Gy and 90% after 50.25 Gy; however, this difference was not statistically significant. Toxicity, as expected, remained limited.
Several papers have described a high local control rate after SBRT for colorectal liver metastases. Recently, Scorsetti et al. reported 2 and 3 year local control rates of 91 and 85% delivering 75 Gy in 3 fractions as a mean dose to the PTV in a similar population to ours (1–3 lesions of <6 cm).6 Toxicity was limited (no grade ≥3). Stintzing et al. reached a local control at 1 and 2 years of 87 and 55%, respectively, after 24 Gy delivered in 1 fraction in a group of patients with 1–2 metastases and <5 cm diameter.20 No side effect was detected. Kim et al. observed a local control chance of 60% at 2 and 3 years after 36–51 Gy in 3 fractions delivered at the 75–80% isodose.21 Patients had 1–3 liver metastases with total tumor volume of ≤500 ml. No grade ≥3 complications were detected. Later on, Chang et al. after a pooled analysis, estimated that a dose of 46–52 Gy (48 Gy) was needed for a 1 year local control of >90%.22 In 2010, our group reported a 74% local control rate at 2 years in a group of patients treated mainly with 37.5 Gy delivered at the 65–67% isodose surrounding the PTV.5 Two episodes of asymptomatic elevation of GGT and one asthenia grade 3 were observed. In our present series, 2 and 3 year local control rates were 74 and 66% for 37.5 Gy and 90 and 81% for 50.25 Gy. After correcting for the risk of death, the chance of local relapse after these two different schemes at 2 years was 18 and 9%, however, no significant difference was found. Toxicity was not increased in the high dose group.
A variety of relatively good results of local control at 2 years between 50 and 91% for colorectal liver metastases after different SBRT fractionation schemes are presented above. Our results fit very well in this range. It may be suggested that large doses of irradiation are needed to ensure a high local control at 2 and 3 years follow-up, and that this does not represent an unsafe option.
The impact of dose escalation in the local control of liver metastases has been published by several authors. In the early years of SBRT, two German groups found a significant improvement in local control related to dose escalation. The investigators from Heidelberg observed that local control was more favorable after 26 Gy than after 14 Gy delivered in 1 fraction.23 The team from Wurzburg found a 2-year local control of 82 vs. 58% after delivering 36–37.5 Gy in 3 fractions or 26 Gy in 1 fraction vs. 30 Gy in 3 fractions.7 Later on, in the North American multiinstitutional phase I/II study, the dose was escalated from 36 to 60 Gy with reported 2 years local control rate of 92%. From the same authors group, a dose of at least 54 Gy in 3 fractions had been proposed to achieve 89% local control at 3 years for targets in lung and liver.24 Accordingly, the authors from the University of Texas Southwestern showed a significant difference in local control between 60 Gy in 5 fractions and 30 Gy in 3 fractions (2 year 100 vs. 56%).8 However, not all groups have identified this significant relationship in their patient populations.10 In 2013, the American Association of Physics in Medicine organized a working group to quantitatively evaluate the impact of different dose fractionation schemes in the local control of liver tumors.25 After a PubMed search, 13 papers met all inclusion criteria and formed the dataset for the analysis. A significant relationship was found between local control for liver metastases after SBRT and a BED >100 Gy10. Although the BED of 37.5 Gy is <100 Gy10 and the BED of 50.25 Gy is >100 Gy10, we have not found this significant relationship in our population. A possible explanation may be the lower number of metastases included in our study compared with the AAPM (55 vs. 290).
The impact of tumor size on local control remains unclear. In univariate analysis Rusthoven et al. showed a significant difference for metastases of ≤3 cm vs. >3 cm (100 vs. 77%).8 No multivariate analysis was performed because of the small number of events. Accordingly, we also found the same result. Chang et al. also showed in univariate analysis a significant impact of tumor diameter on local control in a cohort of colorectal liver metastases.22 However, Scorsetti et al. did not observe this significant relationship.6 We did not find it either in our first analysis published in 2010.5 Perhaps the fact that the number of metastases in our last series has increased may be the reason for this difference.
The number of metastases per patient has been found significantly related to local control in our current analysis. Thirteen patients had 2–3 metastases (8 received 37.5 Gy and 4 received 50.25 Gy). We do not have a clear explanation for this effect. A combination of factors such as tumor biology, a deeper microscopic extension beyond the main metastases border, size of the metastases and a higher number of patients in the low dose group might have influenced this finding.26
Due to the different patient selection criteria for SBRT, the overall survival rates associated with different local treatment techniques are difficult to compare, and therefore we did not consider overall survival as a primary endpoint of this study. Nevertheless, our median survival seems comparable to the recently published 36 months by Shady et al. after RFA for colorectal liver metastases.3
A limitation of this study is the retrospective design. Also, the number of patients is not large; however, it is comparable with the ranges of most liver SBRT studies. The only way to solve this problem is the collaboration of many centers. In the Netherlands a web based database will in the close future collect the data of all patients with liver metastases treated with SBRT from different institutions.
There are no randomized trials between SBRT and other treatment techniques. In an attempt to compare RFA with SBRT, Stintzing and colleagues evaluated the outcomes of these two treatments in a single institution retrospective study.27 The cohort included 60 heavily treated colorectal cancer patients. Median SBRT dose was 24–26 Gy at the 80% isodose. Local control at 1 and 2 years were not significantly different but favored SBRT (85 vs. 65% and 80 vs. 61%). A significant longer local-disease free survival was observed in the SBRT group (34.4 vs. 6 months). No significant difference in median overall survival was found although the difference was almost significant (p = 0.06) with 52.3 vs. 34.4 months, favoring RFA. The authors attribute this difference to a possible patient selection. The 2-year local control after SBRT in our series fit well with the results found in the study. Differences in median survival as mentioned by Stintzing et al. are most probably due to patient selection in the two studies.
We conclude that SBRT for colorectal liver metastases offers a high chance of local control at long term in patients that are not eligible for other local treatments including surgery and RFA. High irradiation doses may contribute to enhance this effect without increasing toxicity.
Authors’ contribution
All authors have contributed to the article.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.van der Pool A.E., Lalmahomed Z.S., Ozbay Y. ‘Staged’ liver resection in synchronous and metachronous colorectal hepatic metastases: differences in clinicopathological features and outcome. Colorectal Dis. 2010;12(10):e229–e235. doi: 10.1111/j.1463-1318.2009.02135.x. [online] [DOI] [PubMed] [Google Scholar]
- 2.Wong S.L., Mangu P.B., Choti M.A. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol. 2010;28(3):493–508. doi: 10.1200/JCO.2009.23.4450. [DOI] [PubMed] [Google Scholar]
- 3.Shady W., Petre E.N., Gonen M. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes – a 10-year experience at a single center. Radiology. 2015:142489. doi: 10.1148/radiol.2015142489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stang A., Fischbach R., Teichmann W., Bokemeyer C., Braumann D. A systematic review on the clinical benefit and role of radiofrequency ablation as treatment of colorectal liver metastases. Eur J Cancer. 2009;45(10):1748–1756. doi: 10.1016/j.ejca.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 5.van der Pool A.E., Mendez Romero A., Wunderink W. Stereotactic body radiation therapy for colorectal liver metastases. Br J Surg. 2010;97(3):377–382. doi: 10.1002/bjs.6895. [DOI] [PubMed] [Google Scholar]
- 6.Scorsetti M., Comito T., Tozzi A. Final results of a phase II trial for stereotactic body radiation therapy for patients with inoperable liver metastases from colorectal cancer. J Cancer Res Clin Oncol. 2015;141(3):543–553. doi: 10.1007/s00432-014-1833-x. [DOI] [PubMed] [Google Scholar]
- 7.Wulf J., Guckenberger M., Haedinger U. Stereotactic radiotherapy of primary liver cancer and hepatic metastases. Acta Oncol. 2006;45(7):838–847. doi: 10.1080/02841860600904821. [DOI] [PubMed] [Google Scholar]
- 8.Rusthoven K.E., Kavanagh B.D., Cardenes H. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27(10):1572–1578. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 9.Rule W., Timmerman R., Tong L. Phase I dose-escalation study of stereotactic body radiotherapy in patients with hepatic metastases. Ann Surg Oncol. 2011;18(4):1081–1087. doi: 10.1245/s10434-010-1405-5. [DOI] [PubMed] [Google Scholar]
- 10.Vautravers-Dewas C., Dewas S., Bonodeau F. Image-guided robotic stereotactic body radiation therapy for liver metastases: is there a dose response relationship? Int J Radiat Oncol Biol Phys. 2011;81(3):e39–e47. doi: 10.1016/j.ijrobp.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 11.Wunderink W., Mendez Romero A., Vasquez Osorio E.M. Target coverage in image-guided stereotactic body radiotherapy of liver tumors. Int J Radiat Oncol Biol Phys. 2007;68(1):282–290. doi: 10.1016/j.ijrobp.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 12.Wunderink W., Mendez Romero A., Seppenwoolde Y., de B.H., Levendag P., Heijmen B. Potentials and limitations of guiding liver stereotactic body radiation therapy set-up on liver-implanted fiducial markers. Int J Radiat Oncol Biol Phys. 2010;77(5):1573–1583. doi: 10.1016/j.ijrobp.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 13.Mendez Romero A., Wunderink W., Hussain S.M. Stereotactic body radiation therapy for primary and metastatic liver tumors: a single institution phase I–II study. Acta Oncol. 2006;45(7):831–837. doi: 10.1080/02841860600897934. [DOI] [PubMed] [Google Scholar]
- 14.Wunderink W., Mendez Romero A., de Kruijf W., de Boer H., Levendag P., Heijmen B. Reduction of respiratory liver tumor motion by abdominal compression in stereotactic body frame, analyzed by tracking fiducial markers implanted in liver. Int J Radiat Oncol Biol Phys. 2008;71(3):907–915. doi: 10.1016/j.ijrobp.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Seppenwoolde Y., Wunderink W., Wunderink-van Veen S.R., Storchi P., Mendez Romero A., Heijmen B.J. Treatment precision of image-guided liver SBRT using implanted fiducial markers depends on marker-tumour distance. Phys Med Biol. 2011;56(17):5445–5468. doi: 10.1088/0031-9155/56/17/001. [DOI] [PubMed] [Google Scholar]
- 16.Lax I., Blomgren H., Naslund I., Svanstrom R. Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta Oncol. 1994;33(6):677–683. doi: 10.3109/02841869409121782. [DOI] [PubMed] [Google Scholar]
- 17.Blomgren H., Lax I., Naslund I., Svanstrom R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34(6):861–870. doi: 10.3109/02841869509127197. [DOI] [PubMed] [Google Scholar]
- 18.Schefter T.E., Kavanagh B.D., Timmerman R.D., Cardenes H.R., Baron A., Gaspar L.E. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys. 2005;62(5):1371–1378. doi: 10.1016/j.ijrobp.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Geskus R.B. CRC Press Taylor & Francis Group; 2015. Data analysis with competing risk and intermediate states. [Google Scholar]
- 20.Stintzing S., Hoffmann R.T., Heinemann V., Kufeld M., Muacevic A. Frameless single-session robotic radiosurgery of liver metastases in colorectal cancer patients. Eur J Cancer. 2010;46(6):1026–1032. doi: 10.1016/j.ejca.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Kim M.S., Kang J.K., Cho C.K. Three-fraction stereotactic body radiation therapy for isolated liver recurrence from colorectal cancer. Tumori. 2009;95(4):449–454. doi: 10.1177/030089160909500407. [DOI] [PubMed] [Google Scholar]
- 22.Chang D.T., Swaminath A., Kozak M. Stereotactic body radiotherapy for colorectal liver metastases: a pooled analysis. Cancer. 2011;117(17):4060–4069. doi: 10.1002/cncr.25997. [DOI] [PubMed] [Google Scholar]
- 23.Herfarth K.K., Debus J., Lohr F. Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol. 2001;19(1):164–170. doi: 10.1200/JCO.2001.19.1.164. [DOI] [PubMed] [Google Scholar]
- 24.McCammon R., Schefter T.E., Gaspar L.E., Zaemisch R., Gravdahl D., Kavanagh B. Observation of a dose-control relationship for lung and liver tumors after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2009;73(1):112–118. doi: 10.1016/j.ijrobp.2008.03.062. [DOI] [PubMed] [Google Scholar]
- 25.Ohri N., Jackson A., Mendez Romero A. Local control following stereotactic body radiotherapy for liver tumors: a preliminary report of the AAPM Working Group for SBRT. Int J Radiat Oncol Biol Phys. 2014;90(1):S52. [Google Scholar]
- 26.Mendez Romero A., Verheij J., Dwarkasing R.S. Comparison of macroscopic pathology measurements with magnetic resonance imaging and assessment of microscopic pathology extension for colorectal liver metastases. Int J Radiat Oncol Biol Phys. 2012;82(1):159–166. doi: 10.1016/j.ijrobp.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 27.Stintzing S., Grothe A., Hendrich S. Percutaneous radiofrequency ablation (RFA) or robotic radiosurgery (RRS) for salvage treatment of colorectal liver metastases. Acta Oncol. 2013;52(5):971–977. doi: 10.3109/0284186X.2013.766362. [DOI] [PubMed] [Google Scholar]