Abstract
Interventional radiology plays a major role in the modern management of liver cancers, in primary hepatic malignancies or metastases and in palliative or curative situations. Radiological treatments are divided in two categories based on their approach: endovascular treatment and direct transcapsular access.
Endovascular treatments include mainly three applications: transarterial chemoembolization (TACE), transarterial radioembolization (TARE) and portal vein embolization (PVE). TACE and TARE share an endovascular arterial approach, consisting of a selective catheterization of the hepatic artery or its branches. Subsequently, either a chemotherapy (TACE) or radioembolic (TARE) agent is injected in the target vessel to act on the tumor. PVE raises the volume of the future liver remnant in extended hepatectomy by embolizing a portal vein territory which results in hepatic regeneration.
Direct transcapsular access treatments involve mainly three techniques: radiofrequency thermal ablation (RFA), microwave thermal ablation (MWA) and percutaneous ethanol injection (PEI). RFA and MWA procedures are almost identical, their clinical applications are similar. A probe is deployed directly into the tumor to generate heat and coagulation necrosis. PEI has known implications based on the chemical toxicity of intra-tumoral injection with highly concentrated alcohol by a thin needle.
Keywords: Transarterial chemoembolization, Transarterial radioembolization, Portal vein embolization, Radiofrequency thermal ablation, Microwave thermal ablation, Percutaneous ethanol injection
1. Background
Interventional radiology became a central element in the treatment of liver cancer, providing multiple possibilities for the management of primary hepatic malignancies or metastases. Technological advances of the two past decades have metamorphosed the prognosis of a number of patients, turning palliative situations into curative hopes. Radiological means are attractive through their potential efficiency, their minimally invasive nature and even more by the fact that they can be used in combined treatment strategies, like granting in situ pathways to heighten chemotherapy effect or allowing a surgical resection by increasing the size of the future liver remnant.
The common characteristic of radiological methods is the organ approach which consists of arterial or venous endovascular access, or a direct transcapsular approach either in a percutaneous way or concurrently with a surgical procedure.
In the following sections, we offer an overview of some radiological methods that can be integrated into liver malignancy therapeutic projects, broaching technical principles and leading applications.
Stereotactic body radiation therapy can also be part of this multidisciplinary approach while it has shown to provide promising results in the treatment of hepatocellular carcinoma and liver metastases.1, 2
2. Endovascular treatments
2.1. Transarterial chemoembolization (TACE)
This treatment is based on the following vascular features: the non-tumor liver parenchyma receives two-third of its blood supply from the portal vein and only one-third from the hepatic artery, whereas hepatic tumors are mainly vascularized by branches of the hepatic artery. Through a peripheral arterial access, a selective catheterization of the targeted arterial branch is performed, allowing the injection of a chemotherapeutic molecule combined with an embolic agent. Beside the hepatic artery, it is crucial to search and to treat extrahepatic arterial supplies of the tumor, such as the right inferior phrenic artery, to ensure an optimal effectiveness. Two types of procedures have been described.
2.1.1. Transarterial oily-chemoembolization
This procedure employs a mixture of highly concentrated chemotherapy agent with ethiodized oil, marketed as Lipiodol®. The latter combines substantial inherent benefits beyond its carrier nature. Its high iodine concentration makes it opaque to X-ray, which is helpful to adapt flow delivery during the injection to prevent the reflux issue and to control the captation rate of the tumor on CT. Indeed, the degree of Lipiodol® uptake appears to be an independent prognosis factor (Fig. 1).3, 4 Furthermore, it has tumor-seeking properties, its viscosity slows down the washout of the chemotherapy agent, and its oily nature provides tropism to small tumor vessels.5, 6 The most widely used chemotherapy agent is doxorubicin but no scientific proof of its superiority has been established, and cisplatin or epirubicin might be as effective.
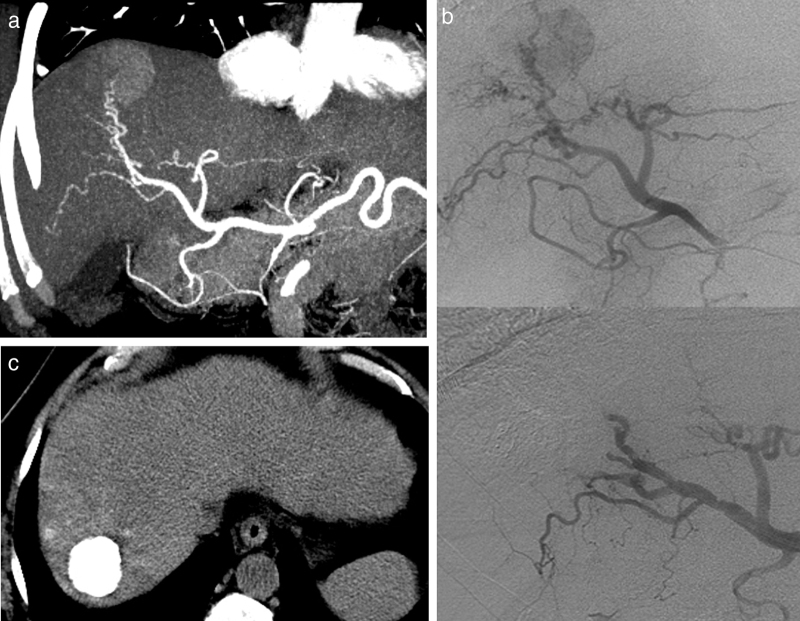
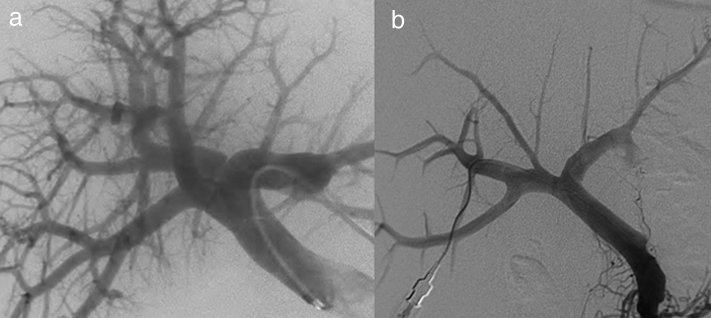
Fig. 1.
Transarterial oily-chemoembolization for hepatocellular carcinoma. (a) Enhanced CT at the arterial phase (same case as in Fig. 3). Hepatocellular carcinoma with arterial enhancement. (b) Corresponding selective angiography of the right branch of the hepatic artery shows tumoral blush (top). Subsequently, a transarterial oily-chemoembolization is performed. The control angiography assessed at the end of the procedure (bottom image) shows the disappearance of the blush. (c) Abdominal non-enhanced CT performed 6 weeks later, shows an homogeneous and intense lipiodol uptake of the tumor.
Afterwards, an embolic agent is injected causing ischemia and necrosis of the tumor and delaying the drug washout. Multiple embolic agents can be employed, the most widely used is gelatin sponge made of purified porcine-derived gelatin which facilitates repetitive procedures by its resorbability.
In patients with hepatocellular carcinoma (HCC), the frequently underlying cirrhosis narrows therapeutic options since TACE can lead to liver decompensation. Thus, functional hepatic reserve becomes a prerequisite for TACE treatment.7 Consequently, patients need to be rigorously selected: a panel of experts has determined contraindications including biliary obstruction, decompensated cirrhosis (Child-Pugh B8 or higher), hepatofugal portal flow or portal vein thrombosis, extensive tumor with replacement of both lobes and severe renal insufficiency.8
Other complications of TACE include non-target embolization, hepatic abscess and bilioma, facilitated by biliary obstruction, renal failure or variceal bleeding.
The post-embolization syndrome, which is a combination of pain, fever, nausea and vomiting lasts a few hours to a few days and is more of an expected consequence than a complication.
2.1.2. TACE with drug-eluting beads
More recently, drug-eluting beads have been developed. These are non-resorbable agents, their chemical structure combines polymeric microspheres doped with sulfonyl groups that provide a reversible ionic binding with polar molecules such as doxorubicin. Benefits reside in a reduced systemic passage of the cytotoxic substances employed, with comparable outcomes to lipiodol (Fig. 2).9, 10, 11 Indeed, the PRECISION V study compared, for a 6 months follow-up, oily-TACE and TACE with DC Beads. The DC Bead group showed non-significantly higher rates of complete response, objective response, and disease control compared with the oily-TACE group (27% vs. 22%, 52% vs. 44%, and 63% vs. 52%, respectively), P = 0.11.11 Nevertheless, the DC Bead group showed improved tolerability, with a reduction in liver toxicity (P < 0.001) and a significantly lower rate of doxorubicin-related side effects (P = 0.0001).11
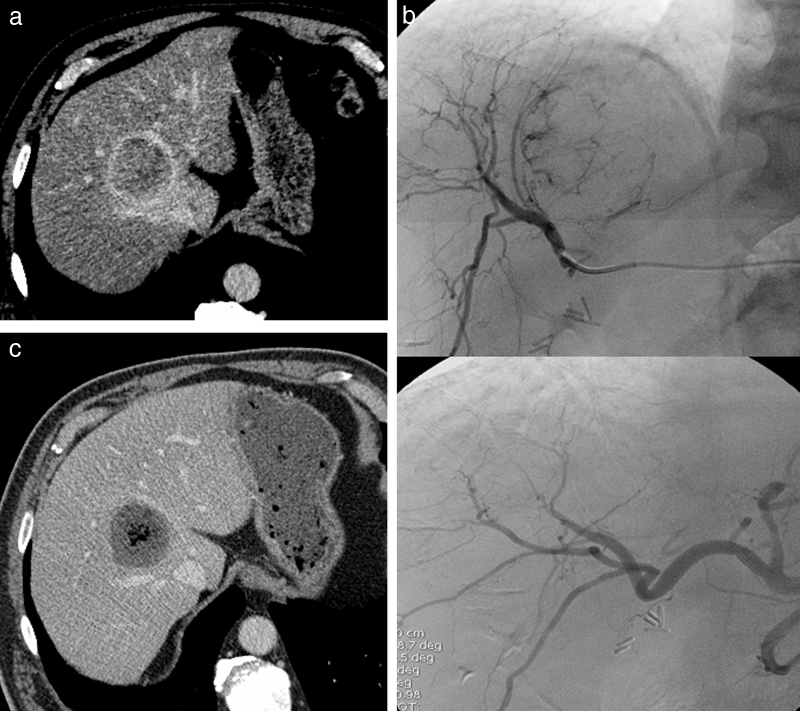
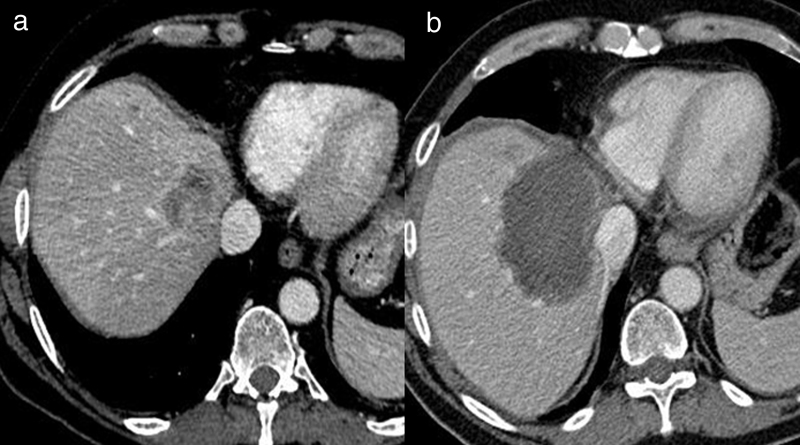
Fig. 2.
Transarterial chemoembolization for hepatocellular carcinoma with drug-eluting beads. (a) Enhanced CT at the venous phase, showing an HCC with wash-out. (b) Selective catheterization and opacification of the right branch of the hepatic artery (top), showing the branches feeding the tumor. The angiography after DC Bead injection (bottom) shows the disappearance of the tumoral blush. (c) Enhanced CT at the portal venous phase 6 weeks later, showing a complete tumoral necrosis.
2.2. Transarterial radioembolization (TARE)
This treatment is based on similar principles to TACE, applied to nuclear medicine. TARE selectively delivers high radiations doses to hepatic tumors and minimizes as much as possible the radiation dose received by the non-tumor liver parenchyma and other organs, especially the lungs, stomach and bowel.12
For this purpose, quite a few combinations of vectors and radionuclides have been proposed.
2.2.1. 131I-Lipiodol®
The first example of this strategy is the use of radiopharmaceutical 131I-Lipiodol®. This combination takes advantages of Lipiodol® properties discussed previously and of the gamma rays emitted by this radioisotope. The therapeutic dose is estimated not to exceed the threshold of 30 Gy to the lungs and to the non-tumor liver.
The preliminary step to perform this technique consists of a procedure achieved with a low radiation dose. The aim of this procedure is to quantify the shunting of microparticles to the lungs or the gastrointestinal tract. It is helpful for patient selection because risks of radiation gastritis, enteritis or pneumonia are supposed to be dose-dependent effects. This first phase also allows the evaluation of the dose necessary for treatment.
Subsequently, the treatment is performed via radiologic techniques comparable to TACE (Fig. 3).
Fig. 3.
Transarterial radioembolization for HCC with 131I-Lipiodol®. (a) Enhanced CT at the arterial phase, MPR reconstructions showing a large heterogeneous hepatocellular carcinoma with marked enhancement. There is a large extension of the tumor into the portal vein (arrow). (b) Enhanced CT at the portal arterial phase, performed 6 weeks after a transarterial radioembolization with 131I-Lipiodol®. MPR reconstructions shows an heterogeneous lipiodol uptake, a shrinkage of the tumor and a substantial reduction of the extension of the tumor into the portal vein.
Main benefits of this method are: a real-time control of the injection allowed by the radio-opacity of the drug and a simplified evaluation of the efficiency which is correlated to the tumoral uptake of Lipiodol® on CT. We can describe several limitations: this procedure seems to be less effective for hypovascularized tumors13 and the biological half-life of 131I is about 8 days and compels a few days of isolation.
2.2.2. Yttrium-90-labeled microspheres
There has been a renewed interest for another radioembolic agent. Direct infusion of yttrium-90-labeled microspheres into the hepatic artery via a catheter from a combination of 90Y, which is mainly a beta-emitter, coupled with microspheres, which are non-resorbable embolic agents that eventually lodge into the microvessels of the tumor.14, 15 Two types of microspheres are commercially available: SIR-Spheres® (Sirtex Medical Limited, Australia) and TheraSphere® (Biocompatibles, UK). TheraSphere® has a higher specific activity (2500 Bq) and lower number of spheres (1.2 million microspheres/3 GBq). Conversely, SIR-Sphere® has a lower specific activity (50 Bq) and greater number of spheres (approximately 40–80 million spheres/3 GBq), the injection of the latter is more challenging due to a higher risk of irradiation of non-targeted organs.
This is a technically more challenging procedure than the former one, requiring an accurate mapping of the hepatic arterial vascularization and the embolization of any collateral vessels of the common hepatic artery in order to prevent gastrointestinal irradiation. Furthermore, in the same angiographic time, 99mTc-labeled macroaggregated albumin is infused into the hepatic artery, which allows the detection and quantification of hepatopulmonary shunting and residual gastrointestinal deposition through a gamma camera (Fig. 4).16 A fraction of hepatopulmonary shunt greater than 10% represents a contraindication to this procedure. The other procedure consists of a therapeutic injection.16, 17, 18
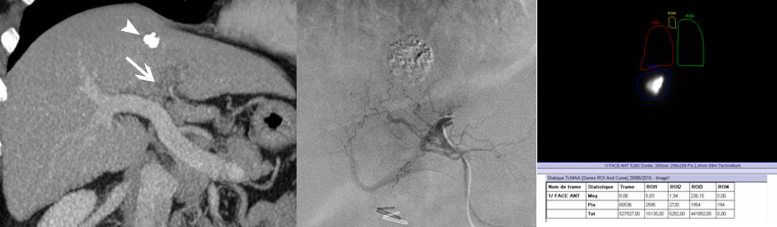
Fig. 4.
Transarterial radioembolization for hepatocellular carcinoma with yttrium-90-labeled microspheres. (a) Enhanced CT at the portal venous phase, performed in order to evaluate the effectiveness of a transarterial chemoembolization for a hepatocellular carcinoma. The primitive tumor shows an incomplete lipiodol uptake (arrowhead) and a portal vein tumor thrombosis (arrow). (b) Selective opacification of the artery feeding the segment IV of the liver, as 1st step of the radioembolization, consisting of the injection of a low dose of 99mTc-labeled macroaggregated albumin. (c) Immediately after the infusion of the 99mTc-labeled macroaggregated albumin, a single-photon emission computed tomography is performed, which also allows to plan the therapeutic dose of 90Y after checking the lack of pulmonary and gastro-intestinal shunting.
2.3. Portal vein embolization
This is not an interventional procedure aiming at ablating hepatic tumors but allowing hepatectomy for patients otherwise contraindicated to surgery because of too small remnant liver. Indeed, performing a hepatic resection is a surgical challenge requiring the preservation of liver parenchyma of a sufficient volume with a maintained hepatic artery, biliary duct, portal and hepatic veins. Predicted future liver remnant (FLR) volume is evaluated on a preoperative computed tomography (CT) using volumetric calculations. Because hepatic volume is correlated with patient body surface area, a standardized ratio of FLR volume to the total functional liver volume is more reliable. Threshold ratios allowing surgical resections differ with different conditions and the values are superior to: 40% for cirrhotic liver or patients suffering a chronic biliary obstruction, 30% for patients with hepatic steatosis or exposed to hepatotoxic chemotherapy and 20% for patients with normal liver function and no other anomaly.19, 20, 21, 22, 23, 24, 25 Below these values, patients are exposed to severe complications including liver insufficiency, especially during the early postoperative period.
The aim of the portal vein embolization (PVE) is to promote liver regeneration, thus hypertrophy of the FLR. Growth of the FLR is a consequence of hyperplasia occasioned by local and systemic growth factors and cytokines provided by an increased portal flow.26, 27, 28
From a technical point of view, the PVE procedure is a direct percutaneous approach to either ipsi- or contralateral distal portal branch (Fig. 5). Subsequently, portography facilitates selective catheterization. It is important to embolize the entire portal targeted vessels, even the distal branches, to prevent portoportal shunts.25 Multiple embolic agents can be used, with a similar efficiency, such as microparticles, n-butyl cyanoacrylate and fibrin glue. The contralateral approach offers the advantage of simpler catheter manipulations and a reduced risk of peritoneal seeding whereas ipsilateral method prevents vascular injury of the FLR. Main indications are represented by right hepatectomy and extended right hepatectomy with an insufficient predicted volume of the FLR (Fig. 6).
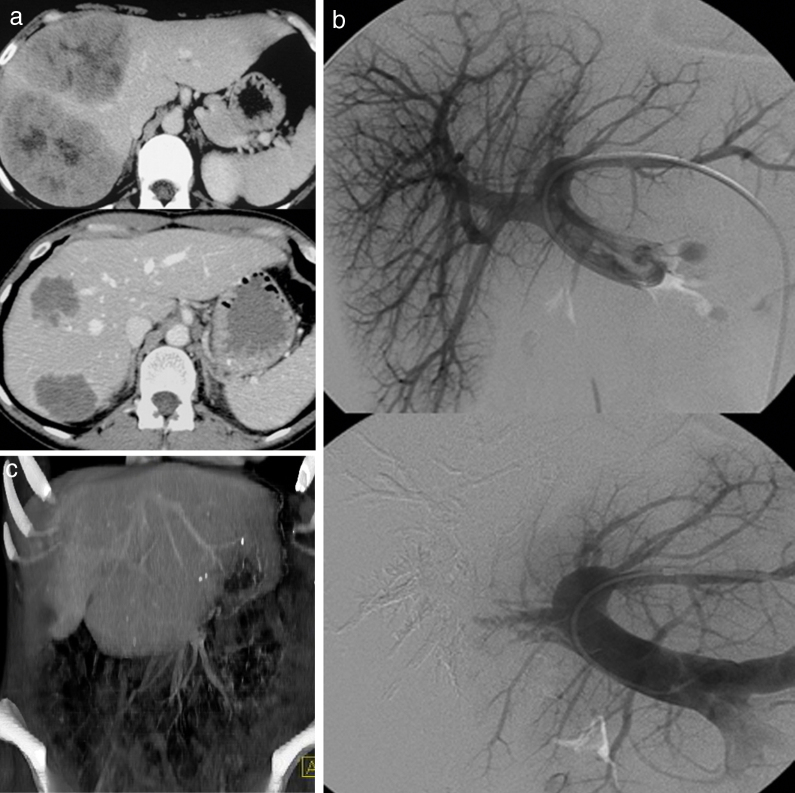
Fig. 5.
Portal vein embolization of the right liver. The procedure can be performed either with a contralateral (a) or a homolateral approach (b).
Fig. 6.
Portal vein embolization of the right liver for colorectal cancer metastasis. (a) Enhanced CT at the portal venous phase before (top) and after (bottom) a systemic chemotherapy treatment showing a good response. The patient could be eligible to a curative right hepatectomy provided that the future liver remnant reaches a sufficient volume. (b) Portal vein embolization of the right liver assessed with a contralateral approach. (c) Enhanced CT at the portal venous phase, volume rendering shows a hypertrophied left lobe allowing surgical resection.
Minor complications occur in 25%, while major complications ensue in 5% of cases, including complete portal vein thrombosis, portal hypertension, ipsilateral portal vein thrombus and hemoperitoneum.29 Expected growth volume of the FLR usually occurs within 4 weeks, normal liver growth rate is 12–21 cm3 a day, whereas cirrhotic livers have a slower and lower potential of regeneration.25, 30
3. Percutaneous treatments
3.1. Radiofrequency thermal ablation
Radiofrequency thermal ablation (RFA) is a minimally invasive percutaneous strategy for solid malignancies that has been expanding widely in the last decades. RFA may be performed with patients under intravenous sedation or general anesthesia.31
During this procedure, the patient is placed in the center of an electromagnetic circuit by means of surface electrodes usually applied on patient's thighs. Then, a thin needle, coupled to a generator, with an insulated shaft and an uninsulated distal tip is introduced and deployed in the tumor, under sonographic, CT or MRI guidance. An alternating current flow generates ionic agitation at the tip of the needle and thus heat in the neighboring tissues. Depending on the size and the shape of the needle's tip, spherical lesions of coagulation necrosis are created, generally from 2 to 5 cm in diameter in about 10 min to half an hour.32
It is important to underline two important aspects. On one hand, as much as surgical resection, a 0.5–1 cm depth rim of safety margin has to be respected and, consequently, the size of the needle has to be oversized in relation to the size of the tumor.31 On the other hand, at the end of the procedure, a slowly managed needle withdrawal has the double benefit of reducing the bleeding risk by a cauterization heat effect and preventing tumor seeding by destroying cells in the needle track.
As the size of lesions increases, its local efficacy is reduced due to a maximum volume of ablation in the range of 4 cm, and in heat loss due to perfusion mediated tissue cooling. The heat-sink effect is a relatively frequent condition that may impair RFA efficiency. Bordering vessels are likely to bring convective cooling effect and reduce the amount of energy locally deposited.31 Several techniques are available to overcome this barrier (Fig. 7, Fig. 8). When vulnerable organs such as the colon and the stomach are threatened by RFA ballistic, instillation of liquid or gas between the targeted area and this structure is a solution to protect them. This latter technique is called hydro or gas dissection.31
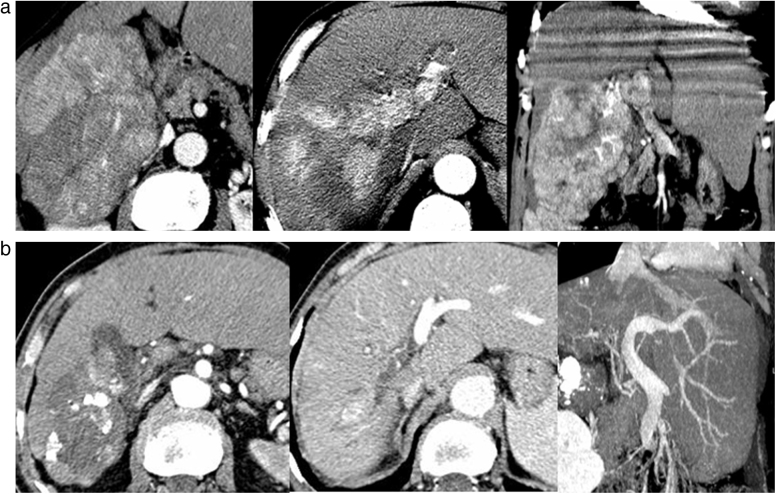
Fig. 7.
Overcoming the heat-sink effect in radiofrequency thermal ablation: a peroperative approach. (a) Enhanced CT at the portal venous phase, showing a colic metastasis in the upper aspect of the right liver, whose posterior aspect has close contact with the right hepatic vein. Patient also required a left lobectomy. Peroperative thermal ablation of the right lesion was performed under Pringle maneuver (clamping of the hepatoduodenal ligament) with a 4-cm diameter needle, to overcome the heat-sink effect. (b) Abdominal CT follow-up at two months shows a complete devascularized thermal lesion, larger than expected.
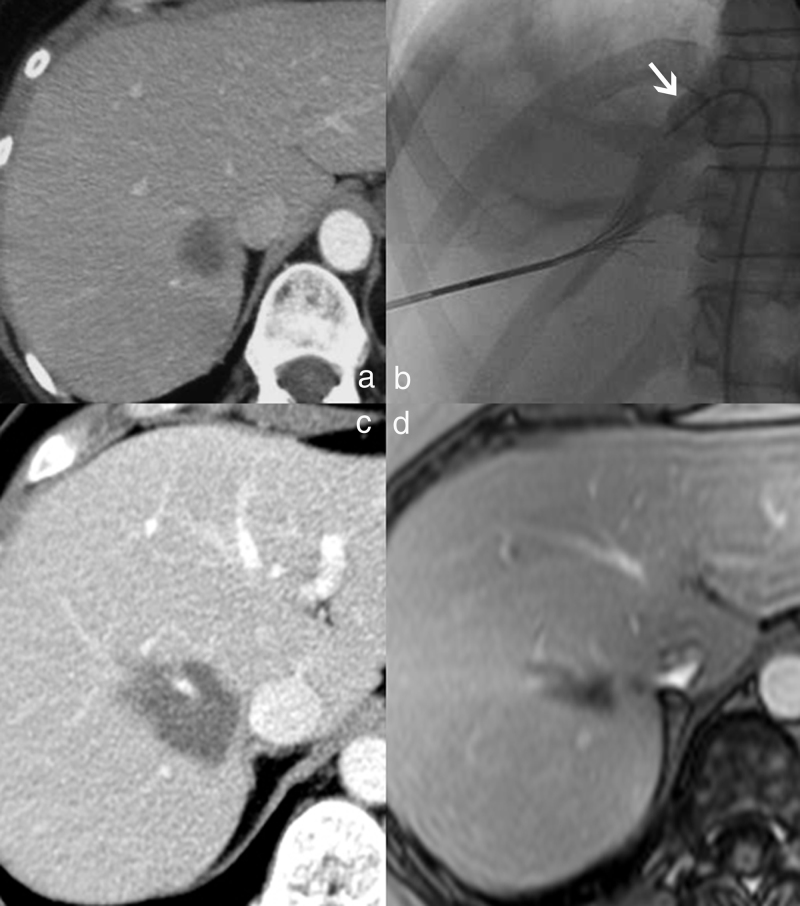
Fig. 8.
Overcoming the heat-sink effect in radiofrequency thermal ablation: a percutaneous approach. (a) Enhanced CT at the portal venous phase showing a breast metastasis whose posterior aspect has close contact with the right hepatic vein. (b) Selective occlusion of the right hepatic vein with a Fogarty balloon during the application of the thermal ablation. (c) Abdominal CT follow-up at three months showing an homogeneous thermal lesion without enhancement. The right hepatic vein remains patent. (d) Liver MRI follow-up at two years, showing the shrinkage of the thermal lesion, without enhancement.
An excessive local temperature, reaching 100–110 °C can provide vaporization of the neighboring tissues.33 Thus, generated gas decreases the volume of tissue destruction.34 Several technical options are available to prevent this effect, such as monitoring temperature or impedance during treatment or assessing instillations of saline solution.35
RFA is a safe technique with very low complication rates. Large studies report 3.5% complications mainly including bile duct injuries (0.7%), liver infarctions (0.5%) and biliomas (0.2%).36
Imaging follow-up is performed at 1, 3, 6, 9 and 12 months after treatment and at 6-month interval thereafter.37 Ablated tumor appears on enhanced imaging (US, CT or MRI) as a non-enhancing area while tumor recurrence shows an irregular nodular enhancement.
3.2. Microwave thermal ablation
Microwave thermal ablation (MWA) is an emerging technique that shares multiple common characteristics with RFA. Indeed, technical features of the procedure are almost identical to those of RFA. The differences arise from the physical phenomenon used to generate heat.
While RFA takes advantage of the resistive heating generated by electric current, MWA refers to devices that use physical waves with frequencies superior or equal to 900 MHz.38 The large majority of the heat is yielded by the agitation of the polar molecules, such as water molecules, induced by microwave pulses; ionic polarization accounts for a much smaller part of the energy deposition.39, 40, 41 Current means are mainly based on two frequency categories: 915 and 2450 MHz. 2450 MHz are the ones most frequently used whereas 915 MHz could present a deeper penetration in the tissues, thus potentially larger ablation zones.40, 41, 42
MWA presents several theoretical advantages over RFA: it can provide larger areas of coagulation necrosis in shorter times as MWA is less affected by the protection of the neighboring tissues provided by vaporization and charring, and is also much less concerned by the heat-sink effect.38, 41, 43 Moreover, it is possible to achieve simultaneously multiple probe deployments.38, 39, 43, 44, 45 However, this efficiency is counterbalanced by a higher risk of injuring surrounding tissues.
It is important to note that MWA, such as RFA, can be part of combined treatments and potentially give rise to a synergic effect with TACE. It has been proved that interrupting hepatic blood flow can increase the thermal ablation zone.46 Furthermore, TACE might control microscopic intrahepatic tumors unreachable for RFA or MWA.47
Despite a relative lack of history for the use of MWA, clinical implications might be similar to RFA indications and can potentially expand to larger tumors than RFA.48 Adverse events and contraindications are also comparable to those of RFA.
3.3. Percutaneous ethanol injection
Percutaneous ethanol injection (PEI) was first described in 1983.49 It is a local treatment based on the chemical properties of alcohol. Intratumoral injection of highly concentrated ethanol induces protein denaturation, microvascular thrombosis, cellular dehydration and coagulative necrosis of the tumor.50 Multiple protocols have been reported. The most frequent one is based on direct and repeated percutaneous intratumoral injections via a thin needle under local anesthesia and intravenous sedation. Comparatively to physical ablation techniques, like RFA or MWA, PEI shows several limitations. Firstly, the requirement to repeat multiple PEI procedures may not be tolerated as well. Secondly, intra-tumoral ethanol diffusion is somewhat random or at least less predictable than thermal ablation, probably because the tumoral capsule and septa make it inhomogenous.51, 52 PEI efficiency is comparable to RFA in the treatment of small tumors.53 However, high tumoral recurrence rates weigh against this method, conferring it worse disease-free and overall survival rates than RFA.54
Nevertheless, PEI also presents obvious advantages due to its low cost, its relative simplicity and safety.
Nowadays, the main remaining indications of PEI are HCC located near an anatomical structure with the risk of heat injury or a heat-sink effect near a vessel, metastatic HCC or even patients presenting contraindications to other ablative methods.55
4. Therapeutic indications
4.1. Hepatocellular carcinoma
Hepatocellular carcinoma represents the third leading cause of deaths from cancer worldwide.56 In Europe and North America, the Barcelona-Clinical Liver Cancer (BCLC) system is the reference for therapeutic decision in HCC.57 This system divides HCC into four groups, based on several criteria: number and size of the nodules, the Child-Pugh score, presence or absence of portal hypertension, World Health Organization performance status, symptomatology, vascular invasion and extra-hepatic spread.
According to BCLC system, indications of RFA include early stage HCC58 with contraindication to orthotopic liver transplantation (OLT) or surgical resection, or as a bridging therapy to those strategies59 while PEI should be considered with suitable candidates with small HCC, particularly for HCC at difficult-to-treat location for RFA. Although initial small randomized clinical trials (RCTs) have failed to show a survival benefit of TACE treatment for HCC patients, two RCTs published in 2002 proved the survival benefits of conventional TACE compared to the best conservative treatment.60, 61 Nowadays, TACE is recommended as first-line therapy for patients who are not candidates for surgery, transplantation or ablation, i.e., who do not have vascular invasion or extrahepatic spread, the latter can also be part of a bridging therapy to OLT.62 Though TACE is not considered as a curative therapy, a recent study comparing hepatic radiofrequency thermal ablation, surgical resection and TACE proved that overall survival rate was similar for all groups for small tumors (<3 cm) without vascular invasion, when balancing liver status.63
TARE seems to be effective in HCC patients who progressed to TACE, for those in the advanced stage because of portal vein invasion or as a bridge to transplantation for HCC.64, 65 In the largest comparative study, all-type adverse events, response rate and time to progression were better in TARE than in conventional TACE but overall survival was not different.66 Many clinical studies with TheraSphere® and SIR-Sphere® are on-going to evaluate feasibility, efficacy and tolerance in primary management of HCC.
4.2. Liver metastases
4.2.1. Colorectal cancer
Despite the absence of controlled studies, RFA is considered as a curative treatment of liver metastases from colorectal cancer, provided that selection criteria (mainly size and location) are respected.67, 68 RFA can be part of a multimodality treatment strategy, especially for some patients presenting bilobar hepatic metastases that would have been ineligible for a curative treatment. Combination of RFA and surgical resection is a possible solution, sequentially69 or at once.70 Moreover, a laparoscopic approach for RFA is sometimes beneficial for hazardous ballistics and provides the advantage of a more accurate intraoperative staging than the percutaneous approach.71, 72
Trans-arterial chemoembolization with drug-eluting beads loaded with irinotecan, combined with systemic chemotherapy73 and TARE74 might be potentially effective for treatment of unresectable colorectal cancer metastases.
4.2.2. Neuroendocrine tumors
Surgery resection is the treatment of choice for hepatic metastases but only 10 to 20% of patients are eligible to this treatment, due to the extension of the disease.75 Eligible patients for these procedures are patients in a metastatic phase, with predominant liver disease, which is judjed not resectable. TACE and TARE are frequently used, especially in patients with refractory, unresectable, or recurrent disease. These treatments are effective both in palliating the hormonal symptoms and achieving objective tumor responses.76, 77 Indeed, in a recent study including 123 patients undergoing an average of 7 TACE cycles each, 62% partial response was seen with overall 3-, 5-, and 10-year survivals of 59, 36, and 20%, respectively, and overall mean survival of 5.47 years.78 TARE also showed good results in a phase II trial.79
4.2.3. Other liver malignancies
TACE might be potentially effective for treatment of cholangiocarcinoma, hepatic metastases from gastric, breast and cutaneous origin.80, 81, 82, 83
Several case series of thermal ablation used to treat metastases from a variety of different primary tumor entities have been reported, including gastric cancer, ovarian cancer, and metastases of unknown primary.84 Despite the limited size of these series, there is a potential survival benefit from percutaneous ablation treatment in patients who are otherwise not eligible for surgery, especially those with breast cancer.83
5. Conclusion
Radiological methods, both endovascular and direct transcapsular approaches, play a determinant role in the modern treatment of liver cancers. Their pros rely on their relative selectivity, safety and their ability to have a synergistic role with surgical, oncological and radiotherapy methods.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Wang P.M., Chung N.N., Hsu W.C., Chang F.L., Jang C.J., Scorsetti M. Stereotactic body radiation therapy in hepatocellular carcinoma: optimal treatment strategies based on liver segmentation and functional hepatic reserve. Rep Pract Oncol Radiother. 2015;20(November–December (6)):417–424. doi: 10.1016/j.rpor.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Comito T., Clerici E., Tozzi A., D’agostino G. Liver metastases and SBRT: a new paradigm? Rep Pract Oncol Radiother. 2015;20(November–December (6)):464–471. doi: 10.1016/j.rpor.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mondazzi L., Bottelli R., Brambilla G. Transarterial oily chemoembolization for the treatment of hepatocellular carcinoma: a multivariate analysis of prognostic factors. Hepatology. 1994;19(May (5)):1115–1123. [PubMed] [Google Scholar]
- 4.Dumortier J., Chapuis F., Borson O. Unresectable hepatocellular carcinoma: survival and prognostic factors after lipiodol chemoembolisation in 89 patients. Dig Liver Dis. 2006;38(February (2)):125–133. doi: 10.1016/j.dld.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 5.Tam K.Y., Leung K.C., Wang Y.X. Chemoembolization agents for cancer treatment. Eur J Pharm Sci. 2011;44(September (1–2)):1–10. doi: 10.1016/j.ejps.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Kan Z., Madoff D.C. Liver anatomy: microcirculation of the liver. Semin Intervent Radiol. 2008;25(June (2)):77–85. doi: 10.1055/s-2008-1076685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goin J.E., Salem R., Carr B.I. Treatment of unresectable hepatocellular carcinoma with intrahepatic yttrium 90 microspheres: factors associated with liver toxicities. J Vasc Interv Radiol. 2005;16(February (2 Pt 1)):205–213. doi: 10.1097/01.rvi.00001142592.89564.f9. [DOI] [PubMed] [Google Scholar]
- 8.Raoul J.L., Sangro B., Forner A. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37(May (3)):212–220. doi: 10.1016/j.ctrv.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Hong K., Khwaja A., Liapi E., Torbenson M.S., Georgiades C.S., Geschwind J.F. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res. 2006;12(April (8)):2563–2567. doi: 10.1158/1078-0432.CCR-05-2225. [DOI] [PubMed] [Google Scholar]
- 10.Varela M., Real M.I., Burrel M. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46(March (3)):474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Lammer J., Malagari K., Vogl T. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33(February (1)):41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy A., Coldwell D., Sangro B., Wasan H., Salem R. Radioembolization for the treatment of liver tumors general principles. Am J Clin Oncol. 2012;35(February (1)):91–99. doi: 10.1097/coc.0b013e3181f47583. [DOI] [PubMed] [Google Scholar]
- 13.Raoul J.L., Guyader D., Bretagne J.F. Prospective randomized trial of chemoembolization versus intra-arterial injection of 131I-labeled-iodized oil in the treatment of hepatocellular carcinoma. Hepatology. 1997;26(November (5)):1156–1161. doi: 10.1002/hep.510260511. [DOI] [PubMed] [Google Scholar]
- 14.Lewandowski R.J., Sato K.T., Atassi B. Radioembolization with 90Y microspheres: angiographic and technical considerations. Cardiovasc Intervent Radiol. 2007;30(July–August (4)):571–592. doi: 10.1007/s00270-007-9064-z. [DOI] [PubMed] [Google Scholar]
- 15.Wang S.C., Bester L., Burnes J.P. Clinical care and technical recommendations for 90yttrium microsphere treatment of liver cancer. J Med Imaging Radiat Oncol. 2010;54(June (3)):178–187. doi: 10.1111/j.1754-9485.2010.02167.x. [DOI] [PubMed] [Google Scholar]
- 16.Bester L., Meteling B., Boshell D., Chua T.C., Morris D.L. Transarterial chemoembolisation and radioembolisation for the treatment of primary liver cancer and secondary liver cancer: a review of the literature. J Med Imaging Radiat Oncol. 2014;58(3):341–352. doi: 10.1111/1754-9485.12163. [DOI] [PubMed] [Google Scholar]
- 17.Cianni R., Urigo C., Notarianni E. Radioembolisation using yttrium 90 (Y-90) in patients affected by unresectable hepatic metastases. Radiol Med. 2010;115(June (4)):619–633. doi: 10.1007/s11547-010-0496-1. [DOI] [PubMed] [Google Scholar]
- 18.Tehranipour N., AL-Nahhas A., Canelo R. Concordant F-18 FDG PET and Y-90 Bremsstrahlung scans depict selective delivery of Y-90-microspheres to liver tumors: confirmation with histopathology. Clin Nucl Med. 2007;32(May (5)):371–374. doi: 10.1097/01.rlu.0000259568.54976.bd. [DOI] [PubMed] [Google Scholar]
- 19.Ribero D., Abdalla E.K., Madoff D.C., Donadon M., Loyer E.M., Vauthey J.N. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94(November (11)):1386–1394. doi: 10.1002/bjs.5836. [DOI] [PubMed] [Google Scholar]
- 20.Abdalla E.K., Barnett C.C., Doherty D., Curley S.A., Vauthey J.N. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137(June (6)):675–680. doi: 10.1001/archsurg.137.6.675. [discussion 80–1] [DOI] [PubMed] [Google Scholar]
- 21.Kishi Y., Abdalla E.K., Chun Y.S. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250(October (4)):540–548. doi: 10.1097/SLA.0b013e3181b674df. [DOI] [PubMed] [Google Scholar]
- 22.de Meijer V.E., Kalish B.T., Puder M., Ijzermans J.N. Systematic review and meta-analysis of steatosis as a risk factor in major hepatic resection. Br J Surg. 2010;97(September (9)):1331–1339. doi: 10.1002/bjs.7194. [DOI] [PubMed] [Google Scholar]
- 23.Shirabe K., Shimada M., Gion T. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188(March (3)):304–309. doi: 10.1016/s1072-7515(98)00301-9. [DOI] [PubMed] [Google Scholar]
- 24.Farges O., Belghiti J., Kianmanesh R. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237(February (2)):208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.May B.J., Madoff D.C. Portal vein embolization: rationale, technique, and current application. Semin Intervent Radiol. 2012;29(June (2)):81–89. doi: 10.1055/s-0032-1312568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5(October (10)):836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 27.Goto Y., Nagino M., Nimura Y. Doppler estimation of portal blood flow after percutaneous transhepatic portal vein embolization. Ann Surg. 1998;228(August (2)):209–213. doi: 10.1097/00000658-199808000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huh C.G., Factor V.M., Sanchez A., Uchida K., Conner E.A., Thorgeirsson S.S. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci U S A. 2004;101(March (13)):4477–4482. doi: 10.1073/pnas.0306068101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denys A., Bize P., Demartines N., Deschamps F., De Baere T. Quality improvement for portal vein embolization. Cardiovasc Intervent Radiol. 2010;33(June (3)):452–456. doi: 10.1007/s00270-009-9737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madoff D.C., Hicks M.E., Vauthey J.N. Transhepatic portal vein embolization: anatomy, indications, and technical considerations. Radiographics. 2002;22(September–October (5)):1063–1076. doi: 10.1148/radiographics.22.5.g02se161063. [DOI] [PubMed] [Google Scholar]
- 31.Patterson E.J., Scudamore C.H., Buczkowski A.K., Owen D.A., Nagy A.G. Radiofrequency ablation in surgery. Surg Technol Int. 1997;6:69–75. [PubMed] [Google Scholar]
- 32.Crocetti L., de Baere T., Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Intervent Radiol. 2010;33(February (1)):11–17. doi: 10.1007/s00270-009-9736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldberg S.N., Gazelle G.S., Mueller P.R. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000;174(February (2)):323–331. doi: 10.2214/ajr.174.2.1740323. [DOI] [PubMed] [Google Scholar]
- 34.Lencioni R., Crocetti L. Radiofrequency ablation of liver cancer. Tech Vasc Interv Radiol. 2007;10(March (1)):38–46. doi: 10.1053/j.tvir.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu A., Ishizaka H., Awata S. Expansion of radiofrequency ablation volume by saturated NaCl saline injection in the area of vaporization. Acta Radiol. 2009;50(January (1)):61–64. doi: 10.1080/02841850802562071. [DOI] [PubMed] [Google Scholar]
- 36.Koda M., Murawaki Y., Hirooka Y. Complications of radiofrequency ablation for hepatocellular carcinoma in a multicenter study: an analysis of 16 346 treated nodules in 13 283 patients. Hepatol Res. 2012;42(November (11)):1058–1064. doi: 10.1111/j.1872-034X.2012.01025.x. [DOI] [PubMed] [Google Scholar]
- 37.McDermott S., Gervais D.A. Radiofrequency ablation of liver tumors. Semin Intervent Radiol. 2013;30(March (1)):49–55. doi: 10.1055/s-0033-1333653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simon C.J., Dupuy D.E., Mayo-Smith W.W. Microwave ablation: principles and applications. Radiographics. 2005;25(October (Suppl. 1)):S69–S83. doi: 10.1148/rg.25si055501. [DOI] [PubMed] [Google Scholar]
- 39.Brace C.L. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol. 2009;38(May–June (3)):135–143. doi: 10.1067/j.cpradiol.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka M., Sato M. Microwave heating of water, ice, and saline solution: molecular dynamics study. J Chem Phys. 2007;126(January (3)):034509. doi: 10.1063/1.2403870. [DOI] [PubMed] [Google Scholar]
- 41.Liang P., Yu J., Lu M.D. Practice guidelines for ultrasound-guided percutaneous microwave ablation for hepatic malignancy. World J Gastroenterol. 2013;19(Septembre (33)):5430–5438. doi: 10.3748/wjg.v19.i33.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Y., Wang Y., Ni X. Comparison of ablation zone between 915- and 2,450-MHz cooled-shaft microwave antenna: results in in vivo porcine livers. AJR Am J Roentgenol. 2009;192(February (2)):511–514. doi: 10.2214/AJR.07.3828. [DOI] [PubMed] [Google Scholar]
- 43.Yu J., Liang P., Yu X., Liu F., Chen L., Wang Y. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. Eur J Radiol. 2011;79(July (1)):124–130. doi: 10.1016/j.ejrad.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Brace C.L., Laeseke P.F., Sampson L.A., Frey T.M., van der Weide D.W., Lee F.T., Jr. Microwave ablation with multiple simultaneously powered small-gauge triaxial antennas: results from an in vivo swine liver model. Radiology. 2007;244(July (1)):151–156. doi: 10.1148/radiol.2441052054. [DOI] [PubMed] [Google Scholar]
- 45.Wright A.S., Sampson L.A., Warner T.F., Mahvi D.M., Lee F.T., Jr. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236(July (1)):132–139. doi: 10.1148/radiol.2361031249. [DOI] [PubMed] [Google Scholar]
- 46.Ishida T., Murakami T., Shibata T. Percutaneous microwave tumor coagulation for hepatocellular carcinomas with interruption of segmental hepatic blood flow. J Vasc Interv Radiol. 2002;13(February (2 Pt 1)):185–191. doi: 10.1016/s1051-0443(07)61937-x. [DOI] [PubMed] [Google Scholar]
- 47.Yang W.Z., Jiang N., Huang N., Huang J.Y., Zheng Q.B., Shen Q. Combined therapy with transcatheter arterial chemoembolization and percutaneous microwave coagulation for small hepatocellular carcinoma. World J Gastroenterol. 2009;15(February (6)):748–752. doi: 10.3748/wjg.15.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin X.Y., Xie X.Y., Lu M.D. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer. 2009;115(May (9)):1914–1923. doi: 10.1002/cncr.24196. [DOI] [PubMed] [Google Scholar]
- 49.Ebara M., Okabe S., Kita K. Percutaneous ethanol injection for small hepatocellular carcinoma: therapeutic efficacy based on 20-year observation. J Hepatol. 2005;43(September (3)):458–464. doi: 10.1016/j.jhep.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 50.Ansari D., Andersson R. Radiofrequency ablation or percutaneous ethanol injection for the treatment of liver tumors. World J Gastroenterol. 2012;18(March (10)):1003–1008. doi: 10.3748/wjg.v18.i10.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shiina S., Tagawa K., Unuma T. Percutaneous ethanol injection therapy for hepatocellular carcinoma. A histopathologic study. Cancer. 1991;68(October (7)):1524–1530. doi: 10.1002/1097-0142(19911001)68:7<1524::aid-cncr2820680711>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 52.Cha D.I., Lee M.W., Rhim H., Choi D., Kim Y.S., Lim H.K. Therapeutic efficacy and safety of percutaneous ethanol injection with or without combined radiofrequency ablation for hepatocellular carcinomas in high risk locations. Korean J Radiol. 2013;14(March–April (2)):240–247. doi: 10.3348/kjr.2013.14.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Germani G., Pleguezuelo M., Gurusamy K., Meyer T., Isgro G., Burroughs A.K. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J Hepatol. 2010;52(March (3)):380–388. doi: 10.1016/j.jhep.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 54.Bouza C., Lopez-Cuadrado T., Alcazar R., Saz-Parkinson Z., Amate J.M. Meta-analysis of percutaneous radiofrequency ablation versus ethanol injection in hepatocellular carcinoma. BMC Gastroenterol. 2009;9:31. doi: 10.1186/1471-230X-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwon J.H. Is percutaneous ethanol injection therapy still effective for hepatocellular carcinoma in the era of radiofrequency ablation? Gut Liver. 2010;4:105–112. doi: 10.5009/gnl.2010.4.S1.S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El-Serag H.B., Rudolph K.L. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(June (7)):2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 57.Forner A., Llovet J.M., Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(March (9822)):1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 58.Peng Z.W., Lin X.J., Zhang Y.J. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology. 2012;262(March (3)):1022–1033. doi: 10.1148/radiol.11110817. [DOI] [PubMed] [Google Scholar]
- 59.Lu D.S., Yu N.C., Raman S.S. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology. 2005;41(May (5)):1130–1137. doi: 10.1002/hep.20688. [DOI] [PubMed] [Google Scholar]
- 60.Llovet J.M., Real M.I., Montana X. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(May (9319)):1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 61.Lo C.M., Ngan H., Tso W.K. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(May (5)):1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 62.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(April (4)):908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 63.Yang H.J., Lee J.H., Lee D.H. Small single-nodule hepatocellular carcinoma: comparison of transarterial chemoembolization, radiofrequency ablation, and hepatic resection by using inverse probability weighting. Radiology. 2014;271(June (3)):909–918. doi: 10.1148/radiol.13131760. [DOI] [PubMed] [Google Scholar]
- 64.Salem R., Lewandowski R.J., Atassi B. Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J Vasc Interv Radiol. 2005;16(December (12)):1627–1639. doi: 10.1097/01.RVI.0000184594.01661.81. [DOI] [PubMed] [Google Scholar]
- 65.Kulik L.M., Atassi B., van Holsbeeck L. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol. 2006;94(December (7)):572–586. doi: 10.1002/jso.20609. [DOI] [PubMed] [Google Scholar]
- 66.Salem R., Lewandowski R.J., Kulik L. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140(February (2)) doi: 10.1053/j.gastro.2010.10.049. 497–507 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim K.H., Yoon Y.S., Yu C.S. Comparative analysis of radiofrequency ablation and surgical resection for colorectal liver metastases. J Korean Surg Soc. 2011;81(July (1)):25–34. doi: 10.4174/jkss.2011.81.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Tilborg A.A., Meijerink M.R., Sietses C. Long-term results of radiofrequency ablation for unresectable colorectal liver metastases: a potentially curative intervention. Br J Radiol. 2011;84(June (1002)):556–565. doi: 10.1259/bjr/78268814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang T., Zeng Y., Huang J., Liao M., Wu H. Combined resection with radiofrequency ablation for bilobar hepatocellular carcinoma: a single-center experience. J Surg Res. 2014;191(October (2)):370–378. doi: 10.1016/j.jss.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 70.Tepel J., Hinz S., Klomp H.J., Kapischke M., Kremer B. Intraoperative radiofrequency ablation (RFA) for irresectable liver malignancies. Eur J Surg Oncol. 2004;30(June (5)):551–555. doi: 10.1016/j.ejso.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 71.Herbold T., Wahba R., Bangard C., Demir M., Drebber U., Stippel D.L. The laparoscopic approach for radiofrequency ablation of hepatocellular carcinoma--indication, technique and results. Langenbecks Arch Surg. 2013;398(January (1)):47–53. doi: 10.1007/s00423-012-1018-5. [DOI] [PubMed] [Google Scholar]
- 72.Hildebrand P., Kleemann M., Roblick U., Mirow L., Birth M., Bruch H.P. Laparoscopic radiofrequency ablation of unresectable hepatic malignancies: indication, limitation and results. Hepatogastroenterology. 2007;54(October–November (79)):2069–2072. [PubMed] [Google Scholar]
- 73.Martin R.C., 2nd, Scoggins C.R., Schreeder M. Randomized controlled trial of irinotecan drug-eluting beads with simultaneous FOLFOX and bevacizumab for patients with unresectable colorectal liver-limited metastasis. Cancer. 2015 doi: 10.1002/cncr.29534. [DOI] [PubMed] [Google Scholar]
- 74.Van Hazel G., Blackwell A., Anderson J. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol. 2004;88(November (2)):78–85. doi: 10.1002/jso.20141. [DOI] [PubMed] [Google Scholar]
- 75.Del Prete M., Fiore F., Modica R. Hepatic arterial embolization in patients with neuroendocrine tumors. J Exp Clin Cancer Res. 2014;33:43. doi: 10.1186/1756-9966-33-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gupta S. Intra-arterial liver-directed therapies for neuroendocrine hepatic metastases. Semin Intervent Radiol. 2013;30(March (1)):28–38. doi: 10.1055/s-0033-1333651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vogl T.J., Gruber T., Naguib N.N., Hammerstingl R., Nour-Eldin N.E. Liver metastases of neuroendocrine tumors: treatment with hepatic transarterial chemotherapy using two therapeutic protocols. AJR Am J Roentgenol. 2009;193(October (4)):941–947. doi: 10.2214/AJR.08.1879. [DOI] [PubMed] [Google Scholar]
- 78.Strosberg J.R., Fine R.L., Choi J. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117(January (2)):268–275. doi: 10.1002/cncr.25425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kennedy A.S., Dezarn W.A., McNeillie P. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol. 2008;31(June (3)):271–279. doi: 10.1097/COC.0b013e31815e4557. [DOI] [PubMed] [Google Scholar]
- 80.Tellez C., Benson A.B., 3rd, Lyster M.T. Phase II trial of chemoembolization for the treatment of metastatic colorectal carcinoma to the liver and review of the literature. Cancer. 1998;82(April (7)):1250–1259. doi: 10.1002/(sici)1097-0142(19980401)82:7<1250::aid-cncr7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 81.Vogl T.J., Gruber T., Balzer J.O., Eichler K., Hammerstingl R., Zangos S. Repeated transarterial chemoembolization in the treatment of liver metastases of colorectal cancer: prospective study. Radiology. 2009;250(January (1)):281–289. doi: 10.1148/radiol.2501080295. [DOI] [PubMed] [Google Scholar]
- 82.Burger I., Hong K., Schulick R. Transcatheter arterial chemoembolization in unresectable cholangiocarcinoma: initial experience in a single institution. J Vasc Interv Radiol. 2005;16(March (3)):353–361. doi: 10.1097/01.RVI.0000143768.60751.78. [DOI] [PubMed] [Google Scholar]
- 83.Vogl T.J., Farshid P., Naguib N.N., Zangos S. Thermal ablation therapies in patients with breast cancer liver metastases: a review. Eur Radiol. 2013;23(March (3)):797–804. doi: 10.1007/s00330-012-2662-4. [DOI] [PubMed] [Google Scholar]
- 84.Foltz G. Image-guided percutaneous ablation of hepatic malignancies. Semin Intervent Radiol. 2014;31(June (2)):180–186. doi: 10.1055/s-0034-1373792. [DOI] [PMC free article] [PubMed] [Google Scholar]