Abstract
Liver metastases from breast cancer are a common occurrence. Local ablative therapies are a promising therapeutic option for these patients, with the potential for a long term disease control in the setting of “oligometastatic patients”. Identification of the perfect candidate for local approaches is still challenging and unclear. Stereotactic body radiation therapy (SBRT) is one of the most valuable local therapy, because of great efficiency, low morbidity and minimal invasiveness. In this paper, we reviewed the state of the art in the care of breast cancer patients with liver metastases, with a focus on SBRT.
Keywords: Breast, Liver, Metastases, SBRT, Radiotherapy
1. State of the art
The standard of care for patients with metastatic breast cancer (BC) remains systemic therapy (chemotherapy, immunotherapy or hormonal therapies), however some of these patients with limited metastatic disease, good performance status and younger age could have an improvement of clinical outcomes with the addition of local therapy.
Firstly in 1995, Hellman and Weichselbaum1 described the state, called oligometastatic disease, characterized by an indolent tumor biology and behavior in which metastases are limited in number and site. In this subgroup of selected patients, the emerging data is that local therapy could have a potential curative role and an impact on survival rate.
The population with oligometastatic BC (OM BC) is estimated to be 1–10% of newly diagnosed patients with metastatic BC2 and is well represented in the subgroup of patients enrolled in recent first line metastatic BC trial; about 50% of them had fewer than two metastases.3
Liver is one of the main site of metastatic disease from BC and other solid tumors. Surgical resection has been recommended in carefully selected patients4, 5 with 5-year survival rates of 30–60% in different studies.6, 7, 8, 9, 10 Most of the experiences describe the role of surgery in liver metastases from colorectal cancer. On the other hand, we have several reports about liver metastasectomy in metastatic BC but these series included small cohort of patients widely different for inclusion criteria and type of systemic therapy received; the reported 5-year survival rate varies from 18% to 61%.11
In the last decade, different local treatment options have been introduced as an alternative to surgery, including radiofrequency ablation (RFA) and stereotactic body radiotherapy (SBRT).
SBRT allows precise delivery of high radiation dose, defined as “ablative dose”, with maximum sparing of normal tissue. This innovative technique can be offered to patients that refuse more invasive procedures or are unable to tolerate them, or when tumors are situated in areas in which invasive procedures would be dangerous.
Published studies on SBRT for metastatic disease included very heterogeneous scenarios; nevertheless, when the authors analyze clinical results according to tumor primary histology, patients with breast cancer have a much better survival as compared with other cancers.12
For liver metastases from OM BC, in different series, the 2-year local control rates varies between 57 and 92%, and radiation-induced liver damage is exceptional.12, 13, 14 Based on these encouraging data, SBRT for liver lesions in OM BC represents a valid alternative local therapy in patients unsuitable for surgery.
In this review we analyzed the literature data about liver oligometastatic breast cancer patients who underwent SBRT. We considered the following points: patients selection and prognostic factors, technical features and literature data on SBRT. Besides, we presented our experience using SBRT in this setting of patients.
We performed a literature search using medical subject heading terms “stereotactic body radiation therapy”, “liver metastases” and “oligometastatic breast cancer”, considering a period of 10 years.
2. Patients selection and prognostic factors
Published previous experiences investigated some factors affecting local control and survival outcomes. Breast histology, long disease-free interval (>6 months), one to three small metastases are the most relevant characteristics of patients that could benefit from SBRT.12
However, selection criteria for oligometastatic patients with liver metastases candidate to SBRT are still debated, therefore a multidisciplinary discussion is recommended for each case.
According to the size and site of the lesions, liver function and patients and disease features, SBRT can be considered as follows: indicated, borderline and contra-indicated.24 Table 1 shows the characteristics for all groups.
Table 1.
Selection criteria for SBRT.
| Selection criteria | Patients categories |
||
|---|---|---|---|
| Indicated | Borderline | Contra-indicated | |
| Performance status | ECOG 0–1 | ECOG 2–3 | ECOG 4 |
| Extra-hepatic disease | Absent | Controlled | Progressing |
| Number of lesions | ≤3 | 4 | ≥5 |
| Size of lesions (cm) | ≤3 | >3 ≤6 | >6 |
| OARs distance (mm) | >8 | ≤8 ≥5 | <5 |
| Liver function | Child A | Child B | Child C |
| Free liver volume (cc) | >1000 | 700–1000 | <700 |
The best candidate for SBRT is an oligometastatic patient unsuitable for surgery with a good performance status (Eastern Cooperative Oncology Group 0–1), absent extra-hepatic disease, number of hepatic lesions ≤3, size lesions ≤3 cm, lesion distance from organs at risk (OARs) >8 mm, good liver function (Child A) and healthy liver volume >1000 cc.
Patients with mild performance status (ECOG 2–3), controlled extra-hepatic disease, 4 liver metastases, diameter ranging from 3 to 6 cm, OARs distance between 5 and 8 mm, moderate liver function (Child B) and healthy liver volume ranging from 700 to 1000 cc, have to be evaluated with caution.
On the other hand, SBRT is contra-indicated for patients with poor PS, extra-hepatic disease progression, ≥5 hepatic lesions, diameter greater than 6 cm, OARs distance less than 5 mm, inadequate liver function (Child C) and healthy liver volume <700 cc.
Age is not included in these criteria because elderly patients can also be treated successfully with this non-invasive and well-tolerated therapy.15
3. Stereotactic body radiation therapy (SBRT) for liver metastases: simulation, target definition, planning and delivery
Practice guidelines published by ASTRO and ACR confirmed the importance of highly precise target definition, planning and delivery to perform SBRT.16, 17
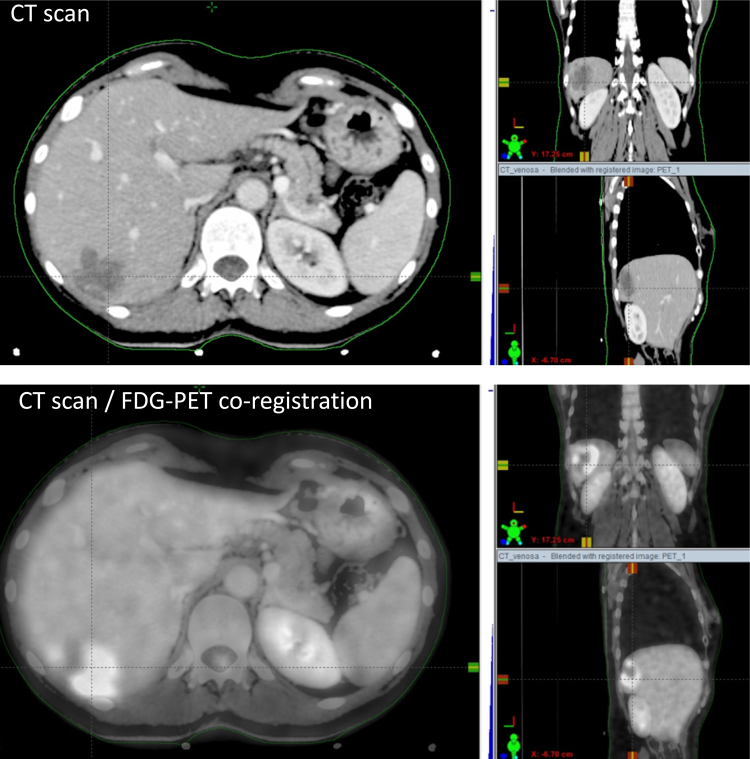
First step of stereotactic treatment is the “simulation phase”. During this phase, the patient is immobilized with a personalized device to maximize reproducibility and accuracy of treatment. An abdominal compression device and a 4D-CT scan should be used to reduce the liver motion related to respiratory cycle.18 A contrast-free computed tomography (CT) scan and a three-phase contrast-enhanced CT scan should be employed, because not all hepatic lesions can be easily identified with a contrast-free CT-scan. To improve target definition, magnetic resonance (MRI) and/or 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) could be necessary. Fig. 1 shows an example of co-registration between CT and PET.
Fig. 1.
Example of simulation procedure for liver metastases from breast cancer.
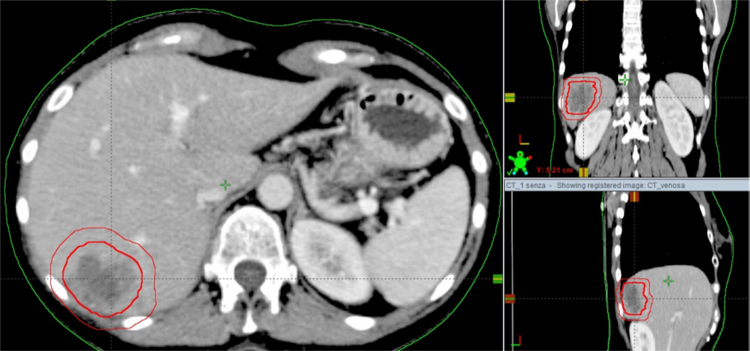
Second step of SBRT performance is the “target definition and contouring”. The clinical target volume (CTV) is defined as equal to the gross tumor volume (GTV). If 4D-CT scan is performed, an internal target volume (ITV) is the volume encompassing all GTVs in the different respiratory phases. ITV is defined as internal margin (IM). A margin to compensate set-up errors is added to ITV to generate the planning target volume (PTV). If 4D-CT scan is not acquired and ITV is not defined, the PTV is generated from CTV.19 Fig. 2 shows an example of these volumes.
Fig. 2.
Clinical target volume and planning target volume for liver metastases from breast cancer.
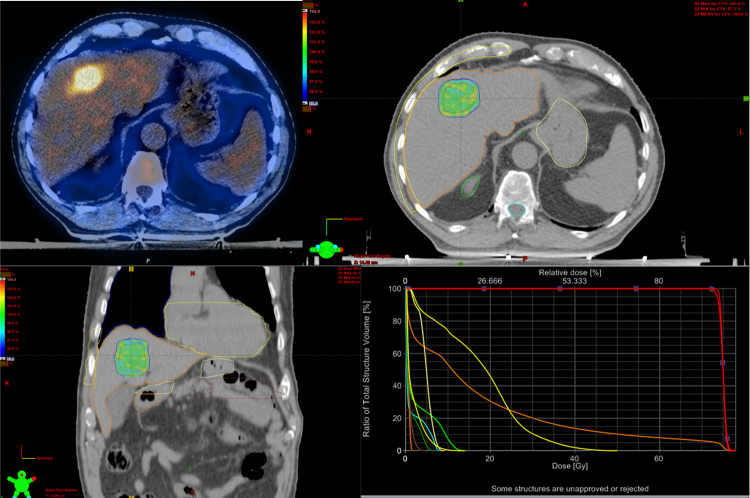
Third step of radiation therapy is the “treatment planning and prescription dose”. SBRT requires a highly conformal dose distribution to deliver high dose of radiation to the tumor and a minimal dose to surrounding critical tissues. Intensity modulated RT (IMRT) and, more recently, volumetric modulated arc therapy (VMAT) improve the homogeneous distribution of dose around concave targets and reduce irradiation of organs at risk (OARs) with multiple beams using either coplanar or non-coplanar geometries.20 Fig. 3 shows an example of dose distribution.
Fig. 3.
Example of dose distribution for the treatment of liver metastases from breast cancer.
Several studies on liver SBRT confirmed the correlation between dose prescription and local control.18, 19, 20, 21, 22
For SBRT in 3 fractions, a prescription dose ranging from 48 to 60 Gy should be considered for lesions with a diameter ≤3 cm.22, 23 A total dose of 60 Gy or higher should be recommended for lesions with a diameter >3 cm.24 Recommended dose constraints for OARs are listed in Table 2.
Table 2.
Dose constraints for organs at risk (OARs).
| Organ | Dose-volume limits | Other conditions |
|---|---|---|
| Healthy liver (defined as total liver volume minus cumulative GTV) | >700 cc at <15 Gy | Healthy liver volume >1000 cc |
| Spinal cord | <18 Gy | |
| Kidneys (R + L) | V15 Gy < 35% | |
| Stomach, duodenum, small bowel | <21 Gy | GTV > 8 mm from the OARs |
| Heart | <30 Gy | |
| Ribs | D30 cm3 <30 Gy |
The last step of SBRT is the “treatment delivery”. Accurate patient positioning check is necessary to reduce set-up uncertainties. Image guided radiation therapy (IGRT) should be performed before each daily session to check patient and tumor position. In selected patients, fiducial markers implantation could be employed for target localization with a related risk of seeding and migration.25 In patients previously submitted to surgery clips could be used as fiducial markers.18
3.1. SBRT for liver metastases in oligometastatic breast cancer patients
3.1.1. Current literature data
Stereotactic body radiotherapy (SBRT) is emerging as an effective and safe treatment for oligometastatic patients, when surgery is not allowed or refused. Retrospective and prospective studies confirmed the safety and efficacy of SBRT also in the setting of liver metastases and its use is steadily increasing.24 However, level of evidence is still weak. Indeed most published experiences are heterogeneous for histology, number and size of metastases, metastatic site and fractionation schedules. Moreover, no randomized trials are yet available.
These limits also apply to the field of SBRT for breast cancer liver metastases. Most of the available data come from single institution experiences and data must be extrapolated from larger series, including different histologies and/or different sites, apart from liver.
Milano et al. in 2009 published their experience on oligometastatic breast cancer patients treated with curative-intent stereotactic body radiation therapy.26 This paper focused only on breast cancer patients. By analyzing their series, 14 patients with 33 liver lesions were treated with SBRT. Authors reported seven local failures in the whole series, three of these occurred in patients with liver metastases. For the whole series of patients treated with curative intent, the 2-year and 4-year OS were 76 and 59%, respectively; the median survival was not reached. The 2-year and 4-year PFS were 44 and 38%, respectively; the median PFS was 23 months.
Rushtoven et al. conducted a phase I/II multi-institutional trial of SBRT, focusing only on liver metastases. All histologies were allowed, except germ cell tumor, leukemia, or lymphoma.22 In the phase I portion of the trial, dose was escalated from 36 Gy to 60 Gy in three fractions, in increments of 6 Gy, without dose-limiting toxicity. In the phase II component, the dose was 60 Gy in three fractions. Only four out of 47 patients were affected by primary breast cancer, therefore an analysis limited to breast oligometastatic patients is not available. For the whole series, median and 2-year OS rates were 20.5 months and 30%. Toxicity was mild.
Once again the Rochester group in 2012 updated results of their trial of oligometastatic patients treated with SBRT.14 The authors enrolled 121 patients with five or fewer clinically detectable metastases, from any primary site, metastatic to one to three organ sites, and treated with SBRT. Limiting the analysis to breast cancer patients (39), the 2-year OS, freedom from widespread distant metastases (FFDM), and local control (LC) rate were 74%, 52%, and 87%, respectively. The 6-year OS, FFDM, and LC rate were 47%, 36%, and 87%, respectively. Thirteen patients were affected by liver metastases. Data restricted to these patients are not detailed. No case of RILD and toxicity events ≥grade 3 were reported.
In 2015 the Italian group of Scorsetti et al. published the preliminary results of a phase II study for unresectable liver metastases treated with SBRT. Among 61 treated patients, eleven had breast primary tumor. Patients were treated with a dose of 75 Gy in 3 fractions and after a median follow-up of 12 months the in-field local response rate was 94%. No correlations were found between local control and histologies and lesion's size.
A retrospective analysis on oligometastatic breast cancer patients treated with local therapies was published by Kobayashi et al.27 From their institutional database, the authors identified 75 patients, treated with chemotherapy and local therapies in case of complete or partial response. This series included ten patients with liver metastases. Local therapies, unfortunately not otherwise specified, showed a significant impact on OS, progression free interval and relapse free interval. However, distinction between surgery and RT is not declared and data specifically focused on liver metastases are not available.
Patients with 1–5 metastatic cancer sites with a life expectancy of >3 months received escalating stereotactic body radiotherapy (SBRT) doses to all known cancer sites in a dose escalation trial published in 2012 by Salama et al.28 Unfortunately, just seven patients had a primary breast tumor. 22 out of 113 lesions were located in the liver. The paper reports results for the whole series. One- and 2-year progression-free survival was 33.3% and 22.0%; 1-year and 2-year overall survival was 81.5% and 56.7%. Treatment was well tolerated.
de Vin et al. treated with SBRT 209 oligometastatic patients.29 There were 77 liver metastases, breast cancer patients represented 11% of the whole series. The median survival of all patients was 24 months. The 3-, 4- and 5-year OS rates were 32%, 25% and 19%, respectively.
3.1.2. Our experience
We recently reviewed our experience on lung and liver SBRT in oligometastatic breast cancer patients.30 Twenty-three patients with a total number of 33 liver lesions were irradiated between 2010 and 2014 in our institution. All but one patient received chemotherapy for metastatic disease before SBRT. Hormonal therapy was administered to 5 patients at the time of SBRT. After SBRT 13 patients continued chemotherapy, 14 patients started or continued targeted therapy with anti HER2 drugs, while hormonal therapies were administered to 15 patients. Chemotherapy was interrupted before SBRT and started again after the treatment, while patients receiving immunotherapy or hormonal therapies continued their usual systemic therapies.
Prescribed RT doses were 75 Gy (22 lesions), 67.7 Gy (5 lesions), 61.8 Gy (4 lesions) or 56.25 Gy (2 lesions) all in three fractions. Mean age at SBRT was 54 years (range 39–78). Mean disease free interval (DFI) between first diagnosis of breast cancer and occurrence of metastases was 7.1 years (range 0.2–26.2). Mean time between diagnosis of metastases and SBRT was 25.6 months (range 0.6–105 months).
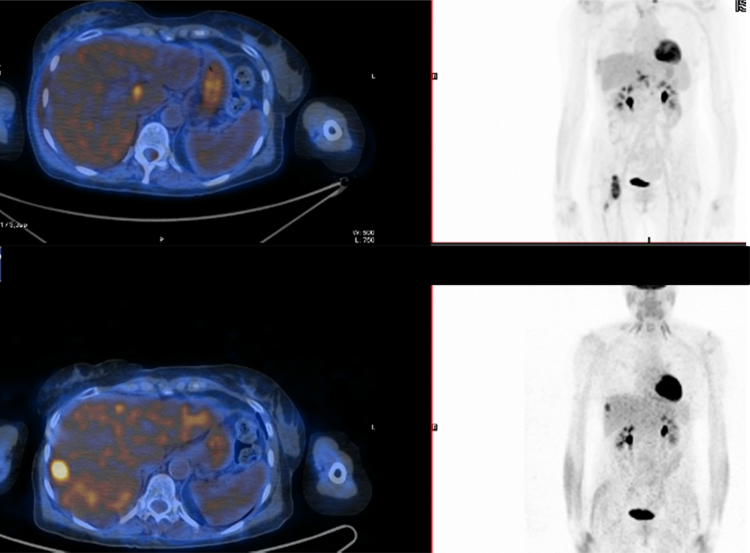
Median follow up was 24 months (range 3–59). At the time of analysis nine patients (39.1%) had died because of disease progression. Both anatomic and metabolic information, where available, were used to define the best response during follow up. Fourteen patients (60.8%) obtained a complete response, according to RECIST criteria, in 6 cases we recorded a partial response (26.1%). Three patients (13.1%) unfortunately had a local progression of the irradiated lesion. Twenty patients (86.9%) experienced a further progression of disease in a different site. Three patients (13.1%) remained disease free at last follow up visit. An example of a complete response of a liver metastases treated with SBRT is reported in Fig. 4.
Fig. 4.
Complete metabolic response of liver metastases from breast cancer treated with 75 Gy in 3 fractions.
One and two years actuarial LC rate were 96% and 87%, respectively. Mean overall survival (OS) was 48 months. Actuarial OS rate at 1 and 2 years was 92% and 66%, respectively.
Mean progression-free survival (PFS) was 11 months, with a PFS rate at 1 and 2 years of 35% and 18%, respectively.
At univariate analysis, DFI > 12 months, hormonal receptor positivity, medical therapies for metastatic disease after SBRT showed a significant impact on OS; extra hepatic disease correlated with a worse PFS. At multivariate analysis, none of the analyzed parameters showed a significant correlation with LC, OS and PFS.
No patients experienced radiation-induced liver disease (RILD, both classic or non-classic) or any grade ≥3 toxicity. In fourteen cases (60.8%) we did not record any acute or late toxicity. Acute nausea and vomiting G1–2 were the most represented side effects (34.8%). Regarding late toxicity, during follow up we recorded only one case of G2 gastritis (4.4%).
4. Conclusion
Local ablative treatments for liver metastases in oligometastatic breast cancer patients represent a promising therapeutic option, with a chance of long term disease control and perhaps a cure in selected cases. In this scenario, SBRT is an excellent choice, being a safe and efficient treatment, as confirmed by our and other published experiences.31 The next challenge will necessarily be the identification of patients that can really benefit from local treatments. Prospective randomized trials are needed and hopefully will clarify this issue. Ongoing trials are NRG BR002 trial, NRG BR001: NCT02206334 trial, the SABR COMET study (NCT01446744), and NCT02581670. Any effort should be done to enroll patients in these and other prospective studies. Only through these trials, will we be able to understand if there is a subset of breast cancer metastatic patients that we can cure with SBRT or other local therapies.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Hellman S., Weichselbaum R.R. Oligometastases. J Clin Oncol. 1995;13(January (1)):8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Pagani O., Senkus E., Wood W. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Inst. 2010;102:456–463. doi: 10.1093/jnci/djq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salama J.K., Chmura S.J. The role of surgery and ablative radiotherapy in oligometastatic breast cancer. Semin Oncol. 2014;41(6):790–797. doi: 10.1053/j.seminoncol.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Yoon S.S., Tanabe K.K. Surgical treatment and other regional treatments for colorectal cancer liver metastases. Oncologist. 1999;4:197–208. [PubMed] [Google Scholar]
- 5.Scheele J., Stangl R., Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–1246. doi: 10.1002/bjs.1800771115. [DOI] [PubMed] [Google Scholar]
- 6.Saltz L.B. Metastatic colorectal cancer: is there one standard approach? Oncology. 2005;19:1147–1154. discussion 1154, 57–58, 60. [PubMed] [Google Scholar]
- 7.Cummings L., Payes J., Cooper G. Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer. 2007;109:718–726. doi: 10.1002/cncr.22448. [DOI] [PubMed] [Google Scholar]
- 8.Wei A.C., Greig P.D., Grant D. Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol. 2006;13:668–676. doi: 10.1245/ASO.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 9.Fong Y., Fortner J., Sun R.L. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomlinson J.S., Jarnagin W.R., Dematteo R.P. Actual 10-year survival after resection of 514 colorectal liver metastases defines cure. J Clin Oncol. 2007;25(29):4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 11.Pockaj P.A., Wasif N., Dueck A.C. Metastasectomy and surgical resection of the primary tumor in patients with stage IV breast cancer: time for a second look? Ann Surg Oncol. 2010;17(September (9)):2419–2426. doi: 10.1245/s10434-010-1016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tree A.C., Khoo V.S., Eeles R.A. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 2013;14:e28–e37. doi: 10.1016/S1470-2045(12)70510-7. [DOI] [PubMed] [Google Scholar]
- 13.Dellas K. Does radiotherapy have curative potential in metastatic patients? The concept of local therapy in oligometastatic breast cancer. Breast Care. 2011;6:363–368. doi: 10.1159/000333115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milano M.T., Katz A.W., Zhang H., Okunieff P. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys. 2012;83:878–886. doi: 10.1016/j.ijrobp.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 15.Scorsetti M., Clerici E., Comito T. Stereotactic body radiation therapy for liver metastases. J Gastrointest Oncol. 2014;5(3):190–197. doi: 10.3978/j.issn.2078-6891.2014.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potters L., Kavanagh B., Galvin J.M. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2010;76(February (2)):326–332. doi: 10.1016/j.ijrobp.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 17.Seung S.K., Larson D.A., Galvin J.M. American College of Radiology (ACR) and American Society for Radiation Oncology (ASTRO) Practice Guideline for the Performance of Stereotactic Radiosurgery (SRS) Am J Clin Oncol. 2013;36(June (3)):310–315. doi: 10.1097/COC.0b013e31826e053d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scorsetti M., Arcangeli S., Tozzi A. Is stereotactic body radiation therapy an attractive option for unresectable liver metastasis? A preliminary report from a phase 2 trial. Int J Radiat Oncol Biol Phys. 2013;86(2):336–342. doi: 10.1016/j.ijrobp.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 19.ICRU ICRU report 83: prescribing, recording, and reporting photon-beam intensity-modulated radiation therapy (IMRT) J ICRU. 2010;10:1–106. doi: 10.1007/s00066-011-0015-x. [DOI] [PubMed] [Google Scholar]
- 20.Høyer M., Swaminath A., Bydder S. Radiotherapy for liver metastases: a review of evidence. Int J Radiat Oncol Biol Phys. 2012;82(3):1047–1057. doi: 10.1016/j.ijrobp.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Lee M.T., Kim J.J., Dinniwell R. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol. 2009;27:1585–1591. doi: 10.1200/JCO.2008.20.0600. [DOI] [PubMed] [Google Scholar]
- 22.Rusthoven K.E., Kavanagh B.D., Cardenes H. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572–1578. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 23.Chang D.T., Swaminath A., Kozak M. Stereotactic body radiotherapy for colorectal liver metastases: a pooled analysis. Cancer. 2011;117(17):4060–4069. doi: 10.1002/cncr.25997. [DOI] [PubMed] [Google Scholar]
- 24.Comito T., Cozzi L., Clerici E. Stereotactic ablative radiotherapy (SABR) in inoperable oligometastatic disease from colorectal cancer: a safe and effective approach. BMC Cancer. 2014;14:619. doi: 10.1186/1471-2407-14-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hennessey H., Valenti D., Cabrera T., Panet-Raymond V., Roberge D. Cardiac embolization of an implanted fiducial marker for hepatic stereotactic body radiotherapy: a case report. J Med Case Rep. 2009;3:140. doi: 10.1186/1752-1947-3-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milano M.T., Zhang H., Metcalfe S.K., Muhs A.G., Okunieff P. Oligometastatic breast cancer treated with curative-intent stereotactic body radiation therapy. Breast Cancer Res Treat. 2009;115:601–608. doi: 10.1007/s10549-008-0157-4. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi T., Ichiba T., Sakuyama T. Possible clinical cure of metastatic breast cancer: lessons from our 30-year experience with oligometastatic breast cancer patients and literature review. Breast Cancer. 2012;19:218–237. doi: 10.1007/s12282-012-0347-0. [DOI] [PubMed] [Google Scholar]
- 28.Salama J.K., Hasselle M.D., Chmura S.J. Stereotactic body radiotherapy for multisite extracranial oligometastases: final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer. 2012;118:2962–2970. doi: 10.1002/cncr.26611. [DOI] [PubMed] [Google Scholar]
- 29.de Vin T., Engels B., Gevaert T. Stereotactic radiotherapy for oligometastatic cancer: a prognostic model for survival. Ann Oncol. 2014;25:467–471. doi: 10.1093/annonc/mdt537. [DOI] [PubMed] [Google Scholar]
- 30.Scorsetti M., Franceschini D., De Rose F. Stereotactic body radiation therapy: a promising chance for oligometastatic breast cancer. Breast. 2016;26:11–17. doi: 10.1016/j.breast.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Comito T., Clerici E., Tozzi A. Liver metastases and SBRT: a new paradigm? Rep Pract Oncol Radiother. 2015;20(November–December (6)):464–471. doi: 10.1016/j.rpor.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]