Abstract
Melatonin confers protective effects on premature ovarian insufficiency (POI) induced by tripterygium glycosides (TG) by reducing oxidative stress. Silent information regulator 1 (SIRT1) signaling is found to be associated with the physiology and pathology of ovary. We hypothesize that melatonin could protect POI via activating SIRT1 signaling. The aim of this study was to investigate the protective effect of melatonin on POI and elucidate its potential mechanisms. Mice were assigned to melatonin treatment with or without SIRT1 inhibitor Ex527 or melatonin receptor antagonist luzindole (Luz) and then subjected to POI. Melatonin conferred a protective effect by improving estrous phase, ovarian and uterus mass and index, increasing ovarian follicles, corpus luteum and anti-mullerian hormone (AMH), decreasing atresia follicles and follicle stimulating hormone (FSH). Melatonin treatment also could reduce malondialdehyde (MDA) level, MDA5, Gp91phox, Caspase3 and Bax expression, and increase total antioxidant activity (TAC), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and Bcl-2 expression by up-regulating SIRT1 signaling. However, these protective effects were blocked by Ex527 and Luz, indicating that SIRT1 signaling and melatonin receptor might be specially involved in these effects. In summary, these findings suggest that melatonin protects POI by reducing oxidative stress and apoptotic damage via activation of SIRT1 signaling in a receptor-dependent manner.
Keywords: Premature ovarian insufficiency, melatonin, melatonin receptor, SIRT1 signaling pathway, oxidative stress, apoptosis
Introduction
Primordial follicles are the basic units of germ cells, which plays a vital role in female fertility. Dysfunctional follicles could lead to some reproductive issues like premature ovarian insufficiency (POI). POI, generally irreversible, which is diagnosed when primary or secondary amenorrhea, elevated gonadotropins and sex steroid hormone deficiency due to reduced number of ovarian follicles are found in women before 40 years old [1,2]. Although numerous studies have reported a variety of etiologies and various adverse reactions of POI, whose mechanisms are still unclear [3-5]. Because of poor feasibility of human studies, a mouse model of POI induced by tripterygium glycosides (TG) has been successfully established in our previous study [6,7]. It was found that oxidative stress might be the potential cause resulting in POI and melatonin could protect ovarian structures and improve its endocrine functions by reducing oxidative stress [8,9]. However, the concrete molecular mechanisms remain unknown, which needs further research.
Melatonin (N-acetyl-5-methoxytryptamine, Mel), the main secrete hormone of pineal gland, is a documented powerful free radical scavenger and a broad-spectrum antioxidant. For reproductive system, melatonin plays an important role in the physiology and pathology of ovary [10]. Tamura and colleagues reviewed previous literatures, summarized new findings related to beneficial effects of melatonin on reproductive physiology and concluded that melatonin as a free radical scavenger in the ovarian follicles contributed to oocyte maturation, embryo development and luteinization of granulosa cells [11,12]. Interesting, recent studies demonstrated that melatonin could ameliorate myocardial ischemia-reperfusion injury, ischemic stroke, multiple sclerosis, early brain injury and acute kidney injury by reducing oxidative stress and apoptotic damage via activation of SIRT1 signaling [13-18]. However, in human tumor cells, melatonin could exert antitumor activity by inhibiting SIRT1 [19,20], which aroused our concerns.
SIRT1 is a conserved nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase, exerts various biological effects in various physiological conditions and diseases [13-20]. Strong experimental evidence supports the notion that SIRT1 plays a crucial role in sensing and modulating the cellular redox state thus providing protective effects in cells and tissues exposed to oxidative stressors in vitro and in vivo [21]. The expression of SIRT1, located in cytoplasm and nucleus, has been observed in mammalian granulosa cells, oocytes, embryos and human ovarian cell lines [21]. Zhao found that SIRT1 was widely detected in non-apoptotic granulosa cells of follicles, but significantly decreased in the process of granulosa cell apoptosis during follicular atresia in porcine ovary [22]. Likewise, SIRT1 was downregulated during aging, while the upregulation of SIRT1 signaling protected the mouse oocytes against oxidative stress [23,24]. In obesity rats, ovarian follicle development and follicle loss were accelerated with decreased expression of SIRT1 [25]. However, caloric restriction inhibited ovarian follicle development and follicle loss through activating SIRT1 signaling and SIRT1 activator could improve the follicle reverse and prolong the ovarian lifespan of diet-induced obesity [26,27]. On the contrary, the SIRT1 level was higher in polycystic ovarian syndrome (PCOS) patients than the healthy controls, which was opposite to another human study of PCOS and the results of PCOS rats model and premature ovarian failure (POF) [28-31]. However, no studies have focused on whether and how melatonin affected SIRT1 and its transcription factors such as forkhead box class O 3a (FoxO3a) and nuclear erythroid 2-related factor 2 (Nrf2), and antioxidant enzyme heme oxygenase-1 (Ho-1) to protect POI induced by TG, which is what we want to elucidate.
Melatonin’s indirect anti-oxidative effects were demonstrated to be receptor dependent either located in cell membrane or within the nucleus [13,14]. Therefore, this study was designed to investigate whether and how melatonin attenuated oxidative stress and apoptosis in POI via SIRT1 signaling pathway in a receptor dependent manner.
Materials and methods
Materials
The chemicals of TG, Mel, Ex527 and Luz were purchased from FudanFuhua Pharmaceutical CO., Ltd., (Shanghai, China), Sigma-Aldrich (St. Louis, MO, USA), Selleck Chemicals (Houston, TX, USA) and Santa Cruz (Paso Robles, CA, USA), respectively. The ELISA kits for AMH, FSH, MDA, SOD and GSH-Px were bought from Huayi Biotechnology (Shanghai, China) and TAC from Jiancheng Biotechnology (Nanjing, China). The primary antibodies of MDA5 and Bcl-2 were obtained from Abcam (Cambridge, UK), GSH-Px from Lab frontier (Korea), Gp91phox, SOD, FoxO3a, Nrf2 and Ho-1 from Santa Cruz, Caspase3, Bax and SIRT1 from Cell Signaling Technology (CST, Beverly, MA, USA), and β-actin and GAPDH from Beyotime Biotechnology (Shanghai, China). The goat anti-rabbit and rabbit anti-goat secondary antibodies, radio-immnuoprecipitation assay (RIPA), protease inhibiter phenylmethaesulfonyl fluoride (PMSF) and the BCA protein assay kit were also purchased from Beyotime Biotechnology. The enhanced chemiluminescence (ECL) reagent and TRIzol reagent were purchased from Merck Millipore of USA and Invitrogen of USA, respectively. PrimeScript™RT Master Mix (Perfect Real Time) and SYBR® Premix Ex Taq II (Perfect Real Time) were obtained from Takara (Takara, Ostu, Japan).
The preparation of TG and Mel was briefly described in our previous studies [6,9]. Ex527 and Luz were first dissolved in dimethyl sulfoxide (DMSO) and then diluted to the final concentration with sterile saline (the DMSO final concentration <2%).
Animals
A total of 112 female Kunming mice (6 weeks old) were obtained from the Department of Laboratory Animal Science of Fudan University (Shanghai, China). Every four mice were housed in per wire cage and all the mice were kept under standard laboratory conditions (12 h of light, 12 h of dark, 22-25°C) with a proper diet chow and water ad libitum. The animal experiments were approved by the Experimental Animal Ethical Committee of Fudan University (Approval No.: 2012-36, Approval Date: 20 February 2012, Shanghai, China).
Experimental groups and treatment
All experimental mice were acclimatized for the first 4 days under standard laboratory conditions before treatment. They were randomly assigned to following experimental groups: (i) NC group: normal mice receiving sterile saline by oral from day 5 to day 42; (ii) TG group: normal mice receiving sterile saline by oral from day 5 to day 7, then sterile saline by oral and TG via subcutaneous injection from day 8 to day 42; (iii) Mel + TG group: normal mice receiving Mel by oral from day 5 to day 7, then Mel by oral and TG via subcutaneous injection from day 8 to day 42; (iv) Mel + Ex527 + TG group: normal mice receiving Mel by oral and Ex527 injection intraperitoneally from day 5 to day 7, then Mel by oral, Ex527 injection intraperitoneally and TG via subcutaneous injection from day 8 to day 42; (v) Ex527 + TG group: normal mice receiving Ex527 injection intraperitoneally from day 5 to day 7, then Ex527 injection intraperitoneally and TG via subcutaneous injection from day 8 to day 42; (vi) Mel + Luz + TG group: normal mice receiving Mel by oral and Luz injection intraperitoneally from day 5 to day 7, then Mel by oral, Luz injection intraperitoneally and TG via subcutaneous injection from day 8 to day 42; (vii) Luz + TG group: normal mice receiving Luz injection intraperitoneally from day 5 to day 7, then Luz injection intraperitoneally and TG via subcutaneous injection from day 8 to day 42. The administration dose of TG, Mel, Ex527 and Luz was 50 mg/kg/day, 20 mg/kg/day, 2.5 mg/kg/day and 1 mg/kg/day [6,9,13].
Body weight and estrous phase analysis
The body weight of mice was monitored twice every week. Vaginal smears of mice were taken during the last 21 days, whose cells were collected via a sterile cotton swab moistened with sterile saline and placed on a clean glass slide. Then, the stages of vaginal smears were analyzed under the light microscope and assessed based on vaginal cytology. The normal estrous cycle is a 4 to 5-day and a cycle duration of >5 days or <4 days is considered as irregular [27]. The abnormal rate of estrous cycle and normal estrous frequency were evaluated.
Organ mass and index assessment
The ovarian and uterine mass and index were determined according to previously described [6].
Preparation of blood samples, ovarian homogenates and ovarian sections
In day 43, the blood samples of all mice were collected and then the mice were sacrificed to harvest the ovaries and uteruses for weight. Of them, 8 left ovaries of each group were homogenized in ice-cold phosphate buffer solution (PBS) to be homogenates at the final concentration of 10%. Then, blood samples and ovarian homogenates were centrifuged at 3,000 rpm for 10 min to separate the supernatants, which were collected and stored at -80°C for further enzyme-linked immunosorbent assay (ELISA) detection. Eight right ovaries were harvested after carefully isolating surrounding adipose tissue and fascia and then fixed in 4% paraformaldehyde at room temperature for 48 hours, flushed under running water for 3 hours, then dehydrated through a series of concentrations of ethanol, cleared in xylene and embedded in paraffin. Ovarian sections of 4 μm were prepared for hematoxylin and eosin (HE) staining and immunohistochemistry (IHC). Another 8 left and 8 right ovaries of each group were stored at -80°C for western blot and quantitative real-time PCR (qRT-PCR) analysis.
HE staining and follicle classification
All sections were deparaffinized in xylene, hydrated with decreasing alcohol concentrations, stained with HE, mounted and then observed under a light microscope. Five representative sections separated by a distance of over 80 μm from each ovary were selected for follicle counting. Ovarian follicles were classified as follows [27]: primordial follicle, an oocyte surrounded by one layer of flatted graunlosa cells; primary follicle, an oocyte surrounded by one layer of cuboidal graunlosa cells; secondary follicle, two or three layers of cuboidal granulose cells with no antral space; and antral follicle, more than four layers of granulosa cells with one or more independent antral spaces. In some cases, antral follicles had no antral space in cross-section analysis, but were considered as antral if they contained more than five granulosa cell layers. Atresia follicles contained at least twenty apoptotic granulosa cells (defined by theapoptotic bodies in the granulosa cell layer), disorganized granulosa cells, a fragmentation of the oocyte nucleus, or a degenerating oocyte. In this paper, we counted numbers of atresia follicles, primordial follicles, developing follicles (primary, secondary and antral follicles), and surviving follicles including primordial and developing follicles.
ELISA quantification of AMH, FSH, MDA, TAC, SOD and GSH-Px
Serum and ovarian homogenates supernatants levels of AMH, FSH, MDA, TAC, SOD and GSH-Px were spectrophotometrically measured by ELISA. All the procedures were strictly done according to the protocols of each kit.
IHC detection for MDA5 and Caspase3
The immunohistochemical analysis was used to detect the expression of MDA5 and Caspase3 in the ovaries. All sections were incubated at 60°C for 1 h, deparaffinized in xylene and rehydrated in a graded series of ethanol routinely. Antigen retrieval was performed in 0.01 M citrate buffer (pH 6.0) and high microwave irradiation for 30 min. Endogenous peroxidase activity was eliminated with 3% H2O2 for 10 min and then non-specific binding was blocked with 10% normal goat serum for 30 min at room temperature. Afterwards, the sections were separately incubated with primary antibodies against MDA5 (1:300) and Caspase3 (1:800) overnight at 4°C, then placed for 45 min at room temperature. The sections were rinsed with PBS, and then secondary antibodies were added for 10 min. After washed with PBS, all sections were incubated with horseradish peroxidase (HRP) for 10 min at room temperature. Following, washed sections were incubated with 3,3-diaminobenzidine (DAB) to visualize the final product and counter-stained. Negative control was treated by substituting PBS for a primary antibody.
Western blot analysis
The protein levels of MDA5, Gp91phox, SOD, GSH-Px, Caspase3, Bax, Bcl-2, SIRT1, FoxO3a, Nrf2 and Ho-1 in the ovaries were measured using western blot. The ovaries were homogenized in RIPA and PMSF on ice. The supernatants were collected for protein analysis after centrifugation (12,000 rpm, 15 min at 4°C) and then the BCA protein assay kit was used to detect protein concentrations. The protein samples were separated by SDS-PAGE and transferred onto polyvinylidenedifluoride (PVDF) membranes. The membranes were blocked in 5% fat free milk for 1 h at room temperature and then overnight at 4°C incubated with primary antibodies (MDA5, 1:300; Gp91phox, 1:500; SOD, 1:800; GSH-Px, 1:500; Caspase3, 1:800; Bax, 1:1000; Bcl-2, 1:1000; SIRT1, 1:1000; FoxO3a, 1:200; Nrf2, 1:400 and Ho-1, 1:500) followed by the corresponding secondary antibodies for 1 h at room temperature. The protein bands were visualized with ECL reagent. The Quantity One software (Bio-Rad Laboratories Pty. Ltd) was used to analyze the band intensities. β-actin and GAPDH were used as the internal loading controls.
Quantitative real-time PCR analysis
The transcript levels of Gp91phox, SOD, GSH-Px, Caspase3, Bax, Bcl-2, SIRT1, FoxO3a, Nrf2 and Ho-1 in the ovaries were analyzed by qRT-PCR. Total RNA was extracted from the ovaries using TRIzol reagent and operated as instructions. Then, 2 μl of RNA was quantified spectrophotometrically by measuring the absorbance at 260 nm and RNA purity was determined by determining the 260/280 nm absorbance ratio. Total RNA (1 μg) was reverse transcribed into complementary DNA (cDNA) using PrimeScript™RT Master Mix (Perfect Real Time) (Code No.: RR036A). To quantify mRNA expression, an amount of cDNA equivalent to 20 ng of total RNA was amplified using SYBR® Premix Ex Taq II (Perfect Real Time) (Code No.: RR820A) according to manufacturer’s instruction. The primers of target gene were listed in Table 1, which were designed using Primer Premier 6.0 software and were synthesized by Shanghai BioTNT Biotechnology Co., Ltd. (Shanghai, China). β-actin and GAPDH served as an internal standard. Relative quantification of the target gene expression levels was conducted using the 2-∆∆Ct method in which ΔΔCt = ΔE - ΔC, ΔE = Ctexp - Ctβ-actin or Ctexp - CtGAPDH, and ΔC = Ctcon - Ctβ-actin or Ctcon - CtGAPDH.
Table 1.
Primers sequence of targeted genes
| Gene name | Forward | Reverse |
|---|---|---|
| Gp91phox | 5’-CCAGTGAAGATGTGTTCAGCT-3’ | 5’-GCACAGCCAGTAGAAGTAGA-3’ |
| SOD | 5’-GAGCACGCTTACTACCTTC-3’ | 5’-AACATTCTCCCAGTTGATTAC-3’ |
| GSH-Px | 5’-GCTCACCCGCTCTTTACC-3’ | 5’-GCCGCCTTAGGAGTTGC-3’ |
| Caspase3 | 5’-GGGTGCCTATTGTGAGGCG-3’ | 5’-CGCCTCACAATAGCACCC -3’ |
| Bax | 5’-GTGAGCGGCTGCTTGTCT-3’ | 5’-GGTCCCGAAGTAGGAGAGGA-3’ |
| Bcl-2 | 5’-GCGGA AGTCA CCGAA ATG-3’ | 5’-AGGAC AGCGA TGGGA AAA-3’ |
| SIRT1 | 5’-GCAACAGCATCTTGCCTGAT-3’ | 5’-GTGCTACTGGTCTCACTT-3’ |
| FoxO3a | 5’-CGGCTCACTTTGTCCCAGAT-3’ | 5’-GCCGGATGGAGTTCTTCCA-3’ |
| Nrf2 | 5’-CAGTGCTCCTATGCGTGAA-3’ | 5’-GCGGCTTGAATGTTTGTCT-3’ |
| Ho-1 | 5’-TGACAGAAGAGGCTAAGACCG-3’ | 5’-AGTGAGGACCCACTGGAGGA-3’ |
| β-actin | 5’-CACTGCCGCATCCTCTTCCTC-3’ | 5’-CTCCTGCTTGCTGATCCACAT-3’ |
| GAPDH | 5’-GGCACAGTCAAGGCTGAGAATG-3’ | 5’-ATGGTGGTGAAGACGCCAGTA-3 |
SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; SIRT1, silent information regulator 1; FoxO3a, forkhead box O 3a; Nrf2, nuclear erythroid 2-related factor 2; Ho-1, heme oxygenase-1.
Statistical analysis
The software of GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA, USA) and SPSS 16.0 (SPSS Inc., Chicago, IL, USA) were used to analyze data in this study. All data were expressed as the mean ± standard error of the mean (SEM). The abnormal rate of estrous cycle among groups was assessed by chi-square test or Fisher’s exact test. Comparisons of other data among multiple groups were assessed by one-way analysis of variance (ANOVA) followed by Bonferroni correction for post hoc t-test and Kruskal-Wallis tests. A P-value less than 0.05 was considered as statistical significance (P<0.05).
Results
All mice were alive at the end of the treatment, and no tumors were found in all parts of the body, but a little superficial abnormalities in the abdomen. Compared with normal mice, the mice with TG treatment seemed like a little thin and appeared a little decreased physical activity.
Melatonin might exert protective effects on POI by attenuating oxidative stress and apoptosis via activation of SIRT1 signaling
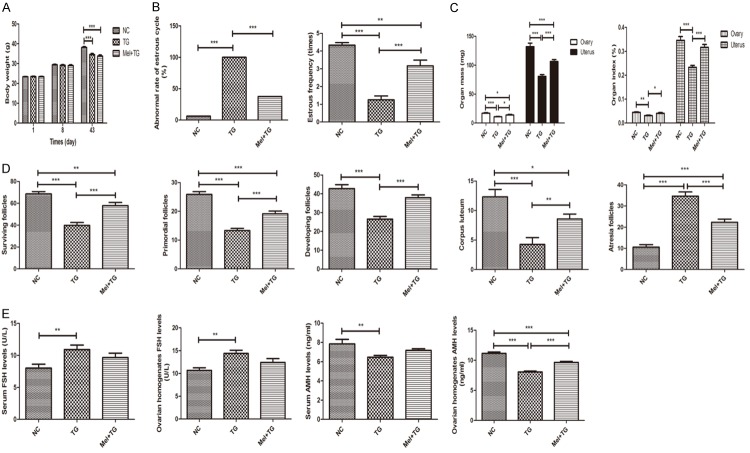
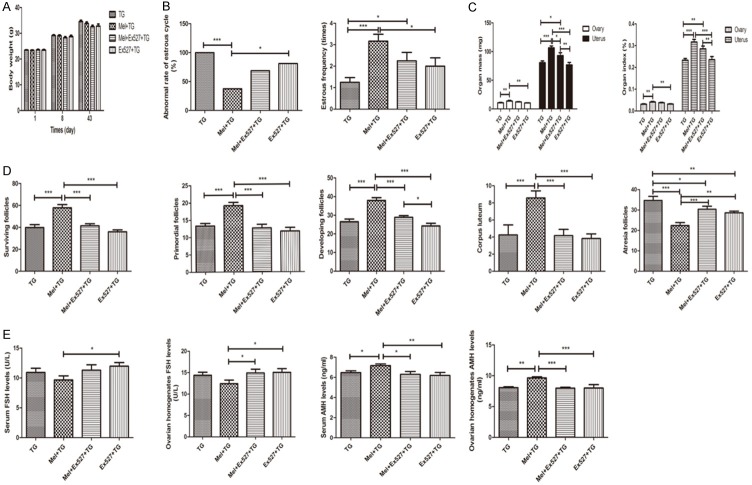
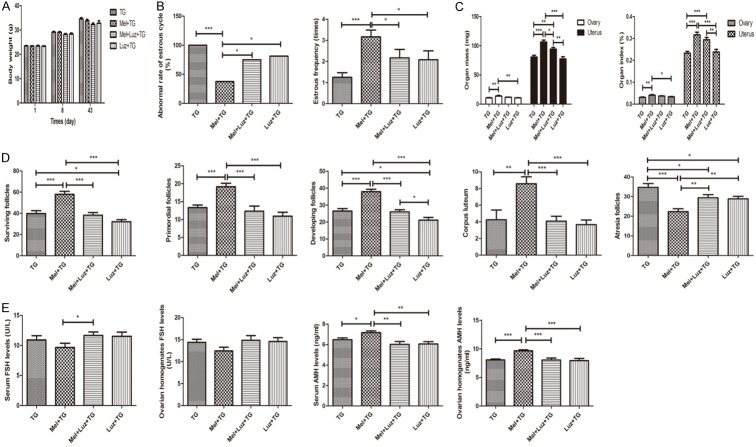
From Figure 1A, it was found that the body-weight gains of mice in normal control (NC) group, TG group and Mel + TG group were normal. Although the mean weight of the later two groups was lower than normal control group, melatonin didn’t increase the body weight of mice with TG. However, melatonin significantly decreased abnormal rate of estrous cycle and increased estrous frequency and ovarian and uterus mass and index (Figure 1B and 1C). For ovarian follicles, the surviving follicles, primordial follicles, developing follicles and corpus luteum remarkably increased after the treatment of melatonin, while the atresia follicles obviously reduced (Figure 1D). Representative images were shown in Supplementary Figure 1A. Compared to NC group, serum and ovarian homogenates FSH levels were significantly higher in TG group. Although no difference was found after melatonin treatment, it displayed a decreasing trend. It was also shown that TG significantly reduced serum and ovarian homogenates AMH levels, and the supplement of melatonin could apparently increase AMH level or have a rising trend at least (Figure 1E).
Figure 1.
Melatonin’s protective effects on premature ovarian insufficiency. A. Melatonin didn’t change body weight of mice. B. Melatonin significantly decreased abnormal rate of estrous cycle and remarkably increased estrous frequency. C. Melatonin obviously increased ovarian and uterus mass and index. D. Melatonin apparently increased surviving follicles, primordial follicles, developing follicles and corpus luteum, and decreased atresia follicles. E. Melatonin significantly reduced ovarian homogenates AMH levels. Data were shown as mean ± SEM or as ratio. *P<0.05, **P<0.01, ***P<0.001.
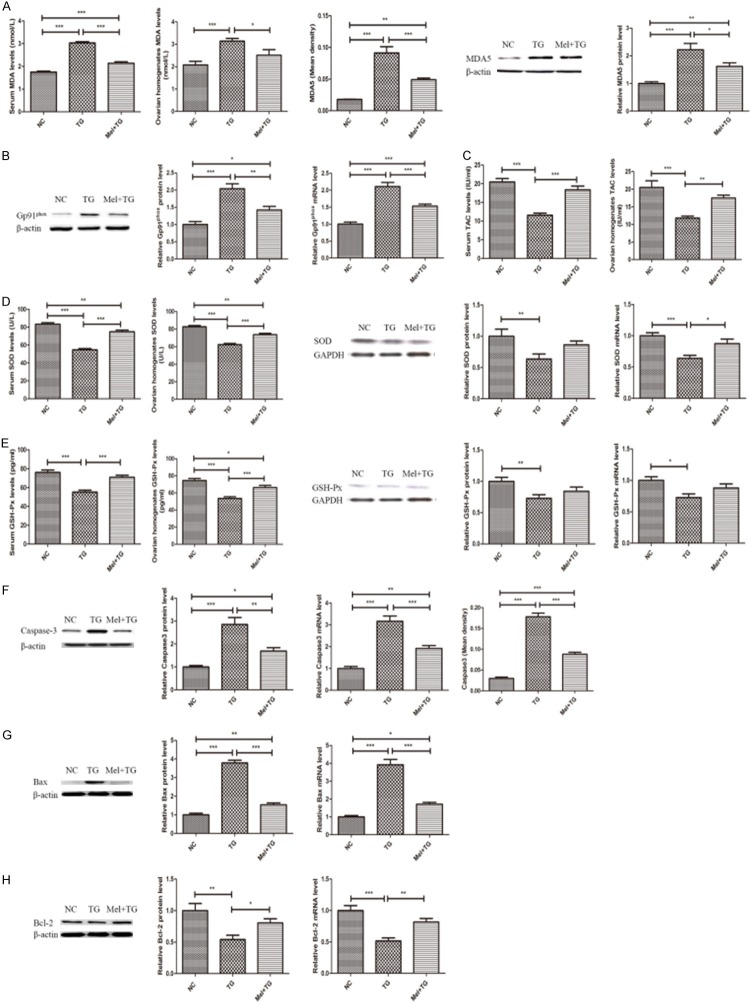
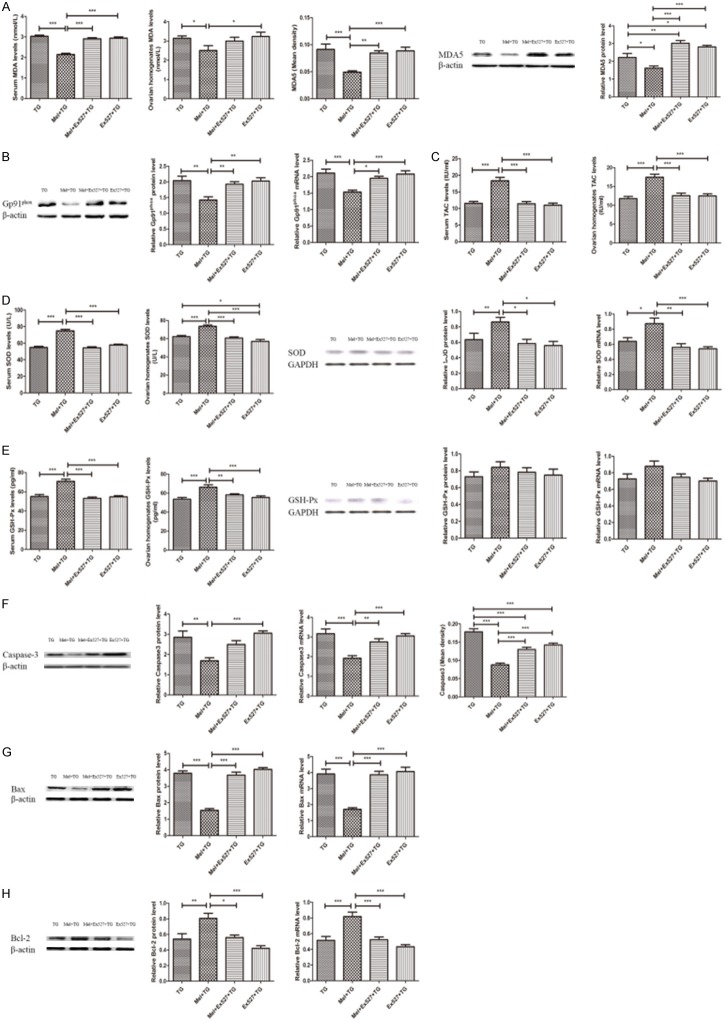
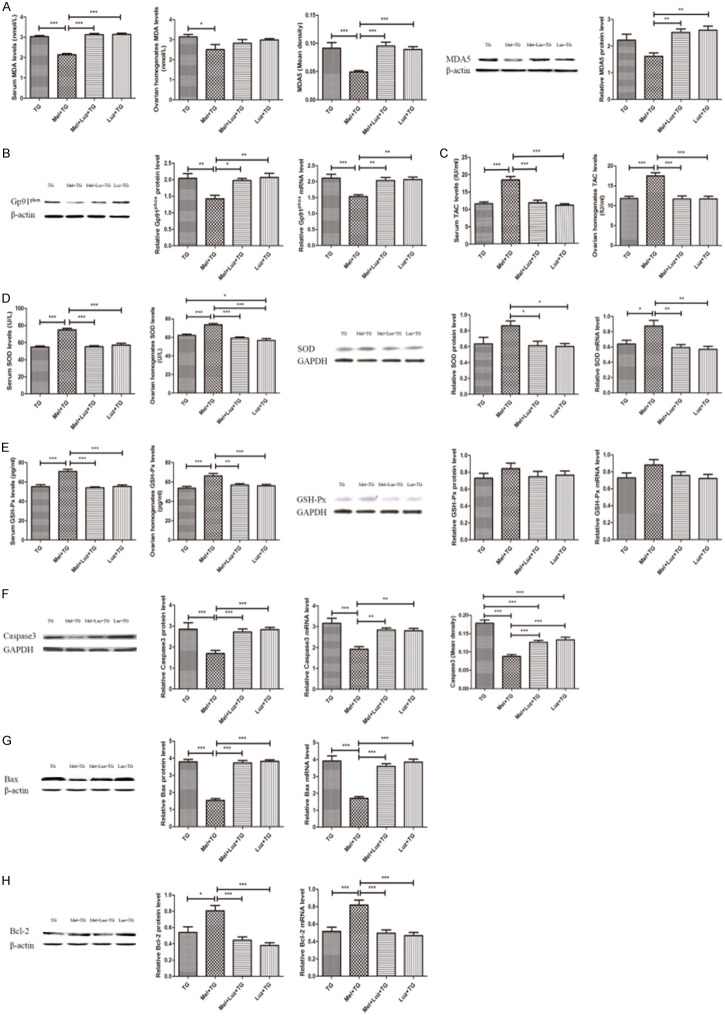
Figure 2 illustrated that TG administration significantly increased oxidative stress and apoptosis. Clearly, the oxidative indexes of serum and ovarian homogenates MDA levels and the MDA5 and Gp91phox expression were higher in TG group than NC group (Figure 2A and 2B). Simultaneously, the anti-oxidative substrates of serum and ovarian homogenates levels of TAC, SOD and GSH-Px, and the protein and mRNA expression of SOD and GSH-Px were lower in TG group when compared to NC group (Figure 2C-E). Also, it was found that TG tremendously promoted the expression of the pro-apoptotic indexes such as Caspase3 and Bax, and inhibited the expression of anti-apoptotic index of Bcl-2 (Figure 2F-H). However, these alterations were significantly improved after the treatment of melatonin (Figure 2). Representative immunohistochemical images of MDA5 and Caspase3 were shown in Supplementary Figure 1B and 1C.
Figure 2.
Melatonin significantly improved oxidative stress and apoptotic damage. A. Melatonin reduced serum and ovarian homogenates MDA level and MDA5 expression in the ovaries. B. Melatonin decreased the protein and mRNA expression of Gp91phox. C. Melatonin increased serum and ovarian homogenates TAC levels. D. Melatonin increased SOD expression. E. Melatonin increased GSH-Px expression. F. Melatonin reduced Caspase3 expression. G. Melatonin decreased Bax expression. H. Melatonin increased Bcl-2 expression. Data were shown as mean ± SEM or as ratio. *P<0.05, **P<0.01, ***P<0.001.
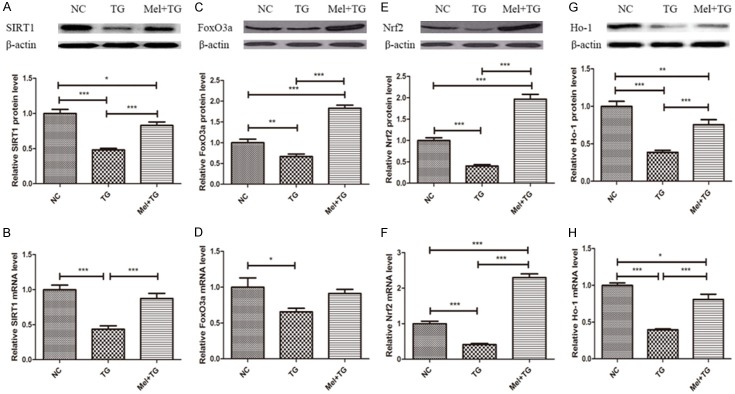
The ovarian protein and mRNA levels of SIRT1 signaling pathway including SIRT1, FoxO3a, Nrf2 and Ho-1 were measured and the results were summarized in Figure 3. Compared with normal mice, the protein and mRNA levels of SIRT1, FoxO3a, Nrf2 and Ho-1 significantly decreased after the treatment of TG. While, after receiving melatonin supplement, all these indexes were significantly higher than those of TG group except for the mRNA level of FoxO3a.
Figure 3.
Melatonin significantly increased SIRT1 signaling pathway. A and B. Melatonin increased the protein and mRNA levels of SIRT1. C and D. Melatonin increased the protein and mRNA levels of FoxO3a. E and F. Melatonin increased the protein and mRNA levels of Nrf2. G and H. Melatonin increased the protein and mRNA levels of Ho-1. Data were shown as mean ± SEM or as ratio. *P<0.05, **P<0.01, ***P<0.001.
SIRT1 inhibitor Ex527 blocked melatonin’s protective effects on POI by aggravating oxidative stress and apoptosis via inactivation of SIRT1 signaling
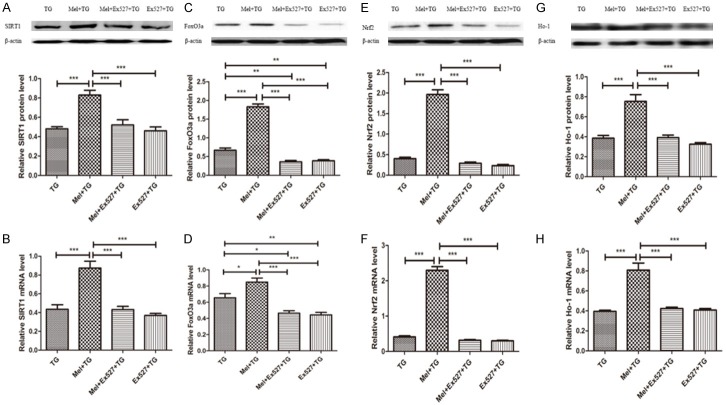
As shown in Figure 4, Ex527, a SIRT1 inhibitor, significantly blocked SIRT1 signaling and decreased the protein and mRNA expression of SIRT1, FoxO3a, Nrf2 and Ho-1. They were close to or lower than the levels of these indexes in TG group.
Figure 4.
SIRT1 inhibitor Ex527 significantly reduced SIRT1 signaling pathway. A and B. Ex527 reduced the protein and mRNA levels of SIRT1. C and D. Ex527 increased the protein and mRNA levels of FoxO3a. E and F. Ex527 reduced the protein and mRNA levels of Nrf2. G and H. Ex527 decreased the protein and mRNA levels of Ho-1. Data were shown as mean ± SEM or as ratio. *P<0.05, **P<0.01, ***P<0.001.
Compared with Mel + TG group, the groups with the supplement of Ex527 displayed an aggravated oxidative and apoptotic damage, which were demonstrated in Figure 5. First of all, serum and ovarian homogenates levels of MDA, the MDA5 and Gp91phox expression of ovarian tissues reflecting the indexes of oxidative damage significantly increased (Figure 5A and 5B). In addition, the anti-oxidative markers of serum and ovarian homogenates levels of TAC, SOD and GSH-Px, and the protein and mRNA levels of SOD were decreased with a significant difference. However, the protein and mRNA levels of GSH-Px were no different (Figure 5C-E). Furthermore, it was found that Ex527 increased the levels of Caspase3 and Bax, and decreased the level of Bcl-2 (Figure 5F-H). At the same time, when compared to TG group, only the protein level of MDA5, ovarian homogenates level of SOD, and the Caspase3 expression detected by IHC in the groups with Ex527 were significantly different (Figure 5A, 5D, 5F). Representative immunohistochemical images of MDA5 and Caspase3 were shown in Supplementary Figure 2B and 2C.
Figure 5.
SIRT1 inhibitor Ex527 significantly aggravated oxidative stress and apoptotic damage. A. Ex527 increased serum and ovarian homogenates MDA level and MDA5 expression in the ovaries. B. Ex527 increased the protein and mRNA expression of Gp91phox. C. Ex527 reduced serum and ovarian homogenates TAC levels. D. Ex527 decreased SOD expression. E. Ex527 reduced GSH-Px expression. F. Ex527 increased Caspase3 expression. G. Ex527 increased Bax expression. H. Ex527 decreased Bcl-2 expression. Data were shown as mean ± SEM or as ratio. *P<0.05, **P<0.01, ***P<0.001.
The parameters demonstrating the effects of Ex527 on POI were summarized in Figure 6. It was found that the body weight of mice among four groups was similar after the intervention of Ex527 (Figure 6A). The abnormal rate of estrous cycle showed an increasing trend in Mel + Ex527 + TG group and were significantly higher in Ex527 + TG group than that in Mel + TG group, but they were no different from TG group (Figure 6B). However, the estrous frequency and ovarian and uterus mass and index after receiving Ex527 treatment decreased with a trend or a significant difference compared with those in Mel + TG group (Figure 6B and 6C). Also, Ex527 could significantly weaken melatonin’s protective effects on ovarian follicles, with characteristics of reduced surviving follicles, primordial follicles, developing follicles and corpus luteum, and added atresia follicles (Figure 6D). Representative images were shown in Supplementary Figure 2A. Moreover, serum and ovarian homogenates levels of FSH and AMH in groups in the presence of Ex527 were significantly different from those in Mel + TG group, but similar to TG group (Figure 6E).
Figure 6.
SIRT1 inhibitor Ex527 blocked melatonin’s protective effects on premature ovarian insufficiency. A. Ex527 didn’t change body weight of mice. B. Ex527 significantly increased abnormal rate of estrous cycle and decreased estrous frequency. C. Ex527 obviously decreased ovarian and uterus mass and index. D. Ex527 apparently reduced surviving follicles, primordial follicles, developing follicles and corpus luteum, and increased atresia follicles. E. Ex527 significantly increased serum and ovarian homogenates FSH levels and decreased serum and ovarian homogenates AMH levels. Data were shown as mean ± SEM. *P<0.05, **P<0.01, ***P<0.001.
Melatonin activated SIRT1 signaling pathway in a receptor-dependent manner against premature ovarian insufficiency
Melatonin receptor antagonist luzindole suppressed SIRT1 signaling pathway (Figure 7). The protein and mRNA levels of SIRT1, FoxO3a, Nrf2 and Ho-1 in Mel + Luz + TG group and Luz + TG group were less than those in Mel + TG group (Figure 7). Simultaneously, the protein and mRNA levels of FoxO3a in Mel + Luz + TG group and Luz + TG group were also significantly lower than those in TG group (Figure 7C and 7D).
Figure 7.
Melatonin receptor antagonist luzindole significantly suppressed SIRT1 signaling pathway. A and B. Luzindole reduced the protein and mRNA levels of SIRT1. C and D. Ex527 increased the protein and mRNA levels of FoxO3a. E and F. Ex527 reduced the protein and mRNA levels of Nrf2. G and H. Ex527 decreased the protein and mRNA levels of Ho-1. Data were shown as mean ± SEM or as ratio. *P<0.05, **P<0.01, ***P<0.001.
The serum MDA levels, the expression of MDA5 and the levels of Gp91phox protein and mRNA in groups with Luz were remarkably higher than those in Mel + TG group (Figure 8A and 8B). However, no significant difference of ovarian homogenates MDA levels was found (Figure 8B). On the contrary, serum and ovarian homogenates levels of TAC, SOD, GSH-Px, and the protein and mRNA levels of SOD in Mel + Luz + TG group and Luz + TG group significantly decreased (Figure 8C-E), but the protein and mRNA levels of GSH-Px were no different (Figure 8E). Moreover, the pro-apoptotic indexes of Caspase3 and Bax in groups with Luz were also obviously higher than those in Mel + TG group (Figure 8F and 8G), while the anti-apoptotic index of Bcl-2 was apparently lower (Figure 8H). Representative immunohistochemical images of MDA5 and Caspase3 were shown in Supplementary Figure 3B and 3C.
Figure 8.
Melatonin receptor antagonist luzindole significantly aggravated oxidative stress and apoptotic damage. A. Luzindole increased serum MDA level and MDA5 expression in the ovaries. B. Luzindole increased the protein and mRNA expression of Gp91phox. C. Luzindole reduced serum and ovarian homogenates TAC levels. D. Luzindole decreased SOD expression. E. Luzindole reduced GSH-Px expression. F. Luzindole increased Caspase3 expression. G. Luzindole increased Bax expression. H. Luzindole decreased Bcl-2 expression. Data were shown as mean ± SEM or as ratio. *P<0.05, **P<0.01, ***P<0.001.
Just like the effects of Ex527, the effects of Luz on body weight of mice among four groups were also no different in the same day (Figure 9A). The abnormal rate of estrous cycle was significantly higher in groups receiving Luz treatment than that in Mel + TG group (Figure 9B).Similarity, the estrous frequency and ovarian and uterus mass and index after the supplement of Luz decreased with a trend or a significant difference compared to those in Mel + TG group (Figure 9B and 9C). Luz also presented an inhibition on ovarian structures, whose typical characterizes were reduced surviving follicles, primordial follicles, developing follicles, corpus luteum and increased atresia follicles compared to Mel + TG group (Figure 9D). Representative images were shown in Supplementary Figure 3A. For serum and ovarian homogenates FSH levels, only serum FSH level in Mel + Luz + TG group was higher than that in Mel + TG group, whereas others within the groups were no different. However, the levels of serum and ovarian homogenates AMH in groups with Luz were significantly decreased compared to Mel + TG group (Figure 9E).
Figure 9.
Melatonin receptor antagonist luzindole blocked melatonin’s protective effects on premature ovarian insufficiency. A. Luzindole didn’t change body weight of mice. B. Luzindole significantly increased abnormal rate of estrous cycle and decreased estrous frequency. C. Luzindole obviously decreased ovarian and uterus mass and index. D. Luzindole apparently reduced surviving follicles, primordial follicles, developing follicles and corpus luteum, and increased atresia follicles. E. Luzindole significantly increased serum FSH levels and decreased serum and ovarian homogenates AMH levels. Data were shown as mean ± SEM. *P<0.05, **P<0.01, ***P<0.001.
Discussion
In the present study, the major findings showed that melatonin could exert protective effects on POI induced by TG by activating SIRT1 signaling, thus reducing oxidative stress and apoptotic damage. Simultaneously, it was found that melatonin’s protective effects on the ovaries might be, at least in part, mediated by melatonin receptor. As far as we know, this is the first study depicting the vital role of SIRT1 signaling pathway and its involvement in melatonin receptor signaling pathway in melatonin’s protective effects on POI.
Melatonin has been shown to be highly effective in reducing oxidative damage in all parts of every cell, whose efficacy derives from its ability to directly scavenge a majority of free radicals and to function as an indirect antioxidant [15]. Previous studies have confirmed that melatonin as a free radical scavenger in the ovarian follicles could contribute to oocyte maturation, embryo development and luteinization of granulosa cells [11,12]. It was also found that melatonin exerted protective effect on premature ovarian insufficiency induced by tripterygium glycosides in our previous study [9]. In the current report, we also observed that melatonin conferred a protective effect on POI by improving estrous phase, ovarian and uterus mass and index, increasing ovarian follicles and corpus luteum, decreasing atresia follicles, and ameliorating ovarian endocrine and reverse functions with decreased FSH level and increased AMH level, which was similar to previous reports [6,7,9,32].
SIRT1, a member of the class III group of histone deacetylases, participants in a wide variety of cellular functions like oxidative stress, apoptosis, aging, DNA repair and cell cycle regulation, whose activity is dependent on the ratio of NAD+ to NADH in the cell [13,14]. It not only deacetylates histones, but also has a wide range of non-histone substrates, such as the forkhead box class O (FoxO) family, p53 and nuclear factor κB (NF-κB), etc. FoxO3a is known as an important substrate of SIRT1, and Nrf2 are the downstream transcription factor of SIRT1. Published papers reported that SIRT1 regulation of antioxidant genes was dependent on the formation of a FoxO3a/PGC-1α complex [33], and melatonin prevented cisplatin-induced primordial follicle loss via suppression of PTEN/AKT/FOXO3a pathway activation in the mouse ovary [34]. Hsu and colleagues confirmed that SIRT1 protects against myocardial ischemia-reperfusion injury by upregulating antioxidants and downregulating pro-apoptotic molecules through the activation of FoxO1, as well as decreasing oxidative stress [35]. Nrf2 played a key role in the antioxidant response by regulating the transcription of a battery of detoxification and antioxidant enzymes to defend against oxidative stress [36]. Some reports also demonstrated that Nrf2 translocates into the nucleus and its signaling is provoked, which further activates downstream cytoprotective genes and upregulates Ho-1 [37].
Activation of SIRT1 by melatonin was reported in many conditions. Previous studies demonstrated that melatonin treatment attenuated myocardial ischemia-reperfusion injury through activation of SIRT1 signaling [13]. Recently, it was reported that melatonin also activated SIRT1 exhibiting possible benefit effects on aging and related diseases [38]. In addition, melatonin can act as a SIRT1 inducer in neuronal primary cultures from neonatal rat cerebellum to exert a neuroprotective effect [39]. For human peripheral blood mononuclear cells, melatonin could act as an antioxidant in hydrogen peroxide-induced oxidative stress [40], and for mesenchymal stem cells, melatonin reversed H2O2-induced premature senescene [41]. However, contrasting findings were reported in cancer and inflammatory responses induced by oxidative stress. Previous studies showed that melatonin exerted antitumor effect in human osteosarcoma cells, promoted hepatic macrosteatosis partially converted to microsteatosis and reduced the proliferative potential of prostate cancer cells by inhibiting SIRT1 expression [19,20,42]. In hydrogen peroxide-stimulated human chondrocytes and rabbit osteoarthritis model, Lim et al. [43] demonstrated that melatonin decreased hydrogen peroxide-induced SIRT1 mRNA and protein expression, thus exerting cytoprotective and anti-inflammatory effects. These previous researches implied that melatonin might exert quite different effects on SIRT1 depending on different pathological conditions and tissues.
In type 2 diabetic rats, reduced SIRT1 exacerbated myocardial ischemia-reperfusion injury, and melatonin ameliorated reperfusion-induced oxidative stress via activation of SIRT1 signaling, thus reducing myocardial ischemia-reperfusion damage and improving cardiac function [14]. Numerous evidences showed the protective effects of SIRT1 by reducing oxidative stress on the physiology and pathology of the ovary, especially for endometriosis, PCOS and POI [11,12,21]. FoxO3a transcript factor was regulated by SIRT1 in many conditions involved in oxidative stress damage [33,34]. Nrf2 was also reported to play a critical role in the antioxidant response against oxidative stress and when Nrf2 translocated into the nucleus, which provoked its signaling and further activated Ho-1 [36,37].
In this study, we investigated the oxidative stress, SIRT1, FoxO3a, Nrf2 and Ho-1 in POI induced by TG treated with or without melatonin. The results showed that melatonin treatment reduced oxidative stress by decreasing MDA level, MDA5 and Gp91phox expression, and increasing TAC, SOD and GSH-Px levels, which were similar to our previous study [9]. Also, decreased SIRT1, FoxO3a, Nrf2 and Ho-1 protein and mRNA expression in POI were attenuated after the supplement of melatonin. However, Ex527 blocked the protective effects of melatonin on oxidative stress by inhibiting the expression of SIRT1 and FoxO3a, which was similar to the melatonin’s protective effect against myocardial ischemia-reperfusion injury by reducing oxidative stress via activation of SIRT1 signaling, while it was abolished by Ex527 [13]. These results suggested that the protection of melatonin involved the activation of SIRT1 signaling, and melatonin-induced SIRT1 signaling activation might promote anti-oxidative stress damage in POI.
It was also demonstrated that melatonin attenuated cerebral ischemia-reperfusion injury in ischemic-stroke mice by reducing mitochondrial dysfunction through the activation of SIRT1 signaling associated with a reduction in the pro-apoptotic factor Bax, and an increase in the anti-apoptotic factor Bcl-2 [15]. Furthermore, the effects of melatonin treatment on the expression of SIRT1 were also reported to be abolished by Ex527 accompanied by increasing Bcl-2 and decreasing Bax and Caspase3 in myocardial ischemia-reperfusion injury [13]. In the current study, we also found that the protein and mRNA levels of Bax and Caspase3 significantly increased and Bcl-2 decreased in POI, which was attenuated by melatonin. As expected, Ex527 abolished melatonin’s anti-apoptotic effect. Taken together, these data suggested that melatonin confers protective effects against MI/R injury via SIRT1 signaling, thus alleviating oxidative stress and reducing apoptosis. However, the reduced apoptosis caused by melatonin is associated with decreased oxidative stress or the activation of SIRT1 signaling pathway remains unclear, which should be interpreted in further research.
So far, melatonin receptor (Mel1a and Mel1b) has been identified as both membrane and nuclear (receptor belonging to the retinoid Z receptor/retinoid-related receptor). Studies have indicated that the direct free radical scavenging actions of melatonin are receptor independent, but the indirect anti-oxidative functions may well be mediated by receptor, either located in the membrane of cells or within the nucleus [13]. In addition, melatonin receptor was found to be located in the ovary. Evidence has been presented for involvement of the melatonin receptor in cardioprotection: the receptor blockers luzindole and n-acetyl-tryptamine abolished the actions of melatonin [13]. In this study, in order to investigate the SIRT1 activation of melatonin, we introduced the melatonin receptor blocker, luzindole. It was found that blockade of melatonin receptor by luzindole significantly downregulated SIRT1 signaling expression, thus aggravating oxidative stress and apoptosis in POI. This was also supported by previous studies reporting that melatonin reduced oxidative stress and apoptotic damage in a melatonin receptor-dependent manner. These findings suggested that melatonin protected POI via activation of SIRT1 signaling pathway in a melatonin receptor-dependent manner.
However, some issues in this study have not been resolved. Whether oxidative stress or downregulated SIRT1 signaling are the main mechanisms resulting in increased apoptosis or not, which couldn’t be answered here. Moreover, whether apoptosis also plays a significant role in oxidative stress, which also needs further research. Furthermore, it is known that Mel1a and Mel1b receptor conduct different signaling pathway in different tissues. Therefore, additional studies are needed to elucidate the specific role of them on SIRT1 signaling. Last but not the least, the melatonin receptor blocker remarkably weakened its effect on SIRT1 signaling. However, whether the alterations of SIRT1 signaling could affect the melatonin receptor expression, which should be interpreted in further studies.
In summary, our findings suggest that melatonin could exert an obviously protective effect on premature ovarian insufficiency induced by tripterygium glycosides by reducing oxidative stress and apoptotic damage, which mainly contributes to the activation of SIRT1 signaling pathway in a melatonin receptor-dependent manner. These data indicated that melatonin might be a promising candidate for the treatment of premature ovarian insufficiency.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no: 81471423) and the Shanghai Municipal Committee of Science and Technology Project (grant no: 14ZR1404200).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67:604–606. [PubMed] [Google Scholar]
- 2.Nelson LM, Covington SN, Rebar RW. An update: spontaneous premature ovarian failure is not an early menopause. Fertil Steril. 2005;83:1327–1332. doi: 10.1016/j.fertnstert.2004.11.059. [DOI] [PubMed] [Google Scholar]
- 3.Gannon AM, Stampfli MR, Foster WG. Cigarette smoke exposure leads to follicle loss via an alternative ovarian cell death pathway in a mouse model. Toxicol Sci. 2012;125:274–284. doi: 10.1093/toxsci/kfr279. [DOI] [PubMed] [Google Scholar]
- 4.Ebrahimi M, Akbari Asbagh F. Pathogenesis and causes of premature ovarian failure: an update. Int J Fertil Steril. 2011;5:54–65. [PMC free article] [PubMed] [Google Scholar]
- 5.De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. Lancet. 2010;376:911–921. doi: 10.1016/S0140-6736(10)60355-8. [DOI] [PubMed] [Google Scholar]
- 6.Chen XY, Gu C, Ma M, Cong Q, Guo T, Ma D, Li B. A mouse model of premature ovarian insufficiency induced by tripterygium glycoside via subcutaneous injection. Int J Clin Exp Pathol. 2014;7:144–151. [PMC free article] [PubMed] [Google Scholar]
- 7.Chen XY, Chen WL, Ma M, Gu C, Xiao XR, Li B. The potential of follicle-stimulating hormone peptide-modified triptolide-loaded nanoparticles to induce a mouse model of premature ovarian insufficiency. Int J Nanomedicine. 2015;10:2765–2774. doi: 10.2147/IJN.S72593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma M, Chen XY, Gu C, Xiao XR, Guo T, Li B. Biochemical changes of oxidative stress in premature ovarian insufficiency induced by tripterygium glycosides. Int J Clin Exp Pathol. 2014;7:8855–8861. [PMC free article] [PubMed] [Google Scholar]
- 9.Ma M, Li RX, Chen XY, Li B. Premature ovarian insufficiency induced by tripterygium glycoside: does melatonin offer protection? Int J Clin Exp Med. 2016;9:10727–10736. [Google Scholar]
- 10.Tamura H, Nakamura Y, Korkmaz A, Manchester LC, Tan DX, Sugino N, Reiter RJ. Melatonin and the ovary: physiological and pathophysiological implications. Fertil Steril. 2009;92:328–43. doi: 10.1016/j.fertnstert.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L, Tamura I, Maekawa R, Asada H, Yamagata Y, Sugino N. Melatonin as a free radical scavenger in the ovarian follicle. Endocr J. 2013;60:1–13. doi: 10.1507/endocrj.ej12-0263. [DOI] [PubMed] [Google Scholar]
- 12.Reiter RJ, Rosales-Corral SA, Manchester LC, Tan DX. Peripheral reproductive organ health and melatonin: ready for prime time. Int J Mol Sci. 2013;14:7231–7272. doi: 10.3390/ijms14047231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu LM, Sun Y, Cheng L, Jin ZX, Yang Y, Zhai MG, Pei HF, Wang XW, Zhang HF, Meng Q, Zhang Y, Yu SQ, Duan WX. Melatonin receptor-mediated protection against myocardial ischemia/reperfusion injury: role of SIRT1. J Pineal Res. 2014;57:228–238. doi: 10.1111/jpi.12161. [DOI] [PubMed] [Google Scholar]
- 14.Yu L, Liang HL, Dong XC, Zhao GL, Jin ZX, Zhai MG, Yang Y, Chen WS, Liu JC, Yi W, Yang J, Yi DH, Duan WX, Yu SQ. Reduced silent information regulator 1 signaling exacerbates myocardial ischemia-reperfusion injury in type 2 diabetic rats and the protective effect of melatonin. J Pineal Res. 2015;59:376–390. doi: 10.1111/jpi.12269. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Jiang S, Dong YS, Fan CX, Zhao L, Yang XM, Li J, Di SY, Yue L, Liang GB, Reiter RJ, Qu Y. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. J Pineal Res. 2015;58:61–70. doi: 10.1111/jpi.12193. [DOI] [PubMed] [Google Scholar]
- 16.Emamgholipour S, Hossein-Nezhad A, Sahraian MA, Askarisadr F, Ansari M. Evidence for possible role of melatonin in reducing oxidative stress in multiple sclerosis through its effect on SIRT1 and antioxidant enzymes. Life Sci. 2016;145:34–41. doi: 10.1016/j.lfs.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Zhao L, Liu H, Yue L, Zhang J, Li X, Wang B, Lin Y, Qu Y. Melatonin attenuates early brain injury via the melatonin receptor/Sirt1/NF-κB signaling pathway following subarachnoid hemorrhage in mice. Mol Neurobiol. 2017;54:1612–1621. doi: 10.1007/s12035-016-9776-7. [DOI] [PubMed] [Google Scholar]
- 18.Bai XZ, He T, Gao JX, Liu Y, Liu JQ, Han SC, Li Y, Shi JH, Han JT, Tao K, Xie ST, Wang HT, Hu DH. Melatonin prevents acute kidney injury in severely burned rats via the activation of SIRT1. Sci Rep. 2016;6:32199. doi: 10.1038/srep32199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng YD, Cai LP, Jiang P, Wang JB, Gao C, Feng HB, Wang CC, Pan HY, Yang Y. SIRT1 inhibition by melatonin exerts antitumor activity in human osteosarcoma cells. Eur J Pharmacol. 2013;715:219–229. doi: 10.1016/j.ejphar.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Stacchiotti A, Favero G, Lavazza A, Golic I, Aleksic M, Korac A, Rodella LF, Rezzani R. Hepatic macrosteatosis is partially converted to microsteatosis by melatonin supplementation in ob/ob mice nonalcoholic fatty liver disease. PLoS One. 2016;11:e0148115. doi: 10.1371/journal.pone.0148115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tatone C, Di Emidio G, Vitti M, Di Carlo M, Santini S Jr, D’Alessandro AM, Falone S, Amicarelli F. Sirtuin functions in female fertility: possible role in oxidative stress and aging. Oxid Med Cell Longev. 2015;2015:659687. doi: 10.1155/2015/659687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao F, Zhao W, Ren S, Fu Y, Fang X, Wang X, Li B. Roles of SIRT1 in granulosa cell apoptosis during the process of follicular atresia in porcine ovary. Anim Reprod Sci. 2014;151:34–41. doi: 10.1016/j.anireprosci.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Fang L, Lu Z, Xiong J, Wu M, Shi L, Luo A, Wang S. Are sirtuins markers of ovarian aging? Gene. 2016;575:680–686. doi: 10.1016/j.gene.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 24.Di Emidio G, Falone S, Vitti M, D’Alessandro AM, Vento M, Di Pietro C, Amicarelli F, Tatone C. SIRT1 signalling protects mouse oocytes against oxidative stress and is deregulated during aging. Hum Reprod. 2014;29:2006–2017. doi: 10.1093/humrep/deu160. [DOI] [PubMed] [Google Scholar]
- 25.Wang N, Luo LL, Xu JJ, Xu MY, Zhang XM, Zhou XL, Liu WJ, Fu YC. Obesity accelerates ovarian follicle development and follicle loss in rats. Metabolism. 2014;63:94–103. doi: 10.1016/j.metabol.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Liu WJ, Zhang XM, Wang N, Zhou XL, Fu YC, Luo LL. Calorie restriction inhibits ovarian follicle development and follicle loss through activating SIRT1 signaling in mice. Eur J Med Res. 2015;20:22. doi: 10.1186/s40001-015-0114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou XL, Xu JJ, Ni YH, Chen XC, Zhang HX, Zhang XM, Liu WJ, Luo LL, Fu YC. SIRT1 activator (SRT1720) improves the follicle reserve and prolongs the ovarian lifespan of diet-induced obesity in female mice via activating SIRT1 and suppressing mTOR signaling. J Ovarian Res. 2014;7:97. doi: 10.1186/s13048-014-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiyak Caglayan E, Engin-Ustun Y, Gocmen AY, Polat MF, Aktulay A. Serum sirtuin 1 levels in patients with polycystic ovary syndrome. J Obstet Gynaecol. 2015;35:608–611. doi: 10.3109/01443615.2014.990428. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Liu J, Zhu K, Hong Y, Sun Y, Zhao X, Du Y, Chen ZJ. Effects of BMAL1-SIRT1-positive cycle on estrogen synthesis in human ovarian granulosa cells: an implicative role of BMAL1 in PCOS. Endocrine. 2016;53:574–584. doi: 10.1007/s12020-016-0961-2. [DOI] [PubMed] [Google Scholar]
- 30.Tao X, Zhang X, Ge SQ, Zhang EH, Zhang B. Expression of SIRT1 in the ovaries of rats with polycystic ovary syndrome before and after therapeutic intervention with exenatide. Int J Clin Exp Pathol. 2015;8:8276–8283. [PMC free article] [PubMed] [Google Scholar]
- 31.Said RS, El-Demerdash E, Nada AS, Kamal MM. Resveratrol inhibits inflammatory signaling implicated in ionizing radiation-induced premature ovarian failure through antagonistic crosstalk between silencing information regulator 1 (SIRT1) and poly (ADP-ribose) polymerase 1 (PARP-1) Biochem Pharmacol. 2016;103:140–150. doi: 10.1016/j.bcp.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 32.Chen XY, Xia HX, Guan HY, Li B, Zhang W. Follicle loss and apoptosis in cyclophosphamide-treated mice: what’s the matter? Int J Mol Sci. 2016;17 doi: 10.3390/ijms17060836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olmos Y, Sánchez-Gómez FJ, Wild B, García-Quintans N, Cabezudo S, Lamas S, Monsalve M. SirT1 Regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1a complex. Antioxid Redox Signal. 2013;19:1507–1521. doi: 10.1089/ars.2012.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jang H, Lee OH, Lee Y, Yoon H, Chang EM, Park M, Lee JW, Hong K, Kim JO, Kim NK, Ko JJ, Lee DR, Yoon TK, Lee WS, Choi Y. Melatonin preventscisplatin-induced primordial follicle loss via suppression of PTEN/AKT/FOXO3a pathway activation in the mouse ovary. J Pineal Res. 2016;60:336–347. doi: 10.1111/jpi.12316. [DOI] [PubMed] [Google Scholar]
- 35.Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170–2182. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Shen L, Luo H. Luteolin ameliorates dextran sulfate sodium-induced colitis in mice possibly through activation of the Nrf2 signaling pathway. Int Immunopharmacol. 2016;40:24–31. doi: 10.1016/j.intimp.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 37.Zhong JQ, Li L. Skin-derived precursors against UVB-induced apoptosis via Bcl-2 and Nrf2 upregulation. Biomed Res Int. 2016;2016:6894743. doi: 10.1155/2016/6894743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramis MR, Esteban S, Miralles A, Tan DX, Reiter RJ. Caloric restriction, resveratrol and melatonin: Role of SIRT1 and implications for aging and related-diseases. Mech Ageing Dev. 2015;146:28–41. doi: 10.1016/j.mad.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Tajes M, Gutierrez-Cuesta J, Ortuño-Sahagun D, Camins A, Pallàs M. Anti-aging properties of melatonin in an in vitro murine senescence model: involvement of the sirtuin 1 pathway. J Pineal Res. 2009;47:228–237. doi: 10.1111/j.1600-079X.2009.00706.x. [DOI] [PubMed] [Google Scholar]
- 40.Emamgholipour S, Hossein-Nezhad A, Ansari M. Can melatonin act as an antioxidant in hydrogen peroxide-induced oxidative stress model in human peripheral blood mononuclear cells? Biochem Res Int. 2016;2016:5857940. doi: 10.1155/2016/5857940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou L, Chen X, Liu T, Gong Y, Chen S, Pan G, Cui W, Luo ZP, Pei M, Yang H, He F. Melatonin reverses H2O2-induced premature senescence in mesenchymal stem cells via the SIRT1-dependent pathway. J Pineal Res. 2015;59:190–205. doi: 10.1111/jpi.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung-Hynes B, Schmit TL, Reagan-Shaw SR, Siddiqui IA, Mukhtar H, Ahmad N. Melatonin, a novel Sirt1 inhibitor, imparts antiproliferative effects against prostate cancer in vitro in culture and in vivo in TRAMP model. J Pineal Res. 2011;50:140–149. doi: 10.1111/j.1600-079X.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim HD, Kim YS, Ko SH, Yoon IJ, Cho SG, Chun YH, Choi BJ, Kim EC. Cytoprotective and antiinflammatory effects of melatonin in hydrogen peroxide-stimulated CHON-001 human chondrocyte cell line and rabbit model of osteoarthritis via the SIRT1 pathway. J Pineal Res. 2012;53:225–237. doi: 10.1111/j.1600-079X.2012.00991.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.