Abstract
Epithelial-mesenchymal transition (EMT) plays crucial role in tumor metastasis and patient prognosis. Previous studies indicated that paired-related homeobox 1 (PRRX1), a newly identified EMT inducer, plays opposing roles in different tumor cell types. However, the function of PRRX1 in lung cancer has not been elucidated. In the present study, we observed that the expression level of PRRX1 was high in A549 cells, and a loss of PRRX1 induced EMT by regulating the EMT markers N-cadherin, E-cadherin and vimentin in A549 cells. This loss also distinctly affected cell morphology, migration and invasion. Moreover, a loss of PRRX1 increased the expression levels of cancer stem cell (CSC) markers and promoted the proliferation of these cells. These findings demonstrated that PRRX1 inhibits EMT and induces CSC-like properties in A549 cells.
Keywords: PRRX1, lung cancer, epithelial-mesenchymal transition, non-small-cell cancer stem cells, metastasis
Introduction
Metastasis is the key feature of tumor cells. In this process, tumor cells break away from extracellular matrix (ECM) and permeate into the systemic circulation. They then disseminate to distant sites and form secondary tumors. Metastasis is associated with 90% of cancer deaths [1]. Therefore, the mechanisms of metastasis need to be further studied to develop strategies to prevent or interrupt the process. Existing studies indicated that the epithelial-mesenchymal transition (EMT) plays a critical role during cancer invasion and metastasis [2]. During EMT, epithelial tumor cell lose their expression of E-cadherin, an important epithelial cell-cell adhesion molecule, and begin to exhibit motility and invasiveness [3,4]. EMT inducers, such as TWIST1 [5], ANAI1 [6], ZEB1 and ZEB2 [7], have been proven to be associated with metastasis and invasion in cancer cells.
Paired-related homeobox 1 (PRRX1), a newly discovered EMT inducer [8], has been shown to correlate with metastasis and prognosis in diverse cancer types, including gastric cancer [9], colorectal cancer [10], and hepatocellular carcinoma [11]. PRRX1 participates in cancer progression in two different manners. Specifically, PRRX1 overexpression induces EMT and promotes metastasis and invasion in tumor cells, and it is an indicator of poor prognosis in gastric cancer [9] and colorectal cancer [10]. Moreover, tumor cells acquire cancer stem cell (CSC)-like properties due to the loss of PRRX1 expression, which results in distant metastasis and indicates a poor prognosis in breast cancer [8] and hepatocellular carcinoma [11]. These contradictory results imply that PRRX1 plays a key role in tumor progression. Although lung cancer has become the principal cause of cancer-related death worldwide [12], and most patients are unfortunately diagnosed in the advanced stages, the function of PRRX1 in lung cancer has not yet been elucidated. Thus, this study aimed analyze the role of PRRX1 in lung cancer cells.
Materials and methods
Cell culture
The human lung cancer cell line A549 was obtained from the Elemental Laboratory of Anhui Medical University. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Invitrogen) supplemented with 10% fetal bovine serum (FBS; Gibco, Invitrogen) in a humidified atmosphere containing 5% CO2 at 37°C.
shRNA transfection
The shRNA sequence specific for PRRX1 was 5’-CCGGCCTGGGAATTTGGCGACGTAACTCGAGTTACGTCGCCAAATTCCCAGGTTTTTG-3’. The sequence of the control shRNA was 5’-GATTCACTCACACCTTCCTTCCAAGGATTGTTTTGGCCACTGACTGACAATCCTTGGAAGGAAGGTGTGAGTG-3’. Cells were transfected with 1.5 μg of shRNA using Lipofectamine 2000 (Invitrogen, Carlsbad, USA) following the manufacturer’s instructions.
Western blot analysis
Total cellular protein was collected and processed as previously described [9]. The primary antibodies were prepared as follows: PRRX1 (dilution 1:1000; OriGene, Rockville, USA), Ecadherin (dilution 1:1000), N-cadherin (dilution 1:1000), vimentin (dilution 1:1000) and β-actin (dilution 1:2000) (Abcam, Cambridge, USA). β-actin was used as an internal control.
MTT colorimetric assay
The colorimetric MTT assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Roche Applied Science, Basel, Switzerland) was used to evaluate cell proliferation. Cells were seeded in 96-well plates at a concentration of 2×103 cells per well and then exposed to the compound for 72 hours (h). The viable cell content was then measured with a MTT assay according to the manufacturer’s instructions. Spectrophotometric absorbance at 490 nm was determined with a microplate reader (Bio-Rad, Hercules, CA, USA). Each sample tested in triplicate.
Soft agar assays
For this assay, 3×103 cells were suspended in 0.35% agarose (DMEM mixed with 0.7% agarose) and cultivated on a 0.6% agarose (DMEM mixed with 1.2% agarose) bed. Colonies were counted after 21 days, and the number of colonies was assessed in 10 randomly selected microscopic fields. The assay was repeated at least three times.
Immunofluorescent analysis
An immunofluorescence analysis was performed to detect E-cadherin and vimentin. In brief, cells were fixed with 4% paraformaldehyde for 30 min. The cells were washed three times with phosphate-buffered saline (PBS) and then incubated with anti-E-cadherin (dilution 1:500; Abcam, Cambridge, USA) and anti-vimentin (dilution 1:250; clone Abcam, Cambridge, USA) antibodies overnight at 4°C. Alexa Fluor 549-conjugated secondary antibody was added for 30 minutes (min). The cells were then counterstained with 4’,6-diamidino-2-phenylindole (DAPI), and images were obtained with a fluorescence microscope.
Wound-healing assay
Cells were seeded in a 24-well plate at an initial density of 3×105 cells/cm2. A uniform monolayer formed within 48 h. All assays were performed in serum-free medium. Briefly, a 10-μl sterile micropipette tip was used to create a wound in the monolayer by scraping, and wound closure was assessed using phase-contrast microscopy (NIKON, Japan) at several time points starting at 0 h until 48 h.
Cell migration and invasion assays
Cell migration and invasion assays were performed using a 12-well Boyden Chamber (Neuro Probe, Gaithersburg, MD, USA) or BD BioCoat Matrigel Invasion Chamber (BD, Franklin Lakes, USA) with 6-μm pore size. Briefly, 4×105 cells were seeded into the upper chamber. After 24 h of incubation, the cells migrating through the pores or invading through the Matrigel were fixed and stained with 0.5% crystal violet. The cells on the lower surface of the chamber were counted in 6 randomly selected fields. The test was repeated three times.
Flow cytometric analysis
Cultured cells were treated with the appropriate amount of FITC-conjugated isotype-specific antibody and analyzed by flow cytometry. The cells were stained with specific lung cancer CSC surface markers: anti-CD133, anti-CD44 and anti-ALDH1 (Abcam, Cambridge, USA). Ten thousand labeled cells were harvested and analyzed using a Cell Sorter SH800 (Sony), and the resultant data were analyzed using the SH800 software (Sony).
Statistical analysis
Differences in continuous variables were assessed using Student’s t test, and data are expressed as the mean values ± standard deviations. Categorical variables were compared using the chi-squared test. All analyses were conducted using SPSS (SPSS Inc., 2003, Chicago, USA) and GraphPad Prism software version 6.0 (GraphPad Software, La Jolla, CA). P values <0.05 were considered indicative of a significant difference.
Results
Silencing PRRX1 induces a mesenchymal phenotype in A549 cells
The expression level of PRRX1 in the lung cancer cell line A549 was estimated using Western blotting. Various concentrations of antibody were used, which all showed that PRRX1 expression was high in A549 cells (data not shown). Therefore, we established a knockdown experiment. A specific shRNA against PRRX1 or control shRNA was transfected into A549 cells using a lentiviral vector, resulting in the A549-PRRX1 and A549-mock cell lines, respectively. The expression level of PRRX1 was significantly lower in A549-PRRX1 cells than that in A549-mock cells (data not shown). A morphological assessment by phase-contrast microscopy after transfection showed that A549-PRRX1 cells exhibited a more spindle-like shape and a less sheet-like architecture than A549-mock cells. These results indicate that A549 cells acquired a mesenchymal phenotype after PRRX1 knockdown (Figure 1).
Figure 1.
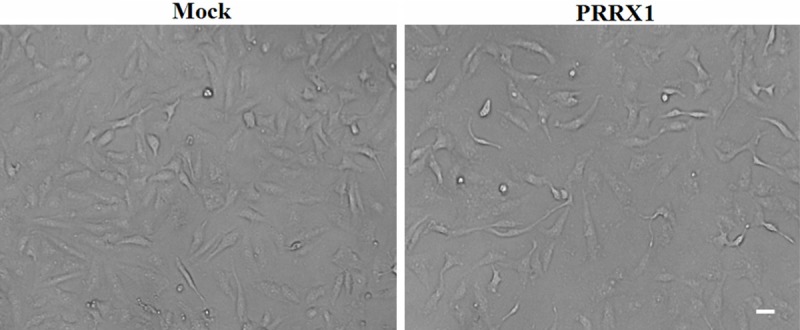
Phase-contrast images of A549 cells. A549 cells acquired a mesenchymal phenotype after the loss of PRRX1. Scale bars: 100 mm.
Silencing PRRX1 promotes EMT in A549 cells
The above results indicated that the loss of PRRX1 was invariably associated with EMT because A549-PRRX1 cells acquired a mesenchymal phenotype. To assess the ability of PRRX1 to induce EMT in A549 cells, the expression levels of EMT markers were quantified by Western blotting 48 h after transfection. The results showed that E-cadherin protein expression was significantly lower and N-cadherin and vimentin protein expression was significantly higher in A549-PRRX1 cells than that in mock cells (Figure 2). These data reveal that PRRX1 attenuated EMT in A549 cells and contradict those reported in previous studies [8-11]. An immunofluorescence analysis was performed to further support for these changes (Figure 3), and similar results were observed.
Figure 2.
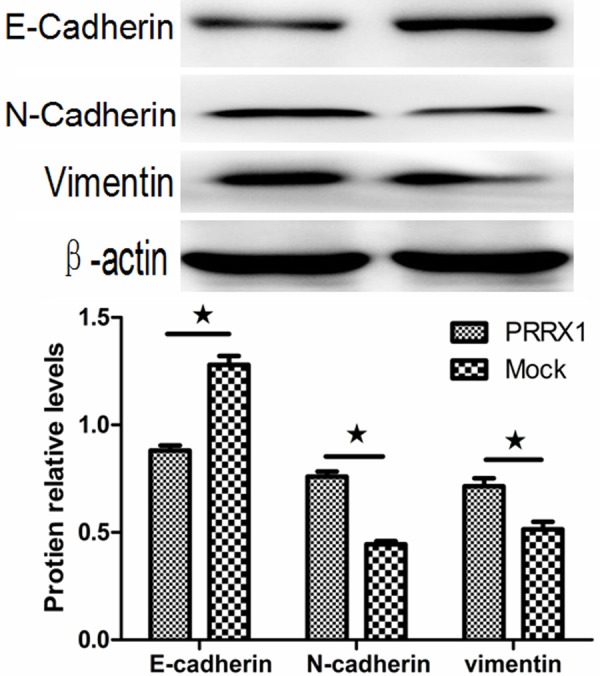
Silencing PRRX1 induced EMT in A549 cells. Western blots showing that E-cadherin protein expression was significantly lower and N-cadherin and vimentin protein expression were significantly higher in A549-PRRX1 cells than in mock cells (★P<0.05 vs. Mock groups).
Figure 3.
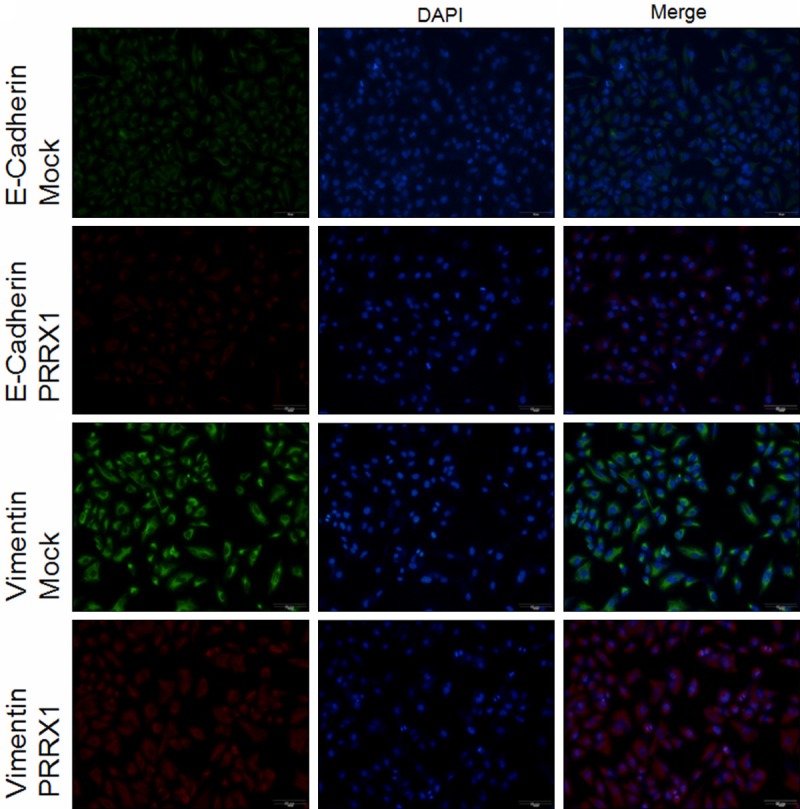
Immunofluorescence analysis of PRRX1 silencing in A549 cells. Targeted proteins are stained green (Mock) and red (PRRX1). Scale bars: 50 mm. Immunofluorescence analysis showing that E-cadherin protein expression was significantly lower and vimentin protein expression was significantly higher in A549-PRRX1 cells than in mock cells.
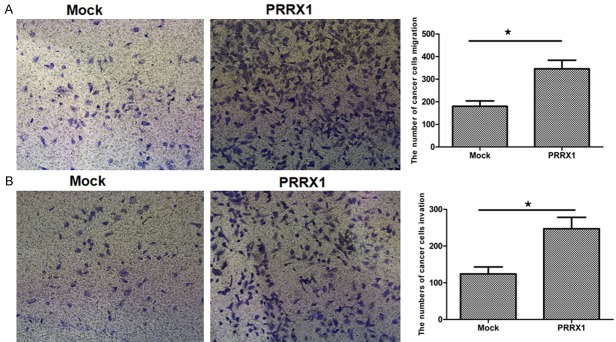
Silencing of PRRX1 modulated invasion and migration in A549 cells
Epithelial cells lose adherent junctions and then acquire the ability to migrate and invade during EMT [13]. To investigate the effect of silencing PRRX1 in A549 cells on migration and invasion, the following experiments were. First, cell migration was assessed in vitro 24 h after transfection. As shown in Figure 4A, the number of A549-PRRX1 cells migrating through the Boyden chamber pores significantly increased compared with the mock cells (P<0.05). Verifying these data, similar results were demonstrated in a wound-healing assay (Figure 5). We further tested the ability of cells to invade using a Boyden chamber assay, the results of which are shown in Figure 4B. The number of A549-PRRX1 cells that permeated through the Matrigel significantly was significantly higher than the number of permeating mock cells (P<0.05). These results suggest that knocking down PRRX1 expression endowed A549 cells with the ability to migrate and invade.
Figure 4.
Silencing PRRX1 promoted migration and invasion in A549 cells. A. Cellular migration was analyzed using a migration assay after silencing PRRX1. B. The invasive properties of A549 cells were examined with a Boyden chamber assay. The number of A549-PRRX1 cells migrating through the Boyden chamber pores was significantly increased compared with the mock cells (★P<0.05 vs. Mock groups).
Figure 5.
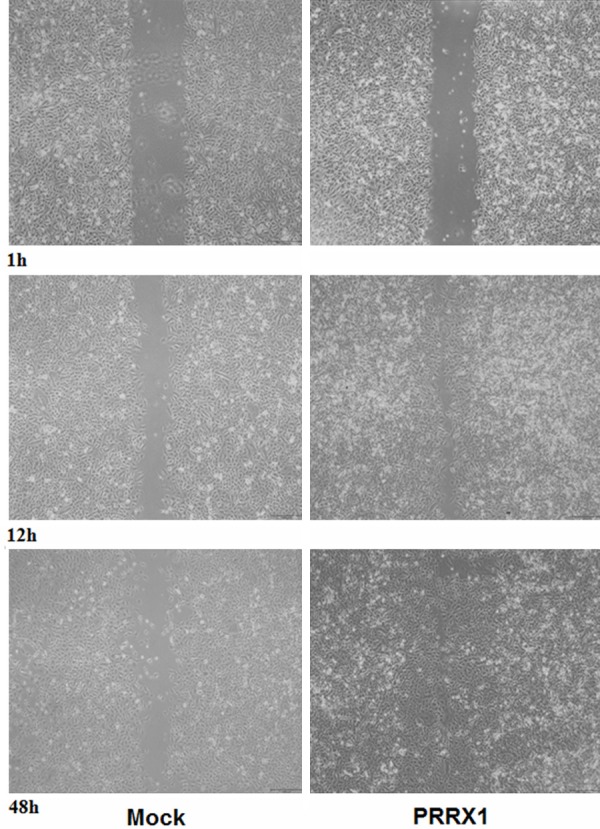
Wound-healing assay indicating healing rates in various groups of A549 cells. Scale bars: 100 mm. The healing rates significantly increased in A549-PRRX1 cells compared with mock cells.
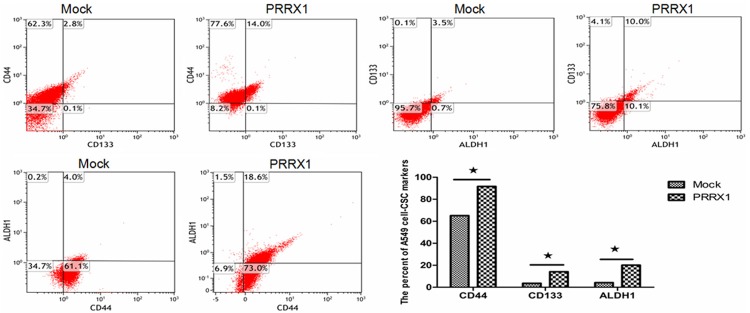
Silencing PRRX1 promotes CSC-like properties and anchorage-independent growth in A549 cells
Breast tumor cells acquire CSC-like properties via the loss of PRRX1 expression [8]. To determine whether similar effects occur in A549 cells, the expression levels of CD133, CD44 and ALDH1 were analyzed by flow cytometry. These markers have all been reported to act as CSC markers in A549 cells [14-16]. A flow cytometric analysis revealed that the populations of CD133+, CD44+ and ALDH1+ cells were larger in A549-PRRX1 cells than in mock cells (Figure 6). The CD133+/ALDH1+, CD133+/CD44+ and CD44+/ALDH1+ ratios were also increased in A549-PRRX1 cells. An MTT colorimetric assay was carried out to investigate the effect of PRRX1 on the CSC-like properties of A549 cells. As shown in Figure 7, proliferation was increased in A549-PRRX1 cells compared with mock cells. Furthermore, we performed soft agar assays to demonstrate the capacity for anchorage-independent growth, which revealed that this capacity was higher in A549-PRRX1 cells than in mock cells (Figure 8). Therefore, these findings imply that a loss of PRRX1 induced a stemness phenotype in A549 cells.
Figure 6.
A549 CSC markers (CD133, CD44, and ALDH1) were analyzed by flow cytometry. Flow cytometry results showed that the expression levels of cancer-stem cell markers were significantly increased when PRRX1 was silenced. (★P<0.05 vs. Mock groups).
Figure 7.
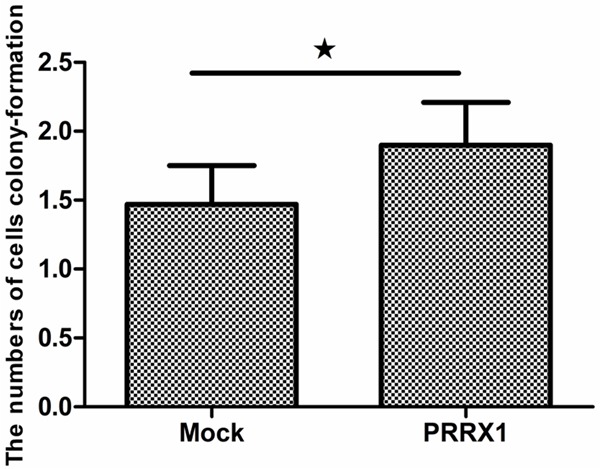
PRRX1 affected A549 cell proliferation based on an MTT assay. Proliferation was significantly increased in A549-PRRX1 cells compared with mock cells (★P<0.05 vs. Mock groups).
Figure 8.
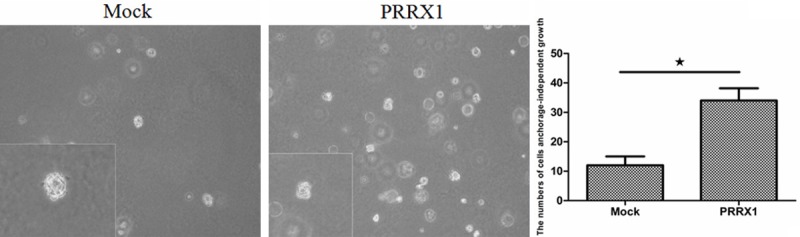
The soft agar assays indicated that A549 exhibited anchorage-independent growth after the loss of PRRX1. Scale bars: 100 mm. A549-PRRX1 cells exhibited higher anchorage-independent growth than mock cells (★P<0.05 vs. Mock groups).
Discussion
In this study, we found that knocking down PRRX1 induced EMT in A549 cells, which implies that PRRX1 is an inhibitor, not inducer, of lung adenocarcinoma. However, PRRX1 was recently reported to induce EMT in other tumor cells [8-11]. This discrepancy may be due to the following. First, the vast majority of previous studies were performed by over-expressing PRRX1, and PRRX1 played opposing roles in different tumor cell types. Moreover, changes in EMT markers, including changes in E-cadherin and vimentin protein expression, were not observed in a PRRX1-silencing experiment in glioblastoma cells [17]. These results indicated that the function of PRRX1 depends on the tumor cell type. Moreover, PRRX1 expression in the human lung is stable from embryonic development to adulthood, unlike in other human tissues [18]. Specifically, PRRX1 plays a critical role in pulmonary vascular development [19], and PRRX1-/- mice are cyanotic and die soon after birth from respiratory distress [20]. These studies indicate that appropriate PRRX1 expression is necessary and valuable for pulmonary development. Therefore, we suspected that PRRX1 plays a different role in lung tissue than in other human tissues. Although the results presented herein suggest a mechanism for PRRX1 in lung cancer, this mechanism warrants further study.
In this study, we demonstrated that A549 cells acquired CSC-like properties and the capacity for anchorage-independent growth after PRRX1 was silenced. These results agreed with a recent report that showed that cancer cells develop stemness properties during the EMT process [21]. However, the mechanism by which PRRX1 regulates the stemness properties of cancer cells remains unclear [22]. One study showed that the knockdown PRRX1 increased reprogramming efficiency [23], whereas other data suggest that stemness properties are closely related to EMT [24]. Notably, EMT and CSC-like properties are significantly associated with a poor prognosis in various cancer types. Our results indicate that knocking down PRRX1 induced EMT and CSC in A549 cell. Moreover, low PRRX1 expression has been associated with tumor progression, metastasis and poor prognosis in squamous cell and adenocarcinomas of the lung based on an analysis of clinicopathological variables [25,26].
In summary, we demonstrated that silencing PRRX1 induced EMT and stemness properties in A549 cells while promoting migration, invasion and cloning. These results indicated that PRRX1 inhibits EMT A549 cells, which contradicts previous studies. We speculate that PRRX1 likely acts as a bidirectional regulation factor in diverse cancer types. However, further study is required to clarify the mechanism.
Disclosure of conflict of interest
None.
References
- 1.Aparicio LA, Blanco M, Castosa R, Concha A, Valladares M, Calvo L, Figueroa A. Clinical implications of epithelial cell plasticity in cancer progression. Cancer Lett. 2015;366:1–10. doi: 10.1016/j.canlet.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Nieto MA, Cano A. The epithelial-mesenchymal transition under control: global programs to regulate epithelial plasticity. Semin Cancer Biol. 2012;22:361–368. doi: 10.1016/j.semcancer.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang LH. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007;67:1979–1987. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- 4.Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7:1267–1278. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- 5.Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Pena C, Garcia JM, Larriba MJ, Barderas R, Gomez I, Herrera M, Garcia V, Silva J, Dominguez G, Rodriguez R, Cuevas J, de Herreros AG, Casal JI, Munoz A, Bonilla F. SNAI1 expression in colon cancer related with CDH1 and VDR downregulation in normal adjacent tissue. Oncogene. 2009;28:4375–4385. doi: 10.1038/onc.2009.285. [DOI] [PubMed] [Google Scholar]
- 7.Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2007;26:711–724. doi: 10.1038/sj.onc.1209808. [DOI] [PubMed] [Google Scholar]
- 8.Ocana OH, Corcoles R, Fabra A, Moreno-Bueno G, Acloque H, Vega S, Barrallo-Gimeno A, Cano A, Nieto MA. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell. 2012;22:709–724. doi: 10.1016/j.ccr.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Guo J, Fu Z, Wei J, Lu W, Feng J, Zhang S. PRRX1 promotes epithelial-mesenchymal transition through the Wnt/beta-catenin pathway in gastric cancer. Med Oncol. 2015;32:393. doi: 10.1007/s12032-014-0393-x. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi Y, Sawada G, Kurashige J, Uchi R, Matsumura T, Ueo H, Takano Y, Akiyoshi S, Eguchi H, Sudo T, Sugimachi K, Doki Y, Mori M, Mimori K. Paired related homoeobox 1, a new EMT inducer, is involved in metastasis and poor prognosis in colorectal cancer. Br J Cancer. 2013;109:307–311. doi: 10.1038/bjc.2013.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirata H, Sugimachi K, Takahashi Y, Ueda M, Sakimura S, Uchi R, Kurashige J, Takano Y, Nanbara S, Komatsu H, Saito T, Shinden Y, Iguchi T, Eguchi H, Atsumi K, Sakamoto K, Doi T, Hirakawa M, Honda H, Mimori K. Downregulation of PRRX1 confers cancer stem cell-like properties and predicts poor prognosis in hepatocellular carcinoma. Ann Surg Oncol. 2015;22(Suppl 3):S1402–1409. doi: 10.1245/s10434-014-4242-0. [DOI] [PubMed] [Google Scholar]
- 12.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 13.Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelialmesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22:725–736. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sowa T, Menju T, Sonobe M, Nakanishi T, Shikuma K, Imamura N, Motoyama H, Hijiya K, Aoyama A, Chen F, Sato T, Kobayashi M, Yoshizawa A, Haga H, Sozu T, Date H. Association between epithelial-mesenchymal transition and cancer stemness and their effect on the prognosis of lung adenocarcinoma. Cancer Med. 2015;4:1853–1862. doi: 10.1002/cam4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung EL, Fiscus RR, Tung JW, Tin VP, Cheng LC, Sihoe AD, Fink LM, Ma Y, Wong MP. Non-small cell lung cancer cells expressing CD44 are enriched for stem cell-like properties. PLoS One. 2010;5:e14062. doi: 10.1371/journal.pone.0014062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, Xing L, Wang H, Liu Z, Su Y, Stass SA, Katz RL. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res. 2009;7:330–338. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugiyama M, Hasegawa H, Ito S, Sugiyama K, Maeda M, Aoki K, Wakabayashi T, Hamaguchi M, Natsume A, Senga T. Paired related homeobox 1 is associated with the invasive properties of glioblastoma cells. Oncol Rep. 2015;33:1123–1130. doi: 10.3892/or.2014.3681. [DOI] [PubMed] [Google Scholar]
- 18.Norris RA, Scott KK, Moore CS, Stetten G, Brown CR, Jabs EW, Wulfsberg EA, Yu J, Kern MJ. Human PRRX1 and PRRX2 genes: cloning, expression, genomic localization, and exclusion as disease genes for Nager syndrome. Mamm Genome. 2000;11:1000–1005. doi: 10.1007/s003350010193. [DOI] [PubMed] [Google Scholar]
- 19.Ihida-Stansbury K, McKean DM, Gebb SA, Martin JF, Stevens T, Nemenoff R, Akeson A, Vaughn J, Jones PL. Paired-related homeobox gene Prx1 is required for pulmonary vascular development. Circ Res. 2004;94:1507–1514. doi: 10.1161/01.RES.0000130656.72424.20. [DOI] [PubMed] [Google Scholar]
- 20.Martin JF, Bradley A, Olson EN. The pairedlike homeo box gene MHox is required for early events of skeletogenesis in multiple lineages. Genes Dev. 1995;9:1237–1249. doi: 10.1101/gad.9.10.1237. [DOI] [PubMed] [Google Scholar]
- 21.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science. 2013;342:1234850. doi: 10.1126/science.1234850. [DOI] [PubMed] [Google Scholar]
- 23.Yang CS, Lopez CG, Rana TM. Discovery of nonsteroidal anti-inflammatory drug and anticancer drug enhancing reprogramming and induced pluripotent stem cell generation. Stem Cells. 2011;29:1528–1536. doi: 10.1002/stem.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen JE, Pavey SJ, Passmore LH, Bowman R, Clarke BE, Hayward NK, Fong KM. Expression profiling defines a recurrence signature in lung squamous cell carcinoma. Carcinogenesis. 2007;28:760–766. doi: 10.1093/carcin/bgl207. [DOI] [PubMed] [Google Scholar]
- 26.Raponi M, Zhang Y, Yu J, Chen G, Lee G, Taylor JM, Macdonald J, Thomas D, Moskaluk C, Wang Y, Beer DG. Gene expression signatures for predicting prognosis of squamous cell and adenocarcinomas of the lung. Cancer Res. 2006;66:7466–7472. doi: 10.1158/0008-5472.CAN-06-1191. [DOI] [PubMed] [Google Scholar]