Abstract
Crizotinib, a small molecule inhibitor of anaplastic lymphoma kinase (ALK), c-ros oncogene 1 (ROS1) and c-MET (also called MET or hepatocyte growth factor receptor), has been approved by the Food and Drug Administration for the treatment of patients with advanced non-small cell lung cancer whose tumors have rearrangements in the ALK or ROS1 gene. However, the anticancer effect of crizotinib on ovarian cancer is still unclear. In this study, our data show that crizotinib can actively induce cell growth inhibition, cell cycle arrest at G2/M phase and apoptosis with the decreasing phosphorylation of the downstream signaling effectors AKT and ERK in human ovarian cancer cells. Crizotinib also increases the intracellular reactive oxidative species (ROS) levels, and pretreating with ROS scavenger N-acety-L-cysteine partially reverses crizotinib-induced apoptosis. Moreover, crizotinib can synergistically inhibit ovarian cancer cells growth in vitro and in vivo when combines with cisplatin. Altogether, crizotinib potently potentiates the activity of cisplatin in ovarian cancer, suggesting the synergistic effect of crizotinib and cisplatin may be valuable for ovarian cancer patients’ treatment.
Keywords: Ovarian cancer, crizotinib, cisplatin, combination therapy
Introduction
Ovarian cancer is the seventh-most common cancer and the eighth-most common cause of death from cancer among women in the worldwide [1]. Although initial response rates to treatment including some combination of surgery, radiation therapy and chemotherapy are high, ovarian cancer recurs in the majority of patients whose 5-years survival rate is approximately close to 36% [2]. Therefore, it is important to develop new therapeutic drugs for the treatment of ovarian cancer.
Crizotinib (PF-2341066, trade name Xalkori), a small molecule inhibitor of anaplastic lymphoma kinase (ALK), c-ros oncogene 1 (ROS1) and c-MET (also called MET or hepatocyte growth factor receptor), has been approved by the Food and Drug Administration for the treatment patients with advanced non-small cell lung cancer (NSCLC) whose tumors have rearrangements in the ALK or ROS1 gene [3]. In the preclinical tests, crizotinib was highly selective for ALK and potently inhibited cell proliferation, which was associated with G1-S phase cell cycle arrest and apoptosis in ALK-positive anaplastic large-cell lymphoma (ALCL) cells [4]. The phase I clinical studies showed that as an oral single agent in 37 patients with advanced cancer (included colorectal, pancreatic, sarcoma, ALCL and NSCLC), crizotinib at 250 mg twice daily in 28-day cycles was generally safe and well tolerated with grade 1 or 2 treatment-related adverse events and achieved a certain efficacy in the ALK-positive lung cancer population [5]. In the subsequent phase II and III clinical studies compared crizotinib (250 mg twice daily) with traditional chemotherapy regimens (pemetrexed 500 mg/m2 plus cisplatin 75 mg/m2 or carboplatin at area under the curve 5 to 6) every 3 weeks for up to six cycles in 347 advanced ALK-positive NSCLC patients the progression-free survival (PFS) and intracranial disease control rate in brain metastases of patients after treatment with crizotinib was significantly better than those with chemotherapy [6,7]. In the study of crizotinib including 50 patients with advanced NSCLC who were tested positive for ROS1 rearrangement, the objective response rate was 72% with 3 complete responses and 33 partial responses, the median duration of response was 17.6 months, and median PFS was 19.2 months [8]. Another clinical trial of crizotinib including 30 patients had stage IV lung adenocarcinoma with ROS1 rearrangement showed 4 patients with disease progression, 2 patients with stable disease, and objective response in 24 patients including 5 complete responses (overall response rate, 80%; disease control rate, 86.7%). Median PFS was 9.1 months, and the PFS rate at 12 months was 44% [9]. Currently, evaluation of crizotinib alone or combined with other chemotherapeutical drugs for multiple types of cancers treatment is still in progress. In this study, we investigate the anticancer effects of crizotinib alone or in combination with cisplatin in human ovarian cancer.
Material and methods
Cell lines, cell culture and reagents
Human ovarian cancer cell lines A2780, SKOV3, ES2, HO8910 and HO8910PM, cultured in Dulbecco’s modified Eagle’s medium (DMEM) including 10% fetal calf serum (FBS), streptomycin (100 ng/ml) and penicillin (100 U/ml) at 37°C in a humidified incubator with 5% CO2. Crizotinib and cisplatin were purchased from ApexBio and Qilu Pharmaceutical, respectively. N-acetly-L-cysteine (NAC) and dihydroethidium (DHE) were ordered from Sigma-Aldrich. Antibodies like Anti-GAPDH (LK9002T) were from Tianjin Sungene Biotech., Anti-PARP (9542), Anti-AKT (4691), Anti-p-AKT S473 (4060), Anti-pERK T202/Y204 (4370) and Anti-ERK (4695) antibodies were bought from Cell Signaling Technologies.
MTT proliferation assay
Cells were seeded in 96-well plates with 5000 cells per well. After 72 h incubated with drugs, cell viabilities were measured by using 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) assays. The cells were allowed to reduce MTT (0.5 mg/ml) into formazan crystals (4 h at 37°C) the amount of which was measured the absorbance at 570 nm by plate reader after lysing the cells in 50 ml of DMSO. The Bliss method was applied to calculate the half maximal inhibitory concentration (IC50) from survival curves [10,11]. For combination index (CI) analysis, cells were treated with crizotinib alone (1, 3 and 10 μM), cisplatin alone (1, 3 and 10 μM) and with combinations of both. CompuSyn software was used to analyze the data by combination index (CI) values (CI>1, =1, and <1 suggested antagonism, additive effect, and synergism, respectively) for drug combination experiments [12,13].
Cell cycle assay
Cells were seeded on 6-well plates with 3.0 × 105 cells per well and treated with crizotinib, cisplatin or in combination. Forty-eight hours post treatment. Cells were harvested and washed by cold phosphate-buffered saline (PBS) twice, fixed them in ice-cold 70% ethanol for 2 h at 4°C. Then resuspended and washed them with 0.5 ml PBS, incubated them with propidium iodide (PI) solution at 50 μg/ml, 0.1% Triton X-100, DNase-free RNase (100 μg/ml), and 0.1% sodium citrate for 30 min at 37°C in the dark. Finally, the stained cells were analyzed by a FL-2 filter (585 nm) at an excitation wavelength of 480 nm, and data were calculated with ModFit LT 3.0 software (Becton Dickinson) [14,15].
Apoptosis assay
Cells were seeded in 12-well plates at 1.5 × 105 cells well and treated with relevant drugs, 48 h later, cells were harvested and washed with PBS. Ethanol-fixed cells were stained with PI and Annexin V-FITC for 15 min at 37°C in the dark, and analyzed by flow cytometry (FCM). Cells with fluorescence were analyzed with FL-1 filter (530 nm) and FL-2 filters (585 nm) at an excitation wave length of 480 nm. The data were quantified using FlowJO software [16,17].
ROS assay
Cells were treated with crizotinib for 48 h for reactive oxygen species (ROS) assay. Cells were observed under fluorescence microscope (Olympus, Japan) after fixed with dihydroethidium (DHE) (10 μM) for 30 min at 37°C in the dark. Five fields were observed randomly for each well [18,19]. ROS activation were analyzed by calculate the percentage of positive cells.
Western blot analysis
The proteins were extracted using RIPA buffer with protease inhibitors to lyse cells. The protein concentrations were determined by Bradford assay. Proteins lysates were subjected to 12% SDS-PAGE and transferred to polvinylidene difluoride (PVDF) membranes. Blocking was performed in 5% BSA for 1 h, followed by probing with primary antibodies overnight at 4°C. The membranes were washed and incubated with horseradish peroxidase-conjugated secondary antibodies [20,21].
In vivo xenograft assay
Female Balb/c nude mice were purchased from the Guangdong Medical Laboratory Animal Six female nude mice (5 weeks old) were used for each group. Briefly, 2 × 106 A2780 cells were suspended in 100 μl PBS and injected subcutaneously under the shoulder of female Balb/c nude mic. When the volume of subcutaneous tumor was about 0.3 × 0.3 cm2, mice were randomly assigned to four groups, receiving 0.9% saline alone, crizotinib alone, cisplatin alone, or a combination of crizotinib, respectively. Crizotinib was dissolved in PBS to a working concentration of 50 mg/kg and cisplatin to a working concentration of 2 mg/kg. The tumor size (the two perpendicular diameters (A and B)) and the body weights were measured every 4 days using digital calipers. The tumor volume (V) was calculated according to the formula:
V = (π/6)((A + B)/2)3
Tumor tissue was weighted after excised from the mice. The rate of inhibition (IR) was calculated according to the formula [22,23]:
IR = 1 - (Mean tumor weight of experimental group/Mean tumor weight of control group) × 100%
Statistical analysis
All results are expressed as mean ± standard deviation (SD). Statistical analysis of the differences between two groups is performed with Student’s t-test. Values of P<0.05 are considered as significant differences.
Result
Crizotinib inhibits the growth of ovarian cancer cells in vitro
The increasing concentrations of crizotinib was used to treat five human ovarian cancer cell lines for 72 h. Crizotinib (0, 1, 3, 10, 30, 100 μM) meaningfully inhibited the growth of five ovarian cancer cells in a dose-dependent trend with the IC50 values range from 2.70 to 7.41 μM. Meanwhile, cisplatin (0, 1, 3, 10, 30, 100 μM) also inhibited the growth of cells with the IC50 values range from 7.05 to 9.67 μM (Figure 1). The growth inhibitory effects of drugs were examined by the MTT assay. The results showed that both crizotinib and cisplatin inhibits the growth of ovarian cancer cells.
Figure 1.
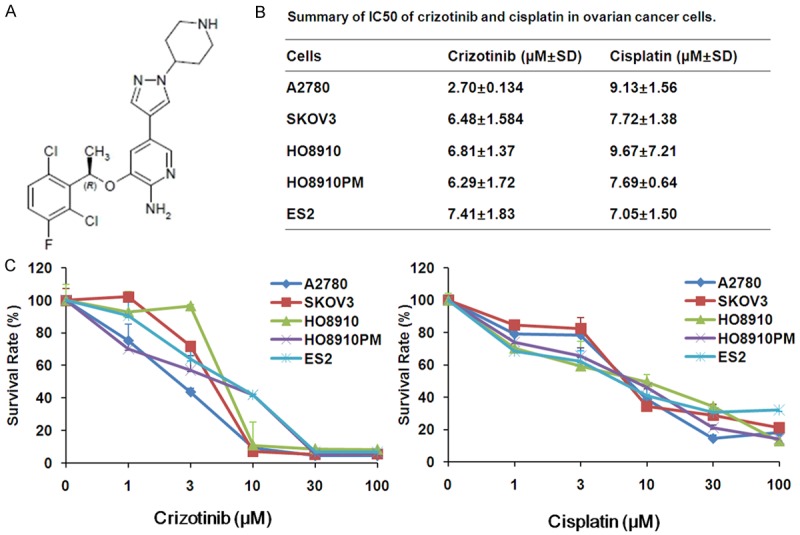
Crizotinib inhibits the growth of ovarian cancer cells in vitro. A. Chemical structure of crizotinib. B. Summary of IC 50 in the different ovarian cancer cells lines treated with crizotinib and cisplatin is shown. Cells were grown in 96-well plates for 24 h and treated with the relevant concentrations of crizotinib or cisplatin for 72 h, and cell survival was determined by MTT assay. C. The representative growth curves of five ovarian cancer cells lines treated with crizotinib and cisplatin are shown. Data are mean ± SD of three independent experiments.
Crizotinib induces cell cycle arrest at G2/M phase in ovarian cancer cells in vitro
A2780 and SKOV3 cells were treated with crizotinib (1, 3 and 10 μM) for 48 h, stained with PI and examined by FCM. As shown in Figure 2A and 2B, crizotinib induced cell cycle arrest at G2/M phase at the low concentration and increases the cell population of sub G1 phase at the high concentration in both cells, the amount of which was analyzed with ModFit LT 3.0 software. These results provided convincing data showing that crizotinib inhibits the growth of ovarian cancer cells is due to induction of cell cycle arrest.
Figure 2.
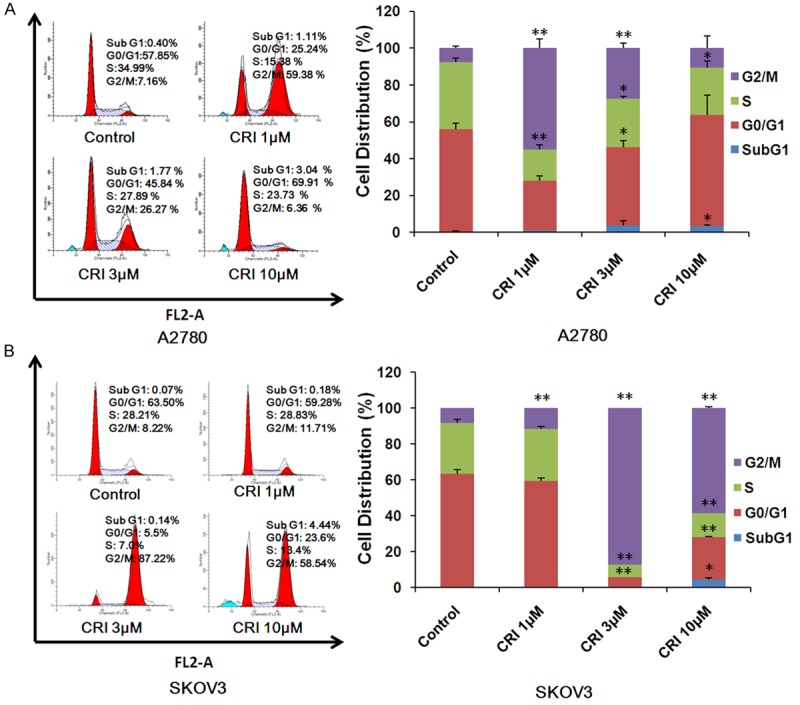
Crizotinib arrests ovarian cancer cell cycle at G2/M phase. A2780 (A) and SKOV3 (B) cells were treated with crizotinib at the indicated concentrations. The distribution of cell cycle was examined by FCM with PI staining. The percentages of subG1, G1/G0, S, G2/M phase were calculated by using ModFit LT 3.0 software. The results of three independent experiments were shown through the representative and quantified charts. CRI: Crizotinib. Statistical analysis of the difference between two groups is performed with Student’s t-test. *P<0.05 and **P<0.01 vs. corresponding control.
Crizotinib induces apoptosis in ovarian cancer cells in vitro
A2780 and SKOV3 cells were treated with crizotinib (1, 3 and 10 μM) for 48 h, stained with Annexin V and evaluated by FCM. As shown in Figure 3A and 3B, crizotinib dose-dependently induced apoptosis in cells. The protein levels of cleaved PARP which is a molecular marker of apoptosis are increased in a dose-dependent manner after volasertib treatment (Figure 3C). In addition, crizotinib dose-dependently suppresses the activities of AKT and ERK by decreasing the expression of pAKT S473 and pERK T202/Y204 in both cells (Figure 3C). The data showed that crizotinib is able to induce apoptosis in ovarian cancer cells.
Figure 3.
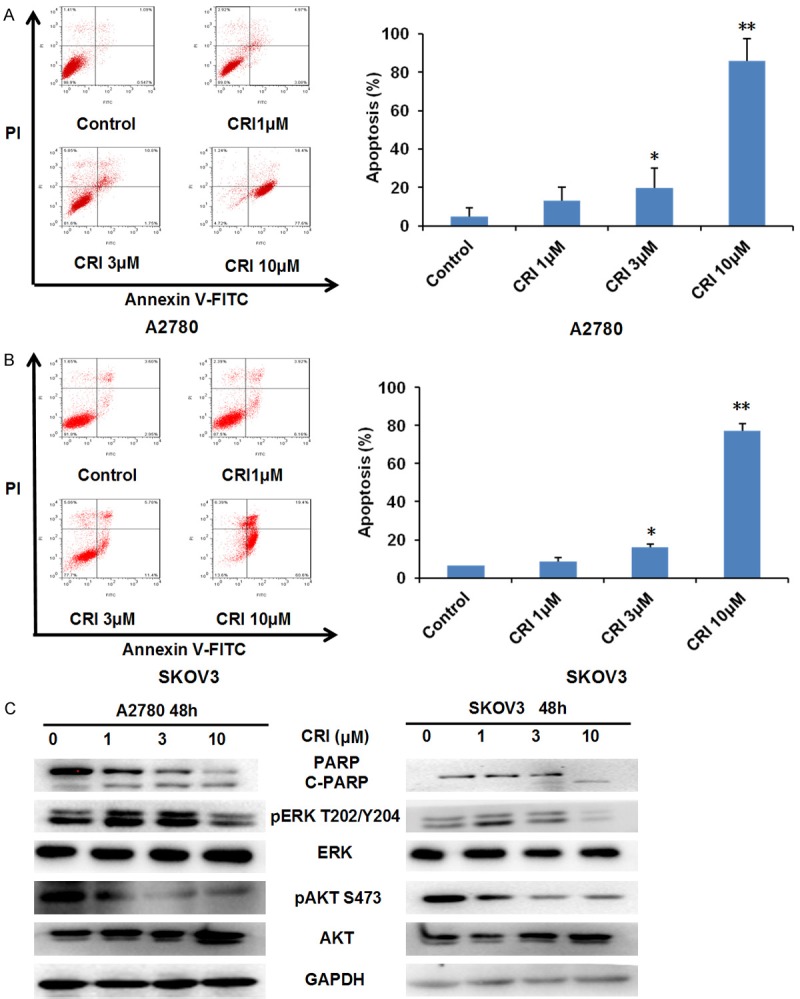
Crizotinib promotes apoptosis in ovarian cancer cells. A2780 (A) and SKOV3 (B) cells were treated with crizotinib at the indicated concentrations. Cell apoptosis was detected by FCM Annexin V/PI staining. The proportions of Annexin V+/PI- and Annexin V+/PI+ cells indicated apoptosis. Western blot was applied to examine protein expression and GAPDH was used as loading control. The representative charts, quantified results and Western blot results (C) of three independent experiments were shown. CRI: Crizotinib. Statistical analysis of the difference between two groups is performed with Student’s t-test. *P<0.05 and **P<0.01 vs. corresponding control.
Crizotinib induces ROS accumulation in ovarian cancer cells
ROS plays a critical role in the response of cancer cells to chemotherapeutic agents. DHE, the ROS fluorescent probe, is oxidized to ethidium that intercalates with DNA and becomes fluorescent in the presence of superoxide [24]. Cells were stained with dihydroethidium (DHE) after treated with crizotinib for 48 h. Crizotinib increased the fluorescent intensity of DHE in both A2780 and SKOV3 cells in a dose- and time-dependent manner (Figure 4). The results provided that crizotinib can increase the intracellular ROS level in ovarian cancer cells.
Figure 4.
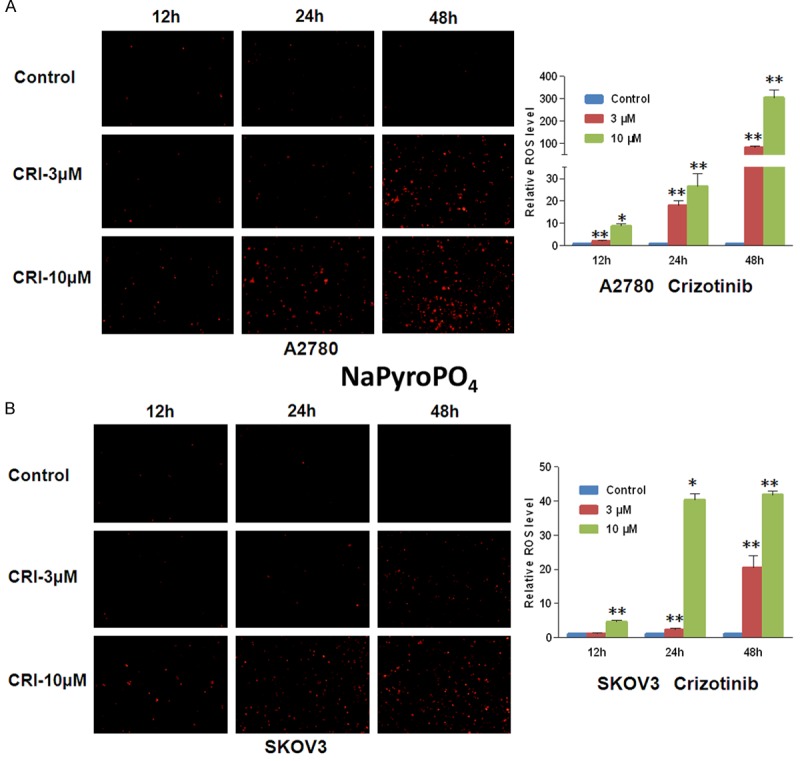
Crizotinib induces ROS accumulation in ovarian cancer cells. A2780 (A) and SKOV3 (B) cells were managed with crizotinib at the relevant concentrations and times, stained with DHE and photographed by florescent microscope. The representative micrographs and quantified results of three independent experiments are shown. CRI: Crizotinib. Statistical analysis of the difference between two groups is performed with Student’s t-test. *P<0.05 and **P<0.01 vs. corresponding control.
Inhibition of ROS partially rescues crizotinib-induced apoptosis in ovarian cancer cells
A2780 and SKOV3 cells were treated with crizotinib at 10 μM for 48 h with or without fixed the ROS scavenger NAC (5 mM) pretreated for 1 h. Cells were stained with DHE before observed under fluorescence microscope (Olympus, Japan). As shown in Figure 5A and 5B, NAC dramatically reversed the crizotinib-induced DHE fluorescent signals in both cells. In addition, cells were treated with crizotinib for 48 h with or without NAC and stained with PI and Annexin V-FITC. The data analyzed by FCM showed that NAC also partly inverted the crizotinib-induced apoptosis in both cells (Figure 5C and 5D). This phenomenon further suggests crizotinib can trigger both ROS dependent and independent apoptosis in ovarian cancer cells.
Figure 5.
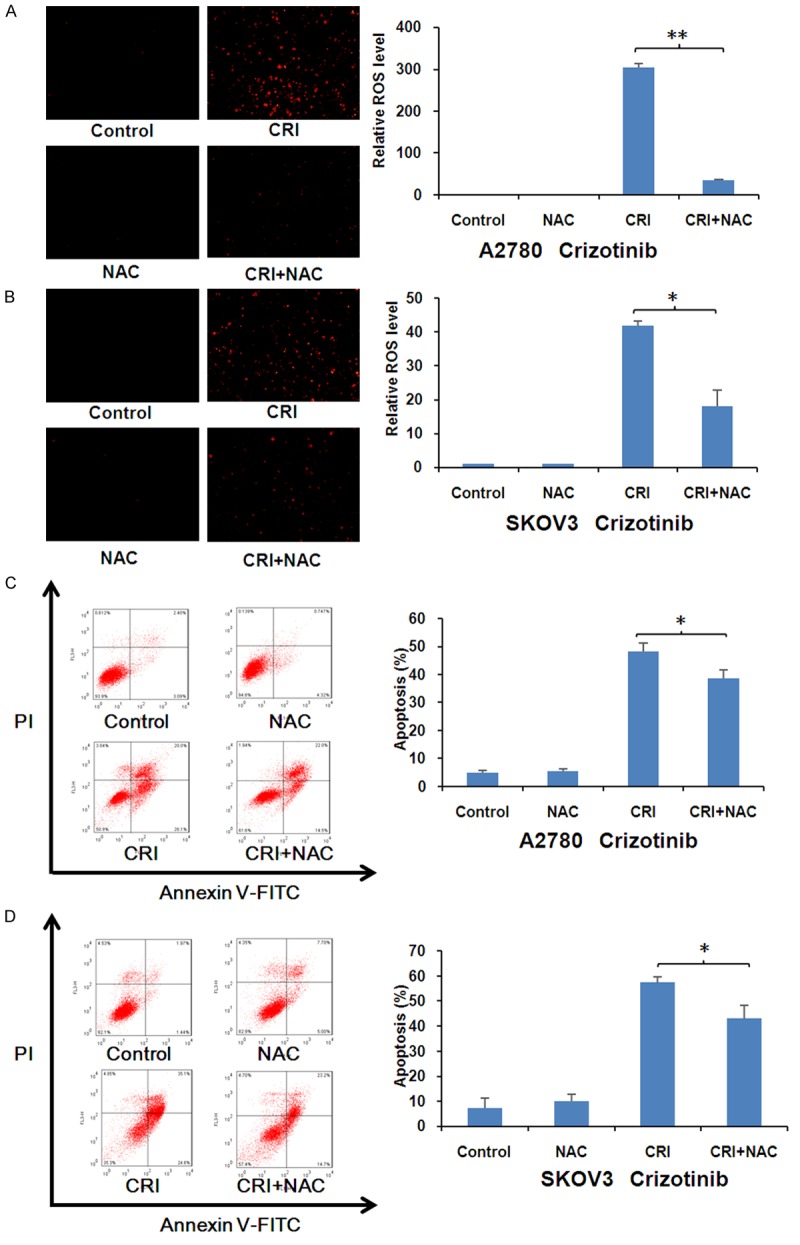
Crizotinib-induced apoptosis was partially rescued by inhibition of ROS in ovarian cancer cells. A2780 (A, C) and SKOV3 (B, D) cells were treated with crizotinib at 10 μM for 48 hr in the presence or absence of 5 mM NAC pretreatment for 1 h, stained with DHE and photographed under fluorescent microscope. The apoptosis was detected by FCM with Annexin V/PI staining. The proportions of Annexin V+/PI- and Annexin V+/PI+ cells suggested apoptosis. The representative micrographs, charts and quantified results of three independent experiments are shown. CRI: Crizotinib. Statistical analysis of the difference between two groups is performed with Student’s t-test. *P<0.05 and **P<0.01 CRI vs. CRI+NAC.
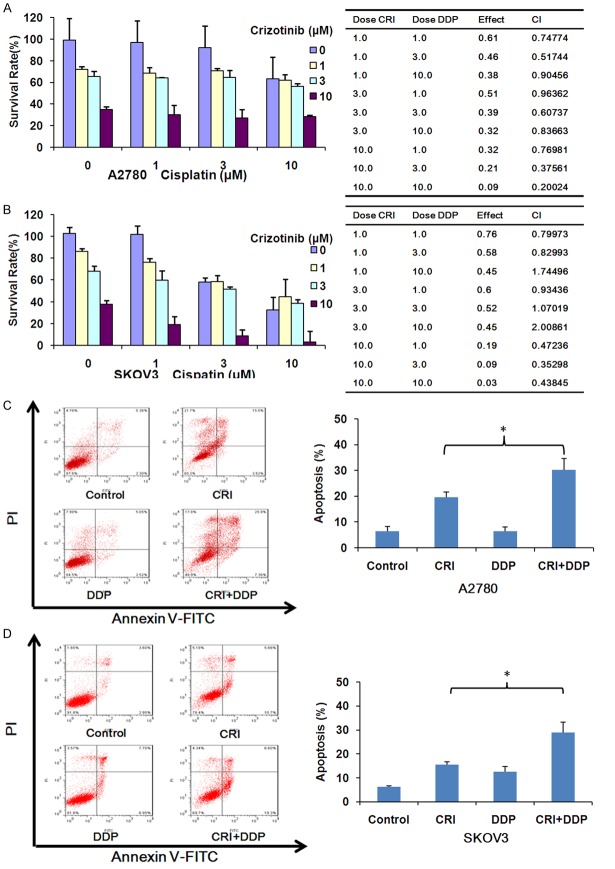
Crizotinib synergizes with cisplatin to inhibit the growth and induce apoptosis of ovarian cancer cells in vitro
Cisplatin currently is one of the first-line chemotherapeutic drugs for ovarian cancer in clinic [25]. Cells were treated with crizotinib alone (1, 3 and 10 μM), cisplatin alone (1, 3 and 10 μM) and with combinations of both for 72 h. As show in Figure 6A and 6B, compared with crizotinib or cisplatin alone treatment in both cells, the survival of ovarian cells was significantly decreased after combined treatment with crizotinib and cisplatin. Meanwhile, CI values of both A2780 and almost all SKOV3 were <1, suggesting that combination of both is synergistic to inhibit the growth of ovarian cancer cells. In addition, to further certify the co-treatment effects of crizotinib and cisplatin, we used FCM to detected cell apoptosis. Combined treatment of crizotinib and cisplatin also effectively promoted apoptosis of both cell lines compared to single treatment with crizotinib and cisplatin (Figure 6C and 6D).
Figure 6.
Crizotinib synergizes with cisplatin to suppress ovarian cancer cells growth and induce apoptosis in vitro. A2780 (A, C) and SKOV3 (B, D) cells were treated with the indicated concentrations of crizotinib and cisplatin for 72 h, and MTT assay was used to detect cell survival. Results were shown as growth histogram, dose-effect curve, CI values and normalized isobologram by CompuSyn software. A2780 were treated with 1 μM crizotinib, 1 μM cisplatin alone or combination for 48 h. SKOV3 treated with with 3 μM crizotinib, 3 μM cisplatin alone or combination for 48 h. The apoptosis was analyzed by FCM Annexin V/PI staining. The proportions of Annexin V+/PI- and Annexin V+/PI+ cells suggested apoptosis. The representative micrographs, charts and quantified results of three independent experiments are shown. CRI: Crizotinib; DDP: Cisplatin. Statistical analysis of the difference between two groups is performed with Student’s t-test. *P<0.05 CRI vs. CRI+DDP.
Crizotinib synergizes with cisplatin to inhibit the subcutaneous xenograft tumor growth of ovarian cancer in nude mice
All four groups of mice were transplanted with A2780 cells to engineer the subcutaneous xenograft tumor models (Figure 7A-C). Co-treatment with crizotinib and cisplatin significantly decreased the substantial tumor volume and weight compared to single treatment with crizotinib and cisplatin in A2780 tumors. The inhibition rates of tumor growth in the combined group were 46.84%, which were significantly higher than those in cisplatin (2.55%) or crizotinib (22.04%) alone group (Figure 7E). In addition, the data also detected that treatment with cisplatin at the indicated dose causes certain toxicities in mice by observing the weight of mice in the cisplatin alone and combined group were lighter than those in control and crizotinib alone group (Figure 7D). These results suggest that combination of crizotinib and cisplatin significantly inhibited tumor growth in vivo.
Figure 7.
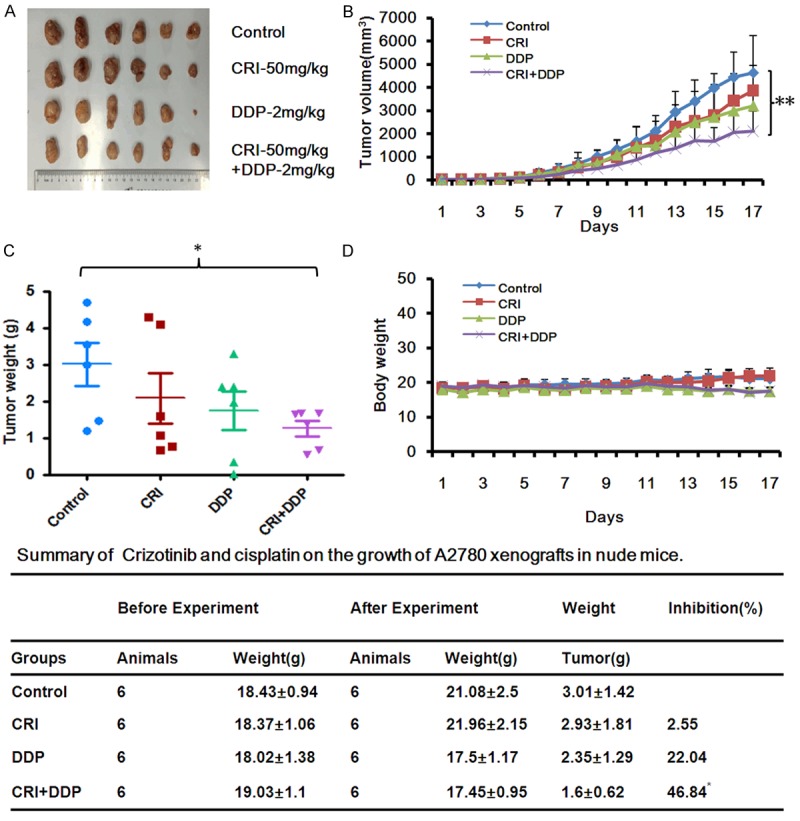
Crizotinib synergizes with cisplatin to suppress the subcutaneous xenograft growth of ovarian cancer in nude mice. A2780 cells (2 × 106 in 100 μl of medium) were injected subcutaneously in each mouse under the axillia. When the subcutaneous tumors were approximately 0.3 × 0.3 cm2 (two perpendicular diameters), mice were randomized into four groups, and were injected intraperitoneally with vehicle alone (20% hydroxypropyl-β-cyclodextrin), crizotinib alone (50 mg/kg), cisplatin alone (2 mg/kg), or a combination of crizotinib and cisplatin every four days. The body weights of mice and tumor volume were monitoring every day. In the end of the experiment, tumor tissue was excised from the mice after anaesthetized and weighted. The original tumors (A), tumor volume (B), tumor weight (C), body weight (D) and summary data (E) were shown. The values presented are the means ± SD for each group. Statistical analysis of the difference between two groups is performed with Student’s t-test. CRI: Crizotinib; DDP: Cisplatin. *P<0.05 and **P<0.01 CRI+DDP vs. corresponding control.
Discussion
In this study, our results show that crizotinib can actively induce cell growth inhibition, cell cycle arrest at G2/M phase and apoptosis with the decreasing phosphorylation of the downstream signaling effectors AKT and ERK in human ovarian cancer cells. This is consistent with the previous report where critizonib reduces tumor burden and metastasis in a preclinical model of ovarian cancer metastasis with the decreasing phosphorylation of AKT and ERK [26]. It has been reported that aberrant ALK expression was detected in 2% to 4% serious ovarian carcinoma patients, and a novel transmembrane ALK fusion gene FN1-ALK found in a stromal sarcoma patient was oncogenic and sensitive to critizonib, suggesting ALK may be a potential therapeutic target in a subset of ovarian cancer patients [27]. Overexpression of c-MET has been found in 11% to 96% of human ovarian cancer tissues, and can be a prognostic factor and an effective therapeutic target for ovarian cancer patients [28-31]. Additionally, we found that critizonib also increased the intracellular ROS levels, and pretreatment with ROS scavenger NAC partially reversed critizonib-induced apoptosis in human ovarian cancer cells. Consistently, crizotinib also can induce the accumulation of intracellular ROS in human alveolar rhabdomyosarcoma cells [32]. Cancer cells usually have the increased basal levels of intrinsic oxidative stress, and a further augment of intracellular ROS may lead to cell death [33]. This oxidative shift results in cancer cells susceptible to chemotherapeutic agents that enhance ROS generation [34]. Consequently, our data suggest critizonib can induce both ROS dependent and independent apoptosis in human ovarian cancer cells.
Combination therapy as the main strategy of cancer chemotherapy can overcome drug resistance and improve treatment efficiency [35]. Several groups have explored the combined anticancer effects of crizotinib and other agents in the preclinical and clinical studies. Combining the HSP90 inhibitor ganetespib with crizotinib synergistically inhibits the activity of c-MET, its downstream signaling pathways, and tumor growth in both crizotinib-sensitive and -resistant MET-driven tumor models [36]. The combination of crizotinib and a selective mTORC1 inhibitor Torin2 or a dual PI3K/mTOR inhibitor PF-05212384 is more effective in reducing tumor growth in ALK-mutated neuroblastoma compared to any of these agents alone [37]. A combination of crizotinib and dasatinib shows the significant cytotoxic across multiple established and primary human glioblastoma multiforme cell lines [38]. Recently, it has been reported that crizotinib synergizes with chemotherapeutic agents topotecan and cyclophosphamide in preclinical models of neuroblastoma [39]. Co-treatment with crizotinib and temozolomide induces synergistic antitumor activity on FIG-ROS1-positive glioblastoma cells [40]. Interestingly, the combined treatment with axitinib and crizotinib reduces bone loss in a mouse model of castration resistant prostate cancer [41]. In our study, the combination of crizotinib with cisplatin can synergistically inhibit the growth of human ovarian cancer cells in vitro and in vivo. However, a recent phase I study in patients with advanced NSCLC has demonstrated that the combination of crizotinib and dacomitinib shows limited antitumor activity due to substantial toxicity [42]. Therefore, the combination of crizotinib with cisplatin for the treatment of ovarian cancer and other types of cancer need to be further investigated in the clinical trials.
Altogether, our study demonstrates that crizotinib can inhibit the growth of ovarian cancer cells by induction of cell cycle arrest and apoptosis and synergize with cisplatin in vitro and in vivo. The combined treatment with crizotinib and cisplatin may be valuable for the treatment of ovarian cancer patients.
Acknowledgements
This work was supported by funds from the National Natural Science Foundation of China No. 31271444 and No. 81201726 (Z. S.), No. 81503293 (X. J. Y.) and No. 81373075 (R.Y.O), the Foundation for Research Cultivation and Innovation of Jinan University No. 21616119 (Z. S.), the Guangdong Natural Science Funds for Distinguished Young Scholar No. 2014A030306001 (Z. S.), the Guangdong Special Support Program for Young Talent No. 2015TQ01R350 (Z. S.) and the Science and Technology Program of Guangdong No. 2016A050502027 (Z. S.).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad S, Qureshi AN, Kazmi A, Rasool A, Gul M, Ashfaq M, Batool L, Rehman RA, Ahmad J, Muniba First cancer statistics report from Hazara division. J Ayub Med Coll Abbottabad. 2013;25:71–73. [PubMed] [Google Scholar]
- 3.Gridelli C, Peters S, Sgambato A, Casaluce F, Adjei AA, Ciardiello F. ALK inhibitors in the treatment of advanced NSCLC. Cancer Treat Rev. 2014;40:300–306. doi: 10.1016/j.ctrv.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Christensen JG, Zou HY, Arango ME, Li Q, Lee JH, McDonnell SR, Yamazaki S, Alton GR, Mroczkowski B, Los G. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther. 2007;6:3314–3322. doi: 10.1158/1535-7163.MCT-07-0365. [DOI] [PubMed] [Google Scholar]
- 5.Camidge DR, Bang YJ, Kwak EL, Iafrate AJ, Varella-Garcia M, Fox SB, Riely GJ, Solomon B, Ou SH, Kim DW, Salgia R, Fidias P, Engelman JA, Gandhi L, Janne PA, Costa DB, Shapiro GI, Lorusso P, Ruffner K, Stephenson P, Tang Y, Wilner K, Clark JW, Shaw AT. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13:1011–1019. doi: 10.1016/S1470-2045(12)70344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, Iyer S, Reisman A, Wilner KD, Tursi J, Blackhall F PROFILE 1014 Investigators. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 7.Solomon BJ, Cappuzzo F, Felip E, Blackhall FH, Costa DB, Kim DW, Nakagawa K, Wu YL, Mekhail T, Paolini J, Tursi J, Usari T, Wilner KD, Selaru P, Mok TS. Intracranial efficacy of Crizotinib versus chemotherapy in patients with advanced ALK-Positive non-small-cell lung cancer: Results From PROFILE 1014. J. Clin. Oncol. 2016;34:2858–65. doi: 10.1200/JCO.2015.63.5888. [DOI] [PubMed] [Google Scholar]
- 8.Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, Riely GJ, Varella-Garcia M, Shapiro GI, Costa DB, Doebele RC, Le LP, Zheng Z, Tan W, Stephenson P, Shreeve SM, Tye LM, Christensen JG, Wilner KD, Clark JW, Iafrate AJ. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazieres J, Zalcman G, Crino L, Biondani P, Barlesi F, Filleron T, Dingemans AM, Lena H, Monnet I, Rothschild SI, Cappuzzo F, Besse B, Thiberville L, Rouviere D, Dziadziuszko R, Smit EF, Wolf J, Spirig C, Pecuchet N, Leenders F, Heuckmann JM, Diebold J, Milia JD, Thomas RK, Gautschi O. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J. Clin. Oncol. 2015;33:992–999. doi: 10.1200/JCO.2014.58.3302. [DOI] [PubMed] [Google Scholar]
- 10.CS W. Tables for convenient calculation of median-effective dose (LD50 or ED50) and instructions in their use. Biometrics. 1952;8:249–263. [Google Scholar]
- 11.Shi Z, Tiwari AK, Shukla S, Robey RW, Singh S, Kim IW, Bates SE, Peng X, Abraham I, Ambudkar SV, Talele TT, Fu LW, Chen ZS. Sildenafil reverses ABCB1- and ABCG2-mediated chemotherapeutic drug resistance. Cancer Res. 2011;71:3029–3041. doi: 10.1158/0008-5472.CAN-10-3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 13.Jiang QW, Cheng KJ, Mei XL, Qiu JG, Zhang WJ, Xue YQ, Qin WM, Yang Y, Zheng DW, Chen Y, Wei MN, Zhang X, Lv M, Chen MW, Wei X, Shi Z. Synergistic anticancer effects of triptolide and celastrol, two main compounds from thunder god vine. Oncotarget. 2015;6:32790–32804. doi: 10.18632/oncotarget.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lv M, Qiu JG, Zhang WJ, Jiang QW, Qin WM, Yang Y, Zheng DW, Chen Y, Huang JR, Wang K, Wei MN, Cheng KJ, Shi Z. Wallichinine reverses ABCB1-mediated cancer multidrug resistance. Am J Trans Res. 2016;8:2969–80. [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Gong L, Ou R, Zheng Z, Chen J, Xie F, Huang X, Qiu J, Zhang W, Jiang Q, Yang Y, Zhu H, Shi Z, Yan X. Sequential combination therapy of ovarian cancer with cisplatin and gamma-secretase inhibitor MK-0752. Gynecol Oncol. 2016;140:537–544. doi: 10.1016/j.ygyno.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Shi Z, Park HR, Du Y, Li Z, Cheng K, Sun SY, Fu H, Khuri FR. Cables1 complex couples survival signaling to the cell death machinery. Cancer Res. 2015;75:147–158. doi: 10.1158/0008-5472.CAN-14-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Z, Li Z, Li ZJ, Cheng K, Du Y, Fu H, Khuri FR. Cables1 controls p21/Cip1 protein stability by antagonizing proteasome subunit alpha type 3. Oncogene. 2015;34:2538–2545. doi: 10.1038/onc.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie FF, Pan SS, Ou RY, Zheng ZZ, Huang XX, Jian MT, Qiu JG, Zhang WJ, Jiang QW, Yang Y, Li WF, Shi Z, Yan XJ. Volasertib suppresses tumor growth and potentiates the activity of cisplatin in cervical cancer. Am J Cancer Res. 2015;5:3548–3559. [PMC free article] [PubMed] [Google Scholar]
- 19.Gong LH, Chen XX, Wang H, Jiang QW, Pan SS, Qiu JG, Mei XL, Xue YQ, Qin WM, Zheng FY, Shi Z, Yan XJ. Piperlongumine induces apoptosis and synergizes with cisplatin or paclitaxel in human ovarian cancer cells. Oxid Med Cell Longev. 2014;2014:906804. doi: 10.1155/2014/906804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo Y, Jiang QW, Wu JY, Qiu JG, Zhang WJ, Mei XL, Shi Z, Di JM. Regulation of migration and invasion by Toll-like receptor-9 signaling network in prostate cancer. Oncotarget. 2015;6:22564–22574. doi: 10.18632/oncotarget.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, Lan SJ, Liu QR, Liu JM, Chen XQ. Neuroglobin, a novel intracellular hexa-coordinated globin, functions as a tumor suppressor in hepatocellular carcinoma via Raf/MAPK/Erk. Mol Pharmacol. 2013;83:1109–1119. doi: 10.1124/mol.112.083634. [DOI] [PubMed] [Google Scholar]
- 22.Mei XL, Yang Y, Zhang YJ, Li Y, Zhao JM, Qiu JG, Zhang WJ, Jiang QW, Xue YQ, Zheng DW, Chen Y, Qin WM, Wei MN, Shi Z. Sildenafil inhibits the growth of human colorectal cancer in vitro and in vivo. Am J Cancer Res. 2015;5:3311–3324. [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu JG, Zhang YJ, Li Y, Zhao JM, Zhang WJ, Jiang QW, Mei XL, Xue YQ, Qin WM, Yang Y, Zheng DW, Chen Y, Wei MN, Shi Z. Trametinib modulates cancer multidrug resistance by targeting ABCB1 transporter. Oncotarget. 2015;6:15494–15509. doi: 10.18632/oncotarget.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue YQ, Di JM, Luo Y, Cheng KJ, Wei X, Shi Z. Resveratrol oligomers for the prevention and treatment of cancers. Oxid Med Cell Longev. 2014;2014:765832. doi: 10.1155/2014/765832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen XX, Xie FF, Zhu XJ, Lin F, Pan SS, Gong LH, Qiu JG, Zhang WJ, Jiang QW, Mei XL, Xue YQ, Qin WM, Shi Z, Yan XJ. Cyclin-dependent kinase inhibitor dinaciclib potently synergizes with cisplatin in preclinical models of ovarian cancer. Oncotarget. 2015;6:14926–14939. doi: 10.18632/oncotarget.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zillhardt M, Christensen JG, Lengyel E. An orally available small-molecule inhibitor of c-Met, PF-2341066, reduces tumor burden and metastasis in a preclinical model of ovarian cancer metastasis. Neoplasia. 2010;12:1–10. doi: 10.1593/neo.09948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren H, Tan ZP, Zhu X, Crosby K, Haack H, Ren JM, Beausoleil S, Moritz A, Innocenti G, Rush J, Zhang Y, Zhou XM, Gu TL, Yang YF, Comb MJ. Identification of anaplastic lymphoma kinase as a potential therapeutic target in ovarian cancer. Cancer Res. 2012;72:3312–3323. doi: 10.1158/0008-5472.CAN-11-3931. [DOI] [PubMed] [Google Scholar]
- 28.Sawada K, Radjabi AR, Shinomiya N, Kistner E, Kenny H, Becker AR, Turkyilmaz MA, Salgia R, Yamada SD, Vande Woude GF, Tretiakova MS, Lengyel E. c-Met overexpression is a prognostic factor in ovarian cancer and an effective target for inhibition of peritoneal dissemination and invasion. Cancer Res. 2007;67:1670–1679. doi: 10.1158/0008-5472.CAN-06-1147. [DOI] [PubMed] [Google Scholar]
- 29.Di Renzo MF, Olivero M, Katsaros D, Crepaldi T, Gaglia P, Zola P, Sismondi P, Comoglio PM. Overexpression of the Met/HGF receptor in ovarian cancer. Int J Cancer. 1994;58:658–662. doi: 10.1002/ijc.2910580507. [DOI] [PubMed] [Google Scholar]
- 30.Ayhan A, Ertunc D, Tok EC. Expression of the c-Met in advanced epithelial ovarian cancer and its prognostic significance. Int J Gynecol Cancer. 2005;15:618–623. doi: 10.1111/j.1525-1438.2005.00117.x. [DOI] [PubMed] [Google Scholar]
- 31.Davies S, Holmes A, Lomo L, Steinkamp MP, Kang H, Muller CY, Wilson BS. High incidence of ErbB3, ErbB4, and MET expression in ovarian cancer. Int J Gynecol Pathol. 2014;33:402–410. doi: 10.1097/PGP.0000000000000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Megiorni F, McDowell HP, Camero S, Mannarino O, Ceccarelli S, Paiano M, Losty PD, Pizer B, Shukla R, Pizzuti A, Clerico A, Dominici C. Crizotinib-induced antitumour activity in human alveolar rhabdomyosarcoma cells is not solely dependent on ALK and MET inhibition. J Exp Clin Cancer Res. 2015;34:112. doi: 10.1186/s13046-015-0228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fruehauf JP, Meyskens FL Jr. Reactive oxygen species: a breath of life or death? Clin Cancer Res. 2007;13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 34.Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10:175–176. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Bozic I, Reiter JG, Allen B, Antal T, Chatterjee K, Shah P, Moon YS, Yaqubie A, Kelly N, Le DT, Lipson EJ, Chapman PB, Diaz LA Jr, Vogelstein B, Nowak MA. Evolutionary dynamics of cancer in response to targeted combination therapy. Elife. 2013;2:e00747. doi: 10.7554/eLife.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyajima N, Tsutsumi S, Sourbier C, Beebe K, Mollapour M, Rivas C, Yoshida S, Trepel JB, Huang Y, Tatokoro M, Shinohara N, Nonomura K, Neckers L. The HSP90 inhibitor ganetespib synergizes with the MET kinase inhibitor crizotinib in both crizotinib-sensitive and -resistant MET-driven tumor models. Cancer Res. 2013;73:7022–7033. doi: 10.1158/0008-5472.CAN-13-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore NF, Azarova AM, Bhatnagar N, Ross KN, Drake LE, Frumm S, Liu QS, Christie AL, Sanda T, Chesler L, Kung AL, Gray NS, Stegmaier K, George RE. Molecular rationale for the use of PI3K/AKT/mTOR pathway inhibitors in combination with crizotinib in ALK-mutated neuroblastoma. Oncotarget. 2014;5:8737–8749. doi: 10.18632/oncotarget.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nehoff H, Parayath NN, McConnell MJ, Taurin S, Greish K. A combination of tyrosine kinase inhibitors, crizotinib and dasatinib for the treatment of glioblastoma multiforme. Oncotarget. 2015;6:37948–37964. doi: 10.18632/oncotarget.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krytska K, Ryles HT, Sano R, Raman P, Infarinato NR, Hansel TD, Makena MR, Song MM, Reynolds CP, Mosse YP. Crizotinib synergizes with chemotherapy in preclinical models of neuroblastoma. Clin Cancer Res. 2016;22:948–960. doi: 10.1158/1078-0432.CCR-15-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das A, Cheng RR, Hilbert ML, Dixon-Moh YN, Decandio M, Vandergrift WA 3rd, Banik NL, Lindhorst SM, Cachia D, Varma AK, Patel SJ, Giglio P. Synergistic effects of Crizotinib and temozolomide in experimental FIG-ROS1 fusion-positive glioblastoma. Cancer Growth Metastasis. 2015;8:51–60. doi: 10.4137/CGM.S32801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eswaraka J, Giddabasappa A, Han G, Lalwani K, Eisele K, Feng Z, Affolter T, Christensen J, Li G. Axitinib and crizotinib combination therapy inhibits bone loss in a mouse model of castration resistant prostate cancer. BMC Cancer. 2014;14:742. doi: 10.1186/1471-2407-14-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janne PA, Shaw AT, Camidge DR, Giaccone G, Shreeve SM, Tang Y, Goldberg Z, Martini JF, Xu H, James LP, Solomon BJ. Combined Pan-HER and ALK/ROS1/MET inhibition with dacomitinib and crizotinib in advanced non-small cell lung cancer: results of a phase i study. J Thorac Oncol. 2016;11:737–747. doi: 10.1016/j.jtho.2016.01.022. [DOI] [PubMed] [Google Scholar]