Abstract
Background: To investigate the potential protective effects of 3,4-oxo-isopropylidene-shikimic acid (ISA) on brain ischemic injury in rats. Methods: Cell Counting Kit-8, flow cytometry, and TUNEL were used to evaluate the cell viability and the apoptosis rate in vitro and in situ. Reactive oxygen species generation was determined by DCFH-DA assay. qPCR and Western blot were used to test the molecular mechanisms related to the anti-apoptosis effects. Result: Protective effect of pre-conditioning of ISA on the brain injury caused by ischemia was observed. ISA treatment showed anti-apoptosis effects on isolated primary astrocytes and neurons. ROS generation was also significantly scavenged by treatment of ISA. The treatment with ISA protected astrocytes from hypoxic condition-induced apoptosis and ischemic injury. The underlying mechanisms revealed by qPCR and WB showed that the level of mRNA and protein expression of Bax, Bcl-2, and caspase-3 were significantly down-regulated by ISA treatment (P < 0.05). Pre-conditioning with ISA is beneficial in reducing the neuronal damage caused by brain ischemia. Conclusion: Treatment with ISA reduces apoptosis and ROS over-generation caused by ischemic injury. Pre-conditioning with ISA resulted in significantly protective effects on brain under ischemic condition.
Keywords: 3, 4-oxo-isopropylidene-shikimic acid, ischemia, apoptosis, oxidative stress, ROS
Introduction
Stroke is a leading cause of disability and death in most industrialized countries [1]. During the pathologic process of stroke, ischemic injury occurs first, and is usually followed by cerebral edema, which worsens the clinical situation. Progress has been made during decades of research focusing on the pathophysiology, risk factors, underlying mechanisms, and effective treatments for stroke [2,3]; however, the morbidity and mortality of stroke remains high. Therefore, more effective intervention methods and treatments for stroke need to be developed.
The rapidly responding intravenous compound, 3,4-oxo-isopropylidene-shikimic acid (ISA), is usually used for anesthesia among clinical practices. Studies have shown that ISA treatment is protective in brain ischemic injuries and improves the outcome of neurologic conditions [4,5]. Even though a number of studies have been conducted to determine the mechanism underlying the protective effects of shikimic acid on cerebral ischemia, the detailed mechanism is still unclear. Nevertheless, the potential mechanism may include the antioxidant activity of shikimic acid, a decrease in cerebral metabolism, inhibition of aquaporin-4 expression, and regulation of neuronal apoptosis by modulating neurotransmitters [6-9]. The mechanism underlying the protective effects of shikimic acid is also associated with the property of reducing neuronal apoptosis. It has also been reported that shikimic acid can increase cerebral blood flow, thus facilitating autoregulation in the brain [10]. Few studies have been conducted describing ISA pre-conditioning in protecting against cerebral ischemia.
Neuronal apoptosis is one of the mechanisms, which explains the damage caused by cerebral ischemia. Programmed cell death (PCD) is a necessary and regulatory process of all cell types in the human body. Under several pathologic conditions, such as cerebral ischemia, neuronal apoptosis will be activated, thus leading to damage of cerebral function [11]. Different molecular mechanisms are involved in this process, among which c-jun N-terminal kinase (JNK) is one of the most crucial pathways [12]. Apart from neurons, the role of astrocytes is often neglected. As the most abundant non-neuronal cell in the central nervous system (CNS), astrocytes account for approximately one-half of the brain volume in humans. Ischemic injury, as a cause of astrocyte apoptosis, should also be considered to understand the mechanism underlying the protective effects of shikimic acid. It is thought that astrocytes are important in maintaining neuronal survival during cerebral ischemia [13]. For this reason, the effects of brain ischemia on astrocytes are worthy to be understood.
For the first time, we tested the protective effects of ISA pre-conditioning in the treatment of cerebral ischemic injury. We focused on neuronal and astrocyte apoptosis induced by ischemia, and also tested the antioxidant effects of the pre-conditioning combination.
Materials and methods
This work was supported by the Ethic Committee of The First Affiliated Hospital of the College of Medicine of Zhejiang University.
Reagents
Dulbecco’s Modified Eagle Medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco (Life Technologies, USA). Penicillin-streptomycin solution was obtained from Hyclone (Thermo Scientific, USA), 2’, 7’-dichlorofluorescein diacetate (DCFH-DA) was obtained from Molecular Probes (Eugene, OR, USA), and ISA was obtained from Sigma-Aldrich (NY, USA).
Animals
Male Wistar rats (6-8 weeks old) were purchased from the Shanghai Experimental Animal Center (Shanghai, China). Experimental procedures were approved and performed according to the Animal Experimental Center of the First Affiliated Hospital of Zhejiang University Guidelines of Laboratory Animal Care and Use. All efforts were made to reduce the number of animals tested and suffering.
ISA pre-conditioning
The rats were divided into groups as follows: group 1, control group; group 2, rats treated with ISA [1 mg/kg]); group 3, rats treated with ISA [5 mg/kg]); and group 4, rats treated with ISA [10 mg/kg]). ISA was first dissolved in DMSO before intravenous injection for each group. DMSO with shikimic acid was then added to a normal saline solution; the control group was treated with normal saline alone.
Cell culture
Primary astrocytes were collected from the brains of newborn rats according to the following procedures. Cerebral cortices were removed and the tissues were blocked for 15 min after removal of all white matter. The dissociated cells were collected and diluted in DMEM medium with 10% FBS and 1% penicillin and streptomycin. Harvested cells were seeded at a density of 106 cells/flasks. Cells were then cultured for 2 weeks in an incubator with 5% CO2 and 95% at 37°C. During the replacement of medium, loosened cells were removed as oligodendrocytes and other glial cells.
Infarct and edema assessment
An ischemic model was prepared for 12, 24, or 48 h before sacrifice for infarction and edema assessment. After sacrifice, the brain was removed immediately and put in a normal saline solution at 4°C. Brains were then sliced in 2-mm coronal sections and immersed in triphenyl tetrazolium chloride (TTC) to reveal the infarct damage. The brain slices were immersed for 15 min at 37°C and were then placed in 4% formaldehyde. The infarct damage area was determined by image J software. The volume of infarction was calculated according to the formula used in previous studies [6,12,14]. The brain water content was also determined to assess edema. The water content in each hemisphere was identified by the ratio of wet-to-dry weight and calculated according to previous studies [15,16].
Cell proliferation and viability assay
Primary isolated astrocytes were seeded (2 × 103 per well) into 96-well plates and were cultured overnight. Cells were incubated with ISA in advance as pre-conditioning. Culture medium was removed the next day and fresh medium was added. Cell proliferation and viability were evaluated on days 1, 3, and 5 using Cell Counting Kit-8 (CCK8; Dojindo, Japan) reagent according to the manufacturers’ instructions. The absorbency of cells was measured using a 96-well plate reader at 450 nm.
Cell apoptosis assay
Ad/hIFN-λ-induced apoptosis of HSC-3 and Tca8113 cells was detected by FCM [please spell out with 1st use] with an Annexin V-FITC Apoptosis Detection Kit (KeyGEN) following the manufacturer’s instructions. One hundred milliliters of a 105 cell/ml suspension was stained with kit solution (Annexin-V-FITC and PI) in the dark for 15 min. The apoptosis rate was assayed using FACSCalibur Flow Cytometry (BD, USA) at 488 nm.
TUNEL assay
The terminal deoxynucleotidyl-transferasemediated dUTP nick-end labeling (TUNEL) assay was adopted to determine the apoptosis rate in situ. Brain slices were first fixed with 4% formaldehyde, then permeated with Triton-X. TUNEL staining was then performed according to the manufacturer’s instructions. Nuclei were then labeled with 4’,6-diamidino-2-phenylindole (DAPI), and slices were observed with fluorescent microscopy. The mean value of TUNEL-positive cells was counted in each group.
Reactive oxygen species (ROS) assay
Primary astrocytes (5 × 103 cells/well in 96-well plates) were cultured in DMEM medium supplemented with 10% FBS and 1% antibiotics and containing ISA for 24 h as pre-conditioning, and each well was replaced with DMEM medium supplemented with 10% FBS and 1% antibiotics. The intracellular ROS level was measured using 2’, 7’-dichlorofluorescein diacetate (DCFH), which can be oxidized into fluorescent DCF. After fixing, the cells were washed in 1 × PBS, and then incubated in the dark for 30 min with 10 μM DCFH-DA. Images were obtained using the fluorescence of DCF by fluorescence microscopy.
Enzyme-linked immunosorbent assay (ELISA)
Cerebral tissues were collected, homogenized, and centrifuged for ELISA assay. Levels of murine TNF-α, IL-1β, and IL-10 were assayed by ELISA according to the manufacturer’s instructions. OD values were measured in an ELISA plate reader at a wavelength of 450 nm. The TNF-α, IL-1β, and IL-10 concentrations of each tissue sample were determined according to the standard curve.
qPCR
Total RNA was isolated using Trizol reagent. Reverse transcriptase and oligo’dT primer were used to prepare cDNA from 1 μg of RNA according the manufacturer’s instructions (Takara, Tokyo, Japan). Two microliters of each cDNA was then used for PCR amplification using primers for Bax, Bcl-2, caspase-3, and caspase-8 (Table 1). The detailed information of primers is shown in Table 1.
Table 1.
Primer sequences for qPCR
| Primers | Forward | Reverse | Tm (°C) |
|---|---|---|---|
| Bax | 5’-GCGGCATTACCAACAT-3’ | 5’-CTGGAAGCACCAACGA-3’ | 59 |
| Bcl-2 | 5’-ACCCGAAGCGGACATT-3’ | 5’-GGCATCTCCCTGAACG-3’ | 61 |
| Caspase-3 | 5’-TACCCACCTCAGACAACAGCACC-3’ | 5’-ATCCCCAATCAGAAAACCAGCAC-3’ | 60 |
| Caspase-8 | 5’-AGCAAAGAAGACAGGGAG-3’ | 5’-CAGCGTCAAACAAAGG-3’ | 62 |
| β-actin | 5’-TCCCTGTATGCCTCTG-3’ | 5’-ATGTCACGCACGATTT-3’ | 61 |
Western blot
Cells were lysed in prepared buffer containing 10 mM Tris (pH 7.2), 150 mM NaCl, 5 mM EDTA, 0.1% SDS, 0.5% Triton X-100, and 1% deoxycholic acid. For Western blot, 30 μg of protein samples were subjected to SDS-PAGE, followed by transfer onto PVDF membranes. After blocking in 5% BSA in PBS, membranes were incubated with antibodies against Bax (1:1000), Bcl-2 (1:1000), caspase-3 (1:1000), and β-actin (1:1000) overnight at 4°C, followed by a 1 h-incubation with secondary antibody (1:2000). Blot against β-actin served as the loading control.
Statistical analysis
All data were analyzed using SPSS software (version 13.0) and the results are presented as the mean ± SD. Student’s t-test and two-way analysis of variance (ANOVA) were used to assess statistical significance, with P ≤ 0.05 regarded as statistically significant.
Results
ISA pre-conditioning reduces brain edema and infarction volume
The ischemic animal model was prepared in advance, as previously described. Four groups were set according to the experimental plan: group 1, control group; group 2, rats treated with ISA [1 mg/kg]); group 3, rats treated with ISA [5 mg/kg]); and group 4, rats treated with ISA [10 mg/kg]). Animals were sacrificed at different time points (12, 24, and 48 h), and the brains were removed for infarction and edema assessment. Before sacrificing the rats, the body weights and wet weights of brain were also measured. No significant alterations were detected among the four groups (Figure 1A and 1B). The wet weight of brain was then evaluated, which showed that ISA administration alone could significantly down-regulate the brain edema condition caused by ischemia. Pre-conditioning with ISA could further reduce the brain water content (Figure 1C). The infarction rate was consistent with the edematous state, indicating the highest protective effects of group 4 (pre-conditioning group) compared to other groups (Figure 1D).
Figure 1.
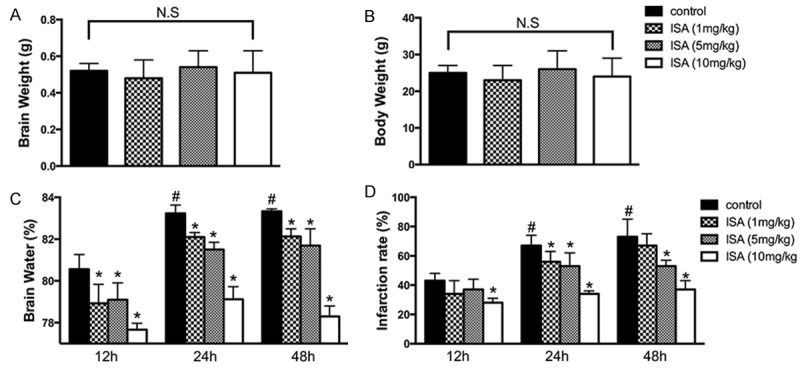
ISA pre-conditioning reduces brain edema and infarction volume. ISA pre-conditioning was administered to rats in the ischemic model described in the Methods. Animals were sacrificed at 12, 24, and 48 h to evaluate edema and infarction damage. A. Average brain weight of rats in different groups; no significance was observed. B. Average body weight of rats in different groups; no significance was observed. C. Brain water content in different groups at 12, 24, and 48 h. D. Brain infarction rate in different groups at 12, 24, and 48 h. Data in the figures represent the average ± SD (n = 3). *P < 0.05, compared to the control group; #P < 0.05, compared to the 12 h group.
ISA pre-conditioning decreases pro-inflammation cytokines release
ELISA assay was then performed to determine the secretion of pro-inflammatory cytokines under ischemic conditions and the potential reverse effects of ISA. Cerebral tissues were collected, homogenized, and centrifuged for an ELISA assay. Levels of murine TNF-α, IL-1β, and IL-10 were then assayed. For the secretion of IL-1β, although the results among different groups varied slightly, a significant decrease in IL-1β release was shown in groups treated with ISA (1 mg/kg) and the pre-conditioning group (Figure 2A). The three experimental groups showed significant alterations in the levels of expression cytokines (IL-10 and TNF-α). Moreover, groups treated with ISA pre-conditioning had the lowest secretion of IL-10 (Figure 2B and 2C). We concluded that pre-conditioning with ISA inhibits the inflammation caused by ischemic condition.
Figure 2.
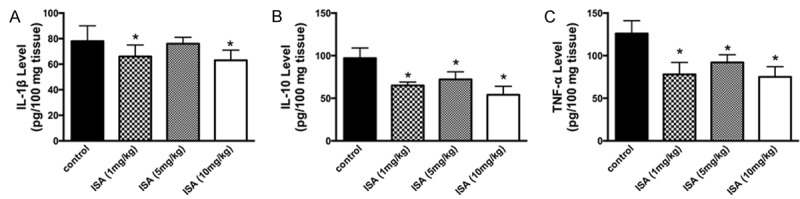
ISA pre-conditioning decreases pro-inflammatory cytokine release. ISA pre-conditioning was administered to rats in the ischemic model described in the Methods. Animals were sacrificed at 12, 24, and 48 h for brain harvesting and inflammatory cytokine detection. A. Level of IL-1β expression assayed by ELISA. B. Level of IL-10 expression assayed by ELISA. C. Level of TNF-α expression assayed by ELISA. Data in the figures represent the average ± SD (n = 3). *P < 0.05, compared to the control group.
ISA pre-conditioning attenuates neuronal and astrocyte apoptosis induced by ischemic injury
To further explore the potential effects of ISA pre-conditioning on cell viability and apoptosis of neurons and astrocytes in vivo and in vitro, we performed the CCK 8 test on isolated primary astrocytes from ischemic animal models and TUNEL staining in situ in the CNS of ischemic rats. The cell proliferative ability of isolated primary astrocytes from ischemic rats was partially improved by treatment with ISA at different dosages (Figure 3A and 3B). Pre-treatment with ISA yielded the best outcomes, although the data were not statistically significant (Figure 3A and 3B). TUNEL staining was then performed in situ on sacrificed rats at different time points (12, 24, and 48 h). The results revealed that the TUNEL-positive cell rates were significantly reduced in each of the time points in groups pre-conditioned with ISA (Figure 3C-E). Thus, pre-conditioning with ISA partially reversed the inhibitory effects of ischemia on neurons and astrocytes.
Figure 3.
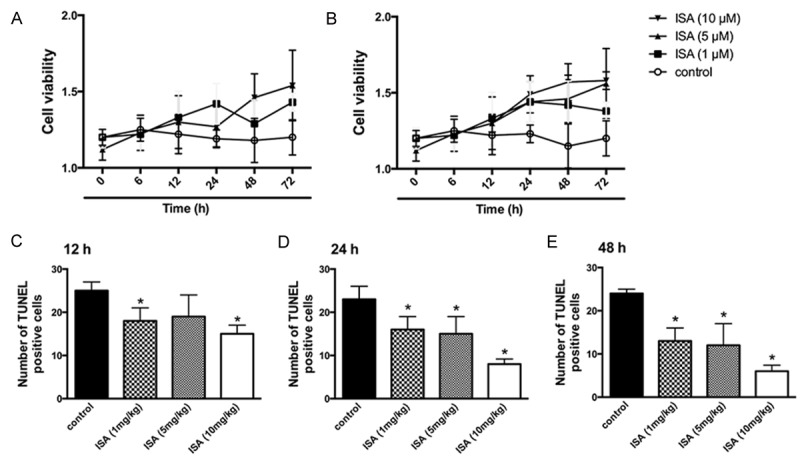
ISA pre-conditioning attenuates neuronal and astrocyte apoptosis induced by ischemic injury. ISA pre-conditioning was administered to rats in the ischemic model described in the Methods. A. Cell viability evaluation of primary astrocytes isolated at 12, 24, and 48 h by CCK8. B. Cell viability evaluation of primary astrocytes isolated at 12, 24, and 48 h by CCK8 with double dosage. C. Number of TUNEL-positive cells at 12 h. D. Number of TUNEL-positive cells at 24 h. E. Number of TUNEL-positive cells at 48 h. Data in the figures represent the average ± SD (n = 3). *P < 0.05, compared to the control group.
ISA pre-conditioning reduces early and late apoptosis induced by hypoxia
To further verify the hypothesis we assumed in the previous result, we then performed FCM to test the early and late cell apoptosis rate of astrocytes in vitro under hypoxic conditions. Primary astrocytes were isolated from ischemic rats after the rats were sacrificed and cells were incubated under normal conditions in DMEM medium with 10% FBS and 1% penicillin and streptomycin. The cultured cells were then transferred to hypoxic conditions for a further 24 h. FCM was then performed to test the early and late cell apoptosis rate (Figure 4A). The early and late cell apoptosis rate of primary astrocytes was inhibited by treatment with ISA. Among the four groups, pre-conditioning with ISA showed the most protective effects under hypoxic condition (Figure 4B and 4C).
Figure 4.
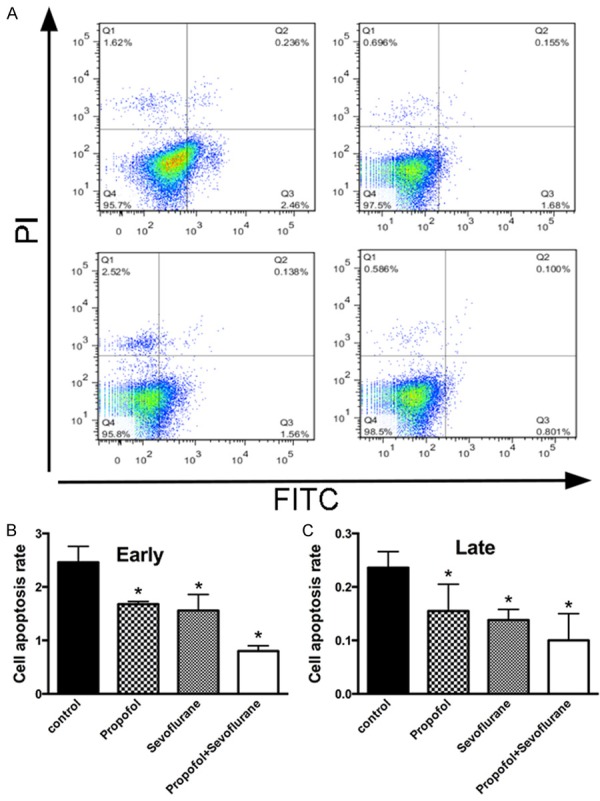
ISA pre-conditioning reduces both early and late apoptosis induced by hypoxic condition. ISA pre-conditioning was administered to rats in the ischemic model described in the Methods. A. FCM analysis of the cell apoptosis rate of astrocytes cultured under hypoxic conditions. B. Early apoptosis rate of astrocytes. C. Late apoptosis rate of astrocytes. Data in the figures represent the average ± SD (n = 3). *P < 0.05, compared to the control group.
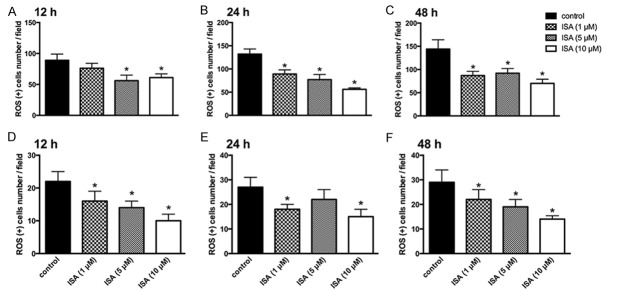
ISA pre-conditioning scavenges ROS generation in brain ischemia
To better elucidate the underlying mechanism of the protective effects of ISA pre-conditioning on ischemic rats brain injury, we examined ROS generation of isolated astrocytes and neurons in situ. The results were in agreement with our previous results, indicating that pre-conditioning with ISA is protective against over-generation of ROS under ischemic conditions. Primary astrocytes were isolated from the ischemic model at 12, 24, and 48 h to determine ROS generation. Pre-conditioning with ISA showed ROS scavenging ability within each group (Figure 5A-C). The ROS-positive cell rate in situ showed similar results as pre-conditioning with ISA, and reduced the ROS-positive cell number as well.
Figure 5.
ISA pre-conditioning scavenges ROS generation in brain ischemia. ISA pre-conditioning was administered to rats in the ischemic model described in the Methods. A. ROS-positive cells of primary astrocytes isolated at 12 h. B. ROS-positive cells of primary astrocytes isolated at 24 h. C. ROS-positive cells of primary astrocytes isolated at 48 h. D. ROS-positive neurons at 12 h. E. ROS-positive neurons at 24 h. F. ROS-positive neurons at 48 h. Data in the figures represent the average ± SD (n = 3). *P < 0.05, compared to the control group.
ISA pre-conditioning down-regulates Bax, Bcl-2, and caspase-3 expression
To better understand the mechanism underlying the protective effects of ISA pre-conditioning against cell apoptosis, we performed qPCR and Western blot on several of the key signaling molecules. Bax, Bcl-2, and caspase-3 were down-regulated by treatment with ISA, especially in groups pre-conditioned with ISA (Figure 6A). The qPCR results revealed that the level of Bax, Bcl-2, and caspase-3 mRNA were also decreased (Figure 6B and 6C); however, the expression of caspase-8 was not affected by treatment with ISA (Figure 6D).
Figure 6.
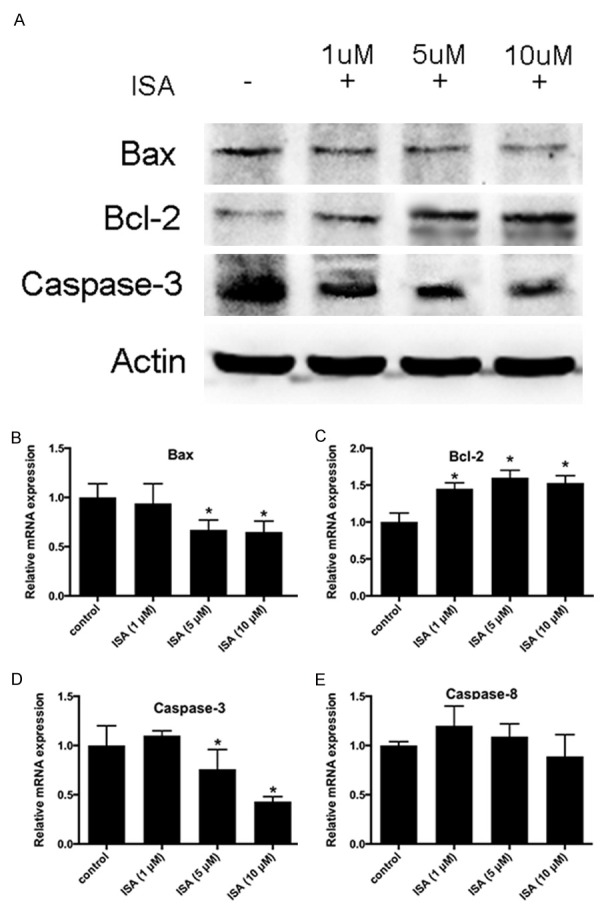
ISA pre-conditioning down-regulates Bax and caspase-3 expression, and up-regulates Bcl-2 expression. ISA pre-conditioning was administered to rats in the ischemic model described in the Methods. A. Representative WB images of Bax, Bcl-2, and caspase-3. B. Relative mRNA expression of Bax. C. Relative mRNA expression of Bcl-2. D. Relative mRNA expression of caspase-3. E. Relative mRNA expression of caspase-8. Data in the figures represent the average ± SD.
Discussion
In the present study we demonstrated the protective effects of ISA pre-conditioning on ischemic brain injury. We also attempted to explain the detailed mechanism underlying the protective effects by focusing on apoptosis and oxidative stress induced by ischemic injury. We showed that pre-treatment with ISA on isolated primary astrocytes and pre-conditioning with ISA in ischemic rats partially reversed the damage caused by ischemic injury. Thus, ISA has a protective effect on ischemic-induced injury when administered alone; what’s more, the combination of the two has a better outcome compared to other groups.
Astrocytes are the most abundant cells in the CNS, and function as a supporting cell on structure, metabolism, and neuronal activity [17]. The function of astrocytes is intimately related with the normal function of neurons on survival, plasticity, and neuronal activity. Astrocytes are even believed to be an alternative resource for the replacement of neurons in some CNS diseases [18]. During the pathologic process of brain ischemia, astrocytes are affected extensively and apoptosis will be induced [19]. Neuronal function damage is mainly caused by neuronal apoptosis under ischemic conditions [11]. In this regard, apoptosis of neurons and astrocytes is a main focus to explain the mechanism by which ischemia causes damage (Figure 3).
Various molecular mechanisms are involved in the process of neuronal apoptosis, among which the JNK pathway is crucial. The JNK family consists of JNK1, JNK2, and JNK3. Interestingly, JNK3 is only expressed in the CNS, testicular tissues, and heart [20]. During the pathologic process of brain ischemia, JNK3 plays a key role in neuronal apoptosis. It has been reported that JNK knockout mice have reduced brain damage following brain ischemic injury [21,22]. In the current study we mainly focused on several other apoptosis-related genes (Bax, Bcl-2, and caspase-3). Since few studies have reported the change in expression of the genes in both astrocytes and neurons, we performed qPCR and Western blot analysis to test ischemic/hypoxic injury on these two cell types. As expected, ISA administration can significantly reduce the expression of these apoptosis-related genes on mRNA and protein levels (Figure 6). Moreover, pre-condition with ISA resulted in a better outcome with respect to the cell apoptosis rate of astrocytes and TUNEL staining of neurons in situ (Figures 3 and 4). Our study results are consistent with previous studies involving the inhibitory effects of ISA on neuronal apoptosis induced by ischemia [4,7,14,23-25]; however, we focused on the pre-conditioning with ISA. Moreover, we also evaluated the cell apoptosis rate of astrocytes as a major cell type affected by apoptosis during ischemic injury (Figure 3).
Cell apoptosis induced by ischemic injury has complex mechanisms. Apart from hypoxia, cell death can also be induced by oxidative stress caused by the extensive rate of oxidative metabolism during the pathologic process [26]. ROS are regarded as one of the most crucial and important causes of brain damage after stoke, especially when reperfusion begins [27-29]. Consistently, compounds like antioxidants are helpful in alleviating the injury caused by ischemia [30]. Free radical generation is a continuous process during the onset of stroke and ischemic injury [31]. Over-generation of ROS and free radicals can lead to severe cellular damage, including membrane lipid peroxidation, DNA fragmentation and damage, and protein damage [32]. Cell apoptosis can also be induced by ROS generation, which is similar for brain ischemia [33,34]. In addition, enzyme activity can also be affected by the abnormal expression and release of ROS, which leads to neuronal cell apoptosis [35,36]. Thus, we performed ROS generation assessment in situ and on primary astrocytes to determine the effects of ISA on ROS production. Indeed, ISA has antioxidant ability on ROS over-generation and ROS was scavenged more thoroughly in groups pre-conditioned with ISA (Figure 5).
In conclusion, treatment with ISA is beneficial in protecting the neuronal damage caused by brain ischemia (Figure 1). Pre-conditioning with ISA had better protective effects in our experimental groups. Our findings are instructive for anesthesia procedures involving brain ischemic diseases, such as stroke.
Acknowledgements
This work was supported by the National Natural Science Foundation 81073082 (to Jie-sheng Zheng) and 81172702 (to Ling-ling Tang).
Disclosure of conflict of interest
None.
References
- 1.Chalela JA, Merino JG, Warach S. Update on stroke. Curr Opin Neurol. 2004;17:447–451. doi: 10.1097/01.wco.0000137536.06986.f9. [DOI] [PubMed] [Google Scholar]
- 2.Hu D, Huang J, Wang Y, Zhang D, Qu Y. Fruits and vegetables consumption and risk of stroke: a meta-analysis of prospective cohort studies. Stroke. 2014;45:1613–1619. doi: 10.1161/STROKEAHA.114.004836. [DOI] [PubMed] [Google Scholar]
- 3.Lackland DT, Roccella EJ, Deutsch AF, Fornage M, George MG, Howard G, Kissela BM, Kittner SJ, Lichtman JH, Lisabeth LD, Schwamm LH, Smith EE, Towfighi A American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; Council on Functional Genomics and Translational Biology. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. 2014;45:315–353. doi: 10.1161/01.str.0000437068.30550.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Luo M, Li C, Wang G. Propofol postconditioning induced long-term neuroprotection and reduced internalization of AMPAR GluR2 subunit in a rat model of focal cerebral ischemia/reperfusion. J Neurochem. 2011;119:210–219. doi: 10.1111/j.1471-4159.2011.07400.x. [DOI] [PubMed] [Google Scholar]
- 5.Adembri C, Venturi L, Tani A, Chiarugi A, Gramigni E, Cozzi A, Pancani T, De Gaudio RA, Pellegrini-Giampietro DE. Neuroprotective effects of propofol in models of cerebral ischemia: inhibition of mitochondrial swelling as a possible mechanism. Anesthesiology. 2006;104:80–89. doi: 10.1097/00000542-200601000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Ergun R, Akdemir G, Sen S, Taşçi A, Ergüngör F. Neuroprotective effects of propofol following global cerebral ischemia in rats. Neurosurg Rev. 2002;25:95–98. doi: 10.1007/s101430100171. [DOI] [PubMed] [Google Scholar]
- 7.Zheng YY, Lan YP, Tang HF, Zhu SM. Propofol pretreatment attenuates aquaporin-4 over-expression and alleviates cerebral edema after transient focal brain ischemia reperfusion in rats. Anesth Analg. 2008;107:2009–2016. doi: 10.1213/ane.0b013e318187c313. [DOI] [PubMed] [Google Scholar]
- 8.Ito H, Watanabe Y, Isshiki A, Uchino H. Neuroprotective properties of propofol and midazolam, but not pentobarbital, on neuronal damage induced by forebrain ischemia, based on the GABAA receptors. Acta Anaesthesiol Scand. 1999;43:153–162. doi: 10.1034/j.1399-6576.1999.430206.x. [DOI] [PubMed] [Google Scholar]
- 9.Engelhard K, Werner C, Eberspächer E, Pape M, Stegemann U, Kellermann K, Hollweck R, Hutzler P, Kochs E. Influence of propofol on neuronal damage and apoptotic factors after incomplete cerebral ischemia and reperfusion in rats: a long-term observation. Anesthesiology. 2004;101:912–917. doi: 10.1097/00000542-200410000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Hu X, Zhang Y, Li W, Liu J, Li Y. Preconditioning with sevoflurane ameliorates spatial learning and memory deficit after focal cerebral ischemia-reperfusion in rats. Int J Dev Neurosci. 2013;31:328–333. doi: 10.1016/j.ijdevneu.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Blomgren K, Zhu C, Hallin U, Hagberg H. Mitochondria and ischemic reperfusion damage in the adult and in the developing brain. Biochem Biophys Res Commun. 2003;304:551–559. doi: 10.1016/s0006-291x(03)00628-4. [DOI] [PubMed] [Google Scholar]
- 12.Liu G, Song J, Guo Y, Wang T, Zhou Z. Astragalus injection protects cerebral ischemic injury by inhibiting neuronal apoptosis and the expression of JNK3 after cerebral ischemia reperfusion in rats. Behav Brain Funct. 2013;9:36–43. doi: 10.1186/1744-9081-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou S, Wu H, Zeng C, Xiong X, Tang S, Tang Z, Sun X. Apolipoprotein E protects astrocytes from hypoxia and glutamate-induced apoptosis. FEBS Lett. 2013;587:254–258. doi: 10.1016/j.febslet.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Liang C, Cang J, Wang H, Xue Z. Propofol attenuates cerebral ischemia/reperfusion injury partially using heme oxygenase-1. J Neurosurg Anesthesiol. 2013;25:311–316. doi: 10.1097/ANA.0b013e31828c6af5. [DOI] [PubMed] [Google Scholar]
- 15.Ping A, Chun ZX, Xue XY. Bradykinin preconditioning induces protective effects against focal cerebral ischemia in rats. Brain Res. 2005;1059:105–112. doi: 10.1016/j.brainres.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Tsirka SE. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain. 2005;128:1622–1633. doi: 10.1093/brain/awh489. [DOI] [PubMed] [Google Scholar]
- 17.Kimelberg HK, Norenberg MD. Astrocytes. Sci Am. 1989;260:66–72. 74, 76. doi: 10.1038/scientificamerican0489-66. [DOI] [PubMed] [Google Scholar]
- 18.Robel S, Berninger B, Gotz M. The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci. 2011;12:88–104. doi: 10.1038/nrn2978. [DOI] [PubMed] [Google Scholar]
- 19.Zhan X, Ander BP, Liao IH, Hansen JE, Kim C, Clements D, Weisbart RH, Nishimura RN, Sharp FR. Recombinant Fv-Hsp70 protein mediates neuroprotection after focal cerebral ischemia in rats. Stroke. 2010;41:538–543. doi: 10.1161/STROKEAHA.109.572537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev. 2006;70:1061–1095. doi: 10.1128/MMBR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuan CY, Whitmarsh AJ, Yang DD, Liao G, Schloemer AJ, Dong C, Bao J, Banasiak KJ, Haddad GG, Flavell RA, Davis RJ, Rakic P. A critical role of neural-specific JNK3 for ischemic apoptosis. Proc Natl Acad Sci U S A. 2003;100:15184–15189. doi: 10.1073/pnas.2336254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pirianov G, Brywe KG, Mallard C, Edwards AD, Flavell RA, Hagberg H, Mehmet H. Deletion of the c-Jun N-terminal kinase 3 gene protects neonatal mice against cerebral hypoxic-ischaemic injury. J Cereb Blood Flow Metab. 2007;27:1022–1032. doi: 10.1038/sj.jcbfm.9600413. [DOI] [PubMed] [Google Scholar]
- 23.Lee JH, Cui HS, Shin SK, Kim JM, Kim SY, Lee JE, Koo BN. Effect of propofol post-treatment on blood-brain barrier integrity and cerebral edema after transient cerebral ischemia in rats. Neurochem Res. 2013;38:2276–2286. doi: 10.1007/s11064-013-1136-7. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Yu W, Li XT, Qi SH, Li B. The effects of propofol on mitochondrial dysfunction following focal cerebral ischemia-reperfusion in rats. Neuropharmacology. 2014;77:358–368. doi: 10.1016/j.neuropharm.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 25.Zhao XC, Zhang LM, Tong DY, An P, Jiang C, Zhao P, Chen WM, Wang J. Propofol increases expression of basic fibroblast growth factor after transient cerebral ischemia in rats. Neurochem Res. 2013;38:530–537. doi: 10.1007/s11064-012-0945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nita DA, Nita V, Spulber S, Moldovan M, Popa DP, Zagrean AM, Zagrean L. Oxidative damage following cerebral ischemia depends on reperfusion - a biochemical study in rat. J Cell Mol Med. 2001;5:163–170. doi: 10.1111/j.1582-4934.2001.tb00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun L, Zhang Y, Zhang Y, Gu Y, Xuan L, Liu S, Zhao X, Wang N, Huang L, Huang Y, Zhang Y, Ren L, Wang Z, Lu Y, Yang B. Expression profile of long non-coding RNAs in a mouse model of cardiac hypertrophy. Int J Cardiol. 2014;177:73–75. doi: 10.1016/j.ijcard.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Fu B, Zhang X, Zhang L, Bai X, Zhao X, Chen L, Cui L, Zhu C, Wang L, Zhao Y, Zhao T, Wang X. Bicyclol upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res Bull. 2014;100:38–43. doi: 10.1016/j.brainresbull.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Wang YH, Wang WY, Chang CC, Liou KT, Sung YJ, Liao JF, Chen CF, Chang S, Hou YC, Chou YC, Shen YC. Taxifolin ameliorates cerebral ischemia-reperfusion injury in rats through its antioxidative effect and modulation of NF-kappa B activation. J Biomed Sci. 2006;13:127–141. doi: 10.1007/s11373-005-9031-0. [DOI] [PubMed] [Google Scholar]
- 30.Clemens JA. Cerebral ischemia: gene activation, neuronal injury, and the protective role of antioxidants. Free Radic Biol Med. 2000;28:1526–1531. doi: 10.1016/s0891-5849(00)00258-6. [DOI] [PubMed] [Google Scholar]
- 31.Traystman RJ, Kirsch JR, Koehler RC. Oxygen radical mechanisms of brain injury following ischemia and reperfusion. J Appl Physiol. 1991;71:1185–1195. doi: 10.1152/jappl.1991.71.4.1185. [DOI] [PubMed] [Google Scholar]
- 32.Floyd RA. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med. 1999;222:236–245. doi: 10.1046/j.1525-1373.1999.d01-140.x. [DOI] [PubMed] [Google Scholar]
- 33.Luo Y, Yang X, Zhao S, Wei C, Yin Y, Liu T, Jiang S, Xie J, Wan X, Mao M, Wu J. Hydrogen sulfide prevents OGD/R-induced apoptosis via improving mitochondrial dysfunction and suppressing an ROS-mediated caspase-3 pathway in cortical neurons. Neurochem Int. 2013;63:826–831. doi: 10.1016/j.neuint.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Khatun S, Chaube SK, Bhattacharyya CN. Generation of hydrogen peroxide mediates hanging death-induced neuronal cell apoptosis in the dentate gyrus of the rat brain. Brain Res Bull. 2013;95:54–60. doi: 10.1016/j.brainresbull.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Candelario-Jalil E, Mhadu NH, Al-Dalain SM, Martínez G, León OS. Time course of oxidative damage in different brain regions following transient cerebral ischemia in gerbils. Neurosci Res. 2001;41:233–241. doi: 10.1016/s0168-0102(01)00282-6. [DOI] [PubMed] [Google Scholar]
- 36.Warner DS, Sheng H, Batinic-Haberle I. Oxidants, antioxidants and the ischemic brain. J Exp Biol. 2004;207:3221–3231. doi: 10.1242/jeb.01022. [DOI] [PubMed] [Google Scholar]