Abstract
Approximately 30% of all breast cancers are caused by a lack of estrogen receptor (ER), which renders the cancer resistant to endocrine-based therapy. Many studies suggest that ubiquitin-specific peptidase 9, X-linked (usp9x) regulates multiple cellular behaviors, such as tumor growth, invasion, and resistance to chemotherapeutic agents. This study aimed to evaluate the anti-tumor effects of WP1130, a partially selective inhibitor of deubiquitinating enzymes, in breast cancer cells. We found that WP1130 enhanced cisplatin cytotoxicity in ER-negative tumor cells (MDA-MB-231 and MDA-MB-468), but had little effect in ER-positive Bcap-37 cells. Western blot analysis revealed that usp9x expression was dramatically lower in ER-positive cells compared to that in ER-negative cells. Furthermore, WP1130 treatment suppressed the expression of usp9x and Mcl-1 in ER-negative cells, but not in ER-positive cells. In addition, we found that knockdown of usp9x diminished the chemosensitization activity of WP1130 on breast cancer cells in the presence of cisplatin. Taken together, these results demonstrated that combined treatment with WP1130 could increase the cisplatin sensitivity in a usp9x-dependent manner in estrogen receptor-negative breast cancer cells.
Keywords: Breast cancer, estrogen receptor, drug resistance, WP1130, usp9x
Introduction
Breast cancer is the leading cause of cancer-related deaths among females worldwide [1]. Estrogen plays a critical role in the growth and differentiation of mammary glands [2]. About 70% of breast cancers are estrogen receptor (ER)-positive, rendering ER an ideal molecular target for hormonal treatment [3]. Nevertheless, approximately 30% of breast cancers fail to express ER protein and are thus resistant to endocrine therapy [4].
Ubiquitination is a covalent modification of cellular proteins at the posttranslational level, which is involved in a wide range of biological processes including cell growth, differentiation, and apoptosis [5,6]. Deubiquitinating enzymes (DUBs) are a large group of ubiquitin-specific proteases that can cleave ubiquitin from proteins [7,8]. Currently, many studies report the differential expression or activation of DUBs in non-small cell lung cancer, breast cancer, and pancreatic ductal adenocarcinoma [9-11].
The ubiquitin specific peptidase 9X (usp9x), a member of the DUBs family, regulates multiple physiological pathways via targeting a variety of substrates [12]. It has been reported that increased usp9x expression predicts poor clinical outcome in patients with esophageal squamous cell carcinoma [13]. In addition, usp9x can promote multiple myeloma cell proliferation by stabilizing myeloid cell leukemia 1 (Mcl-1), a member of the pro-survival BCL-2 family [14]. WP1130, a partially selective DUB inhibitor, has been considered a potential chemosensitizer owing to its inhibiting activity on usp9x deubiquitination [15]. In the current study, we investigated the anti-tumor effects of cisplatin-based therapy combined with WP1130 in breast cancer cells.
Materials and methods
Cell culture
Human breast cancer cell lines, including MDA-MB-231, MDA-MB-468, and Bcap-37, were purchased from American Type Culture Collection (Manassas, VA, USA). All cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C in 5% CO2 incubator.
Cell viability assay
Cell viability was measured by using the Cell Counting Kit-8 (CCK-8) (Dojindo Molecular Technologies, Inc., Rockville, MD, USA) following the manufacturer’s protocol. In brief, cells were seeded onto 96-well plates at a density of 3000 cells/well. After treatment with cisplatin for 48 h, 10 µL of CCK-8 solution (Dojindo, Kumamoto, Japan) was added to each cell. Three hours later, the absorbance was measured at 450 nm using an MRX II microplate reader (Dynex, Chantilly, VA, USA).
EdU incorporation assay
Measurement of inhibitive rate of cell proliferation was carried out using a Click-iT EdU Imaging Kit (Invitrogen, Carlsbad, CA, USA) following the procedure previously described [16].
Flow cytometry
Breast cancer cells were exposed to cisplatin alone or in combination with WP1130. Cells were trypsinized and centrifuged 48 h post the treatment, and the pellet obtained was washed twice with phosphate buffered saline (PBS). Cells were resuspended and then washed with PBS thrice. Apoptotic cells were detected with annexin V-FITC/PI following the protocol of Annexin V-FITC cell Apoptosis Detection Kit (BD, USA).
siRNA transfection
Cells were transfected with usp9x-small interfering RNA (siRNA) or negative control siRNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. The transfection medium (Opti-MEM, Gibco, USA) was replaced with complete medium 12 h post transfection, and the cells were incubated for 48 h.
Western blot analysis
Cells were lysed in 50 μL cell lysis buffer containing protease inhibitors (Sigma, USA), and the cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After transferring proteins to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA), primary antibodies, including anti-usp9x, anti-Mcl-1, and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Abcam, Cambridge, MA, USA), were added. Overnight incubation at 4°C was followed by the membranes being incubated with the appropriate HRP-conjugated secondary antibodies. Protein expression was detected by chemiluminescence (GE Healthcare, Piscataway, NJ, USA) and normalized with the internal control (GAPDH).
Statistical analysis
Data were presented as means ± standard deviation (SD) and analyzed using SPSS 16.0. Analysis of variance (ANOVA) was used to analyze differences between groups with the threshold significance level set at P < 0.05.
Results
Different cisplatin sensitivity and usp9x protein expression in breast cancer cells
First, we performed the CCK-8 assay to measure the viability of breast cancer cell lines (MDA-MB-231, MDA-MB-468 and Bcap-37) exposed to cisplatin for 48 h. Results suggested that the cisplatin sensitivity varied among these three cell lines (Figure 1A). The ER-positive Bcap-37 cells were most sensitive to cisplatin drug treatment; ER-negative MDA-MB-231 and MDA-MB-468 cells were more resistant to cisplatin drug treatment. Western blot analysis revealed that the level of usp9x protein expression in the breast cancer cells was higher in ER-negative MDA-MB-231 and MDA-MB-468 cells compared with that in ER-positive Bcap-37 cells (Figure 1B). These results indicated that the higher expression of usp9x in ER-negative cells may be associated with resistance to cisplatin in breast cancer cells.
Figure 1.
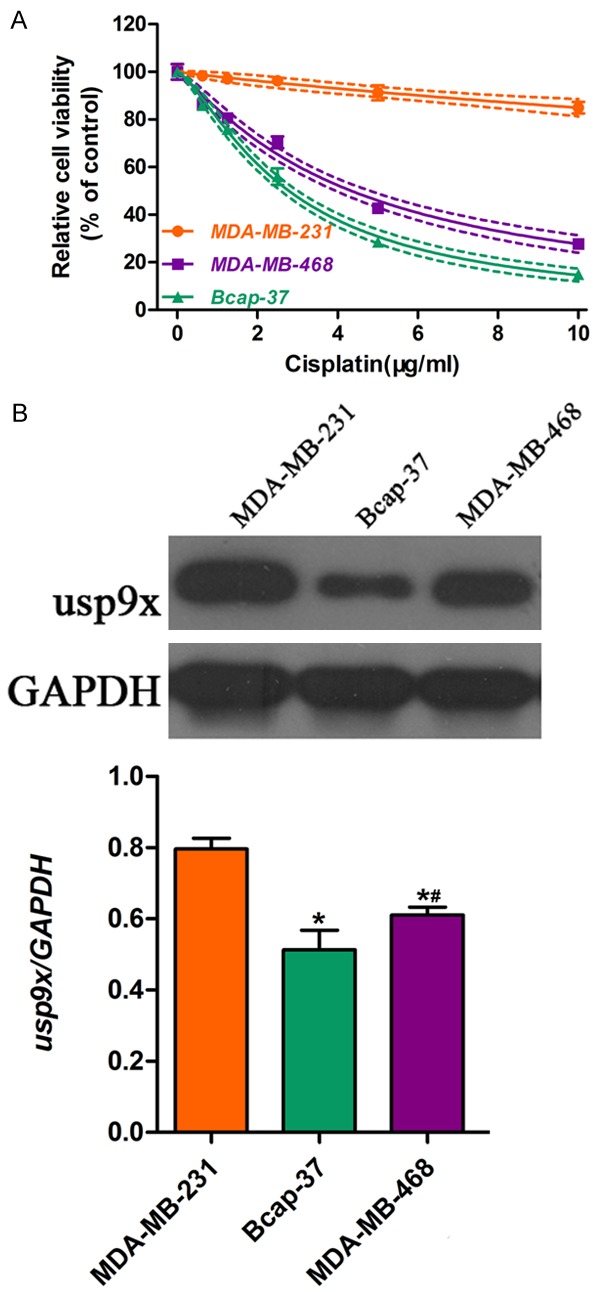
Different cisplatin sensitivity and usp9x expression in breast cancer cells. Breast cancer cell lines including MDA-MB-231, MDA-MB-468, and Bcap-37 were incubated with cisplatin for 48 h. A. CCK-8 assay was performed to measure cell viability of MDA-MB-231, MDA-MB-468, and Bcap-37 cells exposed to cisplatin at different doses (0, 2, 4, 6, 8, 10 μg/mg). B. Western blot was applied to detect the protein expression of usp9x in MDA-MB-231, MDA-MB-468, and Bcap-37 cells. The protein bands were then quantified and normalized to the internal control. * vs. MDA-MB-231 cells, P < 0.05; # vs. Bcap-37 cells, P < 0.05.
Effects of WP1130 on breast cancer cell viability
Tumor cell lines (MDA-MB-231, MDA-MB-468, and Bcap-37) were exposed to different concentrations of WP1130 (0.625, 1.25, 2.5, 5, 10 µM) for 48 h to determine the cytotoxic effects of WP1130. We found that higher doses of WP1130 (5, 10 µM) strongly suppressed the viability of MDA-MB-231 (Figure 2A), MDA-MB-468 (Figure 2B), and Bcap-37 cells (Figure 2C). However, low concentrations of WP1130 (< 2.5 µM) showed negligible cytotoxicity on breast cancer cells. Thus, 2.5 µM WP1130 was used for further co-administration with cisplatin.
Figure 2.
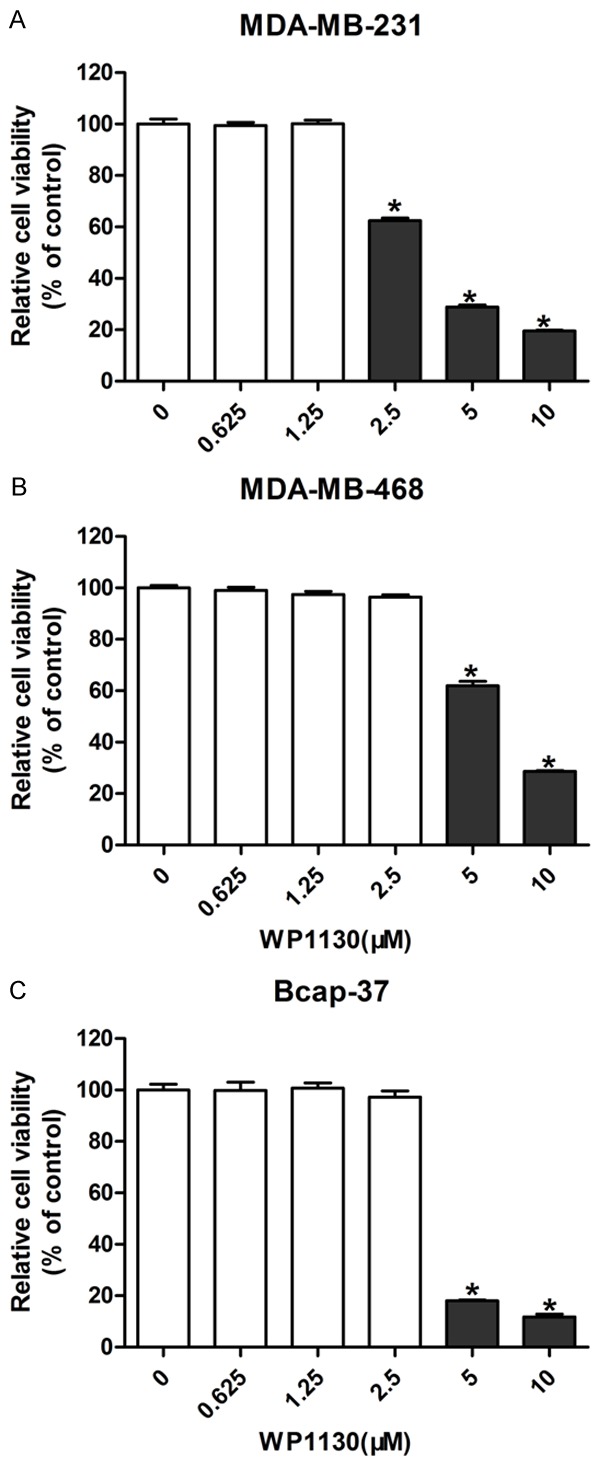
Effects of WP1130 on breast cancer cell viability. Breast cancer cells were exposed to different concentrations of WP1130 (0.625, 1.25, 2.5, 5, 10 µM) for 48 h. CCK-8 assay was performed to measure the cell viability in MDA-MB-231 (A), MDA-MB-468 (B) and Bcap-37 (C). *P < 0.05.
WP1130 increased the cytotoxic effects of cisplatin in ER-negative breast cancer cells
Next, we incubated tumor cells with cisplatin alone or in combination with WP1130. Data from the CCK-8 assay revealed that ER-negative cells (MDA-MB-231 and MDA-MB-468) showed increased sensitivity to cisplatin treatment (Figure 3A and 3B). No significant change in cisplatin cytotoxicity in ER-positive Bcap-37 cells (Figure 3C) was observed.
Figure 3.
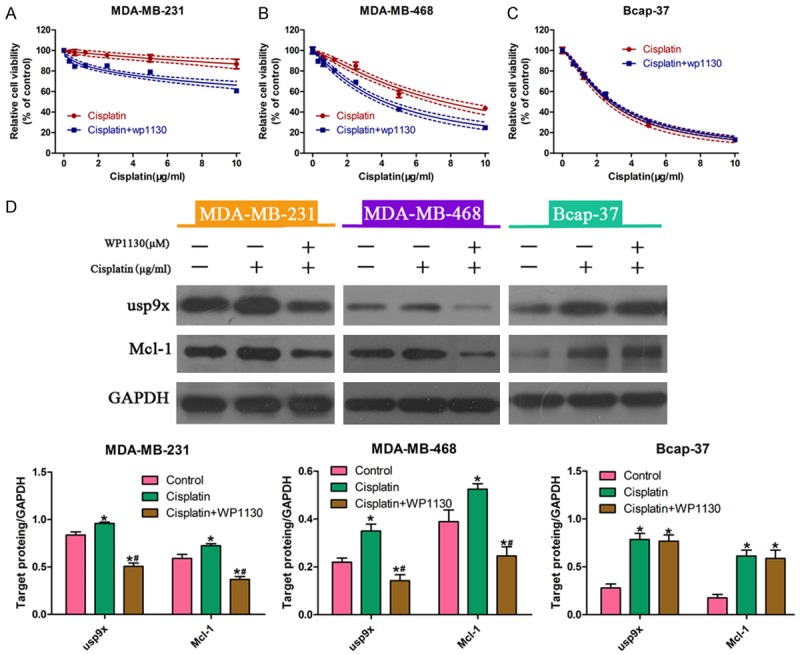
WP1130 increased the cytotoxic effects of cisplatin in breast cancer cells. Breast cancer cells were incubated with cisplatin alone or in combination with WP1130 for 48 h. The CCK-8 assay was performed to determine viability of MDA-MB-231 (A), MDA-MB-468 (B), and Bcap-37 (C) cells. Western blot analysis was performed to measure the protein expression of usp9x and Mcl-1 in MDA-MB-231, MDA-MB-468, and Bcap-37 cells exposed to cisplatin alone or in combination with WP1130 (2.5 µM). The protein bands were then quantified by densitometry with the imaging system (D). * vs. control, P < 0.05; # vs. cisplatin, P < 0.05.
In addition, we investigated the expression of usp9x proteins in cells treated with cisplatin alone or in combination with WP1130. Western blot analysis showed that the protein levels of usp9x and Mcl-1 were significantly reduced in MDA-MB-231 and MDA-MB-468 cells in the presence of WP1130. However, there was no obvious change in usp9x and Mcl-1 protein expression in Bcap-37 cells (Figure 3D). Taken together, these results implied that WP1130 increased cisplatin sensitivity, at least in part, through regulation of usp9x in breast cancer cells.
Effect of WP1130 on cell growth and apoptotic death in breast cancer cells
The DNA synthesis in breast cancer cells were determined using EdU incorporation assay. We found that the proliferation ability of MDA-MB-231, MDA-MB-468, and Bcap-37 cells was significantly reduced after cisplatin treatment. In addition, co-treatment of cells with WP1130 further decreased the DNA synthesis (Figure 4A-C). Moreover, flow cytometry showed no obvious change in the apoptotic rates of tumor cells (Figure 4D). Collectively, these data suggested that combined treatment with WP1130 increased cisplatin cytotoxicity mainly through suppression of cell growth.
Figure 4.

Effect of WP1130 on cell growth and apoptotic death in breast cancer cells. Breast cancer cell lines (MDA-MB-231, MDA-MB-468 and Bcap-37) were incubated with cisplatin alone or in combination with WP1130 for 48 h. Photomicrographs and bar charts depict the EdU staining and relative EdU-positive ratio, respectively, of (A) MDA-MB-231, (B) MDA-MB-468, and (C) Bcap-37 cells after treatment with cisplatin alone or in combination with WP1130 (2.5 µM). Symbols: * vs. control, P < 0.05; # vs. cisplatin, P < 0.05. (D) Flow cytometry was performed to detect the apoptosis rate of MDA-MB-231, MDA-MB-468, and Bcap-37 cells treated with cisplatin alone or combined with WP1130 (2.5 µM).
Down-regulation of usp9x increased cisplatin sensitivity in ER-negative breast cancer cells
RNA interference (RNAi) was used to knockdown the expression of usp9x in the three breast cancer cells to confirm the regulatory role of usp9x on cellular response to cisplatin. Western blot analysis indicated that usp9x protein expression was down-regulated in MDA-MB-231, MDA-MB-468, and Bcap-37 cell lines (Figure 5D). Moreover, reduced protein expression of Mcl-1 was observed in MDA-MB-231 and MDA-MB-468 cells, but not in Bcap-37 cells (Figure 5D). As a result, the usp9x siRNA transfection increased the cisplatin cytotoxicity in MDA-MB-231 (Figure 5A), MDA-MB-468 cells (Figure 5B). Meanwhile, the cell viability in ER-positive Bcap-37 cells exhibited no significant changes regardless of usp9x status (Figure 5C). These data suggested that usp9x played an important role in chemoresistance of breast cancer cells.
Figure 5.
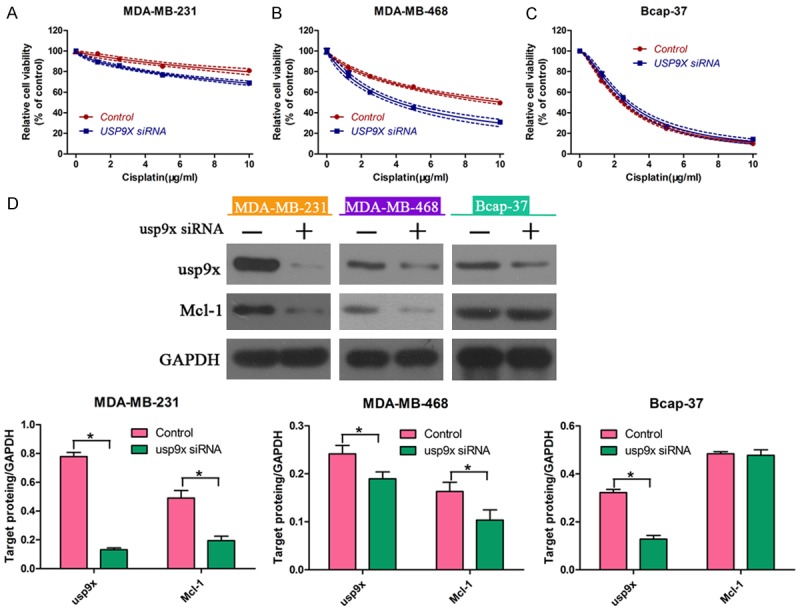
Downregulation of usp9x increased cisplatin sensitivity in breast cancer cells. After transfection with usp9x siRNA or negative control, breast cancer cell lines (MDA-MB-231, MDA-MB-468 and Bcap-37) were exposed to cisplatin at different concentrations (0, 2, 4, 6, 8, 10 μg/mg). Viability of (A) MDA-MB-231, (B) MDA-MB-468, and (C) Bcap-37 cells was determined using CCK-8 assay. (D) Western blot was performed to measure the protein expression of usp9x and Mcl-1 in MDA-MB-231, MDA-MB-468, and Bcap-37 cells. * vs. control, P < 0.05.
Usp9x knockdown diminished the sensitization role of WP1130 in breast cancer cells
Given that usp9x was involved in drug resistance, we hypothesized that the sensitization effects of WP1130 may be dependent on usp9x protein levels. To prove this hypothesis, we transfected usp9x-siRNA into MDA-MB-231 (Figure 6A), MDA-MB-468 (Figure 6B) and Bcap-37 (Figure 6C) cells exposed to cisplatin alone or in combination with WP1130. Consequently, no significant changes in cell viability were observed in cisplatin-treated cells and cisplatin plus WP1130-treated cells. These results suggested that WP1130 increased cellular responses to cisplatin via usp9x in breast cancer cells.
Figure 6.

Usp9x knockdown diminished the chemosensitization role of WP1130. After transfection with usp9x siRNA or negative control, breast cancer cell lines (MDA-MB-231, MDA-MB-468 and Bcap-37) were exposed to cisplatin alone (0, 2, 4, 6, 8, 10 μg/mg) or in combination with WP1130 (2.5 µM). Viability of (A) MDA-MB-231, (B) MDA-MB-468, and (C) Bcap-37 cells was determined using CCK-8 assay.
Discussion
ERs play a critical role in both normal breast development and in breast cancer progression [17]. The presence of ER is considered an effective predictive biomarker for disease-free survival in patients diagnosed with breast cancer. As such, the prognosis is poor for ER-negative patients who typically have a shorter survival time [18]. Currently, it has been recognized that drug resistance to traditional chemotherapeutics greatly contributes to failure in breast cancer therapy [19]. In the current study, we found that WP1130 greatly increased the cisplatin cytotoxicity on ER-negative breast cancer cells.
Protein ubiquitination has been demonstrated as a versatile posttranslational modification, which regulates a wide range of cellular behaviors via the ubiquitylating/deubiquitylating process. The gene usp9x, a member of the DUB family, has been implicated in many biological processes. In pancreatic cells, usp9x plays a role in pro-survival pathway through regulation of autophagy [20]. It has been documented that usp9x promotes tumor cell survival and suppresses apoptosis via deubiquitinating and stabilizing Mcl-1 [14]. Elevated expression of usp9x has been shown to be associated with poor prognosis for patients with multiple myeloma [14]. In addition, RNAi-mediated inhibition of usp9x reduced the tamoxifen sensitivity in breast cancer cells, indicating the association between usp9x and drug resistance [21]. In our study, we found that ER-positive breast cancer cells were more sensitive to cisplatin compared with ER-negative tumor cells. Intriguingly, usp9x protein express was lower in ER-positive cells compared with ER-negative cells, suggesting that increased usp9x protein expression may render breast cancer cells resistant to cisplatin.
WP1130, initially discovered as the inhibitor of JAK and STAT signals, recently has been shown to inhibit the activity of deubiquitinase [22]. It has been reported that WP1130-mediated inhibition of deubiquitinase triggers aggresome formation and promotes tumor cell apoptosis [23]. A recent study has revealed that WP1130 enhances the doxorubicin sensitivity in hepatocellular carcinoma cells through usp9x-dependent p53 degradation [24]. Our study showed that combined treatment with WP1130 increased cisplatin sensitivity in ER-negative cells (MDA-MB-231 and MDA-MB-468), but not in ER-positive Bcap-37 cells. Moreover, molecular investigation revealed WP1130-induced downregulation of usp9x and Mcl-1 in ER-negative cells but not in ER-positive cells. We also found that WP1130-cotreatment increased cisplatin sensitivity mainly through suppression of tumor cell growth. Collectively, these data suggest that WP1130 could increase the sensitivity of breast cancer cells to cisplatin, partly via usp9x, in ER-negative breast cancer cells.
Elevated expression of usp9x protein has been observed in several types of human cancers, including non-small cell lung cancer, esophageal squamous cell carcinoma, colon cancer, and follicular lymphoma [25-27]. On the molecular level, high levels of usp9x protein have been implicated in tumor growth, invasion, and metastasis through regulation of certain genes including TGF-β, Mcl-1, and β-catenin. It has been shown that down-regulation of usp9x increases the sensitivity of tumor cells to chemotherapies via degradation of Mcl-1 proteins [14]. Currently, we interfered with usp9x expression by using RNAi in three breast cancer cell lines. Consequently, the cisplatin sensitivity was increased in ER-negative cells, at least in part, through degradation of Mcl-1 via usp9x inhibition. In addition, we found that knockdown of usp9x diminished the sensitization role of WP1130 on breast cancer cells exposed to cisplatin, suggesting that WP1130 enhanced cisplatin cytotoxicity in a usp9x-dependent way.
In conclusion, the present study demonstrates that combined treatment with WP1130 could sensitize breast cancer cells to cisplatin dependent on usp9x. Thus, our study suggests that combination therapy with WP1130 may improve survival outcomes in patients with breast tumors lacking estrogen receptors.
Acknowledgements
This study was supported by Zhejiang province natural science fund (No. LY13H160006); co-constructed plan by Ministry of Health and Zhejiang Province (No. 201343278); Traditional Chinese medicine scientific research fund project of Zhejiang province (No. 2013ZA076; No. 2012ZA079).
Disclosure of conflict of interest
None.
References
- 1.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 2.Vesuna F, Lisok A, Kimble B, Domek J, Kato Y, van der Groep P, Artemov D, Kowalski J, Carraway H, van Diest P, Raman V. Twist contributes to hormone resistance in breast cancer by downregulating estrogen receptor-alpha. Oncogene. 2012;31:3223–3234. doi: 10.1038/onc.2011.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwarz LJ, Fox EM, Balko JM, Garrett JT, Kuba MG, Estrada MV, Kelley MC, Meszoely IM, Arteaga CL. LYN-activating mutations mediate antiestrogen resistance in estrogen receptorpositive breast cancer. J Clin Invest. 2014;124:5490–5502. doi: 10.1172/JCI72573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santos-Martinez N, Diaz L, Ordaz-Rosado D, Garcia-Quiroz J, Barrera D, Avila E, Larrea F, Garcia-Becerra R. Calcitriol restores antiestrogen responsiveness in estrogen receptor negative breast cancer cells: a potential new therapeutic approach. BMC Cancer. 2014;14:230. doi: 10.1186/1471-2407-14-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou MJ, Chen FZ, Chen HC. Ubiquitination involved enzymes and cancer. Med Oncol. 2014;31:93. doi: 10.1007/s12032-014-0093-6. [DOI] [PubMed] [Google Scholar]
- 6.Voutsadakis IA. Ubiquitination and the ubiquitin-proteasome system as regulators of transcription and transcription factors in epithelial mesenchymal transition of cancer. Tumour Biol. 2012;33:897–910. doi: 10.1007/s13277-012-0355-x. [DOI] [PubMed] [Google Scholar]
- 7.Kapuria V, Peterson LF, Fang D, Bornmann WG, Talpaz M, Donato NJ. Deubiquitinase inhibition by small-molecule WP1130 triggers aggresome formation and tumor cell apoptosis. Cancer Res. 2010;70:9265–9276. doi: 10.1158/0008-5472.CAN-10-1530. [DOI] [PubMed] [Google Scholar]
- 8.McClurg UL, Robson CN. Deubiquitinating enzymes as oncotargets. Oncotarget. 2015;6:9657–9668. doi: 10.18632/oncotarget.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Xu B, Qiang Y, Huang H, Wang C, Li D, Qian J. Overexpression of deubiquitinating enzyme USP28 promoted non-small cell lung cancer growth. J Cell Mol Med. 2015;19:799–805. doi: 10.1111/jcmm.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox JL, Wilder PJ, Wuebben EL, Ouellette MM, Hollingsworth MA, Rizzino A. Context-dependent function of the deubiquitinating enzyme USP9X in pancreatic ductal adenocarcinoma. Cancer Biol Ther. 2014;15:1042–1052. doi: 10.4161/cbt.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuasa-Kawada J, Kinoshita-Kawada M, Rao Y, Wu JY. Deubiquitinating enzyme USP33/VDU1 is required for Slit signaling in inhibiting breast cancer cell migration. Proc Natl Acad Sci U S A. 2009;106:14530–14535. doi: 10.1073/pnas.0801262106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Mancera PA, Rust AG, van der Weyden L, Kristiansen G, Li A, Sarver AL, Knosel T, Goggins M, Pilarsky C, Largaespada DA, Adams DJ, Tuveson DA. The deubiquitinase USP9X suppresses pancreatic ductal adenocarcinoma. Nature. 2012;486:266–270. doi: 10.1038/nature11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng J, Hu Q, Liu W, He X, Cui L, Chen X, Yang M, Liu H, Wei W, Liu S, Wang H. USP9X expression correlates with tumor progression and poor prognosis in esophageal squamous cell carcinoma. Diagn Pathol. 2013;8:177. doi: 10.1186/1746-1596-8-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwickart M, Huang X, Lill JR, Liu J, Ferrando R, French DM, Maecker H, O’Rourke K, Bazan F, Eastham-Anderson J, Yue P, Dornan D, Huang DC, Dixit VM. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature. 2010;463:103–107. doi: 10.1038/nature08646. [DOI] [PubMed] [Google Scholar]
- 15.Kapuria V, Peterson LF, Fang D, Bornmann WG, Talpaz M, Donato NJ. Deubiquitinase inhibition by small-molecule WP1130 triggers aggresome formation and tumor cell apoptosis. Cancer Res. 2010;70:9265–9276. doi: 10.1158/0008-5472.CAN-10-1530. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Feng M, Zheng G, Chen Y, Wang X, Pen B, Yin J, Yu Y, He Z. Chemoresistance to 5-fluorouracil induces epithelial-mesenchymal transition via up-regulation of Snail in MCF7 human breast cancer cells. Biochem Biophys Res Commun. 2012;417:679–685. doi: 10.1016/j.bbrc.2011.11.142. [DOI] [PubMed] [Google Scholar]
- 17.Fishman J, Osborne MP, Telang NT. The role of estrogen in mammary carcinogenesis. Ann N Y Acad Sci. 1995;768:91–100. doi: 10.1111/j.1749-6632.1995.tb12113.x. [DOI] [PubMed] [Google Scholar]
- 18.Schott A, Hayes DF. Adjuvant chemotherapy for elderly women with hormone receptor-positive breast cancer: an old(er) problem. J. Clin. Oncol. 2004;22:4660–4662. doi: 10.1200/JCO.2004.07.961. [DOI] [PubMed] [Google Scholar]
- 19.Natarajan K, Xie Y, Baer MR, Ross DD. Role of breast cancer resistance protein (BCRP/ABCG2) in cancer drug resistance. Biochem Pharmacol. 2012;83:1084–1103. doi: 10.1016/j.bcp.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grasso D, Ropolo A, Lo RA, Boggio V, Molejon MI, Iovanna JL, Gonzalez CD, Urrutia R, Vaccaro MI. Zymophagy, a novel selective autophagy pathway mediated by VMP1-USP9x-p62, prevents pancreatic cell death. J Biol Chem. 2011;286:8308–8324. doi: 10.1074/jbc.M110.197301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oosterkamp HM, Hijmans EM, Brummelkamp TR, Canisius S, Wessels LF, Zwart W, Bernards R. USP9X downregulation renders breast cancer cells resistant to tamoxifen. Cancer Res. 2014;74:3810–3820. doi: 10.1158/0008-5472.CAN-13-1960. [DOI] [PubMed] [Google Scholar]
- 22.Bartholomeusz GA, Talpaz M, Kapuria V, Kong LY, Wang S, Estrov Z, Priebe W, Wu J, Donato NJ. Activation of a novel Bcr/Abl destruction pathway by WP1130 induces apoptosis of chronic myelogenous leukemia cells. Blood. 2007;109:3470–3478. doi: 10.1182/blood-2006-02-005579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapuria V, Peterson LF, Fang D, Bornmann WG, Talpaz M, Donato NJ. Deubiquitinase inhibition by small-molecule WP1130 triggers aggresome formation and tumor cell apoptosis. Cancer Res. 2010;70:9265–9276. doi: 10.1158/0008-5472.CAN-10-1530. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Chen W, Liang C, Chen BW, Zhi X, Zhang S, Zheng X, Bai X, Liang T. WP1130 increases doxorubicin sensitivity in hepatocellular carcinoma cells through usp9x-dependent p53 degradation. Cancer Lett. 2015;361:218–225. doi: 10.1016/j.canlet.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Liu Y, Yang B, Cao H, Yang CX, Ouyang W, Zhang SM, Yang GF, Zhou FX, Zhou YF, Xie CH. Elevated expression of USP9X correlates with poor prognosis in human non-small cell lung cancer. J Thorac Dis. 2015;7:672–679. doi: 10.3978/j.issn.2072-1439.2015.04.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng J, Hu Q, Liu W, He X, Cui L, Chen X, Yang M, Liu H, Wei W, Liu S, Wang H. USP9X expression correlates with tumor progression and poor prognosis in esophageal squamous cell carcinoma. Diagn Pathol. 2013;8:177–181. doi: 10.1186/1746-1596-8-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernassola F, Karin M, Ciechanover A, Melino G. The HECT family of E3 ubiquitin ligases: multiple players in cancer development. Cancer Cell. 2008;14:10–21. doi: 10.1016/j.ccr.2008.06.001. [DOI] [PubMed] [Google Scholar]


