Abstract
Although a series of efficient Akt and ERK inhibitors have been developed to target breast cancer cells, drug resistance can emerge after long-term treatment. Therefore, it is essential to uncover alternative drugs for inhibiting survival pathways in breast cancer cells. Stachydrine hydrochloride, a well-known bioactive ingredients extracted from HerbaLeonuri, has proven to be very efficient for the treatment of various diseases such as prostate cancer. However, whether stachydrine hydrochloride can exert similar prophylactic and therapeutic effects against breast cancer, and the probable underlying molecular mechanism remain unknown. In the present work, the effects of stachydrine hydrochloride on human breast cancer cell lines (T47D and MCF-7) were evaluated. Our results showed that Stachydrine hydrochloride inhibits cell proliferation and induces primary apoptosis and ROS production in T47D and MCF-7 cells in time- and dose-dependent manner. Mechanistically, Stachydrine hydrochloride treatment induced caspase-3 activation and decreased the expression of the anti-apoptotic protein Bcl-2. Moreimportantly, Stachydrine hydrochloride simultaneously inhibited the phosphorylation of Akt and ERK proteins. Overall, our data indicated that Stachydrine hydrochloride induces apoptosis in MCF-7 and T47D cells and exerts inhibitory effects on proliferation by concurrently suppressing Akt and ERK survival signals, suggesting its potential efficiency in treatment of breast cancer.
Keywords: Stachydrine hydrochloride, breast cancer, proliferation, apoptosis, Akt, ERK
Introduction
Breast cancer is one of the major cancers that specifically affect women. In 2013, a total of 232,340 new cases of breast cancer and 39,620 deaths were reported in the United States [1]. Breast cancer is a highly heterogeneous disease with diverse phenotypes, clinical outcomes and responses to therapy that can be divided into several subtypes based on gene expression profiling [2,3]. Several signaling pathways are hyper-activated and play pivotal functions in breast cancer progression and metastasis [4]. Targeting of these signaling pathways via development of selective inhibitors could constitute a very promising approach for treat breast cancer therapy.
Due to the overexpression of human epidermal growth factor 2 (HER2), the mutation of phosphatidylinositide 3-kinases (PI3K) or the inactivation of phosphatase and tensin homolog (PTEN), abnormally high Akt activity is commonly observed in breast cancers [5,6]. Akt serine/threonine kinase, one of the major substrates and signaling mediators of PI3K, plays important roles in cell growth, survival, glycolysis, cell migration and invasion. Moreover, aberrant activation of Akt signaling is generally involved in the pathogenesis of cancer and contributes to poor outcome [7,8]. In recent years, researchers have developed a panoply of Akt inhibitors such as MK-2206 and AZD5363, which proved to effectively inhibit Akt activity in vivo and suppress breast tumor growth, especially HER2-positive breast cancer [9,10]. However, several studies have indicated that some breast cancer cells develop resistance to Akt inhibitors after long-term treatment. For instance, previous studies showed that treatment with AKT inhibitors induces the up-regulation of several receptor tyrosine kinases (RTKs), including human epidermal growth factor 3 (HER3), insulin-like growth factor-1 receptor (IGF-1R), and insulin receptor [11], and that inhibition of HER kinases could dramatically potentiate the anti-cancer effect of Akt inhibitors [11]. It was equally indicated that Akt inhibitor-resistant cells have significantly elevated serum- and glucocorticoid-regulated kinase-1 (SGK-1) levels, which leads to phosphorylation of the SGK1 substrate NDRG1 [N-Myc (neuroblastoma-derived Myc) downstream regulated gene 1] and that SGK1 knockdown could induce growth arrest of Akt-inhibitor-resistant cells [12].
Mitogen-activated protein kinase (MEK)/Extracellular signal-regulated kinase (ERK) pathway is another pivotal survival-related signaling pathway which is frequently activated in cancer cells stimulated by diverse growth factors and cytokines [13]. It has been found that ERK is involved in the regulation of cell proliferation, differentiation and migration processes. ERK signaling is also involved in cell resistance to endocrine therapy, and specific targeting of ERK has been proven to be effective to repress tumor growth [14,15]. Additional findings suggested that Akt and ERK signaling pathways are both vital for breast cancer cell survival and persistent growth, and they are concurrently hyper-activated in breast tumors and could be compensatory for each other when one of them is targeted by specific inhibitors, which is believed to be responsible for drug resistance of breast cancer cells [16,17]. Therefore, in this study, we aimed to uncover potential drugs with potential for dual inhibition of Akt and ERK signaling pathways.
Some extracts from traditional Chinese medicine have been reported to be efficient in breast cancer treatment. For instance, it was found that evodiamine (EVO), an active component of the Chinese herbal medicine EvodiaeFructus, is able to induce apoptosis of doxorubicin-sensitive and -resistant MCF-7 cells by inhibiting the Ras/MEK/ERK cascade [18]. Triptolide, a diterpene triepoxide compound isolated from Tripterygium wilfordii, has been also reported to induce breast cancer cell apoptosis by suppressing the Wnt/β-catenin signaling pathway [19]. An extract purified from Clematis ganpiniana, α-hederin, has been similarly identified as a strong inhibitor of the growth of breast cancer cells and as an apoptosis inducer in these cells [20]. In this study, we sought to identify novel anti-cancer constituents from HerbaLeonuri, a traditional Chinese medicinal plant which has been long used to treat gynecologic diseases and reduce postpartum hemorrhage with low toxicity. Stachydrine Hydrochloride (C7H13NO2·HCL) is the major active constituent of HerbaLeonuri, which is expected to be a potential therapy for cardiovascular diseases. Experimental evidences have suggested Stachydrine hydrochloride as a candidate for alleviating uterine bleeding in RU486-induced abortion [21]. Till now, only one report has conveyed the inhibitory effect of Stachydrine hydrochloride on the viability of prostate cancer cells [22]. Nonetheless, the functional roles of Stachydrine hydrochloride in cancer treatment and the underlying molecular mechanism are still largely unknown.
The present study was designed in order to investigate the effect of Stachydrine hydrochloride on breast cancer cell lines MCF-7 and T47D and explore the underlying molecular mechanism with focus on Akt and ERK signaling pathways. Our present data suggest that Stachydrine hydrochloride is effective in inhibiting proliferation and inducing apoptosis in MCF-7 and T47D cells by dual inhibition of AKT and ERK signaling.
Materials and methods
Cell line, inhibitors and antibodies
Human breast cancer cell lines MCF-7 and T47D were obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured in DMEM medium from Gibco (San Francisco, CA, USA) supplemented with 10% fetal calf serum, penicillin (100 U/mL) and streptomycin (100 µg/mL). The cell culture was maintained at 37°C with 5% CO2 in a humidified atmosphere. Akt inhibitor MK-2206 and ERK inhibitor U0126 were purchased from Selleckchem (Houston, TX, USA). Antibodies for caspase 3 (Ab4051) and Apaf1 (Ab32372) were bought from Abcam (Cambridge, Massachusetts, USA). Antibodies for Akt (#9272S), phospho-Akt (#4058S), ERK (#4376S), phospho-ERK (#4370S), JNK (#9252), phospho-JNK (#4668), p38 (#8690), phospho-p38 (#4511) and GAPDH (#5471) were purchased from Cell Signaling (Danvers, MA, USA). Antibodies for Bcl2 (Sc-492) and Bax (Sc-493) were bought from Santa Cruz (Santa Cruz, CA, USA).
Cell proliferation assay and cell cycle analysis
For CCK-8 counting assay, about 3 × 103 MCF-7 or T47D cells were seeded in each well of a 96-well plate. Cells were then treated with the indicated reagents at indicated time points. On the next day, cells were incubated with CCK-8 (Dojindo, Japan) at 37°C for 1 hour, absorbance was read at 450 nm. For cell cycle analysis, MCF-7 or T47D cells were trypsinized and centrifuged at 1000 rpm for 5 minutes. Cells were then re-suspended in PBS supplemented with 10% FBS and fixed in 700 µL ethanol at -20°C for 24 hours. On the next day, after washing twice with PBS, cells were incubated with RNAase and then mixed with 400 µL of PropidiumIodide (PI) at a concentration of 50 µg/ml for 10 minutes. Cell cycle analysis was then performed by flow cytometry using an apparatus purchased from BD Biosciences (San Jose, CA, USA).
Annexin V/PI dual staining
Cells (1 × 106/ml) were seeded in 6-well plates, cultured until 70-80% confluence and then treated with the indicated reagents for indicated time. Subsequently, cells were subjected to an Annexin V-FITC/PI dual staining kit (Cat. 556547) purchased from BD Biosciences (San Jose, CA, USA) according to the manufacturer’s protocol. Percentage of apoptotic cells was determined using flow cytometry.
Hoechst 33342 staining
MCF-7 and T47D cell nuclei were observed by performing staining with Hoechst 33342. Briefly, cells were incubated with Hoechst 33342 (10 µg/ml) for 15 minutes at room temperature and examined under a fluorescence microscope (Olympus, Beijing, China). Apoptotic cells were characterized by condensation of chromatin and/or nuclear fragmentation.
Mitochondrial membrane potential assay
Cells were washed twice with PBS and then trypsinized. After centrifugation at 1500 rpm for 10 minutes, cell pellets were collected, re-suspended in 100 nM Tetramethylrhodamine, methyl ester (TMRM) for 15 minutes at 37°C and analyzed by flow cytometry according to the manufacturer’s protocol from Immunochemistry technologies (Bloomington, MN, USA).
ROS level detection
Controls and treated MCF-7 and T47D cells were washed twice with PBS and then trypsinized. After centrifugation at 1500 rpm for 10 minutes, collected cells were incubated in diluted Dihydroethidium obtained from Vigorous Biotechnology (Beijing, China) according to the manufacturer’s protocol and analyzed by flow cytometry.
Western blot analysis
Cells were directly lysed in RIPA lysis buffer containing protease and phosphatase inhibitors. The cell lysates were subjected to SDS-PAGE electrophoresis and then blotted onto PVDF membranes. After blocking the membrane with 5% milk fat for 1 hour, the expression of various proteins was detected using corresponding primary antibodies, horseradish peroxidase-conjugated secondary antibody and the enhanced chemiluminescence (ECL) reagents. The concentrations of primary antibodies were as follows: Caspase 3 (1:400), AKT (1:1000), P-AKT (1:1000), ERK (1:1000), P-ERK (1:1000), p38 (1:1000), p-p38 (1:800), Bcl2 (1:100), p-JNK (1:800), JNK (1:1000), Apaf1 (1:500), Bax (1:150) and GAPDH (1:1500).
Statistical analysis
Results are presented as means ± standard deviations (S.D.) for the number of experiments indicated. Student’s t-test and two-way ANOVA followed by the Bofferoni posttest were performed for statistical analysis. The difference was considered significant if P < 0.05.
Results
Stachydrine hydrochloride inhibits proliferation of breast cancer cells
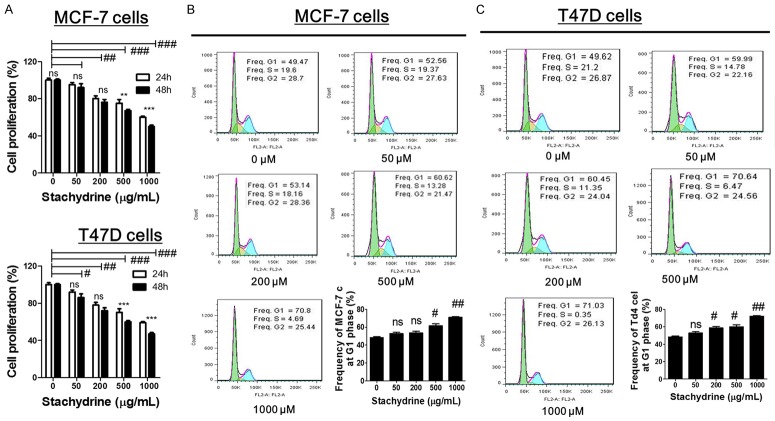
The effect of Stachydrine hydrochloride on the proliferation of MCF-7 and T47D cells when incubated with different concentrations of Stachydrine hydrochloride (0 µM, 50 µM, 200 µM, 500 µM and 1000 µM) was evaluated using the CCK-8 assay. The results showed that treatment with Stachydrine hydrochloride noticeably inhibited MCF-7 and T47D cell proliferation in dose- and time-dependent manner (Figure 1A). Preliminary studies (data not shown) indicated that IC50 of Stachydrine hydrochloride for MCF-7 and T47D is about 500 µM. To further investigate whether Stachydrine hydrochloride treatment regulates cell cycle, flow cytometry was performed for cell cycle analysis. We found that high concentrations (500 µM and 1000 µM) of Stachydrine significantly increased the frequency of both studied cell lines at the G1 phase of cell cycle, suggesting that Stachydrine hydrochloride could cause cell cycle arrest (Figure 1B and 1C). These data suggested that Stachydrine hydrochloride inhibited breast cancer cell growth partly by inducing cell cycle arrest at G1 phase.
Figure 1.
Stachydrine hydrochloride inhibits proliferation and induced G1 phase arrest in MCF-7 and T47D cells. (A) CCK-8 cell counting assay was used to detect proliferation of MCF-7 and T47D cells when treated with Stachydrine hydrochloride at four different concentrations (50, µM 200 µM, 500 µM and 1000 µM) for 24 and 48 hours. Cell cycle analysis of (B) MCF-7 and (C) T47D cells was determined by flow cytometry. Experiments were performed in triplicates and representative images were presented. Data are expressed as mean ± SD from experiments in triplicates. ns: non-significant, **P < 0.01, ***P < 0.001, #P < 0.01 ##P < 0.001 and ###P < 0.001.
Stachydrine hydrochloride induced apoptosis of breast cancer cells
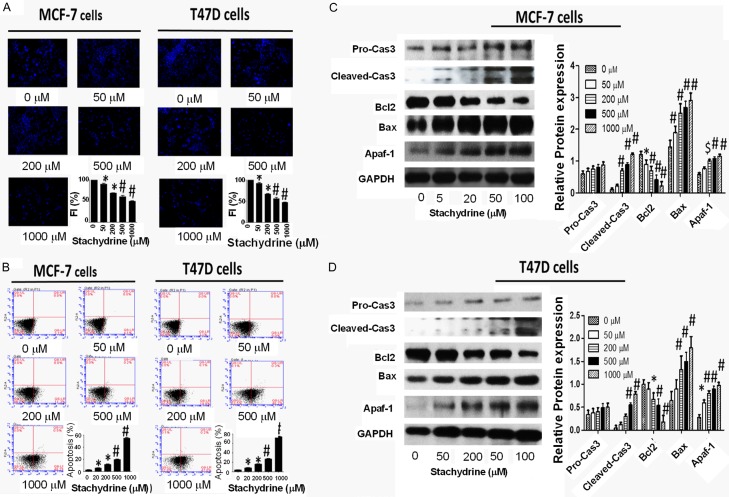
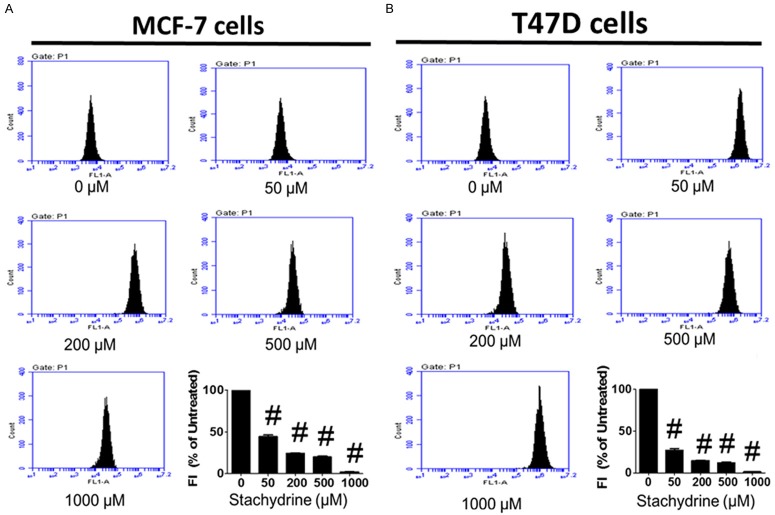
To determine whether Stachydrine hydrochloride exerts its anti-proliferative actions via induction of MCF-7 and T47D cell apoptosis, we first measured its effect on DNA condensation using Hoechst 33342 staining. The results showed that Stachydrine hydrochloride induces DNA condensation in a dose-dependent manner (Figure 2A). Through flow cytometry based Annexin-V/PI double staining analysis, we found that Stachydrine hydrochloride greatly triggered cell death of MCF-7 and T47D cells (Figure 2B), especially by inducing primary apoptosis. To further confirm the pro-apoptotic function of Stachydrine hydrochloride, we measured the expression of several apoptosis-related proteins in MCF-7 and T47D cells treated with different concentration of Stachydrine hydrochloride. The results showed that Stachydrine hydrochloride induced cleaved Caspase-3, Apaf-1 and Bax expression, but downregulated the expression of the antiapoptotic protein Bcl-2 (Figure 2C and 2D). To verify if Stachydrine can effectively induce primary apoptosis, mitochondrial membrane potential detection was performed. The results confirmed that Stachydrine hydrochloride induced primary cell apoptosis (Figure 3). All these data disclosed that Stachydrine hydrochloride markedly induced apoptosis of MCF-7 and T47D cells, especially at high concentration, suggesting its anti-cancer activity.
Figure 2.
Stachydrine hydrochloride induces MCF-7 and T47D cells apoptosis. A. Hoechst 33342 staining showed that Stachydrine hydrochloride induces DNA damage in MCF-7 and T47D cells. Photos were taken at × 400 magnifications. B. Annexin-V/PI double staining was used to detect MCF-7 and T47D cell apoptosis. Experiments were performed in triplicates and representative graphs and their statistical summary were presented. Statistical analysis (mean ± SD) is shown. C. Stachydrine hydrochloride regulates proteins in apoptotic pathways in MCF-7 cells. Western blot analysis showed alterations of expression of several apoptotic markers in MCF-7 cells. MCF-7 cells were treated with Stachydrine hydrochloride at indicated concentrations. Quantitative results are also illustrated. D. Stachydrine hydrochloride regulates proteins in apoptotic pathways in T47D cells. Western blot analysis showed alterations of expression of several apoptotic markers in T47D cells. T47D cells were treated with Stachydrine hydrochloride at indicated concentrations. Quantitative results are also illustrated. *P < 0.05, $P < 0.01 and #P < 0.001.
Figure 3.
Stachydrine hydrochloride induces primary apoptosis in MCF-7 and T47D cells. Mitochondrial membrane potential assay was used to detect early-stage cell apoptosis. MCF-7 and T47D cells were treated with Stachydrine hydrochloride at indicated concentrations. Experiments were performed in triplicates. Statistical analysis (mean ± SD) is shown. #P < 0.001.
Stachydrine hydrochloride induced ROS production in breast cancer cells
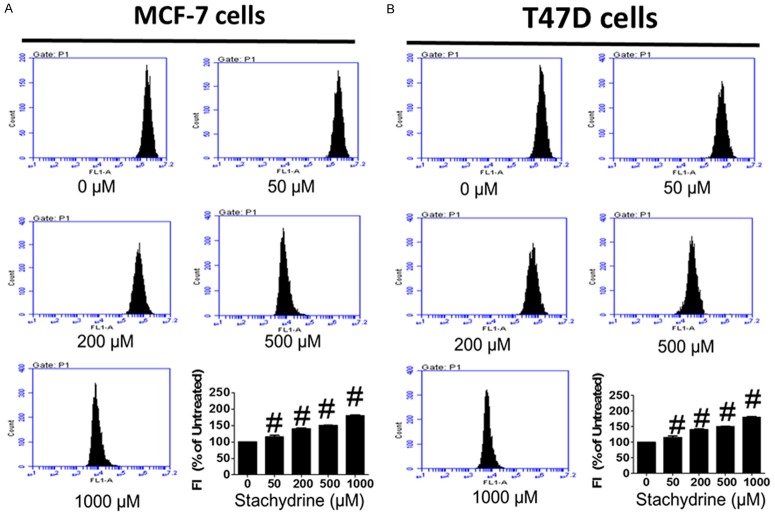
Reactive oxygen species (ROS) is required for apoptosis induction under both physiologic and pathologic conditions by triggering the release of Cytochrome C from mitochondria and downstream caspase activation directly or indirectly [23]. Since Stachydrine hydrochloride effectively induced apoptosis of MCF-7 and T47D cells, to determine whether ROS production is the underlying mechanism of Stachydrine hydrochloride mediated cell death, we measured endogenous ROS levels in these cells with or without Stachydrine hydrochloride treatment. Our result showed that Stachydrine hydrochloride treatment dramatically induced ROS production in both cell lines (Figure 4), suggesting that ROS could be involved in Stachydrine hydrochloride-induced cell apoptosis.
Figure 4.
Stachydrine hydrochloride induces ROS production in MCF-7 and T47D cells. ROS level was detected in MCF-7 and T47D cells when treated with Stachydrine hydrochloride at indicated concentrations. Experiments were performed in triplicates. Statistical analysis (mean ± SD) is shown. #P < 0.001.
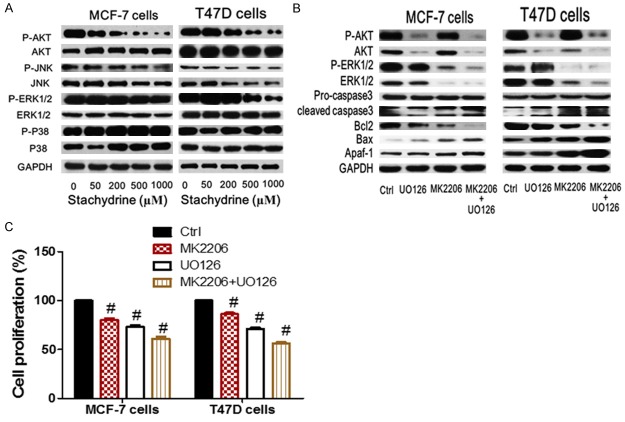
Stachydrine hydrochloride concurrently inhibited Akt and ERK phosphorylation in breast cancer cells
We further evaluated the effect of Stachydrine hydrochlorideon PI3K/Akt and MEK/ERK axes, two vital survival-related signaling pathways in breast cancer cells. Western blot analysis showed that Stachydrine hydrochloride not only significantly suppressed Akt phosphorylation, but also repressed ERK phosphorylation in both MCF-7 and T47D cells. However, Stachydrine hydrochloride treatment did not affect the phosphorylation status of p38 and JNK (Figure 5A). To further confirm the anti-apoptotic roles of Akt and ERK kinase in breast cancer cells, we treated MCF-7 and T47D cells with selective Akt inhibitor MK-2206 and/or ERK inhibitor U0126. As expected, both inhibitors induced Caspase-3 activation and promoted the expression of Apaf-1 and Bax (Figure 5B). On the contrary, these inhibitors repressed Bcl-2 expression. The concurrent treatment with both inhibitors robustly enhanced above changes (Figure 5B). Additionally, CCK-8 cell counting experiment showed that both MK-2206 and U0126 partially inhibited the proliferation of MCF-7 and T47D cells, and combined inhibition of Akt and ERK was more effective than individual inhibitor effect (Figure 5C). Due to the remarkable inhibitory effects of Stachydrine hydrochloride on Akt and ERK signaling pathways, which are both crucial for breast cancer cell survival, we suggested that Stachydrine hydrochloride could be efficient for inhibiting breast cancer cell proliferation through simultaneous suppression of these survival signals, indicating its therapeutic significance in breast cancer treatment.
Figure 5.
Stachydrine hydrochloride concurrently inhibited Akt and ERK phosphorylation in breast cancer cells. A. Western blot analysis of the effect of stachydrine hydrochloride on signaling pathways. B. Effect of Akt inhibitor MK-2206 and/or ERK inhibitor U0126 on apoptotic pathways. C. Effect of Akt inhibitor MK-2206 on cell growth in MCF-7 and T47D cells. #P < 0.001.
Discussion
In the past years, synthesized chemical drugs have been widely used in cancer treatment and some of them have been proved to effectively suppress tumor cell growth and induce their cell death. However, drug development is always accompanied with some severe problems such as side effects and drug resistance. To tackle this issue, researchers are attempting to screen alternative active constituents from natural herbal products as potential candidates with less toxicity for anti-cancer drug development. In this study, we assessed the effect of Stachydrine hydrochloride treatment on breast cancer cell lines MCF-7 and T47D. The results showed that Stachydrine significantly inhibited the proliferation of breast cancer cells, partly by causing cell cycle arrest of MCF-7 and T47D cells at G1 phase, and significantly induced apoptosis of both breast cell lines. The above results were confirmed by western blot analysis of pro- and anti-apoptotic markers and the increased ROS production following Stachydrine hydrochloride treatment. Interestingly, Stachydrine hydrochloride was found to concurrently suppress Akt and ERK phosphorylation in MCF-7 and T47D cells. These data showed that Stachydrine hydrochloride can effectively reduce breast cancer cell growth and their viabilities. This was in corroboration with the data published previously on the effect of Stachydrine on the proliferation or viability of prostate cancer cells [22]. Additionally, we also found that Stachydrine can induce the co-repression of Akt and ERK signaling through which it mediates its anti-cancer effects. Although Stachydrine hydrochloride simultaneously repressed Akt and ERK phosphorylation in a significant manner, the underlying mechanism of de-phosphorylation process is unclear and needs to be explored in further studies. Moreover, whether Stachydrine hydrochloride has similar anticancer effects on other kinds of tumors deserves further in-depth investigations.
Our results also shed light into breast cancer treatment. Aberrant high level of Akt activity is a hallmark of human cancer. Akt regulates cell proliferation and apoptosis by phosphorylating several targeted proteins, including Foxo family members and many cell cycle related proteins. For example, Akt induces foxo3a serine/threonine phosphorylation and blocks its nuclear translocation, leading to repression of foxo3a-induced growth inhibition and expression of apoptosis-related genes [24,25]. Increasing evidence has confirmed that Akt promotes cancer cell proliferation, inhibits cell apoptosis and regulates cell metabolic pathways required for tumor growth [7,8]. Although a series of Akt inhibitors have been proven to effectively induce tumor cell apoptosis, cancer cells can develop resistance to Akt inhibitors via activation of survival pathways. Among Akt feedback signaling molecules, ERK is generally activated with Akt in tumor cells, both of them are pivotal for cell proliferation and evasion to cell apoptosis. In some cases, these two signaling pathways are compensatory for each other. Hence, owing to limited effectiveness of single reagent, a series of clinical trials about combinational usage of inhibitors targeting MEK/ERK and PI3K/AKT pathways are undergoing [16,17]. Similarly, our study demonstrated that combined use of Akt inhibitor MK-2206 and ERK inhibitor U0126 could greatly potentiate the inhibitory effects on MCF-7 and T47D cell proliferation compared to either single treatment. More importantly, Stachydrine hydrochloride exerted a dual inhibitory effect on Akt and ERK activation, suggesting that Stachydrine hydrochloride, as a natural product, is a potential alternative anti-cancer drug that could mimic the combinational use of Akt and ERK inhibitors. However, the anti-proliferative effect of Stachydrine hydrochloride are displayed at high concentrations, which indicates that other cell survival signaling pathways exist besides Akt and ERK signaling pathways. Therefore, it is important to further elucidate the mechanisms underlying cell resistance and to screen those drugs which could potentiate the anti-cancer effects of Stachydrine hydrochloride.
Conclusively, our data demonstrated that Stachydrine hydrochloride significantly inhibited growth and induced apoptosis of MCF-7 and T47D breast cancer cells via activating intrinsic cell death signaling and suppressing cell survival signals, suggesting its potential therapeutic value in the prevention or treatment of breast cancer. Also, the discovery of Stachydrine hydrochloride in breast cancer treatment provides several novel clues which will benefit for future studies, including the potentiality of development of anti-cancer reagents from natural herbal products and possibility of dual inhibition of Akt and ERK activity by a single reagent.
Acknowledgements
We thank all other members of our laboratory for their useful suggestions.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Stingl J, Caldas C. Molecular heterogeneity of breast carcinomas and the cancer stem cell hypothesis. Nat Rev Cancer. 2007;7:791–799. doi: 10.1038/nrc2212. [DOI] [PubMed] [Google Scholar]
- 3.Di Cosimo S, Baselga J. Management of breast cancer with targeted agents: importance of heterogeneity. [corrected] . Nat Rev Clin Oncol. 2010;7:139–147. doi: 10.1038/nrclinonc.2009.234. [DOI] [PubMed] [Google Scholar]
- 4.Nwabo Kamdje AH, Seke Etet PF, Vecchio L, Muller JM, Krampera M, Lukong KE. Signaling pathways in breast cancer: therapeutic targeting of the microenvironment. Cell Signal. 2014;26:2843–2856. doi: 10.1016/j.cellsig.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 5.Rexer BN, Arteaga CL. Optimal targeting of HER2-PI3K signaling in breast cancer: mechanistic insights and clinical implications. Cancer Res. 2013;73:3817–3820. doi: 10.1158/0008-5472.CAN-13-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cidado J, Park BH. Targeting the PI3K/Akt/mTOR pathway for breast cancer therapy. J Mammary Gland Biol Neoplasia. 2012;17:205–216. doi: 10.1007/s10911-012-9264-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciruelos Gil EM. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat Rev. 2014;40:862–871. doi: 10.1016/j.ctrv.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Miller TW, Rexer BN, Garrett JT, Arteaga CL. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res. 2011;13:224. doi: 10.1186/bcr3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, Ueno Y, Hatch H, Majumder PK, Pan BS, Kotani H. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9:1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 10.Davies BR, Greenwood H, Dudley P, Crafter C, Yu DH, Zhang J, Li J, Gao B, Ji Q, Maynard J, Ricketts SA, Cross D, Cosulich S, Chresta CC, Page K, Yates J, Lane C, Watson R, Luke R, Ogilvie D, Pass M. Preclinical pharmacology of AZD5363, an inhibitor of AKT: pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background. Mol Cancer Ther. 2012;11:873–887. doi: 10.1158/1535-7163.MCT-11-0824-T. [DOI] [PubMed] [Google Scholar]
- 11.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J, Rosen N. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sommer EM, Dry H, Cross D, Guichard S, Davies BR, Alessi DR. Elevated SGK1 predicts resistance of breast cancer cells to Akt inhibitors. Biochem J. 2013;452:499–508. doi: 10.1042/BJ20130342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asati V, Mahapatra DK, Bharti SK. PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: structural and pharmacological perspectives. Eur J Med Chem. 2016;109:314–341. doi: 10.1016/j.ejmech.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Neuzillet C, Tijeras-Raballand A, de Mestier L, Cros J, Faivre S, Raymond E. MEK in cancer and cancer therapy. Pharmacol Ther. 2014;141:160–171. doi: 10.1016/j.pharmthera.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 15.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saini KS, Loi S, de Azambuja E, Metzger-Filho O, Saini ML, Ignatiadis M, Dancey JE, Piccart-Gebhart MJ. Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat Rev. 2013;39:935–946. doi: 10.1016/j.ctrv.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Britten CD. PI3K and MEK inhibitor combinations: examining the evidence in selected tumor types. Cancer Chemother Pharmacol. 2013;71:1395–1409. doi: 10.1007/s00280-013-2121-1. [DOI] [PubMed] [Google Scholar]
- 18.Wang S, Wang L, Shi Z, Zhong Z, Chen M, Wang Y. Evodiamine synergizes with doxorubicin in the treatment of chemoresistant human breast cancer without inhibiting P-glycoprotein. PLoS One. 2014;9:e97512. doi: 10.1371/journal.pone.0097512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao H, Ma J, Guo T, Hu R. Triptolide induces apoptosis of breast cancer cells via a mechanism associated with the Wnt/betacatenin signaling pathway. Exp Ther Med. 2014;8:505–508. doi: 10.3892/etm.2014.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng L, Xia TS, Wang YF, Zhou W, Liang XQ, Xue JQ, Shi L, Wang Y, Ding Q, Wang M. The anticancer effect and mechanism of alphahederin on breast cancer cells. Int J Oncol. 2014;45:757–763. doi: 10.3892/ijo.2014.2449. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Wang B, Li Y, Wang L, Zhao X, Zhou X, Guo Y, Jiang G, Yao C. The Th1/Th2/Th17/Treg paradigm induced by stachydrine hydrochloride reduces uterine bleeding in RU486-induced abortion mice. J Ethnopharmacol. 2013;145:241–253. doi: 10.1016/j.jep.2012.10.059. [DOI] [PubMed] [Google Scholar]
- 22.Rathee P, Rathee D, Rathee D, Rathee S. In vitro anticancer activity of stachydrine isolated from Capparis decidua on prostate cancer cell lines. Nat Prod Res. 2012;26:1737–1740. doi: 10.1080/14786419.2011.608673. [DOI] [PubMed] [Google Scholar]
- 23.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 24.Zheng WH, Kar S, Quirion R. Insulin-like growth factor-1-induced phosphorylation of the forkhead family transcription factor FKHRL1 is mediated by Akt kinase in PC12 cells. J Biol Chem. 2000;275:39152–39158. doi: 10.1074/jbc.M002417200. [DOI] [PubMed] [Google Scholar]
- 25.Kumar A, Rajendran V, Sethumadhavan R, Purohit R. AKT kinase pathway: a leading target in cancer research. ScientificWorldJournal. 2013;2013:756134. doi: 10.1155/2013/756134. [DOI] [PMC free article] [PubMed] [Google Scholar]