Abstract
The therapy for the advanced colon cancer (Cca) is unsatisfactory currently. To regulate the immune effector cell function has shown a positive effect on the treatment of advanced cancers. This study tests a hypothesis that administration with curcumin converts the Cca patient-derived regulatory T cells (Treg) to T helper (Th) 1 cells. In this study, a group of patients with advanced Cca was recruited into this study. The patients were treated with curcumin. The peripheral Tregs and Th1 cells were assessed by flow cytometry. The results showed that, after the curcumin therapy, the forkhead box protein (Foxp) 3 positive Treg frequency was markedly reduced, the frequency of Th1 cells was significantly increased in Cca patients. Treating with curcumin repressed the Foxp3 gene transcription in Tregs; the Tregs were then converted into Th1 cells. The results also revealed that Foxp3 bound T-bet to prevent IFN-γ expression in CD4+ T cells, which was abolished by treating with curcumin. In conclusion, the administration of curcumin can convert Tregs to Th1 cells via repressing Foxp3 expression and enhancing IFN-γ production.
Keywords: Colon cancer, curcumin, CD4 T cell, interferon-γ, immunotherapy
Introduction
Colon cancer (Cca) is one of the leading diseases threatening human life [1]. At the advanced stage, Cca often metastases to adjacent tissues or remote tissues, which makes the cancer impossible to be radically removed [2]. Such a condition is indicated suitable for employing the chemotherapy or/and immunotherapy [3,4].
Chemotherapy for cancer is to employ chemical substances, especially one or more anti-cancer drugs to treat cancer. In general, chemotherapy agents are toxic and characterized to kill those cells divided rapidly. Since cancer cells divide rapidly, these drugs kill cancer cells more likely than damage the irrelevant cells in the cancer-bearing hosts [5]. However, a large number of cancer patients still complain plenty of side effects of chemotherapy [6]. Immunotherapy has been employed in the cancer therapy for many years by using immune cells, cytokines and antibodies [7]. Substances are also used to provoke the immune system to attach cancer cells. Curcumin is a phytochemical, with a chemical formula of C21H20O6 (MW: 368.37), is extracted from the plant Curcuma longa, is an antioxidant that exerts antiproliferative and apoptotic effects, and has been employed in the cancer therapy [8]. It is reported that curcumin can modify the various protein expression, including inflammatory cytokines, transcription factors and gene products associating with cell proliferation and survival [9]. Curcumin has been used in the studies of colon cancer [10]; its therapeutic mechanism is not fully understood yet.
Tumor tolerance describes a state of unresponsiveness of the immune system to tumors that have the capacity to elicit an immune response in the body. One of the mechanisms of tumor tolerance is that the population of immune regulatory cells, such as the CD4+ CD25+ Foxp3+ regulatory T cells (Treg), increases in the body [11]. By releasing immune suppressive molecules, such as transforming growth factor (TGF)-β, Tregs suppress the antitumor immune effector cells and thus compromise the immune surveillance on tumors in the body [12]. Yet, how to regulate the Treg population in the cancer-bearing subjects has not been well established.
The immune effector cells are important components of the immune surveillance in the body, including CD8+ T cells, CD4+ T cells, natural killer cells and natural killer T cells. After activation, the antitumor immune cells release antitumor cytokines, such as perforin, granzyme B, interferon (IFN)-γ, to induce cancer cell death [13-15]. The antitumor function of these cells may be disturbed by Tregs in patients with cancers under certain environment [12]. To reconcile such a disturbance between regulatory cells and antitumor cells may contribute to the tumor therapy. In this study, we observed the effect of curcumin, an over-the-counter medicine, in the regulation of the property of Tregs from Cca patients. Curcumin suppressed the expression of forkhead box protein (Foxp) 3, increased the expression of IFN-γ and the frequency of Th1 cells in the peripheral system.
Materials and methods
Reagents
The curcumin (C21H20O6, MW: 368.37, purity: ≥98%) was purchased from Biomart (Beijing, China). The Jurket cells were purchased from the Shanghai Cell Line Bank (Shanghai, China). The antibodies of Foxp3, IFN-γ and T-bet were purchased from Santa Cruz Biotech (Shanghai, China). The fluorochrome-labeled antibodies of Foxp3, CD25, TGF-β and IFN-γ were purchased from BD Biosciences (Shanghai, China). The ChIP kit and luciferase kit were purchased from Sigma Aldrich (Shanghai, China). The reagents for real time RT-PCR and Western blotting were purchased from Invitrogen (Shanghai, China). The immune cell isolation kits were purchased from Miltenyi Biotech (Shanghai, China).
Human subjects and curcumin therapy
Patients with (20 males and 20 females; age: 58.5 ± 22.6) Cca and healthy subjects (15 males and 15 females, age: 55.6 ± 15.2) were recruited into the present study. The diagnosis and surgical removal of the Cca were performed by their surgeons and pathologists. Immediately after the Cca surgery, Cca patients were treated with one month of therapy of 3 g oral capsules of curcumin or an identical placebo in 2 divided doses daily (consisting of 3 capsules twice a day before meals). No other specific Cca treatment was carried out during the period of the curcumin therapy, although the patients were treated with chemotherapy or radiotherapy afterwards (the curcumin therapy did not interfere with the initiation of those Cca therapies). The experiments were approved by the Human Ethic Committee at Tianjin Union Medical Center. All the procedures were performed in accordance with the approved guidelines. An informed, written consent was obtained from each subject.
Isolation of peripheral blood mononuclear cells (PBMC)
The peripheral blood samples (20 ml per subject) were obtained via the elbow vein puncture. PBMCs were isolated from the blood samples by gradient density centrifugation.
Cell culture
The PBMCs or other immune cells were cultured in RPMI1640 medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 0.1 mg/ml streptomycin and 2 mM L-glutamine. The medium was changed in 3 days. The cell viability was checked with the Trypan blue exclusion assay.
Immune cell isolation
The immune cells were purified by the magnetic cell sorting (MACS) with commercial reagent kits following the manufacturer’s instructions. The purity of the isolated cells was checked by flow cytometry. If the purity did not reach or over 95%, the MACS was performed again.
Flow cytometry
For staining of the surface markers, the cells were stained with fluorochrome-labeled antibodies of interest or isotype IgG for 30 min on ice. After washing with buffer, the cells were analyzed by a flow cytometer (FACSCanto II, BD Bioscience, Shanghai, China). For the intracellular molecule staining, after staining with extracellular markers, cells were permeabilized, and fixed, and were stained with fluorochrome-labeled antibodies of interest or isotype IgG for 30 min on ice. After washing, the cells were analyzed with a flow cytometer. The data were analyzed with the software FlowJo with the data of isotype IgG staining as a gating reference.
Preparation of cytosolic and nuclear extracts
Cells were incubated with lysis buffer at 4°C for 15 min, and centrifuged at 500×g for 10 min at 4°C. The supernatant was collected as the cytosolic extract. The pellet was added with nuclear extract buffer and incubated for 15 min at 4°C, followed by centrifugation at 13,000×g for 10 min at 4°C. The supernatant was collected as the nuclear extract. The protein concentrations were determined by the Bradford method.
Chromatin immunoprecipitation (ChIP)
ChIP assay was performed with the reagents and protocol from Sigma Aldrich. In Cells were fixed with 1% formaldehyde for 15 min to cross-link the DNA and protein. The cells were then lysed and sonicated to shear the chromatin DNA. Cell lysate was precleared with protein G-agarose for 2 h at 4°C. The supernatant was incubated overnight at 4°C with 2 μg of specific antibodies or isotype IgG. The precipitated antibody-chromatin complex was collected by incubation with protein G-agarose for 1 h at 4°C, and then washed and eluted in elution buffer. DNA was recovered from the precipitated samples by reverse crosslinking at 65°C for 4 h. The cross-links were reversed at 65°C for 4 h and digested with proteinase K for 1 h at 45°C to remove proteins, then the immunoprecipitated DNA was recovered by phenol/chloroform extraction and ethanol precipitation. The DNA or input (10%, collected before antibody precipitation) was analyzed by qPCR with the following primers: Foxp3 promoter (gcgaaggatgctttgggtag and tcttctccaggttgctgagg). The results were calculated and presented as the folds of change against the input.
Real time quantitative RT-PCR (RT-qPCR)
The total RNA was extracted from the cells with the Trizol reagents. The cDNA was synthesized with the RNA and a reverse transcription kit. The qPCR was performed in a real time PCR device with the SYBR Green Mater Mix and the primers including Foxp3 (cccggatgtgagaaggtctt and cttgtcggatgatgccacag) and IFN-γ (gtgattatcggcagctggtg and tccctttgtttctcccctgg). The results were calculated with the 2-ΔΔCt method and presented as folds of change against a control.
Western blotting
The total proteins were extracted from cells, fractioned by SDS-PAGE and transferred onto a PVDF membrane. After blocking by 5% skim milk, the membrane was incubated with the primary antibodies or isotype IgG overnight at 4°C, and followed by incubating with the second antibodies (labeled with peroxidase) for 1 h at room temperature. Washing with Tris buffered saline-Tween 20 was performed after each time of incubation. The immune blots on the membrane were developed with enhanced chemiluminescence. The results were photographed with an image processing system (KODAK, Shanghai, China).
Reporter gene transfection
The luciferase gene-conjugated reporter genes of Foxp3 and T-bet were provided by Genescript (Nanjing, China) and transfected into Jurket cells or CD4+ T cells with a lipofectamine kit following the manufacturer’s instructions. Cells were lysed 24-h after the transfection in a lysis buffer. Luciferase assays were performed on an Orion II microplate luminometer (Berthold detection systems, Oak Ridge, TN, Germany) with commercial reagent kits following the manufacturer’s instructions. The results were presented as folds of change against a control.
Foxp3 overexpression
The Foxp3 overexpression plasmids and control plasmids were provided by Genescript (Nanjing, China). The plasmids were transfected to CD4+ T cells with a lipofectamine reagent kit following the manufacturer’s instructions. The overexpression results were checked by Western blotting.
Foxp3 polarization
In the RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, 10 mM HEPES and 5 mM β-mercaptoethanol, the naive CD4+ CD25- Foxp3- CD62Lhi T cells were activated with plate-bound anti-CD3 (5 μg/ml) plus soluble anti-CD28 (2 μg/ml), 5 ng/ml rmTGF-β1 and 10 ng/ml rmIL-2. Six days later, the cells were checked by flow cytometry.
Th1 polarization
In the RPMI-1640 medium, supplemented with 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, 10 mm HEPES and 5 mm β-mercaptoethanol, the naive CD4+ CD25- T cells were cultured with plate-bound anti-CD3 (5 μg/ml) plus soluble anti-CD28 (2 μg/ml), 10 ng/ml rmIL-12 and 10 μg/ml anti-IL-4.
Immunoprecipitation (IP)
The protein extracts were pre-cleared by incubation with protein G agarose at 4°C for 2 h. After centrifugation, the supernatant was incubated with specific antibodies at 4°C overnight. The immune complex was precipitated by incubation with protein G agarose at 4°C for 2 h. The immune complex on the agarose beads were eluted with an elution buffer and subjected to Western blotting.
Statistics
The data are presented as mean ± SD. The difference between two groups was determined by Student t test or ANOVA if more than two groups. A P<0.05 was set as a significant criterion.
Results
Administration of curcumin modulates the ratio of CD4+ CD25+ Foxp3+ T cells/CD4+ CD25+ Foxp3- T cells in patients with Cca
We recruited a group of Cca patients in this study. The patients were randomly divided into two groups, the curcumin group and the placebo group. The patients were treated with curcumin or placebo immediately after the Cca surgery and before receiving any additional treatment (such as radiotherapy or chemotherapy). The peripheral blood samples were collected from each patient before and after the treatment as well as from a group of healthy subjects. The samples were analyzed by flow cytometry. The results showed that, compared to the healthy subjects, the frequency of CD4+ CD25+ Foxp3+ T cells (Tregs) was higher, and the frequency of CD4+ CD25+ Foxp3- T cells (Teffs) was lower in the Cca patients, which was comparable with that in the healthy subjects after treatment with curcumin, but not in those treated with placebo, for one month. The results indicate that treatment with curcumin increases the frequency of Teffs and suppresses the frequency of Tregs in patients with the Cca removal.
We then analyzed the phenotypes of the Teffs following the gating procedures. The results showed that the frequency of IL-4+ T cells in Cca patients was similar to that in healthy subjects. The frequency of IFN-γ+ T cell was lower in Cca patients than that in healthy subjects before the curcumin therapy, which was increased to the similar levels of healthy subjects after the one month’s curcumin therapy (Figure 1G). The results implicate that the curcumin therapy suppresses the Treg population and enhances Th1 population in the peripheral system of Cca patients. Possibly, the curcumin therapy converts Tregs to Th1 cells in the Cca patients.
Figure 1.
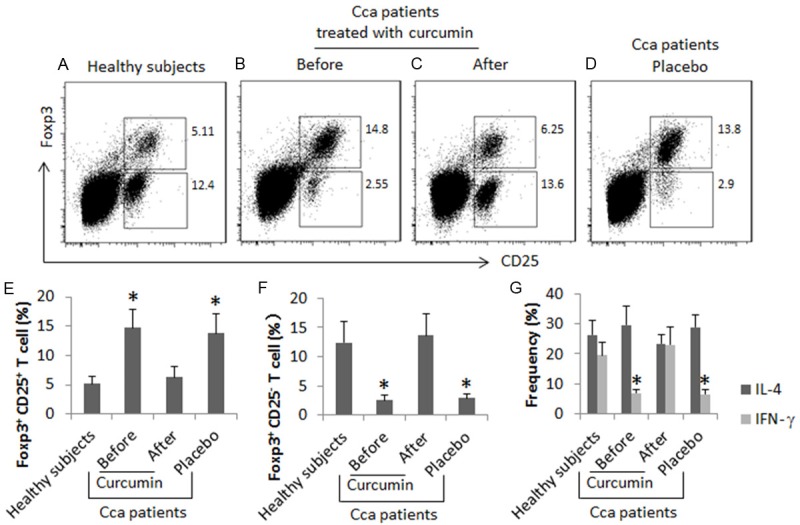
Curcumin modulates the phenotypes of CD4+ T cells in Cca patients. Peripheral mononuclear cells (PBMC) were collected from healthy subjects (n = 10) and Cca patients [treated with curcumin (n = 10) or placebo (n = 10)]. The PBMCs were analyzed by flow cytometry. (A-D) The gated cells show the frequency of Tregs and Teffs. (E-G) The bars indicate the summarized data of Tregs (E) and Teffs (F) in the upper gates of (A-D), or the frequency of IL-4+ cells and IFN-γ+ cells (G) in the lower gates of (A-D). The data of bars are presented as mean ± SD. *, P<0.01, compared to the healthy group. Samples from individual subjects were processed and analyzed separately.
Curcumin induces the chromatin remolding at the Foxp3 promoter locus of the Tregs in Cca patients
The data of Figure 1 suggest that the curcumin therapy suppresses Treg population in Cca patients. To take an insight into the mechanism, we isolated the peripheral CD4+ CD25+ CD127- Tregs from Cca patients before the curcumin therapy and analyzed by ChIP. The results showed that higher levels of p300, acetylated H3K4 and STAT3/5 were detected in the Foxp3 promoter locus, which were gradually declined in the course of the curcumin therapy (Figure 2). The results implicate that the curcumin therapy induces the chromatin remolding at the Foxp3 promoter locus and represses the Foxp3 gene transcription.
Figure 2.
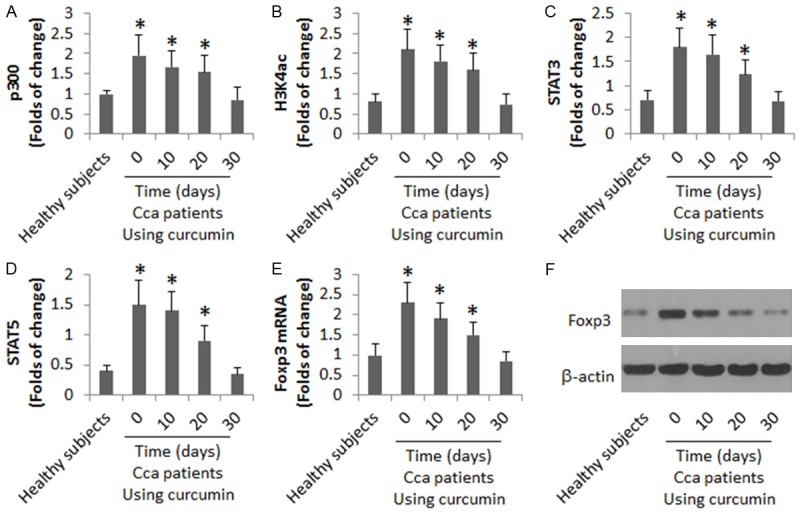
Curcumin represses Foxp3 transcription in Tregs of Cca patients. The CD4+ CD25+ CD127- Tregs were collected from healthy subjects (n = 10) and Cca patients (n = 10; before and 10, 20, and 30 days after the curcumin therapy). The samples were analyzed by ChIP (A-D), RT-qPCR (E) and Western blotting (F). The data of bars are presented as mean ± SD. *, P<0.01, compared to the healthy group. Samples from individual subjects were processed and analyzed separately.
Curcumin represses Foxp3 gene transcription
To enforce the results of Figure 2, we transfected a reporter gene of Foxp3 into Jurket cells, and treated the Jurket cells with the Foxp3 polarization condition with or without the presence of curcumin in the culture. The results showed that the presence of curcumin significantly suppressed the luciferase activity and the Foxp3 levels in the Jurket cells (Figure 3).
Figure 3.
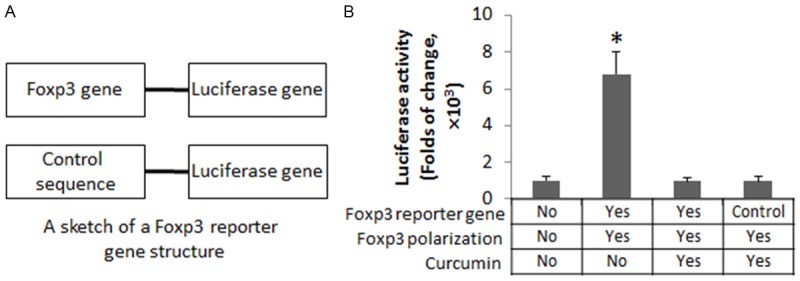
Curcumin represses Foxp3 gene transcription. Jurket cells were transfected with a Foxp3 reporter gene or a control reporter (A). The cells were cultured under a Foxp3 polarization condition for 3 days, and followed by stimulation with curcumin (10 μM) in the culture overnight. (B) The bars indicate the luciferase activity. The data of bars are presented as mean ± SD. *, P<0.01, compared to the control group (the first bar). The data are summarized from 3 independent experiments.
Curcumin converts Foxp3+ Tregs to Th1 cells
The data reported above implicate that curcumin might convert the Tregs to Th1 cells. To this end, we isolated peripheral CD4+ CD25+ CD127- Tregs from Cca patients (Figure 4A, 4B). The cells were treated with curcumin in the culture for 6 days, and then analyzed by flow cytometry. The results showed that more than 90% Tregs were converted to CD4+ IFN-γ+ T cells (Figure 4C-E). No Foxp3+ cells were detected in the curcumin-treated cells (Figure 4F-H). The results were supported by the data of RT-qPCR and Western blotting (Figure 4F, 4G, 4K, 4L).
Figure 4.
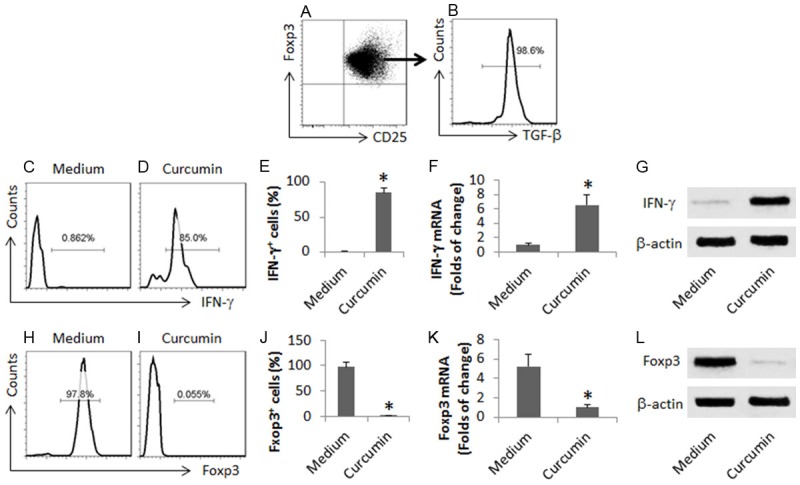
Curcumin converts Cca patient-derived Tregs to Th1 cells. CD4+ CD25+ CD127- T cells were isolated from PBMC (from 10 Cca patients) by MACS (A, B). The cells were cultured in the presence of curcumin (10 mM) for 6 days, and then analyzed. (C, D) The gated histograms indicate the frequency of IFN-γ+ cells. (E) The bars are the summarized data of (C, D). (F) The bars indicate the mRNA levels of IFN-γ. (G) The immune blots indicate the protein levels of IFN-γ. (H, I) The gated histograms indicate the frequency of Foxp3+ cells. (J) The bars are the summarized data of (H, I). (K) The bars indicate the mRNA levels of IFN-γ. (L) The immune blots indicate the protein levels of IFN-γ. The data of bars are presented as mean ± SD. *, P<0.01, compared to the medium group. The data are summarized from 3 independent experiments.
Foxp3 binds T-bet to prevent IFN-γ expression in CD4+ T cells
The above data implicate that the expression of Foxp3 may interfere with the expression of IFN-γ in CD4+ T cells. To test the inference, we overexpressed Foxp3 in CD4+ T cells (Figure 5A). The cells were then exposed to the Th1 polarization condition. Indeed, the expression of Foxp3 prevented the expression of IFN-γ in the CD4+ T cells (Figure 5B). Curiously, we checked if the above CD4+ T cells expressed the T-bet, the IFN-γ transcription factor, after treating with the Th1 polarization. The results showed that the T-bet was still expressed by the CD4+ T cells after treating with Foxp3 overexpression and the Th1 polarization (Figure 5C). The data implicate that although still expressing, the T-bet is dysfunction in the CD4+ T cells. One possibility may be that the Foxp3 prevents the function of T-bet. To test this, we analyzed the extracts of the above CD4+ T cells by immunoprecipitation. The results showed that a complex of Foxp3 and T-bet was detected in the extracts (Figure 5D), suggesting that Foxp3 physically contacts T-bet to prevent the function of T-bet in CD4+ T cells. To support the reasoning, the CD4+ T cells with or without Foxp3 overexpression were transfected with a reporter gene of T-bet (Figure 5E), and then treated with the Th1 polarization condition. The results showed that the Foxp3 overexpression inhibited the T-bet activity in the CD4+ T cells (Figure 5F).
Figure 5.
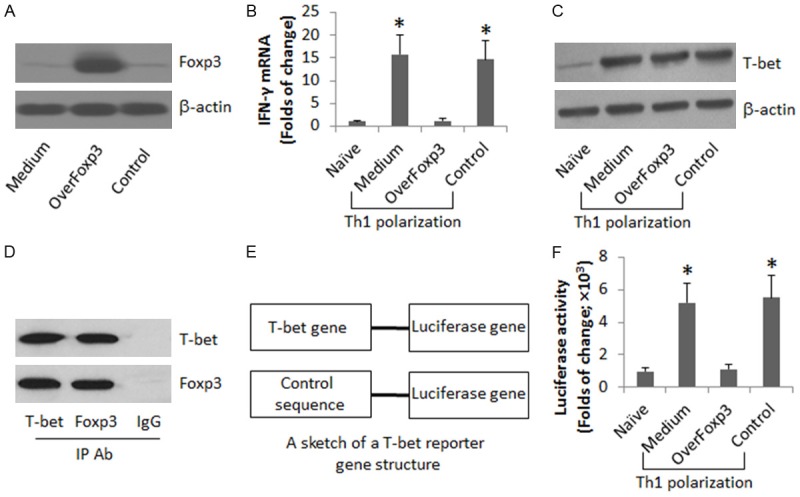
Foxp3 binds T-bet to prevent IFN-γ expression in CD4+ T cells. (A) CD4+ CD25- T cells were collected from 10 healthy human subjects, and transferred with a Foxp3 plasmid or control plasmid (the Control, containing no Foxp3 sequence). The immune blots indicate the Foxp3 overexpression in CD4+ T cells. (B, C) Naïve CD4+ T cells and CD4+ T cells with Foxp3 overexpression were treated with the Th1 polarization condition for 4 days. The cells were analyzed by RT-qPCR (B), Western blotting (C) and IP (D). (E, F) CD4+ T cells with or without Foxp3 overexpression were transfected with a T-bet reporter gene or a control gene, and treated with the Th1 polarization condition for 24 h. The bars indicate the T-bet gene activity. The data of bars are presented as mean ± SD. *, P<0.01, compared to the naive group. The data are representatives of 3 independent experiments.
Discussion
After treating a group of Cca patients with curcumin, we observed that the frequency of Treg was markedly reduced, the Th1 cell population was significantly increased in the peripheral system. The data showed that curcumin repressed the expression of Foxp3 in Tregs. As Foxp3 bound T-bet, the IFN-γ transcription factor, to form a complex, to prevent the IFN-γ expression in CD4+ T cells, the inhibition of Foxp3 by curcumin resulted in the expression of IFN-γ in the CD4+ T cells. Since Th1 cells are one of the important antitumor effector cells [16], the data have expanded our knowledge in the understanding of the antitumor effect of curcumin.
With the property of anti-proliferation and inducing apoptosis, curcumin has the antitumor ability [17]. Curcumin can promote the expression of p53 to induce cancer cell apoptosis, in which the mechanism is to inhibit the expression of insulin-like growth factor-1 (IGF-1) and survivin, since IGF-1 and survivin suppress the function of p53 [18,19]. The present data have revealed a novel functional aspect of curcumin; after taking curcumin for one month, we observed a decrease in Tregs and an increase in Th1 cells in the peripheral system of Cca patients, suggesting that curcumin is capable of regulating the property of Tregs in Cca patients. Tregs are an important cell population in the tumor tolerance [20]. Inhibition of Tregs is one of the approaches to break down the tumor tolerance [21]. Thus, the present data suggest that administration with curcumin may contribute to regulating tumor tolerance.
Th1 cells are one of the antitumor cell population [22]. IFN-γ is regarded as the effective mediator by which the Th1 cells fulfill the antitumor action [23]; such as Imani Fooladi et al reported that administration with Lactobacillus acidophilus increased the production of IFN-γ, which inhibited the experimental breast cancer cell growth [23]. On the other hand, suppressing the production of IFN-γ by Th1 cells promotes cancer growth [24]. Apart from the above published data, the present data have revealed another pathway in the induction of Th1 cells in the cancer bearing hosts. Our data indicate that curcumin can convert Tregs to Th1 cells in the Cca patients. The data are strengthened by the data of in vitro experiments of the present study.
The plasticity of the CD4+ T cells has been reported. Several subtypes of CD4+ T cells have been defined, including Th1, Th2, Th9, Th17 and Tregs. Apart from Tregs, other subtypes of CD4+ T cells have the antitumor capability [13,25,26]. Published data indicate that under a certain environment, Tregs can be converted to other subtypes of CD4+ T cells, such as Singh et al indicate that Tregs can be converted to Th17 cells [27]. Wang et al indicate that Tregs can be converted to Th2 cells [28]. Our data indicate that, after the curcumin therapy, the Cca patient-derived Tregs can be converted into Th1 cells. Supportive data were also reported; Lee et al indicated that transfer with conditioned dendritic cells converted Tregs to Th1 cells [29].
The present data have revealed another indepth mechanism by which the curcumin therapy suppressed the levels of Foxp3 in Tregs. The suppression of Foxp3 resulted in the increase in T-bet levels in the Tregs leading the Tregs to be converted to IFN-γ-producing Th1 cells. Lee et al found that such a conversion was mediated by dendritic cell-derived molecules, which interacted with the Toll like receptors on Tregs. Our data show another aspect of such a conversion. We found that in the Tregs, Foxp3 bound T-bet, the transcription factor of IFN-γ, to prevent the expression of IFN-γ. Since curcumin suppressed the expression of Foxp3, the T-bet was liberated, the IFN-γ was increased in the cells. Zhao et al also observed similar phenomenon; they found that after curcumin stimulation, the nuclear translocation of p65 and c-Rel were markedly decreased, which is critical for Foxp3 and CD25 expression [30].
In summary, the present study showed that the administration of curcumin markedly suppressed the Treg population, and significantly increased the frequency of Th1 in the peripheral system of Cca patients.
Disclosure of conflict of interest
None.
References
- 1.Hemmasi G, Sohrabi M, Zamani F, Ajdarkosh H, Rakhshani N, Khoonsari M, Ameli M, Hatami K. Prevalence of colorectal adenoma in an average-risk population aged 40-50 versus 50-60 years. Eur J Cancer Prev. 2015;24:386–390. doi: 10.1097/CEJ.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Du Y, Liu X, Cho WC, Yang Y. MicroRNAs as regulator of signaling networks in metastatic colon cancer. Biomed Res Int. 2015;2015:823620. doi: 10.1155/2015/823620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu DM, Thakor AS, Baerlocher M, Alshammari MT, Lim H, Kos S, Kennedy AS, Wasan H. A review of conventional and drug-eluting chemoembolization in the treatment of colorectal liver metastases: principles and proof. Future Oncol. 2015;11:1421–1428. doi: 10.2217/fon.15.3. [DOI] [PubMed] [Google Scholar]
- 4.Tudyka V, Blomqvist L, Beets-Tan RG, Boelens PG, Valentini V, van de Velde CJ, Dieguez A, Brown G. EURECCA consensus conference highlights about colon & rectal cancer multidisciplinary management: the radiology experts review. Eur J Surg Oncol. 2014;40:469–475. doi: 10.1016/j.ejso.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Gangadhar T, Schilsky RL. Molecular markers to individualize adjuvant therapy for colon cancer. Nat Rev Clin Oncol. 2010;7:318–325. doi: 10.1038/nrclinonc.2010.62. [DOI] [PubMed] [Google Scholar]
- 6.Saif MW, Relias V, Syrigos K, Gunturu KS. Incidence and management of ZIv-aflibercept related toxicities in colorectal cancer. World J Clin Oncol. 2014;5:1028–1035. doi: 10.5306/wjco.v5.i5.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Childs RW, Carlsten M. Therapeutic approaches to enhance natural killer cell cytotoxicity against cancer: the force awakens. Nat Rev Drug Discov. 2015;14:487–498. doi: 10.1038/nrd4506. [DOI] [PubMed] [Google Scholar]
- 8.Li YH, Niu YB, Sun Y, Zhang F, Liu CX, Fan L, Mei QB. Role of phytochemicals in colorectal cancer prevention. World J Gastroenterol. 2015;21:9262–9272. doi: 10.3748/wjg.v21.i31.9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Talero E, Avila-Roman J, Motilva V. Chemoprevention with phytonutrients and microalgae products in chronic inflammation and colon cancer. Curr Pharm Des. 2012;18:3939–3965. doi: 10.2174/138161212802083725. [DOI] [PubMed] [Google Scholar]
- 11.Janikashvili N, Bonnotte B, Katsanis E, Larmonier N. The dendritic cell-regulatory T lymphocyte crosstalk contributes to tumor-induced tolerance. Clin Dev Immunol. 2011;2011:430394. doi: 10.1155/2011/430394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waldmann TA. Effective cancer therapy through immunomodulation. Annu Rev Med. 2006;57:65–81. doi: 10.1146/annurev.med.56.082103.104549. [DOI] [PubMed] [Google Scholar]
- 13.Galaine J, Borg C, Godet Y, Adotevi O. Interest of tumor-specific CD4 T helper 1 cells for therapeutic anticancer vaccine. Vaccines (Basel) 2015;3:490–502. doi: 10.3390/vaccines3030490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caserta S, Borger JG, Zamoyska R. Central and effector memory CD4 and CD8 T-cell responses to tumor-associated antigens. Crit Rev Immunol. 2012;32:97–126. doi: 10.1615/critrevimmunol.v32.i2.10. [DOI] [PubMed] [Google Scholar]
- 15.Selvan SR, Dowling JP. “Adherent” versus other isolation strategies for expanding purified, potent, and activated human NK cells for cancer immunotherapy. Biomed Res Int. 2015;2015:869547. doi: 10.1155/2015/869547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu HM. Th1 cytokine-based immunotherapy for cancer. Hepatobiliary Pancreat Dis Int. 2014;13:482–494. doi: 10.1016/s1499-3872(14)60305-2. [DOI] [PubMed] [Google Scholar]
- 17.He ZY, Shi CB, Wen H, Li FL, Wang BL, Wang J. Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Invest. 2011;29:208–213. doi: 10.3109/07357907.2010.550592. [DOI] [PubMed] [Google Scholar]
- 18.Tran D, Bergholz J, Zhang H, He H, Wang Y, Zhang Y, Li Q, Kirkland JL, Xiao ZX. Insulinlike growth factor-1 regulates the SIRT1-p53 pathway in cellular senescence. Aging Cell. 2014;13:669–678. doi: 10.1111/acel.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pei SG, Wang JX, Wang XL, Zhang QJ, Zhang H. Correlation of survivin, p53 and Ki67 in laryngeal cancer Hep-2 cell proliferation and invasion. Asian Pac J Trop Med. 2015;8:636–642. doi: 10.1016/j.apjtm.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Lai C, August S, Behar R, Polak M, Ardern-Jones M, Theaker J, Al-Shamkhani A, Healy E. Characteristics of immunosuppressive regulatory T cells in cutaneous squamous cell carcinomas and role in metastasis. Lancet. 2015;385(Suppl 1):S59. doi: 10.1016/S0140-6736(15)60374-9. [DOI] [PubMed] [Google Scholar]
- 21.Whelan MC, Casey G, Larkin JO, Guinn BA, O’Sullivan GC, Tangney M. Oral tolerance to cancer can be abrogated by T regulatory cell inhibition. PLoS One. 2014;9:e97602. doi: 10.1371/journal.pone.0097602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiniwa Y, Li J, Wang M, Sun C, Lee JE, Wang RF, Wang HY. Identification of DRG-1 as a melanoma-associated antigen recognized by CD4+ Th1 cells. PLoS One. 2015;10:e0124094. doi: 10.1371/journal.pone.0124094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imani Fooladi AA, Yazdi MH, Pourmand MR, Mirshafiey A, Hassan ZM, Azizi T, Mahdavi M, Soltan Dallal MM. Th1 cytokine production induced by lactobacillus acidophilus in BALB/c mice bearing transplanted breast tumor. Jundishapur J Microbiol. 2015;8:e17354. doi: 10.5812/jjm.8(4)2015.17354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsukamoto H, Nishikata R, Senju S, Nishimura Y. Myeloid-derived suppressor cells attenuate TH1 development through IL-6 production to promote tumor progression. Cancer Immunol Res. 2013;1:64–76. doi: 10.1158/2326-6066.CIR-13-0030. [DOI] [PubMed] [Google Scholar]
- 25.Ellyard JI, Quah BJ, Simson L, Parish CR. Alternatively activated macrophage possess antitumor cytotoxicity that is induced by IL-4 and mediated by arginase-1. J Immunother. 2010;33:443–452. doi: 10.1097/CJI.0b013e3181cd8746. [DOI] [PubMed] [Google Scholar]
- 26.Vegran F, Apetoh L, Ghiringhelli F. Th9 cells: a novel CD4 T-cell subset in the immune war against cancer. Cancer Res. 2015;75:475–479. doi: 10.1158/0008-5472.CAN-14-2748. [DOI] [PubMed] [Google Scholar]
- 27.Singh K, Gatzka M, Peters T, Borkner L, Hainzl A, Wang H, Sindrilaru A, Scharffetter-Kochanek K. Reduced CD18 levels drive regulatory T cell conversion into Th17 cells in the CD18hypo PL/J mouse model of psoriasis. J Immunol. 2013;190:2544–2553. doi: 10.4049/jimmunol.1202399. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Souabni A, Flavell RA, Wan YY. An intrinsic mechanism predisposes Foxp3-expressing regulatory T cells to Th2 conversion in vivo. J Immunol. 2010;185:5983–5992. doi: 10.4049/jimmunol.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee MK 4th, Xu S, Fitzpatrick EH, Sharma A, Graves HL, Czerniecki BJ. Inhibition of CD4+CD25+ regulatory T cell function and conversion into Th1-like effectors by a Toll-like receptor-activated dendritic cell vaccine. PLoS One. 2013;8:e74698. doi: 10.1371/journal.pone.0074698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao GJ, Lu ZQ, Tang LM, Wu ZS, Wang DW, Zheng JY, Qiu QM. Curcumin inhibits suppressive capacity of naturally occurring CD4+CD25+ regulatory T cells in mice in vitro. Int Immunopharmacol. 2012;14:99–106. doi: 10.1016/j.intimp.2012.06.016. [DOI] [PubMed] [Google Scholar]


