Abstract
Background: We previously showed that miR-301a-3p affects the invasion and migration abilities of pancreatic cancer cells. Here, we explore the role of miR-301a-3p in chemoresistance, which represents a major obstacle in cancer treatment. Methods: We tested the effects of miR-301a-3p ongemcitabine resistance in cytotoxicity assays in vitro and in vivo. We used quantitative real-time PCR (qRT-PCR) to measure miR-301a-3p expression in wild-type and gemcitabine-resistant pancreatic cancer cells. We performed Western blot, qRT-PCR, and luciferase and rescue assays to confirm the direct target of miR-301a-3p. Results: The overexpression and inhibition of miR-301a-3p promoted and reversed, respectively, gemcitabine resistance in pancreatic cancer cells in vitro. The role of miR-301-3p in chemoresistance was dependent on PTEN. The suppression of miR-301-3p expression sensitized pancreatic cancer cells to gemcitabine chemotherapy in a xenograft mouse model. Conclusion: MiR-301a-3p confers resistance to gemcitabine by regulating the expression of PTEN. The co-delivery of miR-301a-3p and gemcitabine might be an effective therapeutic regimen for patients with pancreatic cancer.
Keywords: miRNA, pancreatic cancer, gemcitabine, chemoresistance, PTEN
Introduction
Pancreatic cancer is an extremely aggressive disease in humans. It has a poor prognosis despite recent progress in pharmacological and surgical therapies. Approximately 90% of pancreatic cancers are classified as pancreatic ductal adenocarcinoma (PDAC) and are diagnosed at a later or metastatic stage, offering few therapeutic options. Only one fifth of patients are candidates for surgical resection, after which the 5-year survival rate is less than 5% [1,2]. Although combinational chemotherapies exist, such as gemcitabine with 5-fu or nab-paclitaxel, gemcitabine remains the primary drug for the treatment of pancreatic cancer in most patients [3]. Since 1997, gemcitabine, a nucleoside analog, has been the standard treatment choice for locally advanced and metastatic PDAC [4]. PDAC has proven highly resistant to chemotherapy in clinical practice, however, with primary or acquired drug resistance often leading to treatment failure [5]. Hence, there is an urgent need to understand the mechanism of gemcitabine resistance and to explore new strategies to overcome drug resistance in PDAC.
MiRNAs are endogenous, 18-25 bp, single-stranded, non-coding modulators of the post-transcriptional process [6]. Many biological processes require the functions of miRNAs, and miRNA dysfunction is involved in many pathological processes, including cancers. The links between aberrant miRNA expression and the pathogenesis of several cancers are largely documented [7,8]. There is a growing body of evidence indicating that the deregulation of miRNAs contributes to chemoresistance [9]. The inactivation of oncogenic miRNAs [10] and the restoration of tumor-suppressing miRNAs [11,12] are promising strategies for cancer treatment. Both strategies have been tested in clinical trials [13,14]; however, there are few reports in the literature of miRNA-mediated therapies for PDAC.
In a previous study, we found that miR-301a-3p levels were significantly higher in PDAC tissues and cell lines than in matched non-tumor tissues and normal pancreatic ductal cells. The overexpression of miR-301a-3p in PDAC cells in vitro enhanced colony formation, invasion, and migration but not proliferation [15]. The role of miR-301-3p in chemotherapy has not been investigated. Therefore, we launched a series of experiments to study the effects of miR-301a-3p on gemcitabine resistance in PDAC.
Materials and methods
Cell culture
We obtained human PDAC cell lines (sw1990 and panc-1) from the Department of General Surgery, Shanghai General Hospital (Shanghai, China). We propagated both cell lines in Dulbecco’s modified Eagle medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Invitrogen) at 37°C in humidified air with 5% CO2.
Development of gemcitabine-resistant sw1990 and panc-1 cells
We serially subcultured sw1990 and panc-1 cells for 5 months in medium containing incrementally increasing concentrations of gemcitabine. The starting concentrations for the sw1990 and panc-1 cells were 3 nM and 300 nM, respectively. Thus, we generated resistant cell lines (sw1990/res and panc-1/res) that were able to proliferate in the presence of gemcitabine (8 nM and 600 nM, respectively).
In vitro cytotoxicity tests
We plated sw1990, sw1990/res, panc-1, and panc-1/res cells in triplicate at a concentration of 6×103 cells per well in 96-well plates and maintained the cultures overnight. We prepared fresh gemcitabine before each experiment. We determined the sensitivity of the cells to gemcitabine using the 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) assay. We measured the absorbance of each well at 490 nm (A490) using a spectrophotometer and estimated the concentration of gemcitabine required for 50% growth inhibition (IC50) using relative survival curves. We performed three independent experiments for each treatment and cell type in triplicate.
Plasmids and cell transfection
We purchased a mimic and an inhibitor of miR-301-3p along with their corresponding negative (nonspecific-oligonucleotide) controls from Shanghai GenePharma Co., Ltd. (Shanghai, China). The miR-301-3p mimic consisted of a pcDNA3.1 (+) plasmid with the coding sequence of PTEN inserted into it. The miR-301-3p inhibitor was an siRNA specific for PTEN. We seeded the sw1990 and panc-1 cells in six-well plates at 40% confluence 1 day prior to transfection. We transfected the cells using Invitrogen Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) according to the manufacturer’s protocol. We confirmed the transfection efficiency by Western blot.
Reverse-transcription quantitative polymerase chain reaction (qRT-PCR)
We extracted the total RNA from the cell lines using Invitrogen TRIzol reagent (Thermo Fisher Scientific, Inc.). We performed reverse transcription using the PrimeScript™ RT reagent Kit (Promega, Madison, 21060 WI) according to the manufacturer’s protocol. We performed real-time PCR using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) andan ABI 7900HT fast real-time PCR system (Applied Biosystems). We verified the expression of miR-301-3p by stem-loop qRT-PCR with specific reverse-transcription and PCR primers. We performed qRT-PCR for PTEN and U6 using conditions described previously [15]. We used the comparative CT method, ΔΔCT, to quantify the data. Briefly, we calculated ΔCT by subtracting the CT of U6 or GAPDH mRNA from that of the mRNA of interest and calculated ΔΔCT by subtracting the ΔCT of the negative control from the ΔCT of the samples. We performed the PCRs in triplicate for each sample.
Western blotting
We harvested sw1990 and panc-1 cells in ice-cold PBS and lysed them in radioimmunoprecipitation assay (PIRA) buffer supplemented with protease inhibitors. We determined the protein concentrations using a BCA protein assay kit (Rockford, IL, USA) and analyzed equal amounts of proteins by SDS-PAGE (10% acrylamide gel). The antibodies used for Western blot were β-actin rabbit antibody (CP01, Calbiochem, San Diego, CA) and PTEN rabbit antibodies (Cell Signal Technology, Danvers, MA).
Luciferase assays
We seeded ~1×105 panc-1 cells per well in 24-well plates 24 h before transfection. We transiently co-transfected the panc-1 cells with ~100 ng wild-type or mutant PTEN 3’-UTR psiCHECK-2 plasmid (Promega) and 60 pmol miR-301a-3p mimic, miR-301a-3p inhibitor, or nonspecific-oligonucleotidecontrol using 1.44 μl Lipofectamine reagent (Invitrogen). We harvested the cell lysates 48 h after transfection and measured firefly and Renilla luciferase activities using the Dual-Luciferase Reporter Assay System (Promega) and a Berthold AutoLumat LB9507 rack luminometer. We normalized the Renilla luciferase activity to the firefly luciferase activity to control for transfection efficiency.
Xenograft experiments
All mouse experiments were performed according to protocols approved by the Shanghai Medical Experimental Animal Care Commission. We injected panc-1/res cells (1×106 cells/injection) subcutaneously into both flanks of 5-week-old female nude mice. We injected control miRNA + gemcitabine (20 mg/kg) or miR-301a-3p inhibitor + gemcitabine intraperitoneally into the mice every 4 days starting on day 15. We measured the tumors using calipers and determined the tumor volume [volume = (length×width2)/2] twice per week. We expressed the results as the mean tumor volume and standard deviation (SD).
Statistics
We analyzed the data using SPSS 13.0. We presented the tumor volume as the mean ± SD of triplicate experiments. We assessed differences between groups using Student’s t-tests and considered P<0.05 to be statistically significant.
Results
The intracellular miR-301-3p level affected sensitivity to gemcitabine in PADC cells
To better understand the relationship between miR-301-3p and gemcitabine resistance in PDAC cells, we altered miR-301-3p expression in sw1990 and panc-1 cells. As shown in Figure 1, transfection with the miR-301-3p mimic and inhibitor significantly enhanced and inhibited, respectively, the miR-301-3p expression in the sw1990 cells (Figure 1A) and panc-1 cells (Figure 1C). In cytotoxicity assays, the suppression of miR-301-3p increased the cells’ sensitivity to gemcitabine. The IC50 was 1.625 nM for the suppressed sw1990 cells and 203.4 nM for the suppressed panc-1 cells, which was less than that for the cells transfected with the respective nonspecific-oligonucleotide controls. In contrast, the overexpression of miR-301-3p induced resistance to gemcitabine in both cell types, resulting in IC50 values approximately two times higher than those in cells transfected with the nonspecific-oligonucleotide controls (Figure 1B, 1D).
Figure 1.
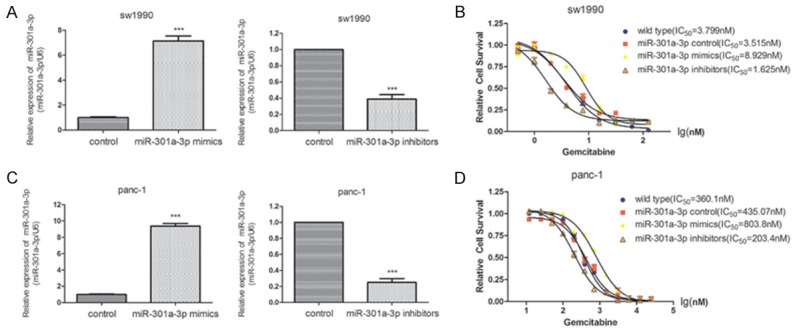
Cytotoxicity of gemcitabine to PDAC cells with altered miR-301a-3p expression. A. The expression of miR-301a-3p in sw1990 cells transfected with nonspecific-oligonucleotide controls or miR-301a-3p mimic or inhibitor. B. Survival curves and IC50 values of sw1990 cells transfected with nonspecific-oligonucleotide controls or miR-301a-3p mimic or inhibitor. C. The expression of miR-301a-3p in panc-1 cells transfected with nonspecific-oligonucleotide controls or miR-301a-3p mimic or inhibitor. D. Survival curves and IC50 values of panc-1 cells transfected with nonspecific-oligonucleotide controls or miR-301a-3p mimic or inhibitor. ***P<0.001 versus the control.
Identification of miR-301a-3p associated with gemcitabine resistance in PDAC cells
The gemcitabine-resistant cell lines were able to tolerate approximately twice the gemcitabine concentrations that the parental cell lines were able to tolerate. The IC50 was 8.058 nM for sw1990/res and 744.0 nM for panc-1/res compared with 3.731 nM for sw1990 and 358.2 nM for panc-1 (Figure 2A, 2C). The qRT-PCR results showed that the levels of miR-301a-3p were strikingly higher in the sw1990/res and panc-1/res cells compared with those in the respective parental cells (Figure 2A, 2C). The inhibition of miR-301a-3p expression in the resistant cells resulted in increased sensitivity to gemcitabine (Figure 2B, 2D).
Figure 2.
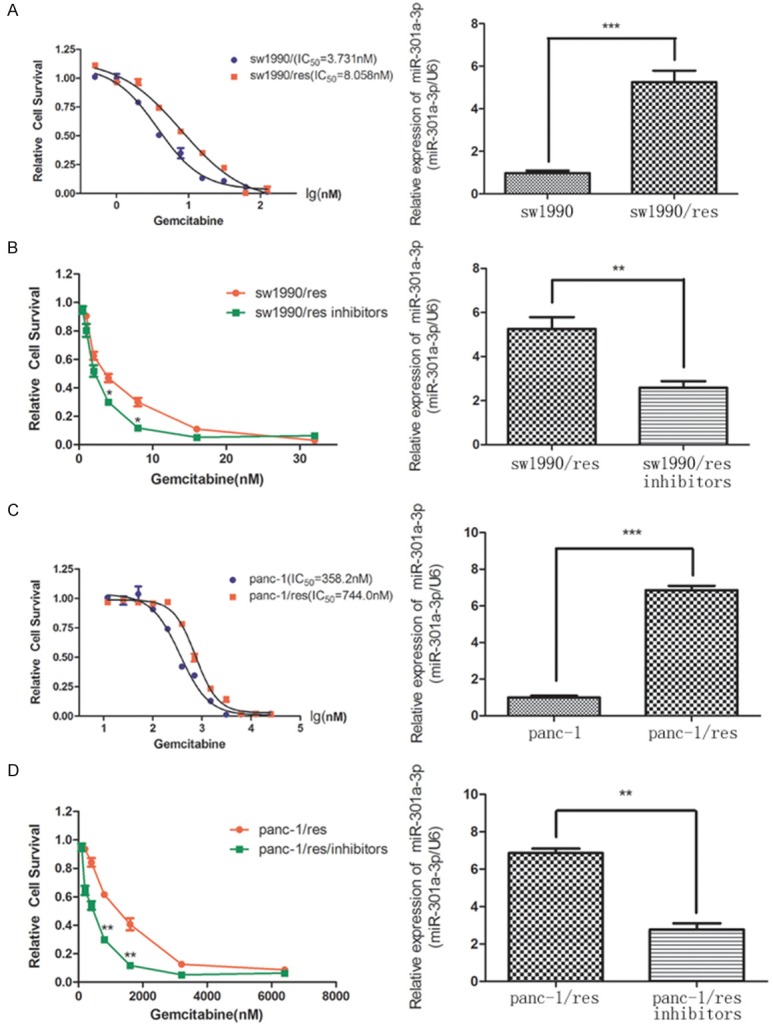
Association of miR-301a-3p expression with resistance to gemcitabine. A. The IC50 value determined by cytotoxicity assay in sw1990 and sw1990/res cells. B. The representative survival curves for sw1990/res cells after transfection with miR-301a-3p inhibitor or nonspecific-oligonucleotide control. C. The IC50 value determined by cytotoxicity assay in panc-1 and panc-1/res cells. D. The representative survival curves for panc-1/res cells after transfection with miR-301a-3p inhibitor or nonspecific-oligonucleotide control. The expression levels of miR-301a-3p in parental, resistant, and inhibitor-transfected cells determined by qRT-PCR (right panel). *P<0.05, **P<0.01 and ***P<0.001 versus the control.
The combination of miR-301a-3p inhibitor and gemcitabine attenuated PDAC tumor formation
Because the miR-301a-3p inhibitor resulted in increased sensitivity to gemcitabine in PDAC cells, we reasoned that the inhibitor might decrease primary tumor formation when administered with gemcitabine in vivo. We established a xenograft mouse model to assess the effect of miR-301a-3p inhibition on chemoresistance in the panc-1/res cells. As shown in Figure 3A-C, gemcitabine treatment alone did not inhibit tumor growth in mice with panc-1/res xenografts, but a combination of miR-301a-3p inhibitor and gemcitabine significantly inhibited tumor formation and reduced the tumor weight. The qRT-PCR results revealed moderate to strong downregulation of miR-301a-3p in the tumors treated with the combination of miR-301a-3p inhibitor and gemcitabine (Figure 3D). Those results indicated that the inhibition of miR-301a-3p could significantly augment the sensitivity to gemcitabine in PDAC cells.
Figure 3.
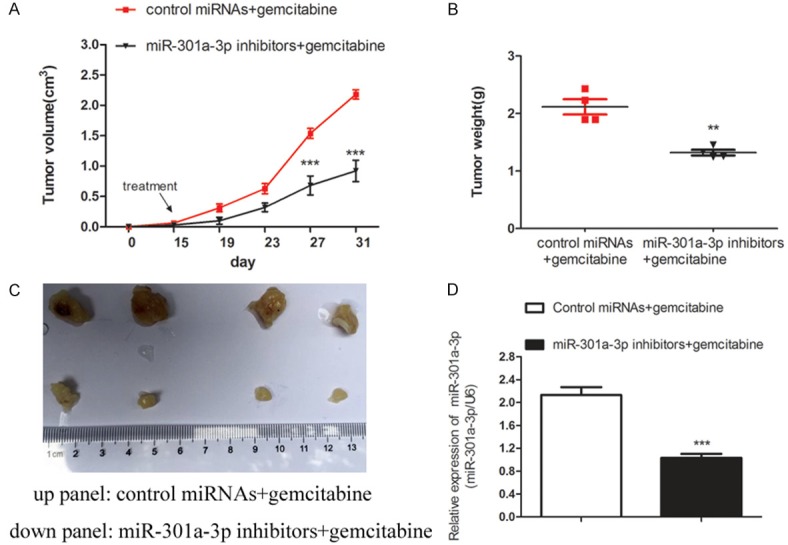
The inhibition of miR-301a-3p increased the chemoresistance of PDAC cells in vivo. A-C. The co-administration of miR-301a-3p inhibitor enhanced the inhibitory effects of gemcitabine on tumor growth in vivo. Nude mice were implanted subcutaneously with panc-1/res cells and received intraperitoneal injections of either control miRNA + gemcitabine (20 mg/kg) or miR-301a-3p inhibitor + gemcitabine. Tumor volumes (mean ± SD) were obtained from four mice in each group and plotted. **P<0.01, ***P<0.001 versus the control. D. MiR-301a-3p expression in tumor tissues was confirmed by qRT-PCR. Data represent the mean ± SD. ***P<0.001 versus the control.
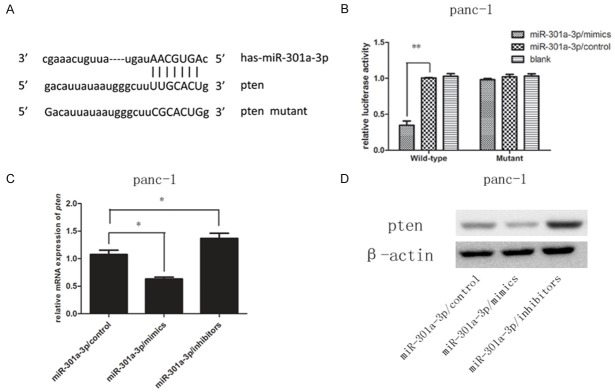
MiR-301a-3p inhibited PTEN expression and directly targeted the PTEN 3’UTR
To elucidate the underlying mechanism by which miR-301a-3p induces gemcitabine resistance, we searched for miR-301a-3p targets using the TargetScan (http://www.targetscan.org) and miRanda (http://www.microrna.org) bioinformatics algorithms. The bioinformatics tools predicted that PTEN was a putative target of miR-301a-3p based on target sequences at the PTEN 3’UTR. We cloned the wild-type 3’UTR sequence of PTEN and a mutated 3’UTR sequence into a luciferase reporter vector (Figure 4A). We then performed a luciferase reporter assay to determine whether miR-301a-3p can directly regulate the expression of PTEN in panc-1 cells. The results showed that the luciferase activity linked with the wild-type 3’UTR of PTEN was repressed in panc-1 cells transfected with the miR-301a-3p mimic compared with that in control cells (Figure 4B). The luciferase activity linked with the mutated 3’UTR of PTEN was not suppressed by the miR-301a-3p mimic (Figure 4B). The results of qRT-PCR indicated that the expression level of PTEN was reduced in the cells transfected with the miR-301a-3p mimic and elevated in the cells transfected with the miR-301a-3p inhibitor (Figure 4C). Similar changes were apparent at the protein level (Figure 4D).
Figure 4.
PTEN was a direct target of miR-301a-3p in panc-1 cells. (A) Schematic graph of the putative binding sites of miR-301a-3p in the PTEN 3’UTR. The PTEN mutant had a mutated 3’UTR to prevent miR-301a-3p binding. (B) Treatment with miR-301a-3p decreased the luciferase reporter activity of the wild-type PTEN 3’UTR (**P<0.01 versus the control) but not that of the mutant PTEN 3’UTR. (C, D) Levels of PTEN mRNA and protein in panc-1 cells after transfection with nonspecific-oligonucleotide control, miR-301a-3p mimic, or miR-301a-3p inhibitor examined by qRT-PCR (C) and Western blot (D), *P<0.05 versus the control.
The effects of miR-301a-3p on gemcitabine resistance were dependent on PTEN
In order to determine whether the effects of miR-301a-3p on gemcitabine sensitivity were dependent on PTEN, we performed rescue experiments with miR-301a-3p and PTEN in sw1990 and panc-1 cells. The overexpression of miR-301a-3p and PTEN blocked the reduction of the PTEN protein level as well as the gemcitabine resistance that resulted from transfection with the miR-301a-3p mimic (Figure 5A, 5B). Conversely, cotransfection with miR-301a-3p inhibitor and siPTEN blocked the augmentation of the PTEN protein level and gemcitabine sensitivity that resulted from miR-301a-3p inhibition (Figure 5C, 5D). Taken together, those results confirmed that miR-301a-3p plays a critical role in gemcitabine resistance in PDAC cells by targeting PTEN.
Figure 5.
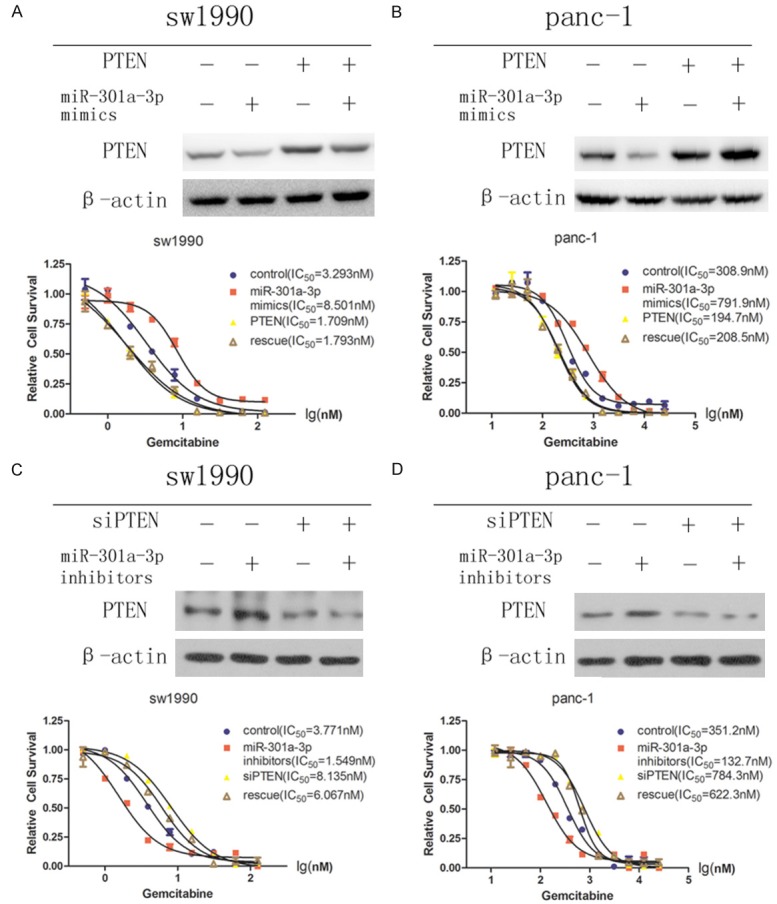
Gemcitabine resistance induced by miR-301a-3p was dependent on PTEN in PDAC cells. Rescue assays were performed by transfection of sw1990 (A) and panc-1 (B) cells with nonspecific-oligonucleotide control, miR-301a-3p mimic, PTEN, or a combination of miR-301a-3p mimic and PTEN. Rescue assays were performed by transfection of sw1990 (C) and panc-1 (D) cells with nonspecific-oligonucleotide control, miR-301a-3p inhibitor, siPTEN, or a combination of miR-301a-3p inhibitor and siPTEN.
Discussion
Resistance to gemcitabine represents a clinical and scientific challenge for patients with PDAC. Several combinations of gemcitabine and other chemotherapeutic regimens without gemcitabine have been evaluated, but few showed better results than gemcitabine alone [16-18]. Therefore, understanding and exploring the underlying mechanisms behind gemcitabine-related chemoresistance is crucial for further clinical developments.
The miRNAs that are often expressed aberrantly in cancers, sometimes referred to as onco-miRNAs or tumor-suppressor miRNAs, are important hallmarks of tumor progression [19,20]. Previously, several studies reported that miR-301a-3p is an onco-miRNA in various cancers including those of the breast [21], stomach [22], and pancreas [15]. Most of the studies found that miR-301a-3p could promote the proliferation, invasion, and migration of cancer cells and might furthermore be a biomarker for diagnosis and prognosis. Little is known, however, about the significance and clinical relevance of miR-301a-3p in regard to chemoresistance. Our findings confirmed that (i) the up regulation and down regulation of miR-301a-3p can attenuate and enhance, respectively, the cytotoxicity of gemcitabine towards human PDAC cells in vitro and in vivo; (ii) the overexpression of miR-301a-3p results in strong gemcitabine resistance; and (iii) the gemcitabine resistance induced by miR-301a-3p is dependent on PTEN.
PTEN, a well-known tumor-suppressor gene, has a suppressive effects on the PI3K/Akt signaling pathway and tumor growth [23,24]. It was also reported that PTEN is a powerful and pleiotropic tumor-suppressor gene that controls many cellular processes such as survival and proliferation [25] and that the impairment of the PTEN tumor-suppressor pathway is a potential causal factor in carcinogenesis. Several mechanisms could account for PTEN down regulation, including genomic loss, epigenetic silencing, transcriptional repression, and negative post-transcriptional regulation by oncogenic miRNAs.
In the last few years, PTEN regulation by miRNAs has been studied extensively. For example, the overexpression of miR-17, miR-21, and miR-128 was shown to induce a malignant phenotype of osteosarcoma via the degradation or translational repression of target PTEN mRNAs [26,27]. MiR-32 could also promote colorectal cancer-cell proliferation and invasion by down regulating PTEN [28]. Our results indicate that PTEN is a target of miR-301a-3p and can be inhibited or overexpressed by means of miR-301a-3p up regulation or down regulation at both the mRNA and protein levels. Meanwhile, the overexpression of PTEN in PDAC could mimic the functions of miR-301a-3p inhibitors as well as rescue the resistance induced by miR-301a-3p mimics and vice versa. MiR-301a-3p up regulation in pancreatic cancer resulted in PTEN suppression and subsequent PI3K/Akt constitutive activation, which led to anti-apoptotic signals caused by the phosphorylation and inactivation of key proteins involved in proliferation and survival, such as BAD, caspase-9, and forkhead [29]. Akt affects cell proliferation by modulating elements of the cell-cycle machinery such as cyclin D1 [30]. MiR-301a-3p modulated gemcitabine chemoresistance by inactivating PTEN, thus causing aberrant PI3K/Aktactivation, resulting in the deregulation of an essential tumor-suppressor pathway.
The treatment of PDAC cells with gemcitabine for 5 months greatly increased miR-301a-3p expression and gemcitabine resistance. The inhibition ofmiR-301a-3p expression in those resistant cells sensitized them to gemcitabine, suggesting a close relationship between miR-301a-3p up regulation and gemcitabine resistance. Those results suggest that miR-301a-3p could be used as a biomarker to screen for patients with pancreatic cancer who would be sensitive to gemcitabine treatment and, furthermore, that miR-301a-3p inhibitors might be an effective therapeutic-intervention target for patients with chemoresistant PDAC in the future.
In conclusion, the results of our investigation of gemcitabine-related mechanisms may help to identify suitable patients for gemcitabine treatment. Novel therapeutic strategies could be tailored according to biomarkers, such as miR-301a-3p, to maximize the clinical efficacy of gemcitabine. In the future, randomized clinical trials will be essential to evaluate whether miR-301a-3p can be the signature for patients with chemorefractive PDAC.
Acknowledgements
This work was Sponsored by the National Natural Science Foundation of China (Grant No. 81372460) and by Shanghai Sailing Program (Grant No. 17YF1415700).
Disclosure of conflict of interest
None.
Authors’ contribution
XX conceived of the study and drafted the manuscript. XX, KZ, GL, GC and JC performed the experiments. ZQ participated in the design of the study and performed the statistical analysis. ZQ and KH supervised all of the work and revised the manuscript. All of the authors have read and approved the manuscript.
References
- 1.Bosetti C, Bertuccio P, Negri E, La Vecchia C, Zeegers MP, Boffetta P. Pancreatic cancer: overview of descriptive epidemiology. Mol Carcinog. 2012;51:3–13. doi: 10.1002/mc.20785. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.De Sousa Cavalcante L, Monteiro G. Gemcitabine: metabolism and molecular mechanisms of action, sensitivity and chemoresistance in pancreatic cancer. Eur J Pharmacol. 2014;741:8–16. doi: 10.1016/j.ejphar.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 4.Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 5.Ying JE, Zhu LM, Liu BX. Developments in metastatic pancreatic cancer: is gemcitabine still the standard? World J Gastroenterol. 2012;18:736–745. doi: 10.3748/wjg.v18.i8.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu WK, Lee CW, Cho CH, Fan D, Wu K, Yu J, Sung JJ. MicroRNA dysregulation in gastric cancer: a new player enters the game. Oncogene. 2010;29:5761–5771. doi: 10.1038/onc.2010.352. [DOI] [PubMed] [Google Scholar]
- 9.Aslam MI, Patel M, Singh B, Jameson JS, Pringle JH. MicroRNA manipulation in colorectal cancer cells: from laboratory to clinical application. J Transl Med. 2012;10:128. doi: 10.1186/1479-5876-10-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obad S, dos Santos CO, Petri A, Heidenblad M, Broom O, Ruse C, Fu C, Lindow M, Stenvang J, Straarup EM, Hansen HF, Koch T, Pappin D, Hannon GJ, Kauppinen S. Silencing of microRNA families by seed-targeting tiny LNAs. Nat Genet. 2011;43:371–378. doi: 10.1038/ng.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams M, Kirschner MB, Cheng YY, Hanh J, Weiss J, Mugridge N, Wright CM, Linton A, Kao SC, Edelman JJ, Vallely MP, McCaughan BC, Cooper W, Klebe S, Lin RC, Brahmbhatt H, MacDiarmid J, van Zandwijk N, Reid G. miR-193a-3p is a potential tumor suppressor in malignant pleural mesothelioma. Oncotarget. 2015;6:23480–23495. doi: 10.18632/oncotarget.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li S, Chowdhury R, Liu F, Chou AP, Li T, Mody RR, Lou JJ, Chen W, Reiss J, Soto H, Prins R, Liau LM, Mischel PS, Nghiemphu PL, Yong WH, Cloughesy TF, Lai A. Tumor-suppressive miR148a is silenced by CpG island hypermethylation in IDH1-mutant gliomas. Clin Cancer Res. 2014;20:5808–5822. doi: 10.1158/1078-0432.CCR-14-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S, Orum H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 15.Xia X, Zhang K, Cen G, Jiang T, Cao J, Huang K, Huang C, Zhao Q, Qiu Z. MicroRNA-301a-3p promotes pancreatic cancer progression via negative regulation of SMAD4. Oncotarget. 2015;6:21046–21063. doi: 10.18632/oncotarget.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheikh R, Walsh N, Clynes M, O’Connor R, McDermott R. Challenges of drug resistance in the management of pancreatic cancer. Expert Rev Anticancer Ther. 2010;10:1647–1661. doi: 10.1586/era.10.148. [DOI] [PubMed] [Google Scholar]
- 17.Veltkamp SA, Beijnen JH, Schellens JH. Prolonged versus standard gemcitabine infusion: translation of molecular pharmacology to new treatment strategy. Oncologist. 2008;13:261–276. doi: 10.1634/theoncologist.2007-0215. [DOI] [PubMed] [Google Scholar]
- 18.Koay EJ, Truty MJ, Cristini V, Thomas RM, Chen R, Chatterjee D, Kang Y, Bhosale PR, Tamm EP, Crane CH, Javle M, Katz MH, Gottumukkala VN, Rozner MA, Shen H, Lee JE, Wang H, Chen Y, Plunkett W, Abbruzzese JL, Wolff RA, Varadhachary GR, Ferrari M, Fleming JB. Transport properties of pancreatic cancer describe gemcitabine delivery and response. J Clin Invest. 2014;124:1525–1536. doi: 10.1172/JCI73455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mardin WA, Mees ST. MicroRNAs: novel diagnostic and therapeutic tools for pancreatic ductal adenocarcinoma? Ann Surg Oncol. 2009;16:3183–3189. doi: 10.1245/s10434-009-0623-1. [DOI] [PubMed] [Google Scholar]
- 20.Rachagani S, Kumar S, Batra SK. MicroRNA in pancreatic cancer: pathological, diagnostic and therapeutic implications. Cancer Lett. 2010;292:8–16. doi: 10.1016/j.canlet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi W, Gerster K, Alajez NM, Tsang J, Waldron L, Pintilie M, Hui AB, Sykes J, P’ng C, Miller N, McCready D, Fyles A, Liu FF. MicroRNA-301 mediates proliferation and invasion in human breast cancer. Cancer Res. 2011;71:2926–2937. doi: 10.1158/0008-5472.CAN-10-3369. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z, Chen L, Zhang X, Xu X, Xing H, Zhang Y, Li W, Yu H, Zeng J, Jia J. RUNX3 regulates vimentin expression via miR-30a during epithelial-mesenchymal transition in gastric cancer cells. J Cell Mol Med. 2014;18:610–623. doi: 10.1111/jcmm.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bunney TD, Katan M. Phosphoinositide signalling in cancer: beyond PI3K and PTEN. Nat Rev Cancer. 2010;10:342–352. doi: 10.1038/nrc2842. [DOI] [PubMed] [Google Scholar]
- 25.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y, Luo LH, Li S, Yang C. miR-17 inhibitor suppressed osteosarcoma tumor growth and metastasis via increasing PTEN expression. Biochem Biophys Res Commun. 2014;444:230–234. doi: 10.1016/j.bbrc.2014.01.061. [DOI] [PubMed] [Google Scholar]
- 27.Shen L, Chen XD, Zhang YH. MicroRNA-128 promotes proliferation in osteosarcoma cells by downregulating PTEN. Tumour Biol. 2014;35:2069–2074. doi: 10.1007/s13277-013-1274-1. [DOI] [PubMed] [Google Scholar]
- 28.Wu W, Yang J, Feng X, Wang H, Ye S, Yang P, Tan W, Wei G, Zhou Y. MicroRNA-32 (miR-32) regulates phosphatase and tensin homologue (PTEN) expression and promotes growth, migration, and invasion in colorectal carcinoma cells. Mol Cancer. 2013;12:30. doi: 10.1186/1476-4598-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hopkins BD, Hodakoski C, Barrows D, Mense SM, Parsons RE. PTEN function: the long and the short of it. Trends Biochem Sci. 2014;39:183–190. doi: 10.1016/j.tibs.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Luca A, Maiello MR, D’Alessio A, Pergameno M, Normanno N. The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin Ther Targets. 2012;16(Suppl 2):S17–27. doi: 10.1517/14728222.2011.639361. [DOI] [PubMed] [Google Scholar]