Abstract
In this paper, the effect of silencing the expression of CXCR4 and CXCR7 by RNAi on the growth of endometrial carcinoma (EC), in vivo, was evaluated. To establish endometrial carcinoma model, thirty nude mice were subcutaneously inoculated with 1 × 107 Ishikawa cells. All tumor-bearing mice were randomly assigned to five groups (six mice in each group) when the tumor xenografts reached 5-7 mm in diameter, and treated with CXCR4-siRNA (5 nmol), CXCR7-siRNA (5 nmol), CXCR4-siRNA (5 nmol) plus CXCR7-siRNA (5 nmol), negative-siRNA (5 nmol) and normal saline, respectively. Following intra-tumor injection, the growth rate of tumor xenografts in the three treatment groups was significantly delayed compared with those in Ne-si and NS group (P<0.05). The results of QRT-PCR and immunohistochemical assessment showed that the expression levels of CXCR4 and CXCR7 could be down regulated by RNA interference. We also observed that treatment with CXCR4-siRNA and CXCR7-siRNA reduced cell proliferation, but there was no significant difference in apoptosis among the five groups. CXCR4 and CXCR7 silencing by RNAi inhibit the growth of human endometrial carcinoma xenografts by inhibiting cancer cell proliferation, in vivo. These results indicate that CXCR4 and CXCR7 could serve as potential alternative targets for gene therapy in endometrial carcinoma.
Keywords: RNA interference, CXCR4, CXCR7, endometrial carcinoma, xenografts
Introduction
Endometrial cancer (EC) is one of the most common gynecological malignant tumors, the mortality of which is only second to ovarian carcinomain developed countries, with 40,000 new cases and 7,500 cancer-related deaths in the US in 2008 [1,2]. Endometrial cancer most often affects postmenopausal women, but the incidence among younger women is increasing in recent years. Despite surgical resection coupled with systemic chemotherapy, it is still difficult to improve the poor prognosis of newly diagnosed endometrial cancer patients due to tumor recurrence and metastasis.
A plethora of studies indicate that the initiation, development, local invasion and distal metastasis of cancer are regulated by factors in the tumor microenvironment, such as cytokines and chemokines [3,4]. As a member of the CXC family of chemokines, stromal cell-derived factor-1 (SDF-1), now also named CXCL12 and its receptors CXCR4 and CXCR7 were found to play important roles in almost all malignancies including endometrial carcinoma [5-17], which may affect the extent of infiltration and phenotype of the leukocyte, angiogenesis, as well as the growth, survival and migration of tumor cells. Yoon et al., [18] found that CXCR4 antagonist TN14003 could suppress primary tumor growth of head and neck cancer, in xenograft mouse models, by inhibiting tumor angiogenesis and prevented lung metastasis. Cho et al., [19] reported that CXCR4 specific antagonist ADM3100 significantly inhibited prostate cancer xenograft growth and reduced microvessel formation, as well as decreased the expression of Ki-67 (a proliferation marker) and Bcl-2 (an anti-apoptotic marker). It is also reported that silencing CXCR7 in the HCCLM3 cells gave rise to tumor inhibition and the number of lung metastases in nude mice [20].
Overall, in the last several years, several studies have focused on CXCR4 and CXCR7 in many malignancies, but their physiological and molecular mechanisms in endometrial cancer are still unclear. Therefore, specifically inhibiting the functions of CXCR4 and CXCR7 may provide clues regarding endometrial carcinogenesis and be of great therapeutic benefit. Our previous studies, in vitro, have demonstrated that knocking down CXCR4 and CXCR7 expression using RNA interference (RNAi) in endometrial cancer cell line could inhibit the proliferation and invasion of tumor cells. In this study, in order to further explore the roles of CXCR4 and CXCR7 in the development of endometrial carcinoma, we evaluated the inhibitory efficacy of CXCR4-siRNA and/or CXCR7-siRNA, in vivo. Following treatment with CXCR4-siRNA and/or CXCR7-siRNA, xenograft tumor growth changes were observed as well as cell proliferation and apoptosis.
Methods
Cell culture and establishment of animal model
Thirty female BALB/c nude mice (4-5 weeks old, 15-18 g) were purchased from Beijing Weitong Lihua Experimental Animal Technology Co. Ltd., with qualified number SCXK (Beijing) 2012-0001. All animals were housed in a specific pathogen free, temperature-controlled isolation conditions, fed with sterilized food and autoclaved water. The human endometrial cancer cell line Ishikawa was obtained from the Central Laboratory of the Affiliated Hospital of Qingdao University (Qingdao, Shandong Province, China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) with 10% heat-inactivated fetal bovine serum (Hyclone, USA) at 37°C in a humidified atmosphere containing 5% CO2. 1 × 107 Ishikawa cells in 200 μL ofPBS were subcutaneously injected into the right flank of each mouse. When the tumor size was 5-7 mm in diameter, the experiments were carried out. All animal experiments were approved by ethical and humane committee of Affiliated Hospital of Qingdao University Medical College and carried out strictly in accordance with the related ethical regulations.
Treatment
Chemically modified siRNA targeting CXCR4 or CXCR7 was designed and validated by RIBIOBIO (Guangzhou, China). The three siRNA sequences were called CXCR4-siRNA, CXCR7-siRNA and Negative-siRNA, whose sequences were same as reported previously by us [21]. Following the establishment of the nude mice tumor xenografts with Ishikawa cells, thirty tumor-bearing nude mice were randomly assigned into five groups (six mice in each group) and there were no significant differences in body weight, implanted tumor volumes, etc. between the five groups. i) CXCR4-siRNA (CX4-si); ii) CXCR7-siRNA (CX7-si); iii) CXCR4/CXCR7-siRNA (CX4/CX7-si); iv) Negative-siRNA (Ne-si); v) normal saline (NS). The five groups received 50 μL intra-tumor injections of CXCR4-siRNA (5 nmol/mouse), CXCR7-siRNA (5 nmol/mouse), CXCR4-siRNA (5 nmol/mouse) plus CXCR7-siRNA (5 nmol/mouse), Negative-siRNA (5 nmol/mouse) and normal saline, respectively. The intra-tumor injections were administered once every three days for six cycles. The longest (a) and shortest (b) diameters of tumors were measured using a caliper every three days and the volumes were calculated using the formula V = ab2/2 and plotted against time. At the end of treatment, the nude mice were sacrificed and tumor tissues were dissected and their weights and volumes were measured. Then, each harvested tumor was divided into two parts, one part was fixed in 10% formalin for measuring mRNA expression of the related genes and the other one was frozen at -80°C for immunohistochemical analysis as described below.
Quantitative RT-PCR for CXCR4 and CXCR7 mRNA in Xenograft tumors
Trizol (Invitrogen, USA) was used for the extraction of total RNA from -80°C frozen transplanted tumor samples, dissected from nude mice, and PrimeScriptTMRT reagent kit with gDNAEraser (Takara, Japan) was used to reverse transcribe the RNA into cDNA, according to the manufacturer’s instructions. To assess the CXCR4 and CXCR7 gene expression, real-time PCR reactions were performed using the SYBR®PremixExTaqTMIIkit (Takara, Japan). The primers for CXCR4, CXCR7 and GAPDH (as internal normalization control) were as follows: CXCR4: 5’-TCATCAAGCAAGGGTGTGAG-3’ (forward) and 5’-TGGCTCCAAGGAAAGCATAG-3’ (reverse); CXCR7: 5’-AGCAGCAGGAGGAAGATGGT-3’ (forward) and 5’-TCTCATTGTTGGACGCAGAC-3’ (reverse); GAPDH: 5’-CTCAGACACCATGGGGAAGGTGA-3’ (forward) and 5’-ATGATCTTGAGGCTGTTGTCATA-3’ (reverse). To ensure the specificity of the related gene primer set, the amplification generated from the PCR reactions were evaluated in terms of specific melting point temperatures using the first derivative primer melting curve software (Applied Biosystems, USA). The expression level of CXCR4 and CXCR7 mRNA were normalized to the expression of the control gene GAPDH and the relative quantification of CXCR4 or CXCR7 mRNA was performed using the comparative cycle threshold method (2-ΔΔCT) [22]. All PCR experiments were repeated two times.
Immunohistochemical study in Xenograft tumors
The transplanted tumor tissues dissected from nude mice were fixed in 10% formalin for 24 h and were then embedded in paraffin and serially cut into sections (4 μm-thickness) for hematoxylin-eosin and immunohistochemical staining. Briefly, tissue sections were deparaffinized and microwaved at 98°C for 10 min in citrate buffer (pH 6.0) after being baked at 64°C for 1 h. After blocking the endogenous peroxidase by immersing the sections in 3% H2O2 for 10 min, the sections were incubated with primary antibodies directed against human CXCR4 (1:200; ABCAM), CXCR7 (1:100; ABCAM) and PCNA (proliferating cell nuclear antigen, 1:100; ABCAM), respectively. The primary antibody was removed and washed with TBS and HRP-conjugated secondly antibody (PV-6001; Zhongshan Bio-tech Co., Ltd., Beijing, China) was then added and samples incubated at 37°C for 30 min. The sections were then visualized using DAB coloration fluid (Zhongshan Bio-tech Co., Ltd., Beijing, China) for 1 minute and then counterstained with hematoxylin. The immunoreactions were viewed under an Olympus microscope CX31 (Japan).
Terminal deoxynucleotidyl transferase-mediated DUTP nick end-labeling (TUNEL) assay
To assess the level of apoptosis of tumor xenograft cells, an in situ cell death detection kit conjugated with horse-radish peroxidase (POD) (RocheApplied Science, USA) was used. The assessment of cell apoptosis was performed by TUNEL analysis according to the manufacturer’s instructions. Five equal-sized positive staining fields in each tissue sections were randomly chosen and the numbers of apoptotic cells were counted. To minimize subjectivity, the process was performed by blinded pathologists.
Statistical analysis
All data are shown as mean ± SD. Statistical analyses were performed using SPSS statistical software (SPSSInc., Chicago, Illinois). Differences between two groups were assessed using at test. A p value <0.05 was considered statistically significant.
Results
Inhibition of tumor Xenografts following treatment with CXCR4-siRNA and/or CXCR7-siRNA
The effect of CXCR4 and/or CXCR7 gene silencing was investigated using the Ishikawa cell tumor xenograft model, in vivo. The representative images of mice and excised tumors from each group are shown in Figure 1A and 1B. The mice which received intra-tumor treatment with CXCR4-siRNA, CXCR7-siRNA and combined therapy exhibited reductions in tumor size in comparison with the Ne-si or NS group mice. Tumors in the three treatment groups were significantly delayed beginning at the third treatment and continued until the end of study (Figure 1C). During the treatment period, the tumors in Ne-si and NS group grew rapidly and the difference between these two groups was notstatistically significant (P>0.05) (Figure 1C). CXCR4-siRNA and CXCR7-siRNA significantly slowed tumor growth (Figure 1C), but the combination treatment of CXCR-siRNA and CXCR7-siRNA could not inhibit tumor growth more than any single administration (Figure 1C). The final average tumor weights were lighter in the CXCR4-siRNA (0.42±0.12 g), CXCR7-siRNA (0.43±0.13 g) and combined administration (0.40±0.08 g) groups than those in the Ne-si (0.90±0.09 g) or NS (0.99±0.11 g) groups and the differences were statistically significant (Figure 1D). These data demonstrated that injection of CXCR4-siRNA and CXCR7-siRNA were able to slow down the growth of Ishikawa-derived xenografts.
Figure 1.
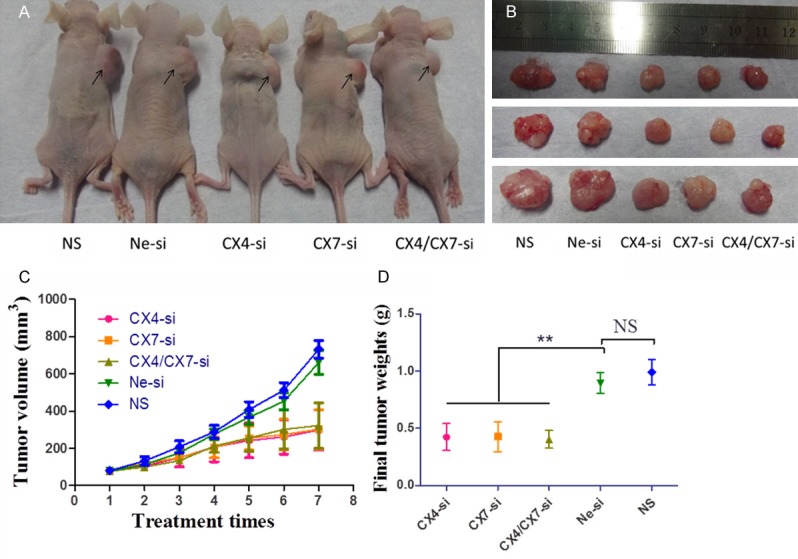
CXCR4-siRNA and/or CXCR7-siRNA inhibit tumor growth in vivo. A. Macroscopic appearance of tumors in nude mice following the completion of therapy. Arrows outline the representative tumors in the right scapular region of the mice. B. Images of representative excised tumors from each group. C. Tumor volumes were averaged for each treatment group and time point over the course of the study (mean ± SD). D. Final tumor weights of the isolated tumors were averaged for each group (mean ± SD). CX4-si, CXCR4-siRNA treatment; CX7-si, CXCR7-siRNA treatment; CX4/CX7-si, CXCR4-siRNA in combination with CXCR7-siRNA treatment; Ne-si, Negative-siRNA treatment; NS, normal saline.
CXCR4 and CXCR7 mRNA expressions were down-regulated by RNAi
Antitumor treatment had significant effects on tumor growth in the present study and subsequently CXCR4 and CXCR7 gene expression was investigated using qRT-PCR. The amount of CXCR4 and CXCR7 mRNA was normalized to the expression of the control gene GAPDH and relative quantification was performed using the 2-ΔΔCT method. The expression of CXCR4 mRNA was significantly reduced following treatment with CXCR4-siRNA (0.38±0.15) and CXCR4/CXCR7 siRNA (0.32±0.09) compared with the Ne-si (1.10±0.49) and NS (1.09±0.49) groups (P<0.05; Figure 2A), while the expression of CXCR7 mRNA was significantly reduced following treatment with CXCR7-siRNA (0.47±0.15) and CXCR4/CXCR7 siRNA (0.52±0.22) compared with the Ne-si (1.05±0.27) and NS (1.07±0.42) groups (P<0.05; Figure 2B).
Figure 2.
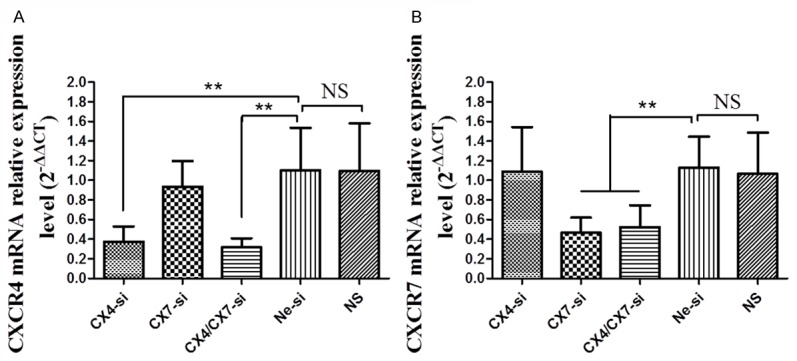
CXCR4-siRNA and/or CXCR7-siRNA decreases CXCR4 and CXCR7 mRNA expression in the tumor tissues (results of Quantitative RT-PCR). Histographs showing (A) relative CXCR4 mRNA expression and (B) relative CXCR7 mRNA expression in the tumor tissues of the five groups. Statistical comparison is with the Ne-si group. CX4-si: CXCR4-siRNA treatment; CX7-si: CXCR7-siRNA treatment; CX4/CX7-si: CXCR4-siRNA in combination with CXCR7-siRNA treatment; Ne-si: Negative-siRNA treatment and NS: normal saline.
CXCR4 and CXCR7 protein expression were down-regulated by RNAi in Xenograft tumors
To assess the effects of CXCR4-siRNA and/or CXCR7-siRNA on CXCR4 and CXCR7 protein expression levels in the treatment groups, xenograft tumors tissues were analyzed by immunohistochemical staining. The results of immunohistochemical staining for CXCR4 and CXCR7 are shown in Figure 3, the cytoplasm of CXCR4 positive cells are stained brown, while the CXCR7 protein are expressed in both the cytoplasm and nuclei. The average optical density was analyzed with Image-Pro plus 5.1 image analysis system (Media Cybernetics, USA). The average optical density (AOD) values of tumor tissues of CX4-si, CX7-si, CX4/CX7-si, Ne-si and NS groups were 0.62±0.17, 2.22±0.63, 0.60±0.21, 2.24±0.57, 2.31±0.46 for CXCR4 and 4.98±1.38, 2.29±0.72, 2.16±0.98, 5.39±1.20, 4.85±0.94 for CXCR7, respectively (Figure 3). These results revealed that the CXCR4 or CXCR7 protein expression levels were significantly reduced by RNAi.
Figure 3.
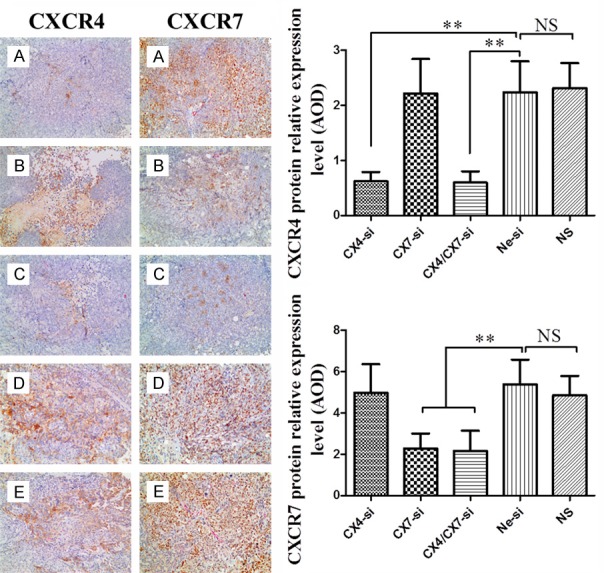
Immunohistochemistry for CXCR4 and CXCR7 protein expression in implanted tumor tissues of nude mice treated with CXCR4-siRNA (A), CXCR7-siRNA (B), CXCR4/CXCR7-siRNA (C), Negative-siRNA (D) or normal saline (E) (magnification, × 200).
Inhibition of tumor cell proliferation following treatment with CXCR4-siRNA and/or CXCR7-siRNA
To determine whether CXCR4-siRNA-mediated down-regulation of CXCR4 or CXCR7 expression was involved in the inhibition of tumor cell proliferation, we used immunohistochemical assessment and probed with anti-PCNA antibody. As shown in Figure 4, the labeled nuclei in tumor sections were regarded as positive. Proliferative tumor cells were counted in 10 randomly microscopic fields. The proliferation index was calculated as follows: (number of positive tumor cells/number of total tumor cells) × 100%. The expression of PCNA in the tumor tissues of CX4-si (40.55±6.06%), CX7-si (37.60±7.94%) and CX4/CX7-si (41.37±8.32%) was poor, but was high in the Ne-si (82.43±8.61%) and NS groups (86.79±7.94%).
Figure 4.
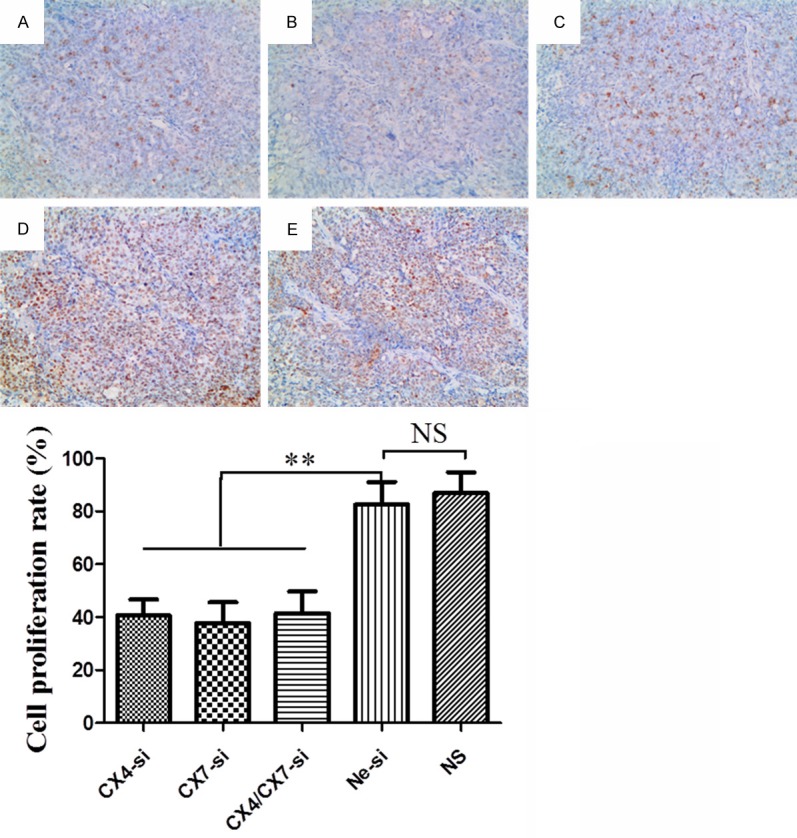
Cell proliferation detected by immunohistochemical staining probed with anti-PCNA antibody in implanted tumors treated with CXCR4-siRNA (A), CXCR7-siRNA (B), CXCR4/CXCR7-siRNA (C), Negative-siRNA (D) or normal saline (E) (magnification, × 200).
TUNEL assay to assess tumor cell death
As shown in Figure 5, dotted or small focal apoptotic cells were found in endometrial cancer tissues, whereas there was no statistically significant difference between the five groups (P>0.05), which are similar to our previous results in vitro. These results indicate that CXCR4 and CXCR7 don not affect endometrial cancer cell apoptosis.
Figure 5.
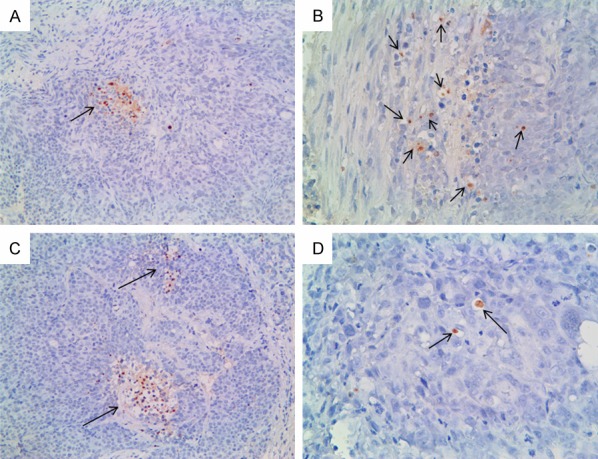
Cell apoptosis detected by TUNEL assay. The nuclei of apoptotic cells are stained brown and arrows indicatea small foci of apoptotic cells. A. Microscopy image from CXCR4-siRNA treatment group (magnification, × 200). B. Microscopy image from CXCR7-siRNA treatment group (magnification, × 400). C. Microscopy image from CXCR4/CXCR7-siRNA treatment group (magnification, × 200). D. Microscopy image from NS group (magnification, × 400). There was no statistically significant difference between the five groups (P>0.05).
Discussion
Since the first report by Muller et al., [17] that chemokine receptors were related to tumor metastasis, a growing number of chemokines and their receptors have been shown to be closely related to tumor development. CXCL12, a member of the CXC family of chemokines and stromal cell derived factor 1 or SDF-1, which has been extensively studied by researchers in recent years are two such members. Some reports have demonstrated that CXCL12/SDF-1 can stimulate multiple signal transduction pathways that are involved in many important biological processes [23-26].
It was believed by researchers that CXCR4 was a unique chemokine receptor of CXCL12 and CXCL12/CXCR4 axis had been identified to play an important role in the growth, invasion and metastasis of many malignancies, including breast cancer [7], prostate cancer [19], ovarian carcinoma [27], colorectal cancer [28], pancreatic cancer [8] and endometrial cancer [5,6]. Yet some reports revealed that CXCR7, also known as, “RDC1” is another specific receptor of CXCL12 [29,30] and that CXCL12/CXCR7 axis was also involved in the onset and development of tumors [9-16].
A previous study [31] suggested that the expression of CXCR4 and CXCR7 was significantly higher in endometrial cancer than those in the corresponding para-tumor and normal tissues, indicating that these two genes are involved in the initiation, development and progression of endometrial carcinoma. Moreover, some reports showed that down-regulating the expression of CXCR4 and CXCR7 using small interfering RNA (siRNA) may inhibit the proliferation and invasion of cancer cells [15,32]. Therefore, specifically inhibiting the abnormal expression of CXCR4 and CXCR7 may be an effective strategy in endometrial cancer therapy.
RNA interference has become widely used for the in vivo knockdown of genes in cancer therapy. However, there are few studies on using RNA interference to silence CXCR4 or CXCR7 expression in endometrial cancer treatment, especially using multiple siRNAs targeting CXCR4 and CXCR7. Our previous in vitro study has demonstrated that both single and combined use of CXCR4-siRNA and CXCR7-siRNA could successfully inhibit cell proliferation and invasion of Ishikawa and HEC-1-A cells [21]. On the basis of the in vitro results, the present study was designed to determine whether CXCR4-siRNA and/or CXCR7-siRNA administration could inhibit the growth of human endometrial cancer xenografts in nude mice.
To our knowledge, this is the first study comparing the inhibitory effects of multiple siRNA with single siRNA (CXCR4-siRNA and/or CXCR7-siRNA) on endometrial cancer, in vivo. Our results indicated that CXCR4-siRNA and/or CXCR7-siRNA significantly delayed the growth of xenografts in nude mice. The tumor weights and the tumor volumes were markedly decreased in the three treatment groups compared with those in the Nesi or NS groups. However, there was no synergy observed in the CXCR4-siRNA and CXCR7-siRNA combined group and this result was consistent with what we observed in our previous study [21], indicating that the chemokine pathway is complicated and they may share the same interference signaling pathway or overlapping function. In addition, tumor cell proliferation potential was significantly inhibited following knocking down of CXCR4 and CXCR7 by RNAi.
Despite some reports [11,33-35] showing that CXCR4 or CXCR7 was involved in the regulation of cells apoptosis via various signaling pathways, there was no change in apoptosis following silencing of CXCR4 and CXCR7 as demonstrated by TUNEL assay. Together with our previous results, these findings suggest that CXCR4 and CXCR7 have different roles in different diseases and that they may not participate in the regulation of cell apoptosis in endometrial cancer. Thus, further research should be done to elucidate their role in the tumor microenvironment. Overall, CXCR4 and CXCR7 may be promising targets for endometrial cancer gene therapy.
Acknowledgements
This work was supported by the Promotive Research Foundation for Excellent Young and Middle-aged Scientists of Shandong (No. BS2009SW002) and the Natural Science Foundation of Shandong Province (No. 2013ZRB14002) for Dr. Yu Huang.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.O’Hayre M, Salanga CL, Handel TM, Allen SJ. Chemokines and cancer: migration, intracellular signaling and intercellular communication in the microenvironment. Biochem J. 2008;409:635–649. doi: 10.1042/BJ20071493. [DOI] [PubMed] [Google Scholar]
- 4.Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267:226–244. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 5.Gelmini S, Mangoni M, Castiglione F, Beltrami C, Pieralli A, Andersson KL, Fambrini M, Taddei GL, Serio M, Orlando C. The CXCR4/CXCL12 axis in endometrial cancer. Clin Expmetas. 2009;26:261–268. doi: 10.1007/s10585-009-9240-4. [DOI] [PubMed] [Google Scholar]
- 6.Felix AS, Stone RA, Chivukula M, Bowser R, Parwani AV, Linkov F, Edwards RP, Weissfeld JL. Survival outcomes in endometrial cancer patients are associated with CXCL12 and estrogen receptor expression. Int J Cancer. 2012;131:E114–E121. doi: 10.1002/ijc.27317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Y, Mao X, Fan C, Liu C, Guo A, Guan S, Jin Q, Li B, Yao F, Jin F. CXCL12-CXCR4 axis promotes the natural selection of breast cancer cell metastasis. Tumour Biol. 2014;35:7765–7773. doi: 10.1007/s13277-014-1816-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koshiba T, Hosotani R, Miyamoto Y, Ida J, Tsuji S, Nakajima S, Kawaguchi M, Kobayashi H, Doi R, Hori T, Fujii N, Imamura M. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin Cancer Res. 2000;6:3530–3535. [PubMed] [Google Scholar]
- 9.Monnier J, Boissan M, L’Helgoualc’h A, Lacombe ML, Turlin B, Zucman-Rossi J, Théret N, Piquet-Pellorce C, Samson M. CXCR7 is upregulated in human and murine hepatocellular carcinoma and is specifically expressed by endothelial cells. Eur J Cancer. 2012;48:138–148. doi: 10.1016/j.ejca.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 10.Iwakiri S, Mino N, Takahashi T, Sonobe M, Nagai S, Okubo K, Wada H, Date H, Miyahara R. Higher expression of chemokine receptor CXCR7 is linked to early and metastatic recurrence in pathological stage I nonsmall cell lung cancer. Cancer. 2009;115:2580–2593. doi: 10.1002/cncr.24281. [DOI] [PubMed] [Google Scholar]
- 11.Hao M, Zheng J, Hou K, Wang J, Chen X, Lu X, Bo J, Xu C, Shen K, Wang J. Role of chemokine receptor CXCR7 in bladder cancer progression. Biochempharmacol. 2012;84:204–214. doi: 10.1016/j.bcp.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Yates TJ, Knapp J, Gosalbez M, Lokeshwar SD, Gomez CS, Benitez A, Ekwenna OO, Young EE, Manoharan M, Lokeshwar VB. C-X-C chemokine receptor 7: a functionally associated molecular marker for bladder cancer. Cancer. 2013;119:61–71. doi: 10.1002/cncr.27661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Shiozawa Y, Wang J, Wang Y, Jung Y, Pienta KJ, Mehra R, Loberg R, Taichman RS. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem. 2008;283:4283–4294. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 14.Miao Z, Luker KE, Summers BC, Berahovich R, Bhojani MS, Rehemtulla A, Kleer CG, Essner JJ, Nasevicius A, Luker GD, Howard MC, Schall TJ. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumorassociated vasculature. Proc Natl Acad Sci U S A. 2007;104:15735–15740. doi: 10.1073/pnas.0610444104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh RK, Lokeshwar BL. The IL-8-regulated chemokine receptor CXCR7 stimulates EGFR signaling to promote prostate cancer growth. Cancer Res. 2011;71:3268–3277. doi: 10.1158/0008-5472.CAN-10-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng K, Li HY, Su XL, Wang XY, Tian T, Li F, Ren GS. Chemokine receptor CXCR7 regulates the invasion, angiogenesis and tumor growth of human hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2010;29:1. doi: 10.1186/1756-9966-29-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verástegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 18.Yoon Y, Liang Z, Zhang X, Choe M, Zhu A, Cho HT, Shin DM, Goodman MM, Chen ZG, Shim H. CXC chemokine receptor-4 antagonist blocks both growth of primary tumor and metastasis of head and neck cancer in xenograft mouse models. Cancer Res. 2007;67:7518–7524. doi: 10.1158/0008-5472.CAN-06-2263. [DOI] [PubMed] [Google Scholar]
- 19.Cho KS, Yoon SJ, Lee JY Cho NH, Choi YD, Song YS, Hong SJ. Inhibition of tumor growth and histopathological changes following treatment with a chemokine receptor CXCR4 antagonist in a prostate cancer xenograft model. Oncol Lett. 2013;6:933–938. doi: 10.3892/ol.2013.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue TC, Chen RX, Han D, Chen J, Xue Q, Gao DM, Sun RX, Tang ZY, Ye SL. Down-regulation of CXCR7 inhibits the growth and lung metastasis of human hepatocellular carcinoma cells with highly metastatic potential. Exp Ther Med. 2012;3:117–123. doi: 10.3892/etm.2011.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long P, Sun F, Ma Y, Huang Y. Inhibition of CXCR4 and CXCR7 for reduction of cell proliferation and invasion in human endometrial cancer. Tumour Biol. 2015:1–8. doi: 10.1007/s13277-015-4580-y. [DOI] [PubMed] [Google Scholar]
- 22.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 23.Park JM, Munoz JL, Won BW, Bliss SA, Greco SJ, Patel SA, Kandouz M, Rameshwar P. Exogenous CXCL12 activates protein kinase C to phosphorylate connexin 43 for gap junctional intercellular communication among confluent breast cancer cells. Cancer Lett. 2013;331:84–91. doi: 10.1016/j.canlet.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Soon PS, Kim E, Pon CK, Gill AJ, Moore K, Spillane AJ, Benn DE, Baxter RC. Breast cancer-associated fibroblasts induce epithelialto-mesenchymal transition in breast cancer cells. Endocr Relat Cancer. 2013;20:1–12. doi: 10.1530/ERC-12-0227. [DOI] [PubMed] [Google Scholar]
- 25.Kerdivel G, Boudot A, Pakdel F. Estrogen represses CXCR7 gene expression by inhibiting the recruitment of NFκB transcription factor at the CXCR7 promoter in breast cancer cells. Biochem Bioph Res Commun. 2013;431:729–733. doi: 10.1016/j.bbrc.2013.01.050. [DOI] [PubMed] [Google Scholar]
- 26.Kryczek I, Wei S, Keller E. Stroma-derived factor (SDF-1/CXCL12) and human tumor pathogenesis. Am J Physiol Cell Physiol. 2007;292:C987–C995. doi: 10.1152/ajpcell.00406.2006. [DOI] [PubMed] [Google Scholar]
- 27.Kajiyama H, Shibata K, Terauchi M. Involvement of SDF-1α/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinoma. Int J Cancer. 2008;122:91–99. doi: 10.1002/ijc.23083. [DOI] [PubMed] [Google Scholar]
- 28.Wang B, Wang W, Niu W, Liu E, Liu X, Wang J, Peng C, Liu S, Xu L, Wang L, Niu J. SDF-1/CXCR4 axis promotes directional migration of colorectal cancer cells through upregulation of integrin αvβ6. Carcinogen. 2013:bgt331. doi: 10.1093/carcin/bgt331. [DOI] [PubMed] [Google Scholar]
- 29.Balabanian K, Lagane B, Infantino S. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 30.Burns JM, Summers C, Wang Y. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walentowicz-Sadlecka M, Sadlecki P, Bodnar M. Stromal derived factor-1 (SDF-1) and its receptors CXCR4 and CXCR7 in endometrial cancer patients. PLoS One. 2014;9:e84629. doi: 10.1371/journal.pone.0084629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lapteva N, Yang AG, Sanders DE. CXCR4 knockdown by small interfering RNA abrogates breast tumor growth in vivo. Cancer Gene Ther. 2005;12:84–89. doi: 10.1038/sj.cgt.7700770. [DOI] [PubMed] [Google Scholar]
- 33.Liang S, Peng X, Li X. Silencing of CXCR4 sensitizes triple-negative breast cancer cells to cisplatin. Oncotarget. 2015;6:1020. doi: 10.18632/oncotarget.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song T, Dou C, Jia Y. TIMP-1 activated carcinoma-associated fibroblasts inhibit tumor apoptosis by activating SDF1/CXCR4 signaling in hepatocellular carcinoma. Oncotarget. 2015;6:12061. doi: 10.18632/oncotarget.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Y, Tan Y, Xi S. A novel mechanism by which SDF-1β protects cardiac cells from palmitate-induced endoplasmic reticulum stress and apoptosis via CXCR7 and AMPK/p38 MAPK-mediated interleukin-6 generation. Diabetes. 2013;62:2545–2558. doi: 10.2337/db12-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]


