Abstract
AIM
To determine the prevalence of QT prolongation in a large series of end stage liver disease (ESLD) patients and its association to clinical variables and mortality.
METHODS
The QT interval was measured and corrected for heart rate for each patient, with a prolonged QT cutoff defined as QT > 450 ms for males and QT > 470 ms for females. Multiple clinical variables were evaluated including sex, age, serum sodium, international normalized ratio, creatinine, total bilirubin, beta-blocker use, Model for End-Stage Liver Disease (MELD), MELD-Na, and etiology of liver disease.
RESULTS
Among 406 ESLD patients analyzed, 207 (51.0%) had QT prolongation. The only clinical variable associated with QT prolongation was male gender (OR = 3.04, 95%CI: 2.01-4.60, P < 0.001). During the study period, 187 patients (46.1%) died. QT prolongation was a significant independent predictor of mortality (OR = 1.69, 95%CI: 1.03-2.77, P = 0.039). In addition, mortality was also associated with viral etiology of ESLD, elevated MELD score and its components (P < 0.05 for all). No significant reversibility in the QT interval was seen after liver transplantation.
CONCLUSION
QT prolongation was commonly encountered in an ESLD population, especially in males, and served as a strong independent marker for increased mortality in ESLD patients.
Keywords: Cirrhosis, Electrophysiology, Arrhythmias, QT prolongation, Mortality, Liver transplantation
Core tip: We performed a case-control retrospective study in a large cohort of patients with end stage liver disease (ESLD) to determine the prevalence of QT prolongation and its association to clinical variables and mortality. Our results showed a high prevalence of QT prolongation in ESLD patients (51%), especially in males, and QT prolongation was a significant independent predictor of mortality. Based on our findings, we recommend close monitoring of the QT interval in ESLD patients with increased attention to any modifiable causes for QT prolongation.
INTRODUCTION
The QT interval on an electrocardiogram (ECG) is a measure of ventricular depolarization and repolarization. Prolongation of the QT interval is associated with ventricular arrhythmias as well as sudden cardiac death in both congenital and acquired conditions. Multiple factors are thought to be responsible for the prolongation of the QT interval in both congenital and acquired conditions, including electrolyte abnormalities, ventricular channelopathies, myocardial ischemia, medications, alcohol toxicity, and autonomic imbalance with sympathetic nervous system hyperactivity[1-11]. Recent studies have shown that end stage liver disease (ESLD) is associated with several electrophysiological changes; specifically, an increased prevalence of QT prolongation is seen in this population[12-30]. While the exact mechanism for QT prolongation is unknown, both improvement in liver function and liver transplantation have been associated with significant shortening in the QT interval in studies with small sample sizes[25-31]. Likewise, previous studies demonstrate reduction in the QT interval for cirrhotic patients who receive beta-adrenergic blockade in both the acute and chronic settings[32-34]. Thus, this may be a modifiable condition and amenable to therapy. Although some studies suggest a prolonged QT interval is related to severity of liver disease, etiology of liver disease, and increased mortality, conflicting results exist regarding these important clinical questions[14,19,24-30,35,36].
We aimed to determine the prevalence and variables associated with QT prolongation in ESLD patients. Furthermore, we assessed whether QT prolongation was associated with increased mortality in these patients.
MATERIALS AND METHODS
The research study was conducted in accordance to the ethical guidelines of the 1975 Declaration of Helsinki as reflected by approval by the institutional review board. After institutional review board approval was obtained, we performed a case-control retrospective study estimating the prevalence of QT prolongation in a cohort of cirrhotic patients with ESLD being evaluated for liver transplant. We then quantified the associations of QT prolongation with multiple clinical variables including etiology of liver disease, sex, age, Model for End-Stage Liver Disease (MELD) score, MELD-Na (MELD with incorporation of serum sodium) score, sodium level, international normalized ratio (INR), creatinine, total bilirubin, beta-blocker use, liver transplantation, and mortality. Variables were collected from the most recent labs collected (within 90 d of baseline ECG) as part of routine outpatient care. We utilized the Organ Transplant Tracking Record (OTTR) database and hospital records to identify our cohort and track their survival.
Patients included men and women above age of 18 with ESLD who were undergoing a liver transplant evaluation for any indication and had ECGs available for visual analysis. For patients who did not undergo a liver transplant, the most recent ECGs were used as baseline. For transplanted patients, the ECG prior to transplant was used as the baseline ECG while the average QT interval from the post-transplant ECGs was used to determine the effects of transplant on QT prolongation. This was done to eliminate artifacts due to the unstable or immediate post transplantation recovery period when the patients’ ECGs are vulnerable to medication changes or electrolyte imbalances. Patients without an interpretable ECG, or with conduction abnormalities, recent myocardial infarction (within the last 30 d by history), or pacemaker use, were excluded from the study.
QT determination
For all 12 lead ECGs, the QT interval based on Bazett’s principle (QTc) was obtained automatically using a computerized ECG machine (General Electronics MAC 5500 HD) to avoid interobserver variability. With Bazett’s principle, the QT interval is measured from the beginning of the QRS complex to the termination of the T-wave and divided by the square root of the R-R interval in seconds[37]. A QTc > 450 ms for males and a QTc > 470 ms for females was considered abnormal or prolonged, as defined by European regulatory guidelines[38] and Goldenburg et al[39] to account for gender differences. Patients with QTc prolongation were subdivided into three categories for analytic purposes: mild (451-470 ms in males; 471-490 ms in females), moderate (471-490 ms in males; 491-510 ms in females), and severe (> 490 ms in males; > 510 ms in females).
Statistical analysis
Patients with (n = 207) and without QTc prolongation (n = 199) were compared on various clinical characteristics by T tests (for continuous variables and after log transformation for INR, creatinine, and bilirubin) and Fisher’s exact tests (for dichotomous variables). Logistic regressions with QTc prolongation as the outcome were performed to identify significant unadjusted and adjusted associations with clinical characteristics identified in the next sentence. The first of three multivariate models included beta blocker use, etiology (viral, ethanol, non-alcohol steatohepatitis), MELD components (sodium, log INR, log creatinine, and log bilirubin), gender, and age as predictors; the second and third multivariate models replaced MELD components by total MELD score and total MELD-Na score respectively. Four logistic regressions with mortality as the outcome were also performed. The first related mortality to degree (mild, moderate, severe) of QTc prolongation. The second through fourth analyses related mortality to QTc prolongation and the aforementioned clinical characteristics, in parallel with the three multivariate models for QTc prolongation. A paired signed rank test was used to examine the change in QTc before and after surgery in a subset of patients (n = 74) receiving liver transplantations. Approximately 25% of the patients did not have routine outpatient labs within the 90 d of their baseline ECG. Therefore, the analyses involving the MELD components and MELD totals were performed using multiple imputation. Microsoft Excel, SAS, and R were used for data analysis and visualization. Statistical significance was declared when P < 0.05.
RESULTS
Patient characteristics
The OTTR database revealed that 729 patients were evaluated for a liver transplant at the University of Kentucky over a recent 12-year period. Of the 729 patients, 406 met the inclusion criteria for this study. In addition, approximately 25% of the patients (102 out of 406) did not have routine outpatient labs within the 90 d of their baseline ECG. The effective sample size for comparison of QT interval pre- and post-transplantation was 74. The estimated prevalence of QT prolongation was 51.0% (207 out of 406).
Table 1 shows that the 207 patients with QT prolongation (QTc by Bazett’s ≥ 450 for males and ≥ 470 ms for females) were generally similar to the 199 patients without QT prolongation based on clinical variables. However, males with QT prolongation had higher creatinine, MELD, and MELD-NA scores than males without QT prolongation. Beta-blocker use was more common in females without QT prolongation.
Table 1.
Patient characteristics n (%)
| Variable |
QTc prolongation |
No QTc prolongation |
||||
| All (n = 207) | Male (n = 150) | Female (n = 57) | All (n = 199) | Male (n = 92) | Female (n = 107) | |
| Beta-blocker use1 | 161 (77.8) | 122 (81.3) | 39 (68.4) | 153 (77.7) | 66 (71.7) | 87 (82.9)3 |
| Viral etiology | 80 (38.7) | 65 (43.3) | 15 (26.3) | 69 (34.7) | 42 (45.7) | 27 (25.2) |
| Ethanol etiology | 90 (43.5) | 78 (52.0) | 12 (21.1) | 68 (34.2) | 46 (50.0) | 22 (20.6) |
| Non-alcohol steatohepatitis etiology | 47 (22.7) | 28 (18.7) | 19 (33.3) | 57 (28.6) | 13 (14.1) | 44 (41.1) |
| Viral and ethanol etiology | 35 (16.9) | 32 (21.3) | 3 (5.3) | 24 (12.1) | 17 (18.5) | 7 (6.5) |
| Sodium2 | 135.6 + 6.2 | 135.4 + 6.4 | 136.3 + 5.3 | 136.2 + 5.6 | 135.4 + 5.6 | 136.9 + 5.6 |
| INR2 (logarithm) | 0.39 + 0.32 | 0.39 + 0.32 | 0.37 + 0.31 | 0.35 + 0.34 | 0.31 + 0.26 | 0.39 + 0.39 |
| Creatinine2 (logarithm) | 0.39 + 0.51 | 0.43 + 0.50 | 0.30 + 0.53 | 0.30 + 0.43 | 0.29 + 0.403 | 0.31 + 0.46 |
| Bilirubin2 (logarithm) | 1.19 + 1.05 | 1.18 + 1.07 | 1.20 + 0.99 | 1.06 + 0.96 | 0.90 + 0.86 | 1.20 + 1.03 |
| MELD-NA2 | 21.0 + 9.5 | 21.6 + 9.5 | 19.5 + 9.3 | 19.3 + 9.1 | 18.6 + 7.73 | 19.8 + 10.2 |
| MELD2 | 19.0 + 9.6 | 19.4 + 9.7 | 18.0 + 9.3 | 17.3 + 9.0 | 16.0 + 7.23 | 18.3 + 10.1 |
| Age | 56.1 + 9.1 | 56.2 + 8.8 | 56.0 + 9.7 | 56.7 + 9.0 | 56.3 + 9.3 | 57.0 + 8.7 |
Entries are number (percent) or mean + SD.
Excluded for two subjects;
Excluded for 102 subjects;
Significantly different (P < 0.05) vs patients of same gender with QTc prolongation.
In logistic regression analysis with QTc prolongation as an outcome variable, there was a significant association with male sex [estimated odds ratio (OR) 3.04, 95%CI: 2.01-4.60, P < 0.001]. The association persisted in all three multivariate models, with adjusted OR between 3.05 and 3.09 (P < 0.001 in all cases); there were no other significant predictors of QTc prolongation in these models.
Of the 406 patients, 187 (46.1%) died during the study period. Any level (mild, moderate, or severe) of QTc prolongation was associated with significantly increased mortality (Table 2). However, the risk of mortality did not exhibit an increasing pattern with respect to the level of prolongation.
Table 2.
Estimated odds ratios for mortality based on levels of QTc prolongation
| OR | P | 95%CI | |
| Mild QTc prolongation vs none | 1.67 | 0.045 | 1.01-2.76 |
| Moderate QTc prolongation vs none | 2.11 | 0.013 | 1.17-3.81 |
| Severe QTc prolongation vs none | 1.83 | 0.044 | 1.02-3.31 |
Mild QTc prolongation: 451-470 ms in males and 471-490 ms in females; Moderate QTc prolongation: 471-490 ms in males and 491-510 ms in females; Severe QTc prolongation: > 490 ms in males and > 510 ms in females.
Figure 1 shows that QTc prolongation is a significant predictor of mortality (OR 1.69, 95%CI: 1.03-2.77, P = 0.039) in a multivariate model which adjusts for the components of MELD and other clinical variables. This model also reveals significant associations with mortality for viral etiology, ethanol etiology, combined viral and ethanol etiology, INR, creatinine, and bilirubin. Figure 2 depicts findings for an additional multivariate model in which MELD replaces its components, while Figure 3 pertains to a model with MELD-Na. In all the models, QTc prolongation and the aforementioned etiologies remain significant predictors of mortality.
Figure 1.
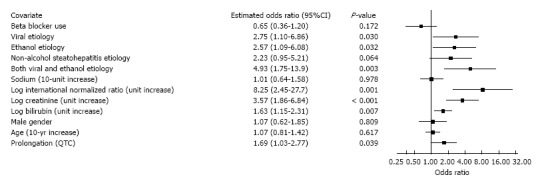
Association of mortality with Model for End-Stage Liver Disease components and clinical variables.
Figure 2.
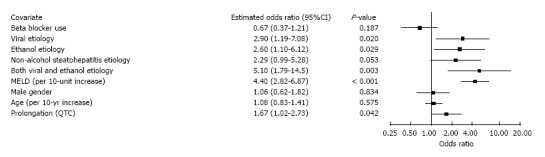
Association of mortality with total Model for End-Stage Liver Disease score and clinical variables.
Figure 3.
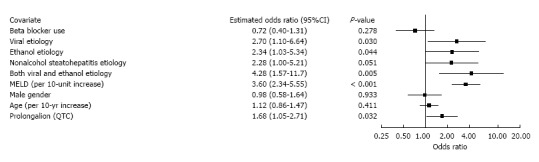
Association of mortality with total Model for End-Stage Liver Disease with incorporation of serum sodium score and clinical variables.
Effective sample size for comparison of QTc interval pre- and post-transplantation was 74. Median pre-transplant QTc was 457 with interquartile range of 435-472 ms. Median post-transplant QTc was 450 with interquartile range of 429-468. Our study showed no significant change in the QTc interval after liver transplantation (P = 0.24).
DISCUSSION
Electrophysiologic cardiac abnormalities are well documented in patients with ESLD. As noted in our study, QT prolongation is a common finding within this population. Although the exact mechanism for QT prolongation in ESLD remains to be established, previous studies have suggested QT prolongation in ESLD may be multifactorial and related to abnormalities in potassium channels involved in repolarization, high plasma concentrations of bile salts, and autonomic dysfunction[12-19,40].
In our study, the majority of patients (51.0%) demonstrated QT prolongation when calculated using Bazett’s formula. Of the 406 patients evaluated, 187 (46.1%) died between years 2000 to 2013, confirming the high mortality in ESLD. An increase in mortality was seen at all levels of prolonged QT. Moreover, some etiologies and MELD components, as well as total MELD and MELD-Na scores, were predictive of mortality. These findings suggest worse outcomes in patients with viral hepatitis or combined viral and ethanol etiology, and they further validate the utility of MELD, MELD-Na, and its components for predicting survival in ESLD[41,42]. Importantly, QT prolongation predicts mortality above and beyond the aforementioned factors.
A number of studies have evaluated the association between QT prolongation and severity of ESLD as measured by Child-Pugh scores[14,19,24,25,28,36]. The majority of such studies have shown an increase in QT prolongation in association with higher Child-Pugh scores with the exception of studies done by Carey et al[27] and Adigun et al[30]. Due to the interobserver variability and use of subjective parameters in Child-Pugh classifications, MELD and MELD-Na scores are now widely viewed as superior indices for predicting mortality and allocating transplants[41,42]. In our study, no significant associations were seen between QT prolongation and these superior indices of ESLD, in accord with the smaller studies by Zurick et al[29] and Day et al[35].
Our study is unique in that we used validated thresholds for QT prolongation. While other studies used a cutoff of 440 msec, our study uses gender specific cutoffs as suggested by Goldenburg et al[39], to account for gender differences in QT prolongation that are typically longer for females[7,38,39,43]. Given our use of higher thresholds (> 450 ms for males and > 470 for females), which allowed higher selectivity for more severe QT prolongation, we may have underestimated the prevalence of QT prolongation while overestimating its association with other variables. Although Adigun et al[30] demonstrated that a physiologic gender difference in the QTc interval is abolished in cirrhosis and that gender hormone concentrations have no effect on the QTc interval, our study reports a much higher prevalence of QT prolongation among males. When QTc prolongation was a dependent variable in our logistic regression models, both unadjusted and adjusted results showed statistically significant associations between QTc prolongation and male sex, while no other variables considered were significantly associated with QTc prolongation. A potential limitation to our study and explanation for these findings may be the use of gender specific thresholds and the corresponding assumption that gender differences in QT interval exist among ESLD patients. In particular, the higher cutoff for females may tend to understate QT prolongation prevalence within that gender.
Due to their utility and wide use in portal hypertension, the effects of acute and chronic beta-blockers on QT prolongation have been evaluated, with results showing a reduced QT interval[32-34]. In addition, partial or full reversal of QT prolongation following liver transplantation has also been noted[14,26-31]. Contrary to these findings, in our study, QTc prolongation was not significantly influenced by etiology, age, beta-blocker use, or transplantation. Although beta-blocker use had a trend towards lower mortality, this did not meet statistical significance. The lack of reversibility in the QT interval following liver transplantation in our study may be due to our small effective sample size.
Several additional limitations need to be considered in our present investigation. As a retrospective study, our results have the potential for unintentional selection bias. Patients from our study were mostly Caucasian residing in rural areas from the state of Kentucky, which may not generalize geographically to the entire country. Our preoperative and postoperative ECGs were obtained during unspecified hospital or clinic visits, exposing our study to sampling bias related to factors such as electrolyte imbalance or concomitant use of QT prolonging drugs. As noted above, MELD variables were unavailable from 102 out of 406 pateints (25%) due to lack of outpatient labs within 90 d of their baseline ECG. Although MELD scores are frequently calculated for cirrhotics as part of their routine assessment, acute biochemical changes are often observed during inpatient hospitalizations, while labs performed outside 90 d of the baseline ECG may not accurately reflect the 90-d mortality rate assessed by MELD score in association to the observed ECG changes. Despite unavailable MELD scores on 102 patients, our analyses re-confirmed what is already well established in current literature that higher MELD scores are associated with higher mortality in ESLD patients. When the MELD components and total score were evaluated in relation to the presence of QT prolongation, our results did show that males with QT prolongation had higher creatinine, MELD, and MELD-NA scores than males without QT prolongation (Table 1). Although males with worse MELD scores (i.e., sicker patients) may have had a higher prevalence of QT prolongation, the retrospective nature of this study does not establish a cause and effect relationship, and the findings do not directly impact the main conclusion of our study where QT prolongation was an independent risk factor for mortality in ESLD.
When examining the severity of QT prolongation and its association with mortality, we defined mild, moderate, and severe levels of prolongation to categorize our patients. However, this categorization may not yield the best discriminatory power. Although the vast majority of heart rates in our study were within an acceptable range, calculation of QT intervals using Bazett’s formula is thought to be less accurate with particularly low or high heart rates[44]. Furthermore, our study relies on the implicit assumptions of accurate data gathering and correct QT interval readings from our ECG machine, which may be prone to systematic error; however, the ECG machine does eliminate interobserver variability. Finally, although QT prolongation has been associated with increased mortality secondary to ventricular arrhythmias, our study did not differentiate specific causes of death.
Our study showed that QT prolongation was common, especially for male patients, in ESLD. Although an association between mortality and QTc prolongation was evident, a greater degree of QTc prolongation did not clearly portend worse outcomes. Finally, while QTc interval may be an independent risk factor for mortality in ESLD patients, the exact mechanism for the increase in mortality remains to be established. Based on our findings, it is reasonable to recommend close monitoring of the QT interval in ESLD patients with attention to any modifiable causes for QT prolongation, such as electrolyte imbalances or medications.
COMMENTS
Background
The QT interval on an electrocardiogram (ECG) is a measure of ventricular depolarization and repolarization. Prolongation of the QT interval is associated with ventricular arrhythmias as well as sudden cardiac death in both congenital and acquired conditions. Multiple factors are thought to be responsible for the prolongation of the QT interval in both congenital and acquired conditions, including electrolyte abnormalities, ventricular channelopathies, myocardial ischemia, medications, alcohol toxicity, and autonomic imbalance with sympathetic nervous system hyperactivity.
Research frontiers
Recent studies have shown that end stage liver disease (ESLD) is associated with several electrophysiological changes; specifically, an increased prevalence of QT prolongation is seen in this population. While the exact mechanism for QT prolongation is unknown, improvement in liver function, beta-blocker use, and liver transplantation have been associated with shortening in the QT interval in studies with small sample sizes. Although some studies suggest a prolonged QT interval is related to severity of liver disease, etiology of liver disease, and increased mortality, conflicting results exist regarding this important clinical question.
Innovations and breakthroughs
The authors aimed to determine the prevalence of QT prolongation in a large series of ESLD patients and its association to clinical variables and mortality. The QT interval was measured and corrected for heart rate (QTc) for each patient, with prolongation defined as QTc > 450 ms for males and QTc > 470 ms for females. Multiple clinical variables were evaluated including sex, age, serum sodium, international normalized ratio, creatinine, total bilirubin, beta-blocker use, Model for End-Stage Liver Disease (MELD), MELD-Na, and etiology of liver disease. Among 406 ESLD patients analyzed, 207 (51.0%) had QT prolongation. The only clinical variable associated with QT prolongation was male gender (OR = 3.04, 95%CI: 2.01-4.60, P < 0.001). During the study period, 187 patients (46.1%) died. QT prolongation was a significant independent predictor of mortality (OR = 1.69, 95%CI: 1.03-2.77, P = 0.039). In addition, mortality was also associated with viral etiology of ESLD, elevated MELD score and its components (P < 0.05 for all). No significant reversibility in the QTc interval was seen after liver transplantation.
Applications
QTc interval may be an independent risk factor for mortality in ESLD patients and thus the authors recommend close monitoring of the QT interval in ESLD patients and increased attention to any modifiable causes for QT prolongation such as electrolyte imbalances or medications.
Terminology
ESLD: End stage liver disease; MELD: Model for End-Stage Liver Disease; MELD-Na: Model for End-Stage Liver Disease score with serum sodium; OTTR: Organ Transplant Tracking Record; QTc: QT interval corrected for heart rate.
Peer-review
This is an interesting and important finding, as QT measurement is not formally considered in liver transplant clinics. The results of this study are interesting and relevant.
Footnotes
Institutional review board statement: This study was reviewed and approved by the institutional review board at University of Kentucky.
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used clinical data without storage of patient identifiers that were obtained after each patient agreed to treatment by written consent. Waiver of informed consent was also obtained because the research involved no more than minimal risk and met criteria specified by federal regulations.
Conflict-of-interest statement: The authors of this manuscript have no conflict of interest to disclose.
Data sharing statement: No additional data are available.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: November 18, 2016
First decision: November 30, 2016
Article in press: February 20, 2017
P- Reviewer: Barili F, Dourakis SP, Sinclair M S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.Karjalainen J, Reunanen A, Ristola P, Viitasalo M. QT interval as a cardiac risk factor in a middle aged population. Heart. 1997;77:543–548. doi: 10.1136/hrt.77.6.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sawicki PT, Dähne R, Bender R, Berger M. Prolonged QT interval as a predictor of mortality in diabetic nephropathy. Diabetologia. 1996;39:77–81. doi: 10.1007/BF00400416. [DOI] [PubMed] [Google Scholar]
- 3.Gowda RM, Khan IA, Wilbur SL, Vasavada BC, Sacchi TJ. Torsade de pointes: the clinical considerations. Int J Cardiol. 2004;96:1–6. doi: 10.1016/j.ijcard.2003.04.055. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz PJ, Periti M, Malliani A. The long Q-T syndrome. Am Heart J. 1975;89:378–390. doi: 10.1016/0002-8703(75)90089-7. [DOI] [PubMed] [Google Scholar]
- 5.Surawicz B. The QT interval and cardiac arrhythmias. Annu Rev Med. 1987;38:81–90. doi: 10.1146/annurev.me.38.020187.000501. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz PJ, Wolf S. QT interval prolongation as predictor of sudden death in patients with myocardial infarction. Circulation. 1978;57:1074–1077. doi: 10.1161/01.cir.57.6.1074. [DOI] [PubMed] [Google Scholar]
- 7.Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, Deckers JW, Kingma JH, Sturkenboom MC, Stricker BH, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–367. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 8.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 9.Schooling CM, Zhao J, Zhang Y. The association of androgens with QT interval and heart rate in US men. Int J Cardiol. 2014;177:592–594. doi: 10.1016/j.ijcard.2014.08.146. [DOI] [PubMed] [Google Scholar]
- 10.Detta N, Frisso G, Zullo A, Sarubbi B, Cozzolino C, Romeo E, Wang DW, Calabrò R, Salvatore F, George AL. Novel deletion mutation in the cardiac sodium channel inactivation gate causes long QT syndrome. Int J Cardiol. 2013;165:362–365. doi: 10.1016/j.ijcard.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarapués M, Cereza G, Arellano AL, Montané E, Figueras A. Serious QT interval prolongation with ranolazine and amiodarone. Int J Cardiol. 2014;172:e60–e61. doi: 10.1016/j.ijcard.2013.12.061. [DOI] [PubMed] [Google Scholar]
- 12.Henriksen JH, Fuglsang S, Bendtsen F, Christensen E, Møller S. Dyssynchronous electrical and mechanical systole in patients with cirrhosis. J Hepatol. 2002;36:513–520. doi: 10.1016/s0168-8278(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 13.Ward CA, Ma Z, Lee SS, Giles WR. Potassium currents in atrial and ventricular myocytes from a rat model of cirrhosis. Am J Physiol. 1997;273:G537–G544. doi: 10.1152/ajpgi.1997.273.2.G537. [DOI] [PubMed] [Google Scholar]
- 14.Mohamed R, Forsey PR, Davies MK, Neuberger JM. Effect of liver transplantation on QT interval prolongation and autonomic dysfunction in end-stage liver disease. Hepatology. 1996;23:1128–1134. doi: 10.1002/hep.510230529. [DOI] [PubMed] [Google Scholar]
- 15.Theocharidou E, Krag A, Bendtsen F, Møller S, Burroughs AK. Cardiac dysfunction in cirrhosis - does adrenal function play a role? A hypothesis. Liver Int. 2012;32:1327–1332. doi: 10.1111/j.1478-3231.2011.02751.x. [DOI] [PubMed] [Google Scholar]
- 16.Al Hamoudi W, Lee SS. Cirrhotic cardiomyopathy. Ann Hepatol. 2006;5:132–139. [PubMed] [Google Scholar]
- 17.Lazzeri C, La Villa G, Laffi G, Vecchiarino S, Gambilonghi F, Gentilini P, Franchi F. Autonomic regulation of heart rate and QT interval in nonalcoholic cirrhosis with ascites. Digestion. 1997;58:580–586. doi: 10.1159/000201505. [DOI] [PubMed] [Google Scholar]
- 18.Kempler P, Szalay F, Váradi A, Keresztes K, Kádár E, Tánczos E, Petrik J. Prolongation of the QTc-interval reflects the severity of autonomic neuropathy in primary biliary cirrhosis and in other non-alcoholic liver diseases. Z Gastroenterol. 1993;31 Suppl 2:96–98. [PubMed] [Google Scholar]
- 19.Bernardi M, Calandra S, Colantoni A, Trevisani F, Raimondo ML, Sica G, Schepis F, Mandini M, Simoni P, Contin M, et al. Q-T interval prolongation in cirrhosis: prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepatology. 1998;27:28–34. doi: 10.1002/hep.510270106. [DOI] [PubMed] [Google Scholar]
- 20.Marafioti V, Benetti V, Montin U, Carbone V, Petrosino A, Tedeschi U, Rossi A. QTc interval prolongation and hepatic encephalopathy in patients candidates for liver transplantation: A valid inference? Int J Cardiol. 2015;188:43–44. doi: 10.1016/j.ijcard.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 21.Møller S, Hove JD, Dixen U, Bendtsen F. New insights into cirrhotic cardiomyopathy. Int J Cardiol. 2013;167:1101–1108. doi: 10.1016/j.ijcard.2012.09.089. [DOI] [PubMed] [Google Scholar]
- 22.Trevisani F, Merli M, Savelli F, Valeriano V, Zambruni A, Riggio O, Caraceni P, Domenicali M, Bernardi M. QT interval in patients with non-cirrhotic portal hypertension and in cirrhotic patients treated with transjugular intrahepatic porto-systemic shunt. J Hepatol. 2003;38:461–467. doi: 10.1016/s0168-8278(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 23.Josefsson A, Fu M, Björnsson E, Kalaitzakis E. Prevalence of pre-transplant electrocardiographic abnormalities and post-transplant cardiac events in patients with liver cirrhosis. BMC Gastroenterol. 2014;14:65. doi: 10.1186/1471-230X-14-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosar F, Ates F, Sahin I, Karincaoglu M, Yildirim B. QT interval analysis in patients with chronic liver disease: a prospective study. Angiology. 2007;58:218–224. doi: 10.1177/0003319707300368. [DOI] [PubMed] [Google Scholar]
- 25.Mimidis KP, Papadopoulos V, Thomopoulos K, Tziakas D, Ritis K, Dalla V, Kotsiou K, Nikolopoulou V, Hatseras D, Kartalis G. Prolongation of the QTc interval in patients with cirrhosis. Ann Gastroenterol. 2003;16:155–158. [Google Scholar]
- 26.Finucci G, Lunardi F, Sacerdoti D, Volpin R, Bortoluzzi A, Bombonato G, Angeli P, Gatta A. Q-T interval prolongation in liver cirrhosis. Reversibility after orthotopic liver transplantation. Jpn Heart J. 1998;39:321–329. doi: 10.1536/ihj.39.321. [DOI] [PubMed] [Google Scholar]
- 27.Carey EJ, Douglas DD. Effects of orthotopic liver transplantation on the corrected QT interval in patients with end-stage liver disease. Dig Dis Sci. 2005;50:320–323. doi: 10.1007/s10620-005-1603-3. [DOI] [PubMed] [Google Scholar]
- 28.Bal JS, Thuluvath PJ. Prolongation of QTc interval: relationship with etiology and severity of liver disease, mortality and liver transplantation. Liver Int. 2003;23:243–248. doi: 10.1034/j.1600-0676.2003.00833.x. [DOI] [PubMed] [Google Scholar]
- 29.Zurick AO, Spier BJ, Teelin TC, Lorenze KR, Alberte C, Zacks S, Lindstrom MJ, Pfau PR, Selzman K. Alterations in corrected QT interval following liver transplant in patients with end-stage liver disease. Clin Cardiol. 2010;33:672–677. doi: 10.1002/clc.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adigun AQ, Pinto AG, Flockhart DA, Gorski JC, Li L, Hall SD, Chalasani N. Effect of cirrhosis and liver transplantation on the gender difference in QT interval. Am J Cardiol. 2005;95:691–694. doi: 10.1016/j.amjcard.2004.10.054. [DOI] [PubMed] [Google Scholar]
- 31.Zamirian M, Tavassoli M, Aghasadeghi K. Corrected QT interval and QT dispersion in cirrhotic patients before and after liver transplantation. Arch Iran Med. 2012;15:375–377. [PubMed] [Google Scholar]
- 32.Henriksen JH, Bendtsen F, Hansen EF, Møller S. Acute non-selective beta-adrenergic blockade reduces prolonged frequency-adjusted Q-T interval (QTc) in patients with cirrhosis. J Hepatol. 2004;40:239–246. doi: 10.1016/j.jhep.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 33.Zambruni A, Trevisani F, Di Micoli A, Savelli F, Berzigotti A, Bracci E, Caraceni P, Domenicali M, Felline P, Zoli M, et al. Effect of chronic beta-blockade on QT interval in patients with liver cirrhosis. J Hepatol. 2008;48:415–421. doi: 10.1016/j.jhep.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 34.Kim YK, Hwang GS, Shin WJ, Bang JY, Cho SK, Han SM. Effect of propranolol on the relationship between QT interval and vagal modulation of heart rate variability in cirrhotic patients awaiting liver transplantation. Transplant Proc. 2011;43:1654–1659. doi: 10.1016/j.transproceed.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Day CP, James OF, Butler TJ, Campbell RW. QT prolongation and sudden cardiac death in patients with alcoholic liver disease. Lancet. 1993;341:1423–1428. doi: 10.1016/0140-6736(93)90879-l. [DOI] [PubMed] [Google Scholar]
- 36.Puthumana L, Chaudhry V, Thuluvath PJ. Prolonged QTc interval and its relationship to autonomic cardiovascular reflexes in patients with cirrhosis. J Hepatol. 2001;35:733–738. doi: 10.1016/s0168-8278(01)00217-3. [DOI] [PubMed] [Google Scholar]
- 37.Bazett HC. An analysis of the time relations of electrocardiograms. Heart. 1920;7:353–367. [Google Scholar]
- 38.Committee for Proprietary Medicinal Products. London: Committee for Proprietary Medicinal Products; 1997. The assessment of the potential for QT interval prolongation by non-cardiovascular medicinal products. [Google Scholar]
- 39.Goldenberg I, Moss AJ, Zareba W. QT interval: how to measure it and what is “normal”. J Cardiovasc Electrophysiol. 2006;17:333–336. doi: 10.1111/j.1540-8167.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 40.Zambruni A, Trevisani F, Caraceni P, Bernardi M. Cardiac electrophysiological abnormalities in patients with cirrhosis. J Hepatol. 2006;44:994–1002. doi: 10.1016/j.jhep.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 41.Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, Benson J, Therneau T, Kremers W, Wiesner R, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130:1652–1660. doi: 10.1053/j.gastro.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Angermayr B, Cejna M, Karnel F, Gschwantler M, Koenig F, Pidlich J, Mendel H, Pichler L, Wichlas M, Kreil A, et al. Child-Pugh versus MELD score in predicting survival in patients undergoing transjugular intrahepatic portosystemic shunt. Gut. 2003;52:879–885. doi: 10.1136/gut.52.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rautaharju PM, Mason JW, Akiyama T. New age- and sex-specific criteria for QT prolongation based on rate correction formulas that minimize bias at the upper normal limits. Int J Cardiol. 2014;174:535–540. doi: 10.1016/j.ijcard.2014.04.133. [DOI] [PubMed] [Google Scholar]
- 44.Zambruni A, Di Micoli A, Lubisco A, Domenicali M, Trevisani F, Bernardi M. QT interval correction in patients with cirrhosis. J Cardiovasc Electrophysiol. 2007;18:77–82. doi: 10.1111/j.1540-8167.2006.00622.x. [DOI] [PubMed] [Google Scholar]


