Abstract
Background:
The need to understand the contribution of bovine tuberculosis (BTB) to the general tuberculosis burden in a poor resource setting is paramount. The aim of this study is to determine the burden of BTB among herdsmen and cattle in the North Tongu district of Volta Region in Ghana.
Materials and Methods:
A cross- sectional study was conducted in the North Tongu District of the Volta Region between the period of October 2011- March 2012. A well-structured questionnaire was used to collect socio-demographic information and possible risk factor information on cattle from participants. Sputum samples from 68 herdsmen and blood samples from 200 cattle belonging to these herdsmen were also collected. Sputum samples were analyzed using Ziehl- Neelsen staining while Anigen Rapid BTB Test was used for Cattle blood samples.
Results:
Ninety percent (61/68) of respondents were also found to consume fresh milk while 84% (57/68) do not use protective clothing. Of a total of 1580 cattle owned by the herdsmen, 200 cattle consisting of 14 bulls and 186 cows were screened where the prevalence of bovine TB was 19% (38/200) and those affected were all females. All (100%) human sample tested negative for Acid-Fast Bacilli (AFB). However, the seropositivity of cattle and kraal density were statistically associated (p= 0.001).
Conclusion:
Bovine TB is prevalent in cattle in North Tongu district. Although herdsmen indulge in risky lifestyles that expose them to BTB, a zero prevalence of BTB was observed, further study is envisaged using a larger sample size.
Keywords: Bovine tuberculosis, Ghana, Herdsmen, North Tongu
Introduction
Tuberculosis (TB) is an infectious disease of warm-blooded animals caused by Mycobacterium tuberculosis (MTB)complex which includes Mycobacterium tuberculosis complex, Mycobacterium bovis, Mycobacterium tuberculosis, Mycobacterium avium (Kaneene and Theon, 2004). The rate of decline of in incidence of the disease is very slow due to the emergence of the Multi- Drug Resistance TB (MDR-TB) (Cosivi et al., 1998). There is also the problem of bovine tuberculosis (BTB) whose numerical contribution currently to the general TB burden is unknown (Ayele et al., 2004). Human M. bovis infection was a major public health risk and an important source of TB in humans in the 1930s as a result of a high prevalence of bovine tuberculosis in cattle but the practice of the test and slaughter programme as well as the introduction of pasteurisation of milk reduced the incidence (Shitaye et al., 2007). Currently bovine tuberculosis is mainly endemic in developing countries including sub-Saharan Africa where the test and slaughter policy has not been properly implemented (Cosivi et al., 1998). As a result, bovine TB is either only partially controlled or not controlled at all which makes people working with cattle such as herdsmen, veterinarians and livestock workers to be at high risk of BTB infection (Georghiou et al., 1989).
Humans often get exposed to Mycobacterium bovis the causative agent of BTB through direct inhalation from animals, consumption of uncooked infected meat or infected unpasteurized milk (Ayele et al., 2004). The close co-existence of farmers and animals is exemplified by the herdsmen, who live their entire lives with their animals, offering ample opportunity for zoonotic transmission of infection.
At the peripheral level of the health system in the Ghana, the Ziehl-Neelsen method is used for the diagnoses of tuberculosis. Detection of BTB in cattle in the country is also carried out most commonly on the basis of tuberculin skin testing using the Purified Protein Derivative (PPD), abattoir meat inspection and rarely on bacteriological techniques (Abubakar et al., 2007). In recent years, there are many diagnostic methods for screening cattle for BTB. One of such methods is the Anigen Rapid BTB Ab Test Kit which saw a tremendous patronage over the years in other countries due to its specificity (98 %), sensitivity (85%), as well as being easy to use (Danbirni et al., 2010).
In Ghana, cattle testing by the tuberculin skin test is sporadic. Between the period of 2005 to 2010, 516 TB cases were diagnosed at abattoirs and slaughter houses in Ghana (Veterinary monthly summary reports, 2010). Currently, the contribution of BTB to human tuberculosis is unknown in Ghana. In a study conducted at the Korle-bu Teaching Hospital, 3% of TB positive human samples were M. bovis (Addo et al., 2007).
Although bovine tuberculosis cases were reported in cattle at slaughter (2005-2009) according to the Veterinary monthly summary reports in 2010, an unknown proportion of cattle in the country is still infected with bovine TB because the test and slaughter policy for the control of the disease has sporadic and not well articulated. Since people who attend to these animals such as herdsmen and veterinarians as well as the general public who consume fresh milk or infected meat are exposed to bovine TB from cattle (Bilal et al., 2010). There is therefore the need to ascertain the burden of BTB in cattle and in human in Ghana using the field approach, rather than abattoir and hospital based approaches targeting herdsmen in rural settings. This study therefore evaluates the burden of BTB among herdsmen and cattle in the North Tongu district of the Volta Region.
Study Area
A cross-sectional study was conducted between the periods of October 2011 to March 2012 in the North Tongu District of the Volta Region. It lies within latitude 5°47’ North to 60 North and longitude 00 5’ East. The total area of the district is 1460 square km, which is about 7.1% of the Volta Region. The district lies within the Tropical Savannah Grassland zone. The total population of the area is130,388. Livestock productions in the district include large ruminants (cattle), small ruminants (sheep and goats) and monogastrics (pigs, rabbits) as well as poultry. Cattle production is mainly done by the semi-intensive system where animals are herded by local cattle boys and Fulani herdsmen for grazing and watering in the mornings and return in late afternoon. Most kraals are situated in the communities and in close proximity to households to minimize theft.
North Tongu was purposively chosen for the study based on the fact that it is the main cattle producing area in the Volta Region and from Veterinary monthly summary reports, 2010, 2% (9/516) of national bovine TB cases are observed at slaughter, According to the District profile of the area, TB is among the top five human diseases (Malaria, HIV/AIDS, Cardiovascular Accident, Anaemia and Tuberculosis) in North Tongu and also a high human and cattle interaction alongside risky lifestyle was observed in the area.
Variables
The dependent variables in the study were positive/ negative test result for bovine tuberculosis in both human and cattle. The independent variables in the human group were socio-demographic characteristics such as age, sex, educational level, religion, ethnicity and occupation as well as occupational characteristics such as experience (years), consumption of raw milk and overcrowding whiles the independent variables in cattle were age, sex, breed, herd size, antimicrobial usage, and husbandry practices.
Sampling of Cattle
The study population for cattle reared in the North Tongu District which was 34,564 (projected livestock census, 2010) and all cattle of age 6 months between October 2011 and March 2012 were included in the study. All cattle that were moved from elsewhere to the study location after the commencement of the study were excluded.
The sample size was 200 using the following formula; N=z2 p(1-p)/d2, where N= sample size, z= risk of Type 1 error (=1.96 at 95% confidence level) p= prevalence of BTB =13.8% d= precision (allowable error) = 5% = 0.05
Using an estimated prevalence of BTB in cattle of value 13.8 % (Bonsu et al., 2003) a minimum sample size of 183 was computed and rounded up to 200.
The population of cattle herdsmen in the North Tongu District which was estimated to be 1182 constituted the study population for humans. All herdsmen of 10 years and above in North Tongu District between October 2011 to March 2012. People who are physically and mentally unable to give consent were excluded from the study.
The sample size for herdsmen which was 80 was computed using the formula; N=z2 p(1-p)/d2, where N= sample size, z = risk of Type 1 error (=1.96 at 95% confidence level) p= prevalence of BTB in human = 3% d= absolute precision = 5% = 0.05
Using a prevalence of BTB in human of 3% found in a study done by Addo and others in Ghana (Addo et al., 2007) a minimum sample size of 44 was computed and rounded up to 80.
All persons living in households with the selected kraals who fall within the inclusion criteria and who consented were sampled by simple random sampling using the ballot method. The study protocol was reviewed and approved by the Ethical Review Board of the Ghana Health Service. The protocol was also approved by the Veterinary Services Directorate of the Ministry of Food and Agriculture. The District Health Management Team (DHMT), the District Assembly and traditional rulers also permitted the study. Verbal and written consent was duly sought from the study participants for voluntary participation in the study. Confidentiality was ensured.
Questionnaire administration
Study questionnaire was pre-tested in a community which is similar to the selected area to train the Research assistants. Structured questionnaires were administered to eligible and consenting participants for socio-demographic, risk (predicting) factors and to assess their knowledge on the occurrence of BTB and its control. The owners of the selected cattle were also interviewed on herd composition and risk (predicting) factors of the disease in their animals.
Sample collection, staining techniques and Anigen Rapid Test
3- 5ml of sputum samples were collected from herdsmen for laboratory testing for BTB. About 5 ml of blood was collected from iugular vein of each cattle for laboratory testing for BTB.
For the diagnoses oi tuberculosis in human samples, Ziehl-Neelsen staining was used because this is the currently used method for tuberculosis diagnoses in the country at the peripheral levels of the health system as recommended by OIE Manual of diagnostic tests and vaccines for terrestrial animals (mammals, birds and bees) (OIE; 2008)
For the diagnoses of BTB in cattle, Anigen Rapid BTB Ab Test Kits was used and the test was as described by Danbirni, et al., (2010). Anigen Rapid Test Kit is a chromatographic immunoassay for the qualitative detection of Mycobacterium bovis antibody in plasma and serum which has many advantages over the tuberculin skin test. The Anigen Rapid BTB test kit has a ‘T and C’ line as Test and control Lines on the surface of the kit. Both the ‘T and the C’ lines in the result window are invisible before any sample is applied. The ‘control Line’ is used as the procedural control (Figure 1). Two bands of purple colour within the result window indicated a positive result. A single purple band within the result window indicates a negative result.
Figure 1.
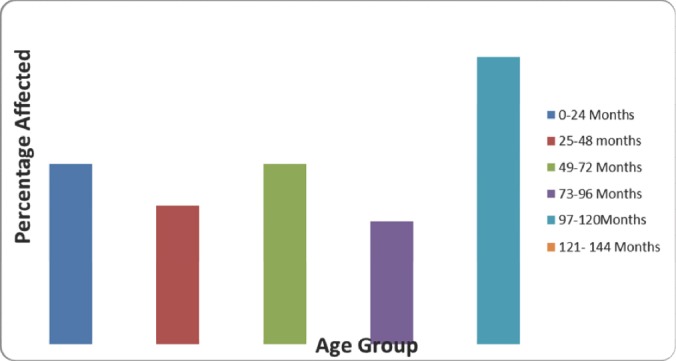
Seropositivity of BTB in cattle by age groups, North Tongu District, October 2011- March 2012.
Data Analysis
Data was entered into Epi Data, coded and exported into SPSS version 16.0 for analysis. Data was cleaned and descriptive and statistical analysis was done.
Pearson’s Chi-Square test was used to test associations between demographic data and dependent variables. Tabulations of frequencies (and percentages) and graphical presentations were done using Statistical Package for Social Sciences software (SPSS, version16). All statistical tests were declared significant for p-value < 0.05.
Results
Cattle population
A total of 200 cattle were sampled. The prevalence of bovine TB in cattle was found to be 19% (Table 1). All cattle samples that tested positive came from cows.
Table 1.
Laboratory result of cattle for bovine TB, North Tongu District, October 2011- March 2012.
| Species | Status | Counts | % |
|---|---|---|---|
| Cattle | Positive | 38 | 19 |
| Negative | 162 | 81 |
Of the 200 cattle, 14 were bulls and were 186 cows. Ten were exotic breeds, 95 were local breeds, and 95 cross breeds. Ninety nine percent (193/194) of cattle were kept under the semi- intensive system and the rest under the intensive system. Eighty four percent (164/195) of cattle were routinely treated with antibiotics (Table 2).
Table 2.
Possible Risk Factors of BTB in cattle, North Tongu District, October 2011- March 2012
| Variable | Item | Counts | % |
|---|---|---|---|
| Husbandry System | Semi-intensive | 193 | 99.5 |
| Intensive | 1 | 0.5 | |
| Total | 194 | ||
| Breed | Local | 95 | 47.5 |
| Exotic | 10 | 5.0 | |
| Crossbreed | 95 | 47.5 | |
| Antibiotic Usage | Yes | 164 | 84.1 |
| No | 31 | 15.9 | |
| Total | 195 | ||
| Sex | Male | 14 | 7.0 |
| Female | 186 | 93.0 | |
| Total | 200 | 100 |
Kraal density is statistically associated with seropositivity of cattle to bovine TB (p=0.001) but all the other risk factors considered were not statistically associated (Table 3).
Table 3.
Seropositivity of cattle to bovine TB and related possible Risk factors, North Tongu District, October 2011-March 2012.
| Factor | P-Value | Odd Ratio | 95% Confidence Interval | |
|---|---|---|---|---|
| Lower | Upper | |||
| Kraal density | 0.001 | - | - | - |
| Age of cattle | 0.936 | - | - | - |
| Sex of cattle | 0.235 | 0.306(male/female) | 0.039 | 2.411 |
| Type of breed | 0.650 | - | - | - |
| Type of husbandry | 0.621 | - | - | - |
| Cough/Running nose | 0.552 | 0.778(yes/no) | 0.339 | 1.784 |
| Treated with antimicrobial | 0.313 | 1.765(yes/no) | 0.578 | 5.388 |
Cattle between the ages of 4-6years (97- 120months) were most infected with bovine TB (Fig 1).
Human population
A total of 68 herdsmen were sampled. Almost 68% (46/68) of participants were males. All human samples tested Negative for Acid Fast Bacilli (AFB) (Table 4). Majority of respondents were between the ages of 11-20 years and the median age group of respondents was 31-40 years (Table 5).
Table 4.
Laboratory result of humans for TB, North Tongu District, October 2011- March 2012.
| Species | Status | Counts | % |
|---|---|---|---|
| Human | Positive | 0 | 0 |
| Negative | 68 | 100 |
Table 5.
Characteristics of participants, North Tongu District, October 2011- March 2012.
| Variable | Item | Count | % |
|---|---|---|---|
| Age | 1-10 | 1 | 1.5 |
| 11-20 | 18 | 26.5 | |
| 21-30 | 5 | 7.3 | |
| 31-40 | 13 | 19.1 | |
| 41-50 | 16 | 24 | |
| 51-60 | 7 | 10.3 | |
| 61-70 | 5 | 7.3 | |
| 71-80 | 3 | 4.0 | |
| Sex | Male | 46 | 68 |
| Female | 22 | 32 |
The majority of the respondents were illiterates. Among those who had education, middle school/JSS was the highest level of education attained (Table 6).
Table 6.
Characteristics of participants, North Tongu District, October 2011- March 2012. Continued.
| Variable | Item | Count | % |
|---|---|---|---|
| Educational Level | Primary | 16 | 24 |
| Middle/JSS | 17 | 25 | |
| Secondary/SSS | 2 | 2.5 | |
| Tertiary | 2 | 2.5 | |
| None | 31 | 46 | |
| Ethnic group | Mole Dagbani | 13 | 19 |
| Ewe | 49 | 72.1 | |
| Krobo | 1 | 1.5 | |
| Other | 5 | 7.4 | |
| Religion | Christian | 28 | 41.2 |
| Moslem | 20 | 29.4 | |
| Traditional | 8 | 11.8 | |
| None | 8 | 11.8 | |
| Other | 4 | 5.8 |
Most of the participants indulge in risky practices such as consuming fresh milk, sharing bulls for breeding as well work without protective clothing that could expose them to contracting bovine TB (Table.7).
Table 7.
Possible risk factors of bovine TB in human, North Tongu District, October 2011- March 2012.
| Variable | Response | Count | % |
|---|---|---|---|
| Fresh milk consumption | Yes | 61 | 89.7 |
| No | 7 | 10.3 | |
| Protective clothing usage during herding and milking | Yes | 11 | 16.2 |
| No | 57 | 83.8 | |
| Bull sharing with other farms | Yes | 48 | 70.6 |
| No | 20 | 29.4 |
Most of the participants indulge in risky practices such as consuming fresh milk, sharing bulls for breeding as well work without protective clothing that could expose them to contracting bovine TB (Table.7).
Discussion
The need to determine the contribution of bovine tuberculosis (BTB) to the general tuberculosis burden in a poor resource setting is paramount. This study evaluates the burden of BTB among herdsmen and cattle in the North Tongu district of the Volta Region in Ghana.
The 19% prevalence rate of BTB in cattle that was found from this study indicates the existence of the disease in the area. The prevalence found in this study is higher than what was found by other workers whose results was based on routine data from slaughter houses. This could mean that active search other than passive surveillance yielded more results (Bonsu et. al., 2003).
The strong association found between seropositivity and the kraal density demonstrates the effect of overcrowding on the degree of transmission of BTB within the flocks and agrees with the findings of Humblet et al., (2010) that seropositivity of cattle to BTB and animal density were statistically significant. The increase in seropositivity of cattle to BTB with age which was though found to be statistically insignificant agrees with the fact that BTB manifests most at the later stage of the disease especially between 4-6 years opined by Kazwala et al., (2001) and Folitse et al. (2013).
Although the fulanis usually treat their animals with antimicrobial to reduce the level of infection in the herd, this was not effective probably because the antimicrobials employed were not effective against M. bovis which further confirmed that mycobacterium bovis is resistant to many antibiotics (Bilal et al., 2010).
The study result which shows cows were thrice infected as bulls by BTB which agrees with the findings of Bonsu et al., (2003) and Folitse et al., (2013) where cows were twice affected as bulls by BTB. In practice this could mean that cows are more at risk of contracting BTB especially during pregnancy when they have reduced immunity since tuberculosis flourishes well in immuno-compromised host. In addition, for the purpose of increasing production, most cattle farmers have more cows than bulls on their farms which gave room for exposure.
In the human population, the zero prevalence of BTB that was found does not necessarily indicate the absence of the disease, as humans are exposed in diverse ways to bovine TB infection. This can be seen in our results where 90% (61/68) of respondents consume fresh milk and 84% (57/68) do not use protective clothing coupled with close proximity of hu0ans and animals. It could also mean that milk may be infected with Mycobacterium bovis but there were other underling factors that made it impossible for us to detect the pathogen among humans or herdsmen might have strong immune systems that combat the disease.
Conclusion
Bovine TB is prevalent in cattle in North Tongu district. The total number of animals in a kraal (Kraal density) is a risk factor for cattle contracting bovine tuberculosis. Although herdsmen indulge in risky lifestyles that expose them to BTB, a zero prevalence of BTB was found among them. Hence further study is envisaged using a larger sample size and milk from cattle in the area should be tested for M. bovis.
References
- 1.Abubakar I.A. Molecular Epidemiology of human and BTB in the federal capital territory and Kaduna State. UK: Nigeria, PhD Thesis, Plymouth University; 2007. [Google Scholar]
- 2.Addo K.K, Owusu-Darko O.K, Yeboah-Manu D, Caulley P, Minamikawa M, Bonsu F, Leinhardt C, Akpedou P, Ofori-adjei D. Mycobacterial species causing pulmonary tuberculosis at the Korle- bu Teaching Hospital, Accra. Ghana Medical Journal. 2007;41:52–57. doi: 10.4314/gmj.v41i2.55293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayele W.Y, Neill S.D, Zinsstag J, Weiss G. M, Pavlik I. BTB: an old disease but a new threat to Africa. The International Journal of Tuberculosis and Lung Disease. 2004;8:924–937. [PubMed] [Google Scholar]
- 4.Bilal S, Igbal M, Murphy P, Power J. Human bovine tuberculosis- remains in the differential. Journal of Medical Microbiology. 2010;59:1379–1382. doi: 10.1099/jmm.0.020511-0. [DOI] [PubMed] [Google Scholar]
- 5.Bonsu O.A, Laing E, Akanmori B.D. Prevalence of Tuberculosis in Dangme- West District of Ghana, Public Health implications. Acta Tropica. 2003;76:9–14. doi: 10.1016/s0001-706x(00)00082-6. [DOI] [PubMed] [Google Scholar]
- 6.Cosivi O, Daborn C.J, Grange J.M, Raviglione M.C, Fujikura T. Zoonotic Tuberculosis due to Mycobacterium bovis and M. Tuberculosis in goats, Nigeria. Emerging Infectious Diseases. 1998;4:59–70. doi: 10.3201/eid0401.980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danbirni S, Sackey A.K.B, Ayo J.O, Baw E.K, Kudi A.C, Okaiyeto S.O, Pewan S.B. Exposure and shedding in milk of Mycobacterium tuberculosis in Dairy herds using One-Step Anigen Rapid Bovine Tuberculosis Antibodies Test and Ziehl-Neelsen Stain. Veterinary Research. 2010;3:38–43. [Google Scholar]
- 8.Folitse R.D, Boi-Kikimoto B. B, Emikpe B.O, Atawalna J. The prevalence of Bovine tuberculosis and brucellosis in cattle from selected herds in Dormaa and Kintampo Districts, Brong Ahafo region, Ghana. Archives of Clinical Microbiology. 2014;5(2) http://imedpub.com/ojs/index.php/acmicrob/article/view/838 . [Google Scholar]
- 9.Georghiou P, Patel A.M, Konstantinos A. Australian and New Zealand Journal of Medicine. 1989;19:409–410. [Google Scholar]
- 10.Humblet M. F, Gilbert M, Govaerts M, Fauville-Dufaux M, Walravens K, Saegerman C. New Assessment of BTB Risk Factors in Belgium Based on Nationwide Molecular Epidemiology. Clinical Veterinary Microbiology. 2010;48:2802–2808. doi: 10.1128/JCM.00293-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaneene J.B, Theon C.O. Tuberculosis. Journal of the American Veterinary Medical Association. 2004;224:685. doi: 10.2460/javma.2004.224.685. [DOI] [PubMed] [Google Scholar]
- 12.Kazwala R.R, Kambarage D.M, Daborn C.J, Nyange J, Jiwa S.F.H, Sharp J.M. Risk Factors Associated with the Occurrence of BTB in Cattle in the Southern Highlands of Tanzania. Veterinary Research. 2001;25:609–614. doi: 10.1023/a:1012757011524. [DOI] [PubMed] [Google Scholar]
- 13.Office International des Epizooties (OIE) Manual of diagnostic tests and vaccines for terrestrial animals (mammals, birds and bees) 2008 [PubMed] [Google Scholar]
- 14.Shitaye J.E, Tsegaye W, Pavlik I. Bovine Tuberculosis infection in animal and human populations in Ethiopia: a review. Veterinaria Medicina. 2007;52:317–332. [Google Scholar]


