Abstract
Background and Objective
A prior meta-analysis revealed that higher doses of transcranial direct current stimulation (tDCS) have a better post-stroke upper-extremity motor recovery. While this finding suggests that currents greater than the typically used 2 mA may be more efficacious, the safety and tolerability of higher currents have not been assessed in stroke patients. We aim to assess the safety and tolerability of single session of up to 4 mA in stroke patients.
Methods
We adapted a traditional 3+3 study design with a current escalation schedule of 1≫2≫2.5≫3≫3.5≫4 mA for this tDCS safety study. We administered one 30-minute session of bihemispheric montage tDCS and simultaneous customary occupational therapy to patients with first-ever ischemic stroke. We assessed safety with pre-defined stopping rules and investigated tolerability through a questionnaire. Additionally, we monitored body resistance and skin temperature in real-time at the electrode contact site.
Results
Eighteen patients completed the study. The current was escalated to 4 mA without meeting the pre-defined stopping rules or causing any major safety concern. 50% of patients experienced transient skin redness without injury. No rise in temperature (range 26°C–35°C) was noted and skin ba rrier function remained intact (i.e. body resistance >1 kΩ).
Conclusion
Our phase I safety study supports that single session of tDCS with current up to 4 mA is safe and tolerable in stroke patients. A phase II study to further test the safety and preliminary efficacy with multi-session tDCS at 4 mA (as compared with lower current and sham stimulation) is a logical next step.
Keywords: high-dose tDCS, stroke recovery, dose escalation, 3+3 design, non-invasive brain stimulation
Introduction
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique [1–4] that can modulate the cortical excitability of targeted brain regions [5] as well as cerebral blood flow [6] in a polarity-dependent manner. tDCS has been extensively tested in stroke patients with motor impairments [7–13]. Although these studies are mostly “proof of concept” with relatively small sample sizes, they do suggest that tDCS can induce behavioral changes and improve motor function. A meta-analysis of prior post-stroke tDCS studies demonstrated such efficacy, and the meta-regression further revealed a positive dose-response relationship between the current density (i.e., current intensity ÷ pad size) and the motor improvement (measured by Fugl-Meyer Upper Extremity scale) [14]. For a given size of tDCS pads, current density is linearly related to current intensity. While it is not practical or realistic to fine-tune the tDCS pad size, regulating the current intensity by turning a dial has been the most common practice among tDCS researchers. Existing tDCS studies have been limited to current intensity not exceeding 2 mA, possibly due to safety concerns from a historical case who suffered mutism and autonomic disturbances as a result of high-dose stimulation at 3 mA with a montage predominantly affecting temporal lobe and brainstem[15]. Note that such a montage would induce current flow that is different from that typically used for stroke recovery studies, additionally the subject received about 10-times higher intensity than intended. [16]
No systematic investigation has been done to determine the most efficacious current that is safe and tolerable for stroke patients. There is empirical evidence that tDCS with current intensity around 2 mA for 20–40 minutes for either single or multiple sessions can safely be administered to healthy subjects. It is likely safe for stroke patients as well, but there are no safety guidelines in this vulnerable population [17, 18]. Animal studies [18, 19] have established a much higher safety limit (>50×) than the human protocols (Fig. 1). Safety and tolerability is a critical issue to explore because it is possible that a higher current is more efficacious in increasing cortical excitability, and hence motor improvement, in stroke patients. Safety and tolerability need to be established first before the clinical efficacy of higher tDCS current can be tested. Here we aim to use a traditional 3+3 dose-escalation trial design to determine the safety and tolerability of incrementally higher current up to 4 mA.
Figure 1. Established brain lesion threshold from an animal study is more than 50-times higher than typical (≤2 mA) human tDCS setup.

tDCS current of 2 mA delivered for 30 minutes using 35 cm2 is much smaller than the charge that was reported to incur brain damage (1029 vs 52400 C/m2). Tested safety limit of 4 mA proposed in this study is still about 25× smaller (2057 vs 52400 C/m2). Adapted from [19], note that charge density on azimuth is projected on a logarithmic scale.
Material and methods
Overview
This is a Phase I [20] single-site dose-escalation study investigating the safety of incrementally higher tDCS currents in stroke patients. The study protocol was approved by the IRB Committee at the Medical University of South Carolina. All the enrolled subjects in this study signed the informed consent. The trial design is adapted from the classical, gold-standard 3+3 drug trial design [21–23], which is commonly used to find the maximal tolerable dose for chemical drug (Fig. 2). Specifically, the study starts with 1 mA: (a) if no major response (defined below) occurs in any of the three subjects, a current at 2 mA will be used next; (b) if a major response occurs in 2–3 study subjects, then the trial will stop at this current level; (c) if major response occurs in 1 subject, then 3 additional subjects will be entered to further test the safety of the same current; if no additional major response occurs, then the current will be escalated to the next current level; if additional major response occurs, then the trial will terminate and the previous current level is defined as the maximal tolerable current (dose). The schedule of advancing current is: 1≫2≫2.5≫3≫3.5≫4 mA. The 4 mA upper dose was chosen mainly due to the upper limit of the available device that can deliver direct current.
Figure 2. 3+3 dose escalation trial design.
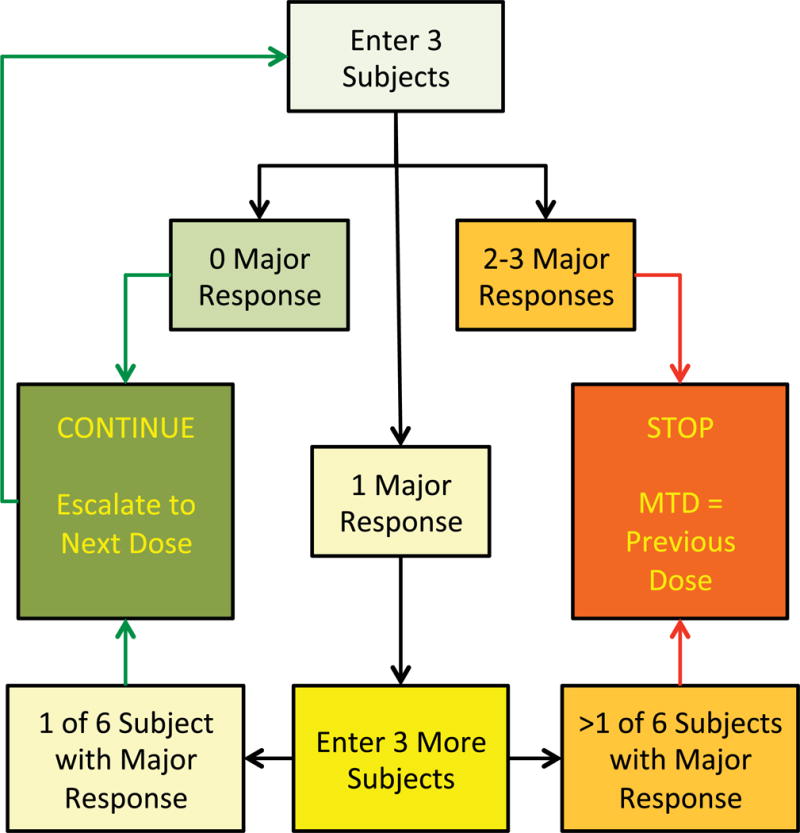
Subjects were tested at incremental dose/current levels of 1.0, 2.0, 2.5, 3.0, 3.5 and 4.0 mA, making six dose/current levels with minimum three subjects at each dose/current level.
Major Response or Stopping Rule and Data Safety Monitoring Board
A major response was pre-defined before the start of the trial by any of the following “serious adverse events” occurring during the study period. It served as the “stopping rule” (Fig. 2).
Second-degree scalp burn at the site of electrode pad; or
Clinical seizure; or
New lesion(s) on Diffusion Weighted Imaging (DWI)/Apparent Diffusion Coefficient (ADC) sequence of MRI scan by visual inspection and the lesion(s) is not explained by any other cause(s); or decreased ADC under the electrode stimulating the motor cortex area; or
Subject discontinues from the study due to any reason above.
An independent Data Safety Monitoring Board (DSMB) was set up for this study to review all of safety profiles according to the NIH guideline. Besides any major response described above, any serious adverse event whether it is tDCS related or not, is required to report to IRB within 24 hours once it occurs, DSMB is subsequently notified to review the event after study principal investigator provide relevant data associated with the event.
Selection criteria and recruitment process and procedures
We recruited first-ever ischemic stroke patients with unilateral motor impairment that fit the inclusion criteria of most stroke recovery trials. Specifically, the inclusion criteria are as followed: 1) 18–80 years old of any race; 2) First-ever ischemic stroke that occurred ≥6 months ago; 3) Completed rehabilitation therapy (including inpatient or outpatient PT/OT/SP) at least two weeks ago; 4) Unilateral limb weakness with Fugl-Meyer Upper Extremity Scale score ≤ 56 (out of 66); 5) Inducible motor evoked potential (MEP) on abductor pollicis brevis (APB) muscle on both sides by transcranial magnetic stimulation (TMS). Subjects meeting any of the following exclusion criteria were excluded:1) Bihemispheric ischemic strokes; 2) History of prior stroke or old infarct, as demonstrated on the CT or MRI or documented in medical records; 3) Other concomitant neurological disorder(s) affecting upper extremity motor function; 4) Documented history of dementia before/after stroke; 5) Documented history of uncontrolled depression or psychiatric disorder either before or after stroke, which could affect the subject’s ability to participate in the experiment; 6) Uncontrolled hypertension despite treatment, specifically SBP/DBP≥180/100 mmHg at baseline; 7) Presence of skin injury or disease at tDCS application sites on scalp; 8) Pregnancy, since the effect of tDCS on the fetus is unknown; 9) Presence of any MRI/tDCS/TMS risk factors: An electrically, magnetically or mechanically activated metal or nonmetal implant including cardiac pacemaker, intracerebral vascular clips or any other electrically sensitive support system; Non-fixed metal in any part of the body, including a previous metallic injury to eye; History of seizure disorder or post-stroke seizure; Preexisting scalp lesion, bone defect or hemicraniectomy.
On the Recruitment Day, the subject was consented first, vital signs were taken, and clinico-demographics were collected. A urine pregnancy test was performed, if applicable. A therapist performed the upper-extremity Fugl-Meyer (UE-FM) scale. The scalp was examined to exclude any skin injury or disease. Transcranial magnetic stimulation (TMS) was applied on the lesioned side of the motor cortex to ensure a motor evoked potential (MEP) could be detected at Abductor Pollicis Brevis (APB) muscle on the affected side. On the Stimulation Day (which can be the same day as the Recruitment Day), the subject underwent a brain MRI and TMS assessments to assess MEP response of APB on both sides. Vital signs were taken and a structured questionnaire was conducted before tDCS session to determine if the subject experienced any symptoms like headache, nausea, burning, tingling, itching, neck pain, electric shock, inattention, etc. The purpose of this questionnaire was to record a baseline, pre-tDCS status of the subject. Each symptom was rated as mild, moderate or severe. Scalp skin was visually inspected and any skin redness was quantified as mild, moderate or severe. The subject then received tDCS stimulation (see below) along with simultaneous customary upper extremity therapy for 30 minutes. Subjects were closely monitored by a neurologist throughout the tDCS session (RC or WF) to detect any sign of clinical seizure or other safety issues. Immediately after tDCS, the subject’s scalp was inspected for injury/skin redness, vital signs were taken and a structured questionnaire regarding potential side-effects of tDCS (see above) was performed immediately after the tDCS session, and any changes in responses were determined by comparing it with pre-tDCS questionnaire. Within 60 minutes of tDCS stimulation ending, the subject underwent four consecutive TMS assessments to test for changes in MEPs (tDCS after-effects can last as long as 90 minutes[4]). A second MRI scan followed within 3–4 hours.
Sample size
Given that this is a 3+3 trial with 6 dose levels (1, 2, 2.5, 3, 3.5 and 4 mA), the minimum number of subjects we would require is 18 (6×3) assuming no major response at any dose level. The maximum number of subjects would be 36 (6×6) assuming one major response was recorded at each dose level. Realistically, with an already established safety profile for up to 2 mA, we did not anticipate any major response for the first two dose levels (1 and 2 mA). Therefore, the number of subjects that would participate in a successful trial would be between 18 to 30 subjects.
tDCS stimulation protocol
We used a Chattanooga Dual-Channel Iontophoresis Device (Chattanooga Group, Hixson, TN) to deliver direct current to study subjects. We used a bihemispheric montage with the center of electrodes placed at C3 and C4 positions according to the 10/20 EEG system. The subject was advised by team staff to trim his/her hair before enrollment, and further advised to take a shower or to wash hair in the morning of the stimulation day. The electrode pads (5×7 cm2 with active area of ~30 cm2) were saline-soaked sponges impregnated with biocarbon material (Soterix Medical, New York, NY). The sponges came pre-soaked with normal saline by the manufacturer, and typically required 6 cc of normal saline to ensure adequate soaking without dripping when they were gently squeezed by fingers or when under the EEG cap. We positioned the anodal electrode on the lesional hemisphere and the cathodal electrode on the contralesional hemisphere. Electrode pads were further secured using a 10/20 EEG cap (Rhythmlink International LLC, Columbia, SC). Hermetically sealed tip insulated T-type thermocouples (Omega Engineering, Inc., Norwalk, CT) were positioned at the skin-electrode interface at both anode and cathode for real-time monitoring of skin temperature. The blunt, smooth and sealed tip of the thermocouples ensured that skin injury does not happen by preventing direct skin contact with any bare wires of the thermocouple. Body resistance was monitored in real-time as a ratio of measured voltage across tDCS electrodes and the current passed through them using data acquisition unit DI-245 (Dataq Instruments Inc., Akron, OH). The electrode pads were disposed after the study and not recycled.
Brain MRI protocol
The following MR images were acquired using a 3T Siemens TIM Trio: a whole brain T1-weighted magnetization-prepared rapid acquisition gradient-echo (MPRAGE) image (TR: 1900 ms, TE: 2.26 ms, TI: 900 ms, matrix: 256 × 256, band width: 200 Hz/Px, voxel size: 1 × 1 × 1 mm3, no gaps, acquisition time: 4:26), a turbo inversion recovery magnitude (TIRM) T2-image (TR: 9000 ms, TE: 93 ms, TI: 2500 ms, voxel size: 0.9 × 0.9 × 4 mm3, gap: 1.2 mm, band width: 287 Hz/px, acquisition time: 4:50). Diffusion-weighted images were acquired at b = 0, 1000, 2000 s/mm2 with 30 directions using a twice-refocused spin-echo (TRSE) echo planar imaging sequence (TR: 6400 ms, TE: 96 ms, matrix = 82 × 82, band width = 1356 Hz/Px, voxel size: 2.7 × 2.7 × 2.7 mm3).
The subject’s chronic stroke lesions were manually outlined by an imaging analyst (RD) under supervision of a stroke neurologist (WF). Lesion delineation was performed on T2 images using multiple slice orientations, with concurrent visualization of other sequences. In general, the lesions were delineated using the following guidelines: 1) microangiopathic changes outside the predominant stroke lesion’s vascular territory were excluded (typically white-matter hyperintensities), 2) remote white-matter signal changes or atrophy outside the stroke lesion were excluded (typically Wallerian degeneration), 3) when portions of the thalamus and caudate nucleus were degraded and replaced by dilated ventricles, the regions corresponding to the lesioned thalamus and caudate were included in the stroke lesion, 4) when the complete cortical layers were missing, the outline of the inner skull was used as the outer boundary of the lesion.
This lesion mask with T1 and T2 images were used for grey/white matter and CSF segmentation by cost function non-linear registration to an elderly template (mean age 72 years old, included in Clinical Toolbox) using the Clinical Toolbox[24] for Statistical Parametric Mapping 8 software (University College London, UK). The manual ROIs (5 cm × 7 cm) were drawn on the T1 image following the curvature of the skull and centered over the central sulcus. ADC maps were calculated using Diffusional Kurtosis Estimator [25] (version 2.6.0). The ROIs and GM/WM segmentation masks were linearly co-registered into individual’s diffusion space using FSL (FMRIB Software Library v4.1.7 Oxford, UK). The skull and CSF were excluded in the ROI and the ADC values were averaged using inhouse MATLAB scripts (R2015a, The MathWorks, Inc., Natick, MA). ADC maps from the MRI taken after tDCS were then subtracted from the ones before tDCS to detect any change in ADC values[26] (Fig. 3).
Figure 3. Quantitative analysis of change in Apparent Diffusion Coefficient (ADC) map as a result of tDCS.
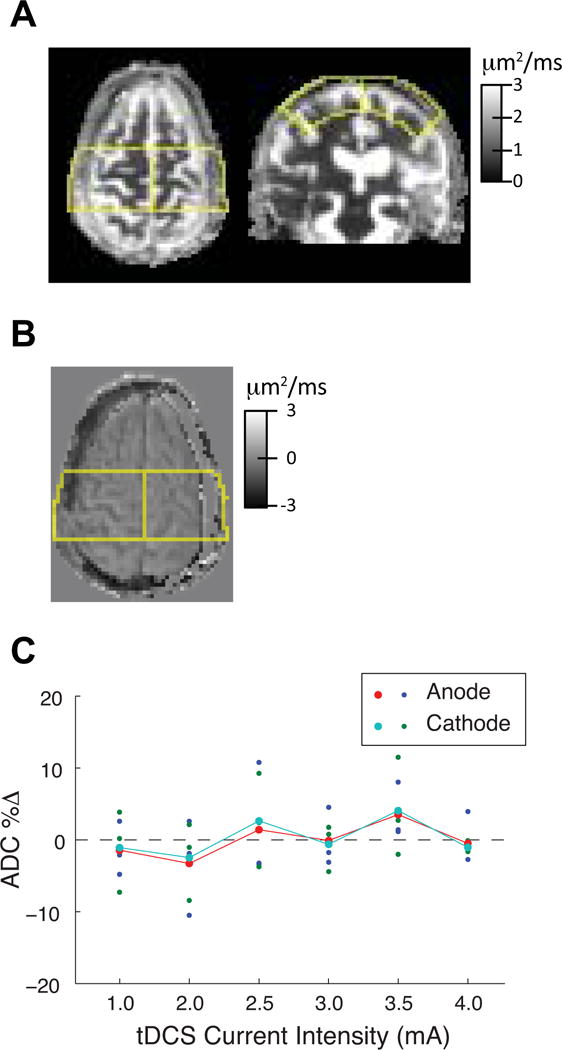
A, Axial and coronal view of the 5 × 7 cm2 mask (yellow outline) to calculate ADC values after excluding scalp, skull and cerebrospinal fluid (CSF). B, Axial view of the subtraction ADC map of the same subject presented in A with the mask overlay. C, Percent change in ADC values of individual subjects at anode (blue) and cathode (green) at each tDCS dose level and also mean ADC change (dots joined by lines). One subject who received 2 mA dose level had mismatched MRI image and therefore could not have accurate percent change ADC calculation and therefore presented with ~10% decrease in ADC as a result.
Results
Eighteen subjects in total (11 males and 7 females, aged 55.3±13.2 years, mean±SD) participated in the study, each receiving one dose level, with 3 subjects at each dose level as the current level was escalated to 4 mA without meeting the stopping rule. Since no major response occurred at any current level, the trial completed with 18 subjects. Skin redness without skin injury at the eletrode site is common as we observed this in 50% of subjects (9 subjects or 12 incidences). Nine incidences occurred at the anodal site and 3 at the cathodal site. Four happened at ≤2 mA vs. 8 at >2 mA. No subject sustained redness beyond a few minutes after tDCS stimulation. No subject had any tolerability issues with tDCS. We did not visually identify any new brain lesion on DWI/ADC sequence or observe clinically meaningful changes in the anisotropy of the brain under tDCS electrode locations (Table 1, Fig. 3). Real-time monitoring of body resistance and temperature at skin-electrode interface confirmed safe delivery of tDCS. Skin temperatures remained below body temperature (26 °C–35 °C). The highest recorded temperature was 35 °C with median temperatures of ~32 °C. No difference in temperature rise was noticed between anode and cathode. There was no abrupt decrease in body resistance (<400 Ω), a parameter we monitor during stimulation for any breach in the skin barrier function [27] (Fig. 4).
Table 1.
Safety and tolerability profiles at each dose level
| tDCS current | ||||||
|---|---|---|---|---|---|---|
| 1.0 mA | 2.0 mA | 2.5 mA | 3.0 mA | 3.5 mA | 4.0 mA | |
| Baseline characteristics | ||||||
| Subjects (n) | 3 | 3 | 3 | 3 | 3 | 3 |
| Females (n) | 1 | 0 | 2 | 2 | 1 | 1 |
| Age (years, Mean) | 50 | 57 | 53 | 62 | 58 | 52 |
| FM-UE (affected side, Mean) | 40 | 51.3 | 38 | 54.7 | 47 | 42.7 |
| rMT (affected side, Mean) | 42.7 | 45.3 | 69.3 | 36.7 | 48 | 39.3 |
| rMT (non-affected side, Mean) | 40.3 | 35.3 | 36.7 | 32.7 | 44.3 | 44.7 |
| Safety profile | ||||||
| Second degree skin burn (n) | 0 | 0 | 0 | 0 | 0 | 0 |
| Clinical seizure (n) | 0 | 0 | 0 | 0 | 0 | 0 |
| New DWI lesion (n) | 0 | 0 | 0 | 0 | 0 | 0 |
| Subject discontinuation (n) | 0 | 0 | 0 | 0 | 0 | 0 |
| Tolerability profile | ||||||
| Headache (n) | 0 | 0 | 0 | 0 | 0 | 0 |
| Neck pain (n) | 0 | 0 | 0 | 0 | 0 | 0 |
| Scalp pain (n) | 0 | 0 | 0 | 0 | 0 | 0 |
| Tingling (n) | 0 | 0 | 0 | 0 | 0 | 0 |
| Itching (n) | 0 | 0 | 0 | 0 | 0 | 0 |
| Burning (n) | 0 | 0 | 0 | 0 | 0 | 0 |
| Electric shock sensation (n) | 0 | 0 | 0 | 0 | 0 | 0 |
| Sleepiness (n) | 0 | 0 | 0 | 0 | 0 | 0 |
| Trouble concentrating (n) | 0 | 0 | 0 | 0 | 0 | 0 |
| Mood change (n) | 0 | 0 | 0 | 0 | 0 | 0 |
| Other issues (n) | 0 | 0 | 0 | 0 | 0 | 0 |
| Skin redness at Anode (n) | 2 | 2 | 1 | 0 | 2 | 2 |
| Skin redness at Cathode (n) | 0 | 0 | 0 | 0 | 1 | 2 |
FM-UE: Fugl Meyer Upper Extremity Scale; rMT: Resting Motor Threshold; DWI: Diffusion Weighted Imaging
Figure 4. Real-time monitoring of tDCS delivery confirmed safety.

The skin temperature at electrode-skin contact sites remained well below normal body temperature value of 37°C (range 26°C–35°C). Body r esistance was always higher than 2 kΩ suggesting no breach in the skin barrier function.
Discussion
We used a traditional 3+3 dose-escalation trial design (Fig. 2) and demonstrated that single session of tDCS up to 4 mA for the duration of 30 minutes is safe and tolerable in stroke subjects. The outcome of a 3+3 design is that the maximal tolerable current (dose) is defined as the current at which ≥2/6 of the stroke subjects experience pre-defined current (dose)-limiting major response (i.e., meeting the stopping dose). The design is simple, clear, has been accepted and widely used in drug trials, even though the selection of one-third for stopping is admittedly arbitrary. While none of the subjects experienced any major response, there is nevertheless a small possibility this could be due to a statistical chance. However, given that 4 mA for 30 minutes represents an order of magnitude smaller charge density than the charge density that demonstrated brain injury in animal models (Fig. 1), we do believe our trial conceptually establishes safety and tolerability. A next logical step is to continue to monitor the safety profile of higher current with multiple sessions and to test preliminary efficacy in a phase II study.
We present this study as the first step towards establishing the efficacy of higher-dose tDCS. Post-stroke motor recovery trials typically use currents ≤2 mA. While the meta-regression analysis revealed a positive dose-response relationship [14], suggesting higher tDCS current (or current density, given same size of tDCS pads are used) may offer better efficacy, prior efficacy studies have not been conducted with currents >2 mA. Other factors in addition to tDCS dose may contribute to the efficacy as well. Before efficacy studies at higher current can be conducted, it is essential to establish the safety profile first. Tolerability is also a critical issue for tDCS, as intolerability can lead to poor subject recruitment and retention. We arbitrarily selected 4 mA as our targeted high-current tDCS value as it was the upper-limit of the devices available at the time of the project planning, and we speculate that even higher currents could be safe and tolerable and should be investigated in the future once such device is available. Regardless, our study has pushed the boundaries of present tDCS practice, as it establishes safety and tolerability of a dose that is two-fold higher than the conventional dose.
Skin redness without injury was common. We believe that skin redness is the result of vasodilation of cutaneous and subcutaneous vessels, analogous to increased cerebral blood flow as measured using MRI imaging [6]. While skin redness was common even at lower current on anodal site, we observed skin redness on both sites, but redness on cathodal sites was noticed only at higher doses at 3.5 and 4.0 mA. Skin redness was a transient phenomenon, and none of the subjects sustained skin redness after one hour of tDCS application. Other tolerability issues (e.g., tingling, itching, burning, electric shock-like sensations, altered mood, sleepiness, trouble concentrating) were absent after tDCS application. Some subjects did report tingling, itching or burning sensations at the beginning or for the early part of tDCS stimulation but such sensations were transient and did not sustain after the end of DC stimulation. Such sensations, if any, were mild at most, and did not interfere with participation in therapy throughout the session, nor did they cause subjects to discontinue from the study.
Skin temperature values below normal body temperature at electrode locations exclude the possibility of tDCS-related thermal injury. Although thermal heating is the square of applied voltage [27], the thermal mass of saline-soaked electrode pads is big enough to absorb any thermal transformation of electrical energy delivered to the skin by tDCS, thus preventing thermal skin injury. Sub-normal skin temperatures rule out any thermal damage to the skin, but direct electrical skin injury remains a theoretical possibility. The transient and reversible erythema or redness found at the anode and/or cathode locations should not be interpreted as skin injury, but rather as vasomotor responses at the skin level, which can be compared to changed vasomotor reactivity at the brain level, as a result of tDCS [28].
We are confident about the integrity of skin barrier function against the tDCS doses as confirmed by >2 KΩ body resistance at each dose level, which is substantially higher than typical human internal body resistance of 300 Ω [27]. Additionally, this finding of intact skin barrier function concurs with the theoretical premise that given a small contact area of a 35 cm2 electrode pad, a much higher voltage (>500 V) would be necessary to disrupt the high resistance of the outer skin layer, [29]. In cases of contact with much larger areas of skin (e.g., a body fully immersed in water) the human body can offer resistance of as little as ~400 Ω, virtually eliminating skin resistance [30]. However, in our case, any possibility of skin barrier function disruption was excluded due to high resistance (>2 kΩ) offered by a limited area of scalp contact (35 cm2 each for entry and exit of tDCS with voltage application in the order of 20 V successfully delivered up to 4 mA).
One subject passed away two weeks late after he received tDCS session at 3.5 mA dose level. As a result, the study was voluntarily placed on hold by the study team. The Data Safety Monitoring Board (DSMB) reviewed medical records and the death certificate, and they determined that the death was likely related to the subject’s preexisting cardiac issues and side effects from three medications with QT prolongation potential. The death was determined to be unrelated to this tDCS study. The study, therefore, was resumed. Data from this subject is included in the final analysis.
Although this study demonstrated that it is safe to escalate current up to 4 mA using a 3+3 design, the most commonly used phase I drug trial design for defining the maximal tolerable drug dose, several cautions should be exercised when interpreting the data. Serious adverse event(s) may occur at low odds, and it is possible that we did not observe any major response simply due to statistical chance with a small sample size of 18 subjects. For example, statistical simulations have demonstrated that a trial using the 3+3 design identifies the maximum tolerated dose (MTD) in about 30% of trials [31]. An alternative 6+6 trial design may partially mitigate this issue. Regardless, our study conceptually demonstrated higher current up to 4 mA can be safely applied to stroke subjects. It is important to emphasize the needs to further assess the safety of higher current with multiple-sessions as a majority of rehab trials used multiple-session tDCS to reduce stroke-related impairments. While safety and tolerability of tDCS for up to 2 mA current intensity is well established, another limitation could be that we did not start the dosing with sham stimulation which can be important to compare tolerability and efficacy with various dose levels. Additionally, our results are specific to the tested bihemispheric montage as an anodal or cathodal montage with the other electrode placement at forehead could result in stronger electric fields in frontal lobes or with the other electrode placed extracephalic (i.e., shoulder) could result in stronger electric fields in the brainstem area [32].
Conclusions
We demonstrated that it is safe and tolerable to deliver up to 4 mA tDCS current with saline-soaked 35 cm2 pads in a bihemispheric montage configuration for one 30-minute session to stroke subjects. Using real-time biometric monitoring of body resistance and skin temperature at tDCS application sites, we also exclude the possibility of thermal injury or disruption of skin barrier function. It is a logical step to further assess the safety and preliminary efficacy of 4 mA with multiple sessions in a phase II study to systematically investigate tDCS application in stroke recovery.
Highlights.
tDCS currents >2 mA have not been investigated in stroke patients
This phase I dose escalation study establishes safety of up to 4 mA in stroke patients
No predefined major response was noted at any current level
Skin temperature did not rise, and skin barrier function remained intact
Transient skin redness without injury was a common finding irrespective of dose level
Acknowledgments
Authors thank William DeVries, Ha Min Lee, Fay Davis, Drs. Hernan Bayona, Shimeng Liu, Robert Adams and Yumei Liu for their generous assistance with various parts of the study.
Sources of funding:
This project is supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (P20GM109040). PYC acknowledges fellowship grant support from SC-CoAST/NIH StrokeNet (U10NS086490) and American Heart Association (15SFDRN24480016); SAK acknowledges grant support from the Rehabilitation Research and Development Service of the VA, I01RX001935); WF, PYC, MG and SAK acknowledge grant support from National Center of Neuromodulation for Rehabilitation (P2CHD086844). WF also acknowledges grant support from American Heart Association (14SDG1829003).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov Identifier: NCT02763826
References
- 1.Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. 2007;72:208–14. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Schlaug G, Renga V. Transcranial direct current stimulation: a noninvasive tool to facilitate stroke recovery. Expert Rev Med Devices. 2008;5:759–68. doi: 10.1586/17434440.5.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol. 2003;114:2220–2. doi: 10.1016/s1388-2457(03)00235-9. author reply 2–3. [DOI] [PubMed] [Google Scholar]
- 4.Nitsche MA, Liebetanz D, Antal A, Lang N, Tergau F, Paulus W. Modulation of cortical excitability by weak direct current stimulation–technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003;56:255–76. doi: 10.1016/s1567-424x(09)70230-2. [DOI] [PubMed] [Google Scholar]
- 5.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng X, Alsop DC, Schlaug G. Effects of transcranial direct current stimulation (tDCS) on human regional cerebral blood flow. Neuroimage. 2011;58:26–33. doi: 10.1016/j.neuroimage.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010;75:2176–84. doi: 10.1212/WNL.0b013e318202013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fregni F, Boggio PS, Mansur CG, Wagner T, Ferreira MJ, Lima MC, et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16:1551–5. doi: 10.1097/01.wnr.0000177010.44602.5e. [DOI] [PubMed] [Google Scholar]
- 9.Kim DY, Lim JY, Kang EK, You DS, Oh MK, Oh BM, et al. Effect of transcranial direct current stimulation on motor recovery in patients with subacute stroke. Am J Phys Med Rehabil. 2010;89:879–86. doi: 10.1097/PHM.0b013e3181f70aa7. [DOI] [PubMed] [Google Scholar]
- 10.Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–9. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- 11.Hesse S, Werner C, Schonhardt EM, Bardeleben A, Jenrich W, Kirker SG. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: a pilot study. Restor Neurol Neurosci. 2007;25:9–15. [PubMed] [Google Scholar]
- 12.Hesse S, Waldner A, Mehrholz J, Tomelleri C, Pohl M, Werner C. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: an exploratory, randomized multicenter trial. Neurorehabil Neural Repair. 2011;25:838–46. doi: 10.1177/1545968311413906. [DOI] [PubMed] [Google Scholar]
- 13.Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. 2007;25:123–9. [PubMed] [Google Scholar]
- 14.Chhatbar PY, Ramakrishnan V, Kautz S, George MS, Adams RJ, Feng W. Transcranial Direct Current Stimulation Post-Stroke Upper Extremity Motor Recovery Studies Exhibit a Dose-Response Relationship. Brain Stimul. 2016;9:16–26. doi: 10.1016/j.brs.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lippold OC, Redfearn JW. Mental Changes Resulting from the Passage of Small Direct Currents through the Human Brain. Br J Psychiatry. 1964;110:768–72. doi: 10.1192/bjp.110.469.768. [DOI] [PubMed] [Google Scholar]
- 16.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1:206–23. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimul. 2016;9:641–61. doi: 10.1016/j.brs.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bikson M, Datta A, Elwassif M. Establishing safety limits for transcranial direct current stimulation. Clin Neurophysiol. 2009;120:1033–4. doi: 10.1016/j.clinph.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liebetanz D, Koch R, Mayenfels S, Konig F, Paulus W, Nitsche MA. Safety limits of cathodal transcranial direct current stimulation in rats. Clin Neurophysiol. 2009;120:1161–7. doi: 10.1016/j.clinph.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Storer BE. Design and analysis of phase I clinical trials. Biometrics. 1989;45:925–37. [PubMed] [Google Scholar]
- 21.Penel N, Isambert N, Leblond P, Ferte C, Duhamel A, Bonneterre J. “Classical 3 + 3 design” versus “accelerated titration designs”: analysis of 270 phase 1 trials investigating anti-cancer agents. Invest New Drugs. 2009;27:552–6. doi: 10.1007/s10637-008-9213-5. [DOI] [PubMed] [Google Scholar]
- 22.Ratain MJ, Mick R, Schilsky RL, Siegler M. Statistical and ethical issues in the design and conduct of phase I and II clinical trials of new anticancer agents. J Natl Cancer Inst. 1993;85:1637–43. doi: 10.1093/jnci/85.20.1637. [DOI] [PubMed] [Google Scholar]
- 23.Hansen AR, Graham DM, Pond GR, Siu LL. Phase 1 trial design: is 3 + 3 the best? Cancer Control. 2014;21:200–8. doi: 10.1177/107327481402100304. [DOI] [PubMed] [Google Scholar]
- 24.Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath HO. Age-specific CT and MRI templates for spatial normalization. Neuroimage. 2012;61:957–65. doi: 10.1016/j.neuroimage.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tabesh A, Jensen JH, Ardekani BA, Helpern JA. Estimation of tensors and tensor-derived measures in diffusional kurtosis imaging. Magn Reson Med. 2011;65:823–36. doi: 10.1002/mrm.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nitsche MA, Niehaus L, Hoffmann KT, Hengst S, Liebetanz D, Paulus W, et al. MRI study of human brain exposed to weak direct current stimulation of the frontal cortex. Clin Neurophysiol. 2004;115:2419–23. doi: 10.1016/j.clinph.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Fish RM, Geddes LA. Conduction of electrical current to and through the human body: a review. Eplasty. 2009;9:e44. [PMC free article] [PubMed] [Google Scholar]
- 28.Vernieri F, Assenza G, Maggio P, Tibuzzi F, Zappasodi F, Altamura C, et al. Cortical neuromodulation modifies cerebral vasomotor reactivity. Stroke. 2010;41:2087–90. doi: 10.1161/STROKEAHA.110.583088. [DOI] [PubMed] [Google Scholar]
- 29.Grimnes S. Dielectric breakdown of human skin in vivo. Med Biol Eng Comput. 1983;21:379–81. doi: 10.1007/BF02478510. [DOI] [PubMed] [Google Scholar]
- 30.Smoot A, Bentel C. Electric shock hazard of underwater swimming pool lighting fixtures. IEEE Transactions on Power Apparatus and Systems. 1964;83:945–64. [Google Scholar]
- 31.Reiner E, Paoletti X, O’Quigley J. Operating characteristics of the standard phase I clinical trial design. Computational Statistics & Data Analysis. 1999;30:303–15. [Google Scholar]
- 32.Truong DQ, Huber M, Xie X, Datta A, Rahman A, Parra LC, et al. Clinician accessible tools for GUI computational models of transcranial electrical stimulation: BONSAI and SPHERES. Brain Stimul. 2014;7:521–4. doi: 10.1016/j.brs.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


