Abstract
Background:
The daily use of Trimethoprim-Sulfamethoxazole (TMP-SMX) prophylaxis reduces morbidity and mortality among patients infected with Human Immunodeficiency Virus (HIV) but its impact on increasing antimicrobial resistance rates has been of public concern globally. This study investigated the effect of daily TMP-SMX prophylaxis on faecal carriage rates of resistant isolates of Escherichia coli in HIV-infected adult patients in Lagos.
Methods:
A total of 550 HIV-infected patients with CD4-cell count of less than 350 cell/mm3 and were eligible for TMP-SMX prophylaxis attending Lagos University Teaching Hospital, Lagos, Nigeria, were recruited. Stool/rectal swab samples were aseptically collected from the patients and processed using standard methods for culture and sensitivity.
Results:
There was a baseline Trimethoprim-Sulfamethoxazole resistance rate of 54% which increased to 77.9% in first 3 months, rising to 96.1% by 6 months and all isolates were resistant by the 9th month. There was also evidence of cross-resistance to other antibiotics with significant association with TMP-SMX resistance (p<0.0001). The Escherichia coli isolates showed a progressive increase in resistance to the tested antibiotics over the 12-month period. The resistance was in the following order: Ampicillin (74% to 82.6% in the first 3 months; 98.3% by the 6th month and 99.4% by the 9th month; all isolates were resistant by the 12th month). Augmentin (32.5% to 47.7% in first 3 months; 76.1% by the 6th month; 86.3% by the 9th month; all isolates were resistant by 12 months). Ceftriaxone (2.0% to 10.8% in first 3 months; 20.6% by the 6th month; 24.2% by the 9th month; 54.3% by the 12 months).
Conclusion:
The carriage rate of faecal E. coli resistant to TMP-SMX is common before TMP-SMX prophylaxis. Initiation of TMP-SMX leads to further increase in resistance to TMP-SMX and cross-resistance to other antimicrobials.
Keywords: Trimethoprim- Sulfamethoxazole, HIV, Escherichia coli, Drug resistance, Prophylaxis
Introduction
Patients infected with Human immunodeficiency virus (HIV) are at increased risk of opportunistic infections mainly due to Mycobacterium tuberculosis, Staphylococcus aureus, Streptococcus pneumoniae, and Enterobactereceae. In sub-Saharan Africa, these infections account for over 90% of morbidity and mortality found in these patients (WHO, 2006). However, the conference convened by the Joint United Nations Program on HIV/AIDS (UNAIDS) and the World Health Organization in April 2000 recommended that Trimethoprim-Sulfamethoxazole (septrin) be used for prophylaxis in adults and children living with HIV/AIDS in Africa as part of a minimum package of care for the prevention of opportunistic infections especially Pneumocystis carrinii pneumonia (PCP) which is the most common presenting manifestation in Africa (UNAID/WHO, 2000).
This recommendation was based on the evidence from trials on Trimethoprim-Sulfamethoxazole prophylaxis conducted in Africa, including Cote d’Ivoire, South Africa, Malawi, Zambia, and Uganda which showed significant reductions in mortality of between 25% and 46% morbidity. This effect was also significant even in areas with high bacterial acquired resistance (Grimwade et al., 2005; Werarak et al., 2006). This resulted in decreased frequency of clinic visits and hospitalizations. Other studies carried out in Uganda and Zambia showed a beneficial effect of Trimethoprim-Sulfamethoxazole prophylaxis on CD4 cell count and HIV viral load (Chintu et al., 2004; Mermin et al., 2004). In addition, it is inexpensive, readily available and relatively safe (Castebon et al., 2001; Grimwade et al., 2001). Septrin and the related compound sulfadoxine-pyrimethamine play central roles in the management of common clinical syndromes in Africa. These drugs are frequently used to treat dysentery, lower respiratory tract infection, and fever in which Shigella spp., non-Typhi serotypes of Salmonella enterica, Streptococcus pneumoniae, and Plasmodium spp., respectively, play major roles. It follows that increases in resistance to septrin among these pathogens could reduce the effectiveness of empiric treatment strategies, leading to more illness and death. Therefore concern was raised that the widespread use of SXT may substantially increase the prevalence of antimicrobial resistance in common community-acquired pathogens.
The continuous exposure of bacteria to antibiotics leads to antibiotic resistance from selective pressure. Trimethoprim-Sulfamethoxazole prophylaxis creates such a selective pressure (Cotton et al., 2008) on Escherichia coli which is a normal commensal in the gastrointestinal tract (GIT) of humans. The large microbial population in the GIT (over 109/ml of faeces) allow for the easy transmission of antimicrobial resistant elements between various organisms and E. coli has been shown to be a source of resistant gene to Salmonella, Shigella, Yersina, and Vibro species as well as potentially pathogenic Escherichia coli (Chiller et al., 2003; Chikwendu et al., 2008). We therefore decided to evaluate the impact of Trimethoprim-Sulfamethoxazole prophylaxis on antimicrobial resistance using Escherichia coli as an indicator.
Materials and Methods
Study Area and Population
The study was carried out in the Adult HIV Clinics of the Lagos University Teaching Hospital (LUTH) and the National Institute for Medical Research (NIMR), Yaba both in Nigeria.
Study Design
The study was an incidence and a cross-sectional study.
Cross-sectional study
This was carried out over a 3 month period between February and April 2009 at the Adult HIV Clinics in LUTH Idi-araba and NIMR Yaba, Lagos. A total of 550 (82.2%) participants out of the 669 patients that were diagnosed with HIV in that period were recruited. Using the clinic register, every second patient that consented was recruited.
Incidence study
The 200 (20%) newly registered patients who had not commenced TMP-SMX prophylaxis out of the 2012 patients attending the clinic that year were randomly recruited and followed up for a period of one year on outpatient basis. Also 120 participants who were not on TMP-SMX prophylaxis were recruited and followed up for one year as control. These were siblings or spouses of the HIV-infected patients who regularly accompanied them to the clinic. They were HIV-negative and not on TMP-SMX.
Ethical Consideration
The study was reviewed and approved by the ethical committee of the Lagos University Teaching Hospital according to Helsinki Declaration. All patients were required to sign an informed consent and those that gave their consent were recruited. Samples, data and results of patients were treated with utmost confidentiality and used for the purpose of this study only.
Sample collection
Stool samples were collected in sterile universal bottles. Three rectal swabs were collected from patients who could not produce faeces. No patient was sampled more than once in the cross-sectional study while a repeat stool sample were collected from all the new patients that were followed up as a cohort at their 3th, 6th, 9th and 12th month hospital visit. A two week widow of opportunity before and after the expected date of collection was accepted. Stool and rectal swabs were immediately taken to the laboratory of the Department of Medical Microbiology and Parasitology of the College of Medicine, University of Lagos, Nigeria, and processed within one hour of collection.
Isolation and Identification of Escherichia coli
Faecal samples were inoculated directly onto MacConkey agar (OXOID) and incubated for 24 hours at 37°C. From each plate, three to five smooth, dry and flat colonies that were lactose fermenting were picked, and sub-cultured for purity. Pure colonies were gram stained. If Gram negative, a part of one colony was picked and tested for motility. If motile, a part of the same colony was sub-cultured and tested for indole. All indole positive, motile gram negative bacilli were further characterized on the basis of their reactions to citrate, oxidase, urease, and growth characteristics on Kliager Iron Agar. All motile gram-negative, oxidase and urea negative, indole positive, producing acid in both the butt and slant on KIA with greenish metallic-sheen colonies on Eosin methylene blue (EMB) agar were presumed to be E. coli. These isolates were then stored on nutrient agar (OXOID) slants for antibiotic susceptibility testing.
Antimicrobial Susceptibility Testing
The antimicrobial susceptibility tests were carried out using Kirby-Bauer disk diffusion method as recommended by the Clinical and Laboratory Standards Institute (CLSI, 2009a). The antibiotics tested were: ceftriaxone (30<xg), ciprofloxacin (5<xg), amoxicillin/clavulanate (20/10ug), gentamicin (10ug), ampicillin (10ug), imipenem (30ug) and trimethoprim-sulfamethoxazole (1.25/23.75ug). Escherichia Coli ATCC 25922 strain was used as a control.
All antibiotic discs were allowed to equilibrate to room temperature before use. Isolates with zones of inhibition to TMP-SMX above 16mm were considered sensitive while those less than 10mm were considered resistant. The Minimum inhibitory concentrations (MIC) of these resistant isolates were carried out using E-test method to confirm resistance of these drugs in accordance to the CLSI methodology (CLSI, 2009b).
TMP-SMX resistant isolates were further screened for j3-lactamase production using a nitrocephin disc. All isolates that were resistant to 3rd generation cephalosporin were tested for extended j3-lactamase (ESBL) production (CLSI, 2009b). ESBL was determined according to the criteria of the Clinical and Laboratory Standards Institute (CLSI) by means of a double disk diffusion method12.
Statistical analysis
The Epi-Info software 6.04 version (World Health Organization) was used for data analysis.
Results
Socio-Demographic Characteristics
A total of 550 were recruited into a cross sectional study and the effect of TMP-SMX prophylaxis on intestinal isolates of E. coli evaluated at pre-TMP-SMX prophylaxis (0) and at 3rd, 6th, 9th, and 12th month prophylaxis. The mean age of the participants was 35.6 ± 9.1, about half (52%) were married while the male to female ratio was 1:1.4. All participants came from socio-economically deprived settings with 18.2 % being Unemployed, 11.1 % Artisans and 11.6% Traders. The participant’s ability to read and write was low; 40.4 % attended primary school, 43.4 % secondary school and 8.6% had no formal education. The median income level was ₦6,500.00 per month, with on average of 5 persons per household, translating into ₦217.00/person/day. Nine (1.6%) patients were hospitalized within the last three months, 28 (5.1%) took over the counter antimicrobial use in the preceding month, and 17 (3.1%) took herbal medications within the last three months. Among the controls, the mean age was 34.9 ± 4. 57.5% were married while the male to female ratio was 1:1.6. Also all participants came from socio-economically deprived settings with 14.2 % being Unemployed, 12.5 % Artisans, and 17.5% were Traders. The participant’s ability to read and write was low; 34.0 % attended primary school, 51.0% secondary school and 7.0% had no formal education. The median income level was ₦6,250.00 per month, with on average of 5 persons per household, translating into ₦208.00/person/day. Sixty-two (11.3%) took over the counter antimicrobial use in the preceding month, and 34 (6.2%) took herbal medications within the last three months.
Cross Sectional Study
Carriage rate of TMP-SMX resistance of faecal Escherichia coli.
The carriage rate of TMP-SMX resistant isolates of E. coli (PLATE 1) varied between the different groups of patients. One hundred and eight (54%) of the 200 isolates (0 month) were resistant. And of the each of 100 participants, 80% of the isolates were resistant at 3 month visit, 97% at 6 month visit, 99% at 9 month visit, and 100% at 12 month visit (Figure 1). There was an increasing trend in TMP-SMX resistance among each group of patients. However, there is statistically significant difference between TMP-SMX resistance at 3 months and that of 0 month (p=0.001) and also between TMP-SMX resistance at 6 months and that of 3 months (p=0.001). There was no statistically difference between TMP-SMX resistance at 9 months and 6 months (p=0.07) as well as between 12 months and 9 months.
Figure 1.
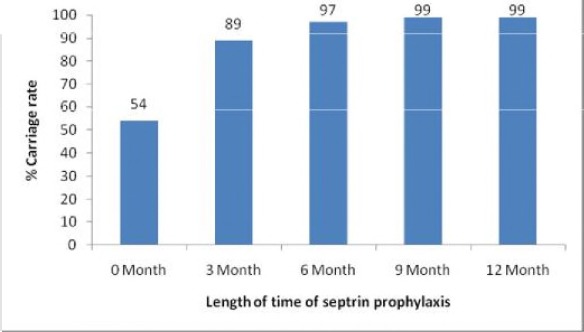
Carriage rate of resistant E. coli among the various groups of patients.
TMP-SMX resistance and antibiotic resistance profile.
Co-selection of antimicrobial resistance was assessed among all the isolates. With the commencement of TMP-SMX prophylaxis, TMP-SMX resistance was found to be strongly associated with cross resistance to all other antimicrobial tested except Imipenem (Figure 2) (p<0.000001).
Figure 2.
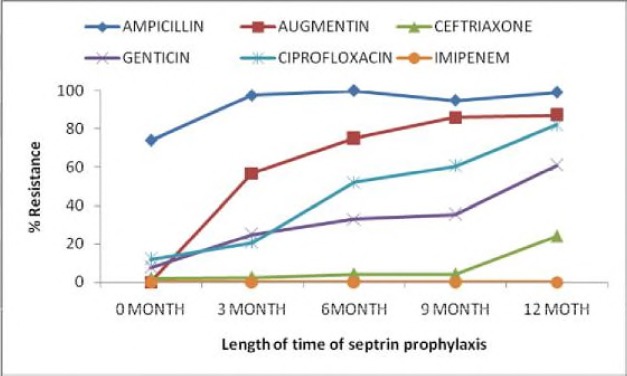
Association of TMP-SMX resistance and antibiotic resistance profile.
Risk factors for Acquisition of TMP-SMX Resistance.
Multivariate logistics was done which showed that the use of Ampiclox and Sulfadoxine-pyrimethamine at the preceding month predict TMP-SMX resistance (OR 8.05, CI 1.78-36.49, p< 0.007) and (OR 12.14, CI 1.53-96.07, p<0.018) respectively. This was irrespective of the patients’ educational or income level, occupation; length of hospital stay or intake of herbal medicine and acquisition of either j3-lactamase or extended spectrum j3-lactamases (ESBL).
Cohort study
Of the 200 patients recruited, loss to follow-up occurred in both the study participants and the control. Five (2.5%) were lost at three month visit, 15(7.7%) at six month, 19 (10.5%) at nine month, and 10(6.6%) at twelve month visit. Of the 120 controls recruited, 1 (0.8%) were lost at three month visit, 8 (6.7%) at six month, and 11 (9.9%) at nine and none at twelve month visit.
Carriage rate of resistant Escherichia coli isolates due to TMP-SMX prophylaxis.
The carriage rate of TMP-SMX resistance varied at each hospital visit between the participants and the control. Among the participants, the carriage rate of TMP-SMX resistant isolates was 108 (54.0%) at 0 month, 152 (77.9%) at 3 months, 173 (96.1%) at 6 months and 161 (100.0%) at 9 months, and 151(100%) at 12 months while that of the control was 35(29.2%) at 0 month, 33 (27.7%) at 3 months, 34 (30.6%) at 6 months and 51 (51.0%) at 9 months, and 53(53.0%) at 12 month Figure 3. The difference in the increasing trend in TMP-SMX resistance and selection of TMP-SMX resistance among the participants were because these patients were on TMP-SMX prophylaxis. However, there is statistically significant difference between TMP-SMX resistance among the participants when compared with the control at each hospital visit starting from the baseline (p=0.001), at 3 months (p=0.0001), at 6 months (p=0.0001), at 9 months (p=0.001), and at 12 months (p=0.0001).
Figure 3.
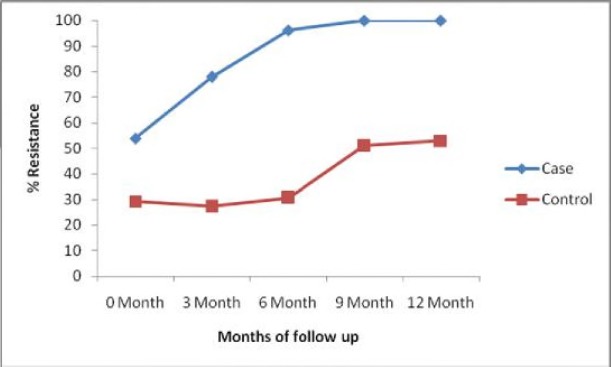
Carriage rate of resistant Escherichia coli isolates due to TMP-SMX prophylaxis.
Effect of TMP-SMX prophylaxis on antibiotic resistance profile.
Co-selection of antimicrobial resistance due to TMP-SMX prophylaxis was also assessed among all the isolates of both the study participants each hospital visit. The association of other antibiotic tested to septrin prophylaxis is shown in Figure 4. This was found to be statistically significant. This revealed that the longer the patient is on TMP-SMX, the higher chance of developing resistance (p=0.0001).
Figure 4.
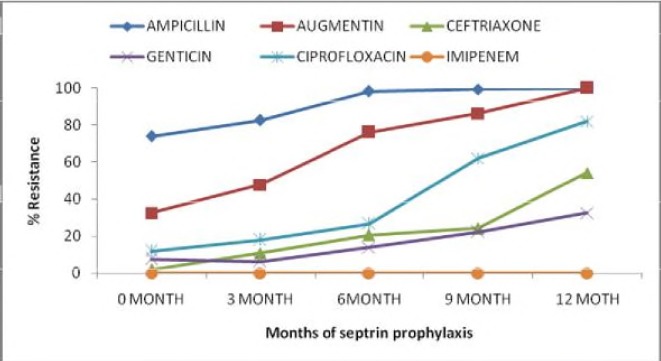
Effect of TMP-SMX prophylaxis on antibiotic resistance profile.
Risk factors for Acquisition of Resistance.
Among the controls, there was a gradual increase in trend of TMP-SMX resistance (Figure 3). This increase was attributed to the use of Ampiclox and Sulfadoxine-Pyrimethamine in the preceding month which was found to be strongly associated with TMP-SMX resistance (OR 10.05, CI 1.92-36.49, p< 0.005) and (OR 11.04, CI 1.45-94.06, p<0.002) respectively. This was also irrespective of the patients’ educational or income level, occupation; length of hospital stay or intake of herbal medicine and acquisition of either j3-lactamase or extended spectrum j3-lactamases (ESBL) (p< 0.0001).
Effect of TMP-SMX prophylaxis on Acquisition of resistance.
The association of acquisition of resistance to TMP-SMX resistance is shown in Figure 5. This was also found to be statistically significant among the participants group than the control (p=0.001). This revealed that the longer the patient is on TMP-SMX, the higher chance of developing resistance. The sensitivity test plates are shown before (PLATES 2, 3 and 4).
Figure 5.
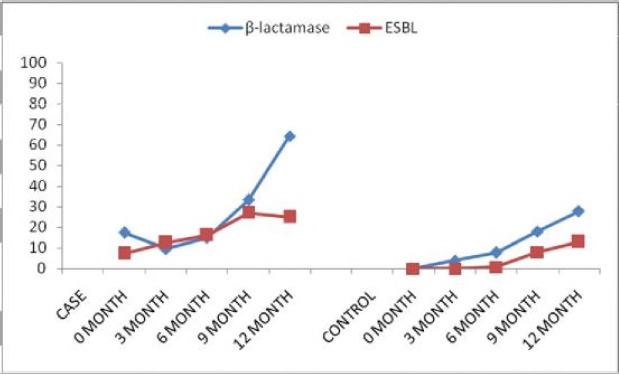
Effect of TMP-SMX prophylaxis and Acquisition of resistance.
Comparison of the carriage rate and the effect of TMP-SMX prophylaxis between cross sectional and cohort study.
In both studies, it was shown clearly that there was increasing trend in carriage rate of resistance E. coli isolates; Table 1. There was also an increasing trend in resistance between TMP-SMX prophylaxis and other antibiotics tested; Table 2. These validate the hypothesis that initiation of TMP-SMX prophylaxis in HIV-infected individuals would lead to rapid and widespread resistance of faecal E. coli to TMP-SMX.
Table 1.
Carriage rate of resistant Escherichia coli isolates due to TMP-SMX prophylaxis.
| Months of follow up | Resistance Carriage rate | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cross sectional Study | Control Study | ||||||||
| N | Freq | % | Case | Control | |||||
| N | Freq | % | N | Freq | % | ||||
| 0 | 200 | 108 | 54.0 | 200 | 108 | 54.0 | 120 | 35 | 29.2 |
| 3 | 100 | 89 | 89.0 | 195 | 152 | 77.9 | 119 | 33 | 27.7 |
| 6 | 100 | 97 | 97.0 | 180 | 173 | 96.1 | 111 | 34 | 30.6 |
| 9 | 100 | 99 | 99.0 | 161 | 161 | 100 | 100 | 51 | 51.0 |
| 12 | 100 | 99 | 99.0 | 151 | 151 | 100 | 100 | 53 | 53.0 |
N = total number
Table 2.
The Effect of septrin prophylaxis on antibiotic resistance profile.
| Antibiotics tested | % Resistance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cross sectional study | Cohort study | |||||||||
| 0 | 3 | 6 | 9 | 12 | 0 | 3 | 6 | 9 | 12 | |
| Ampicillin | 74.0 | 97.7 | 100.0 | 94.9 | 99.1 | 74.0 | 82.6 | 98.3 | 99.4 | 100.0 |
| Augmentin | 32.5 | 56.8 | 75.0 | 85.9 | 87.0 | 32.5 | 47.7 | 76.1 | 86.3 | 100.0 |
| Ceftriaxone | 2.0 | 2.3 | 4.0 | 4.0 | 24.0 | 2.0 | 10.8 | 20.6 | 24.2 | 54.3 |
| Genticin | 7.5 | 25.0 | 33.0 | 35.4 | 61.0 | 7.5 | 6.2 | 13.9 | 22.4 | 32.5 |
| Ciprofloxacin | 12.0 | 20.5 | 52.0 | 60.6 | 82.0 | 12.0 | 17.9 | 26.7 | 62.1 | 82.1 |
| Imipenem | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
Discussion
The effect of TMP-SMX prophylaxis on faecal carriage rates of resistant E. coli was studied to assess the role that TMP-SMX in driving antimicrobial resistance. The carriage rate of TMP-SMX resistant E. coli even before the commencement of TMP-SMX prophylaxis was 54%. This was similar with the recent observational cohort study done in Moshi, Tanzania on the effect of TMP-SMX prophylaxis on antimicrobial resistance of faecal Escherichia coli in HIV- infected patients. Fifty-seven faecal E. coli isolated from HIV-infected patients that were on TMP-SMX prophylaxis, 37(64.9%) were already resistant to TMP-SMX before the commencement of TMP-SMX prophylaxis (Zachariah et al., 2002). Studies in East Africa and southern Africa have also demonstrated such resistance in Enterobacteriaceae (Murray, 1987; van Oosterhout et al., 2005; WHO, 2007). Concern about the impact of TMP-SMX resistance among key HIV bacterial copathogens on the efficacy of TMP-SMX prophylaxis has been raised (Murray et al., 1983). Although large observational studies done in East Africa and southern Africa have shown that TMP-SMX prophylaxis significantly reduces morbidity and mortality, even in settings where TMP-SMX prophylaxis is more common (Buskin et al., 1999; Mwaungulu et al., 2004). No study that has investigated the magnitude of the effect of TMP-SMX prophylaxis on resistance pattern of intestinal faecal flora.
In this study, most of those patients on TMP-SMX prophylaxis continued to carry TMP-SMX resistant E. coli at a proportion higher than baseline. Furthermore, initiation of TMP-SMX prophylaxis rapidly leads to further loss of susceptibility not only to TMP-SMX but to other important antimicrobial agents. This finding suggests that the impact of TMP-SMX prophylaxis on antimicrobial resistance of bacterial flora occurs rapidly and that it is sustained as long as TMP-SMX prophylaxis is continued. Although only the faecal indicator organism E. coli was studied there also is evidence from East Africa that antimicrobial use is associated with the frequency of resistance among bacterial enteric pathogens. In Kenya, resistance to antimicrobials among diarrheal non-Typhi Salmonella and Shigella spp. was inversely proportional to the frequency with which the antimicrobials were prescribed, with TMP-SMX being the most common treatment prescription and the least effective agent (Mermin et al., 2005a).
We demonstrated that the initiation of TMP-SMX prophylaxis not only selects for TMP-SMX resistant E. coli but also seems to select for ampicillin, augmentin, ceftriaxone, genticin, and ciprofloxacin resistant. It is likely that a mechanism for the coselection of resistance is by means of mobile genetic elements such as integrons in plasmids and transposons. Research on enteroinvasive or enteroaggregative E. coli in Senegal showed that trimethoprim and other antimicrobial resistance was common and that the mechanism was likely within the class 1 integron-containing plasmids that may be horizontally transferred from gut commensal organisms (Hall et al., 1995; Gassama et al., 2004). In Tanzania, a study examining prevalence and mechanisms of antimicrobial resistance among Shigella spp. from pediatric stool cultures found that resistance to ampicillin, chloramphenicol, tetracycline, and TMP-SMX was common (Navia et al., 1999). Ampicillin resistance was most frequently related to an integron-borne OXA-1-type b-lactamase, and resistance to TMP-SMX was attributable to the presence of an integron-borne dhfr Ia gene (Gassama et al., 2004). Studies in Europe and Canada on uropathogenic E. coli that was resistant to trimethoprim, sulfamethoxazole, or both found dfr or sul gene-containing integrons in 59% of isolates. Analysis of the regional distribution of these integrons indicated that horizontal gene transfer was the main mechanism of resistance spread rather than clonal expansion (Muray et al., 1983). These studies suggest that coselection of resistance by means of mobile genetic elements in faecal E. coli attributable to TMP-SMX use is likely to occur and that these genetic elements can disseminate from faecal flora to bacterial enteric pathogens.
This study also revealed that among the control group (those were not on TMP-SMX prophylaxis), gradual increase in trend of TMP-SMX resistance was seen over time (Figure 4). This is in contrast to the study by Mermin et al. (2005b) in Uganda which showed that antimicrobial resistance among diarrheal pathogens infecting family members did not increase. This increase was attributed to the use of Ampiclox and Sulfadoxine-Pyrimethamine in the preceding month. This is due to abuse and misuse of these antibiotics which are readily available at every patent medicine store of the country and are readily given out to these patients by these unqualified medical personnel (Okeke et al., 1999). This study demonstrated that the carriage rate of faecal E. coli resistant to TMP-SMX is common among HIV patients before the commencement of TMP-SMX prophylaxis. The carriage rate went from 54% at baseline to 99% and 100% after one year in cross-sectional and cohort study respectively. This study also revealed that septrin prophylaxis induces an accelerated selection of multi-drug resistant strains and co-selection to other antibiotic classes as well (ampicllin, augmentin, ceftriaxone, ciprofloxacin, and genticin).
Finally, use of Ampiclox and Sulfadoxine-Pyrimethamine in the preceding month was found to predict Trimethoprim-Sulfamethoxazole resistance among the case and control groups. This was irrespective of other risk factors like the patients. educational or income level, occupation; length of hospital stay or intake of herbal medicine and acquisition of either j3-lactamase or extended spectrum j3-lactamses (ESBL).
Footnotes
Competing Interests: No conflicting interests exist either financially or otherwise.
References
- 1.Buskin S.E, Newcomer L.M, Koutsky L.A, Hooton T.M, Spach D.H, Hopkins S.G. Effect of trimethoprim-sulfamethoxazole as Pneumocystis carinii pneumonia prophylaxis on bacterial illness Pneumocystis carinii pneumonia, and death in persons with AIDS. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1999;20:201–206. doi: 10.1097/00042560-199902010-00014. [DOI] [PubMed] [Google Scholar]
- 2.Castetbon K, Anglaret X, Attia A, Toure S, Dakoury-Dogbo N, Messou E, Dri-Dabis N. F, Salamon R, Cotrimo C.L Study Group. Effect of early chemoprophylaxis with Trimethoprim-Sulfamethoxazole on nutritional status evolution in HIV-1-infected adults in Abidjan, Cote d’Ivoire. AIDS. 2001;15(7):869–76. doi: 10.1097/00002030-200105040-00007. [DOI] [PubMed] [Google Scholar]
- 3.Chikwendu C.I, Nwabueze R.N, Anyanwu B.N. Antibiotic resistance profile ofEscherichia coli from clinically healthy pigs and their commercial farm environments. African Journal of Microbiology Research. 2008;2:012017. [Google Scholar]
- 4.Chiller T, Polyak C, Brooks J, Kumar L, Ochieng B, Greene C, Hemel M, Ouma P, Slutsker L, Vulule J, Shi Y, Wells J, Mintz E. Trimethoprim-sulfamethoxazole (TMP-SMX) prophylaxis in HIV-infected adults in Kenya increases TMP-SMX resistance in Escherichia coli Does it predispose to resistant Salmonella and Shigella? Antimicrob. Agents Chemother. 2003;43:14–17. [Google Scholar]
- 5.Chintu C, Walker A.S, Mulenga V, Siyinza F, Lishimpi K, Kaganson N, Zumla A, Gillespie S.H, Nunn A.J, Gibb D.M. Trimethoprim-Sulfamethoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomized placebo-controlled trial. Lancet. 2004;364:1865–71. doi: 10.1016/S0140-6736(04)17442-4. [DOI] [PubMed] [Google Scholar]
- 6.Clinical Laboratories Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard-Tenth Edition Informational Supplement. Wayne, PA: CLSI; 2009a. [Google Scholar]
- 7.Clinical Laboratories Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Sixteenth Informational Supplement. Wayne, PA: CLSI; 2009b. [Google Scholar]
- 8.Cotton M.F, Wesserman E, Whitelaw A, Zar H.J. High incidence of antimicrobial resistant organisms including extended beta-lactamase producing Enterobacteriaceae and methicillin-resistant Staphylococcus aureus in nasopharyngeal and blood isolates of HIV-infected children from Cape Town, South Africa. BMC Infect. Dis. 2008;8(1):40–47. doi: 10.1186/1471-2334-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gassama A, Aidara-Kane A, Chainer D. Integron-associated antibiotic resistance in enteroaggregative and enteroinvasive Escherichia coli Microb Drug Resist. 2004;10:27–30. doi: 10.1089/107662904323047763. [DOI] [PubMed] [Google Scholar]
- 10.Grimwade K, Gilks C. Trimethoprim-Sulfamethoxazole prophylaxis in adults infected with HIV in low income countries. Curr. Opin. Infect. Dis. 2001;14(5):507–12. doi: 10.1097/00001432-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Grimwade K, Sturm A.W, Nunn A.J, Mbatha D, Zungu D, Gilks F.C. Effectiveness of prophylaxis on mortality in adults with tuberculosis in rural South Africa. AIDS. 2005;19(2):163–168. doi: 10.1097/00002030-200501280-00008. [DOI] [PubMed] [Google Scholar]
- 12.Hall R.M, Collis C.M. Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Molecular Microbiology. 1995;15(4):593–600. doi: 10.1111/j.1365-2958.1995.tb02368.x. [DOI] [PubMed] [Google Scholar]
- 13.Mermin J, Lule J, Ekwaru J.P, Downing R, Huges P, Bunnell R, Malamba S, Ransom R, Kaharuza F, Coutinho A, Kigozi A, Ouick R. Trimethoprim-Sulfamethoxazole prophylaxis by HIV-infected persons in Uganda reduces morbidity and mortality among HIV-infected family members. AIDS. 2005;19(10):1035–42. doi: 10.1097/01.aids.0000174449.32756.c7. [DOI] [PubMed] [Google Scholar]
- 14.Mermin J, Lule J, Ekwaru J.P, Malamba S, Downing R, Kizito F, Culver D, Bunnell R, Ransom R, Kaharuza F, Nakanjako D, Kigozi A, Wafula W, Ouick R. Effect of Trimethoprim-Sulfamethoxazole prophylaxis on morbidity, CD4-cell count, and viral load in HIV-infection in rural Uganda. Lancet. 2004;364(9443):1428–1434. doi: 10.1016/S0140-6736(04)17225-5. [DOI] [PubMed] [Google Scholar]
- 15.Mermin J, Lule J.R, Ekwaru J.P, Pitter C. Should Trimethoprim-Sulfamethoxazole prophylaxis be taken by all adults with HIV in Africa? AIDS. 2005;19:841–846. doi: 10.1097/01.aids.0000168986.12832.a4. [DOI] [PubMed] [Google Scholar]
- 16.Murray B.E. Emergence of high-level trimethoprim resistance in faecal Escherichia coli during oral administration of trimethoprim or trimethoprim-sulfamethoxazole. N. Engl J. Med. 1987;306:130–5. doi: 10.1056/NEJM198201213060302. [DOI] [PubMed] [Google Scholar]
- 17.Murray B.E, Rensimer E.R. Transfer of trimethoprim resistance from faecal Escherichia coli isolated during a prophylaxis study in Mexico. J. Infect. Dis. 1983;147(4):724–8. doi: 10.1093/infdis/147.4.724. [DOI] [PubMed] [Google Scholar]
- 18.Mwaungulu F.B.D, Floyd S, Crampin A.C. Trimethoprim-Sulfamethoxazole prophylaxis reduces mortality in human immunodeficiency virus-positive tuberculosis patients in Karonga District, Malawi. Bull World Health Organisation. 2004;82:354–363. [PMC free article] [PubMed] [Google Scholar]
- 19.Navia M.M, Capitano L, Ruiz J. Typing and characterization of mechanisms of resistance of Shigella spp. isolated from faeces of children under 5 years of age from Ifakara, Tanzania. J Clin Microbiol. 1999;37:3113–3117. doi: 10.1128/jcm.37.10.3113-3117.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okeke I.N, Lamikanra A, Edelman R. Socioeconomic and Behavioural Factors Leading to Acquired Bacterial Resistance to Antibiotics in Developing Countries. Emerging Infectious Disease. 1999;5:18–27. doi: 10.3201/eid0501.990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.UNAIDS/WHO. Provisional WHO/UNAIDS Secretariat Recommendations on the use of Trimethoprim- Sulfamethoxazole Prophylaxis in Adults and children living with HIV/AIDS in Africa. Geneva, Switzerland: UNAIDS; 2000. [Google Scholar]
- 22.van Oosterhout J.J, Laufer M.K, Graham S.M, Thumba F, Perez M.A, Chimbiya N, Wilson L, Chagomerana M, Molyneux M.E, Zijlstra E.E, Taylor T.E, Plowe C.V. A community-based study of the incidence of trimethoprim-sulfamethoxazole preventable infections in Malawian adults living with HIV. J Acquir Immune Defic Syndr. 2005;39:626–631. [PubMed] [Google Scholar]
- 23.Werarak P, Ratanasiri S, Sungkanuparph S, Kiertiburanakul S. Poor response to trimethoprim-sulfamethoxazole in HIV-infected patients with pneumocystis jiroveci pneumonia and concurrent opportunistic infections. European Society of Clinical Microbiology and Infectious Disease. 2006;4:616–620. [Google Scholar]
- 24.World Health Organization. Facts on ACTs. Geneva: World Health Organization; 2006. [Google Scholar]
- 25.World Health Organization. Global tuberculosis control: surveillance, planning, financing. Geneva, Switzerland: World Health Organization; 2007. [Google Scholar]
- 26.Zachariah R, Harries A.D, Spielmann M.P, Arendt V, Nchingula D, Mwenda R, Courtielle O, Kirpach P, Mwale B, Salaniponi F.M.L. Changes in Escherichia coli resistance to Trimethoprim-Sulfamethoxazole in tuberculosis patients and in relation to Trimethoprim-Sulfamethoxazole prophylaxis in Thyolo, Malawi. Royal Society of Tropical Medicine and Hygiene. 2002;96:202–204. doi: 10.1016/s0035-9203(02)90306-8. [DOI] [PubMed] [Google Scholar]


