Abstract
Introduction:
Klebsiella pneumoniae is a gram negative enterobacteriaciae commonly associated with nosocomial infections. Multidrug resistant strains are increasingly being reported with corresponding increase in morbidity and mortality. The study outlines the epidemiology and antibiotic resistance pattern of K. pneumonia over a 10 year period in Moi Teaching and Referral Hospital, Eldoret, Kenya.
Methodology and Study Design:
This is a retrospective analysis of all the blood culture results for K. pneumoniae isolates in the hospital for the period 2002-2013.
Results:
K. pneumoniae accounted for 23% of the hospital isolates (231/1356) during the study period; of these, 82.6% were from the New Born Unit. Most of the isolates were multi drug resistant with highest resistance of over 80% to Penicillins, Cephalosporins, Macrolides, Tetracyclines, Sulphonamides, Lincosamides and Chloramphenicol. Aminoglycoside and Quinolone resistance was also high at 49.2% and 41.3% respectively. The lowest resistance rates were documented for Carbapenems (23.2%). For specific antibiotics, there was high resistance to commonly used antibiotics (over 80% for Ceftriaxone, Cefipime, Gentamycin and Ceftazidime). The antibiotics with least resistance were Amikacin and Meropenem (21% and 7 % respectively).
Conclusion:
There was a high prevalence of multidrug resistant K. pneumoniae isolates in the hospital, the majority originated from the New Born Unit. Resistance to third generation Cephalosporins and Gentamycin was high while Meropenem and Amikacin had the least resistance.
Keywords: Klebsiella pneumoniae, Antibiotic resistance, Multi drug resistance, Nosocomial infections
Introduction
Klebsiella pneumoniae is a gram negative, rod shaped bacterium belonging to the family Enterobacteriaceae. It has in recent years become an important pathogen in nosocomial infections worldwide. Increasingly, many strains that are extended-spectrum P-lactamase (ESBL) producing as well as Carbapenem resistant are being reported as causing outbreaks in hospitals and particularly in intensive care units (ICUs) and New Born Units (NBUs) where there is great antibiotic pressure (Centers for Disease Control and Prevention, 2003; George AJ et al., 2005). The spread of these nosocomial infections occur from patient to patient, healthcare workers to patients and vice versa as well as contaminated hospital environment and equipment. Most of the infections occur in neonates, in immunocompromised patients such as critically ill patients in ICU, patients with malignancies, patients on chemotherapy, HIV infected patients and diabetic patients (Adamski J et al., 2008; Richards MJ et al., 1999; Winokur PL et al., 2001).
Treatment options for multi-drug resistant K. pneumonia (MDR KP) are limited; more so in resource constrained settings. Most studies on MDR KP are from developed countries with scanty data from resource limited settings. The study objective was to describe the epidemiology and antibiotic resistance pattern and trends for K. pneumoniae at Moi Teaching and Referral Hospital (MTRH) in Eldoret, Kenya.
Materials and Methods
Moi Teaching and Referral Hospital (MTRH) is the second largest public hospital in Kenya. It hosts Moi University School of Medicine and serves a catchment with population of about 16 million people. It has a microbiology laboratory that handles the culture and sensitivity specimen in the hospital. Blood specimens were cultured in BACTEC 9120 and BACTEC 9050 (Becton-Dickinson, New Jersey, USA) automated systems. Antibiotic sensitivity at the facility was performed by disc diffusion method and susceptibility reported based on Clinical and Laboratory Standards Institute (CLSI) susceptibility criteria. The laboratory is International Organization for Standardization (ISO 15189) certified and has internal quality management systems in place. The culture results are manually documented in a microbiology register provided by the Kenya Ministry of Health.
Ethical approval was sought from the MTRH/ Moi University Ethics and Review Board and from the Director of MTRH before the study was conducted. The data was anonymized through unique study numbers and no patient identifiable information was collected.
This was a retrospective analysis of K. pneumoniae isolates in patients of MTRH for the period 2002 to 2013. Data is analyzed using SAS version 9.3 (SAS Institute Inc., Cary, NC). Normality test is conducted using Shapiro and Wilks normality test. Categorical variables are presented as frequencies and percentages while continuous variables are expressed as mean and standard deviation. Bar charts and line graphs are used to present the pictorial distribution of organisms.
Results
Majority of the study samples were from female patients (64.8%). The median age was 5 days with interquartile range of 3-15 days (Table i). K pneumoniae accounted for 23 % of the total isolates during the study period (281/1231). It was the most prevalent pathogenic isolate. It constituted 65.5% of NBU growths (205/313) and 50% of ICU growths (5/10). It constituted 17%, 15% and 14 % of the growths in the pediatric wards (22/130), medical wards (13/88) and obstetrics/gynecology wards (1/7) respectively. There was no K. pneumoniae isolated from the surgical wards (Fig i). The NBU K. pneumoniae isolates contributed 83% of all the hospital KP isolates (205/281). The pediatric and medical wards contributed 8% and 5% of the hospital K. pneumoniae isolates respectively (Fig i). K. pneumoniae was constantly a significant growth in the hospital over the 11 year period (Fig ii).
Table i.
Demographic characteristics of patients with K. pneumoniae isolates at MTRH 2002-2013
| Characteristics | n (%)N=281 |
|---|---|
| Age, yrs; mean(std) | 4.8 (11.5) |
| Gender | |
| Male | 99 (35.2) |
| Female | 182 (64.8) |
| Wards* | |
| Adults | 13 (5) |
| Pediatric | 22 (8) |
| NBU | 205 (83) |
| Obstetrics/Gynecology | 1 (0) |
| ICU | 5 (2) |
| Others | 6 (2) |
NBU-New born unit; ICU- Intensive care unit
Percentages reflect distribution of K. pneumoniae among the wards. 83% of the K. pneumoniae isolates originated from the NBU.
Figure i.
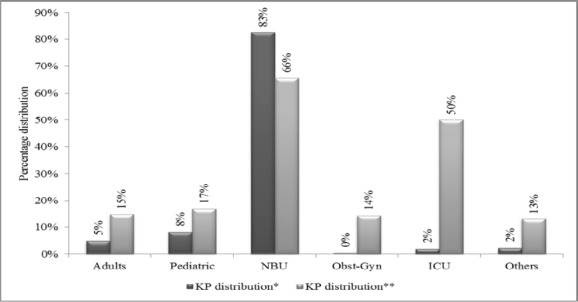
K. pneumoniae distribution within wards at MTRH 2002-2013
Figure ii.
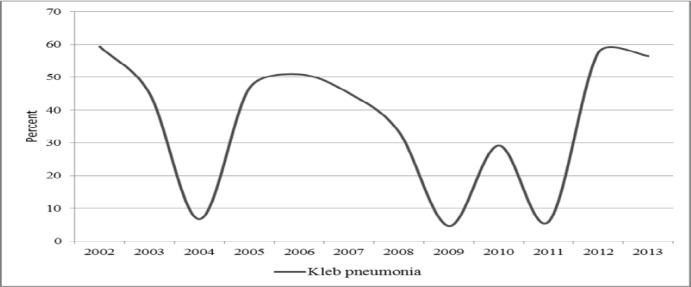
Prevalence of K. pneumoniae by year at MTRH 2002-2013 as a percentage of the total hospital blood culture isolates.
Most of the isolates were multidrug resistant with highest resistance of over 80% to Penicillins, Cephalosporins, Macrolides, Tetracyclines, Sulphonamides, Lincosamides and Chloramphenicol (Table ii). Aminoglycoside and quinolone resistance was at 49.2% and 41.3% respectively. The lowest resistance rates were documented for Carbapenems (23.2 %). An analysis of the resistance levels to individual commonly prescribed antibiotics indicated resistance of over 80% to Ceftriaxone, Cefipime, and Gentamycin (Table iii). Amikacin and Meropenem had least resistance (21% and 7 % respectively).
Table ii.
K. pneumoniae resistance to antibiotic groups at MTRH 2002-2013
| ANTIBIOTIC GROUP | RESISTANT n (%) | SENSITIVE n (%) | INTERMEDIATE n (%) | TOTAL NO TESTED |
|---|---|---|---|---|
| Penicillin | 226 (84.6) | 37 (13.9) | 4 (1.5) | 267 |
| Cephalosporins | 511 (81.8) | 90 (14.4) | 24 (3.8) | 625 |
| Aminoglycosides | 204 (49.2) | 161 (38.8) | 50 (12) | 415 |
| Macrolides | 20 (87) | 3 (13) | 0 (0) | 23 |
| Carbapenems | 44 (23.2) | 143 (75.3) | 3 (1.6) | 190 |
| Tetracyclins | 38 (95) | 2 (5) | 0 (0) | 40 |
| Sulphonamides | 79 (88.8) | 9 (10.1) | 1 (11) | 89 |
| Quinolones | 50 (41.3) | 71 (58.7) | 0 (0) | 121 |
| Lincosamides | 4 (80) | 1 (20) | 0 (0) | 5 |
| Vancomycin | 29 (87.9) | 4 (12.1) | 0 (0) | 33 |
| Oxazolidinones | 18 (75) | 6 (25) | 0 (0) | 24 |
| Minocycline | 2 (40) | 1 (20) | 2 (40) | 5 |
| Chloramphenicol | 46 (93.9) | 3 (6.1) | 0 (0) | 49 |
Table iii.
K. pneumoniae resistance pattern to specific antibiotics at MTRH 2002-2013
| ANTIBIOTIC GROUP | RESISTANT n(%) | SENSITIVE n(%) | INTERMEDIATE n(%) | TOTAL NO TESTED |
|---|---|---|---|---|
| Ceftriaxone | 68 (87.2) | 9 (11.5) | 1 (1.3) | 78 |
| Ciprofloxacin | 32 (44.4) | 40 (55.6) | 0 (0.0) | 72 |
| Gentamycin | 106 (82.8) | 19 (14.8) | 3 (2.3) | 128 |
| Amikacin | 48 (21.0) | 135 (59.0) | 46 (20.1) | 229 |
| Meropenem | 8 (7.0) | 106 (92.2) | 1 (0.9) | 115 |
| Cefipime | 105 (85.4) | 12 (9.8) | 6 (4.9) | 123 |
| Ceftazidime | 108 (69.7) | 37 (23.9) | 10 (6.5) | 155 |
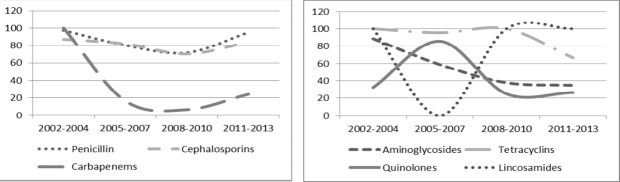
We assessed for resistance pattern over the 11 year period for possible trends. Antibiotic class resistance pattern analysis showed persistent high resistance (>70%) to Cephalosporins and Penicillins throughout the study period. Quinolone resistance was below 40% for the study period except for 2005-2007 when it peaked to above 80%. Cephalosporin resistance declined from 100% in the year 2002-2004 to below 10% for the next 6 years followed by an increase to 20% in the period 2011-2013 (Fig iii).
Figure iii.
K. pneumoniae resistance pattern to antibiotic groups over time at MTRH 2002-2013. Left pane showing trend for Penicilin, Cephalosporins, and Carbapenems; Right pane showing trend for Aminoglycosides, Tetracyclins and Quinolones and Lincosamides antibiotic groups
Figure iv.
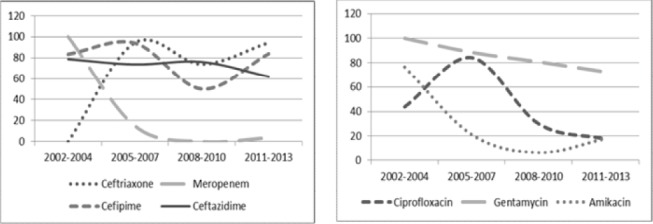
K. pneumoniae resistance pattern to individual antibiotics over time at MTRH 2002-2013. Left pane showing trend for Ceftriaxone, Meropenem, Cefipime and Ceftazidimine; Right pane showing trend for Ciprofloxacin, Gentamycin and Amikacin antibiotics.
Among the Cephalosporins, resistance to Ceftriazone showed a steady rise over the study period from no resistance recorded in 2002-2004 to remain constantly above 70% for the remaining period. Resistance to Cefipime and Ceftazidime was high (above 50% and 60% respectively) throughout the study period. For the Aminoglycosides, Gentamycin resistance was constantly above 70% while that of Amikacin dropped from above 70% at the beginning to below 20% in the last 6 years. Likewise, Meropenem resistance steadily reduced from 100% resistance in the first 3 years to below10% in the last 6 years. Ciprofloxacin resistance was at 40% in the first 3 years, peaked during the period 2005-2007 at 84.6% then rapidly declined thereafter.
Discussion
Klebsiella pneumonia is normal human intestinal enterobacteria. It is considered an opportunistic human pathogen and is responsible for severe nosocomial infections in immunocompromised patients, in patients with prolonged hospital stay and in patients with various implants (Adamski J et al., 2008; Mehrgan H et al., 2010; Velasco E et al., 2004). It has been found to be a significant cause of neonatal sepsis in the developing countries (Mathure NB et al., 2002; Stephen EM et al., 2013; Tiwari DK et al., 2013; Tumaini VM et al., 2012). In the developed world, multi drug resistant K. pneumoniae (MDR KP) has been documented to cause disease outbreaks leading to significant morbidity and mortality (Lee Ket al., 2010; Patricia AB et al., 2004). It was the commonest pathogenic organism isolated in our hospital during the study period and was most prevalent in the New Born Unit (NBU), the pediatric wards and Intensive Care Unit (ICU). Most of the KP isolates were multidrug resistant with high resistance to Cephalosporins, Penicillins and Gentamycin, relatively high resistance to the Quinolones and relatively moderate sensitivity to Carbepenems. Our study suggests a high prevalence of MDRKP in the hospital with possible cross contamination between the wards.
MDR KBP has been found to be resistant to third generation Cephalosporins, Aminoglycosides and Quinolones but often sensitive to Carbapenems (Bradford PA, 2001; Hirsch EB et al., 2010). In our study, resistance to Cephalosporins was very high (>80% resistance) possibly due to widespread use of ceftriaxone especially in our NBU where the largest burden of this organism was present. Ceftriaxone resistance increased rapidly during the study period to remain constantly above 70%. Cefipime and Ceftazidime, although not frequently prescribed in our set up, had very high resistance throughout the study period. Cephalosporin resistance is conferred from ^-lactamase production, which is plasmid mediated and transferable to other Cephalosporins and to other gram negative enterobacteriacea such as E. coli (Bradford PA, 2001; George AJ et al., 2005). Similar findings were documented in Tanzania where Cefotaxime was the most prescribed antibiotic in the NBU resulting in high overall third generation Cephalosporin resistance (Stephen EM et al., 2013). Data from Korea for the year 2007 showed lower Cephalosporin resistance rates than in our study (Cefotaxime 25%, Cefepime 22%, Ceftazidime 29%, Cefoxitin 21%)) although during an ESBL KP outbreak Ceftazidime resistance rose to 47% (Lee K et al., 2010; Roh KH et al., 2008).
Development of bacterial resistance to Aminoglycosides has been documented to be slowest amongst the antibiotics (Rennie RP et al., 1977). In Toronto, the first Gentamycin resistance was documented 7 years after first use (Curie K et al., 1978). This resistance was transferrable to other Gram Negatives, particularly E. coli. Gentamycin is part of many first line regimens in both developed and developing countries. In our hospital, it is often used as first line antibiotic in neonatal sepsis and has been used in the unit for over 10 years. It recorded a high level of resistance of 83% compared to Amikacin (21% resistance) which is preserved for second-line treatment. In Korea, both Gentamycin and Amikacin had relatively lower resistance of about 30% (Lee K et al., 2010). Data from Tanzania was similar to ours for Gentamycin (77% resistance) and slightly lower for Amikacin (1.45 % resistance) (Tumaini VM et al., 2012). Gentamycin resistant KP species has been found to have higher carriage in the intestinal and urinary tracts and longer durations of shedding than Gentamycin sensitive KP resulting in nosocomial hospital outbreaks (Hart CA et al., 1982).
K. pneumoniae resistance to Quinolones was 41.3% with Ciprofloxacin resistance being at 44.4 %. Similar findings were reported in India where K. pneumoniae resistance to Ciprofloxacin amongst children below ten years was 35.71% (Tiwari DK et al., 2013). In our study, Ciprofloxacin resistance reduced from over 80% in 2005-2007 to below 30% in 2011 -2013 possibly due to reduction in prescription in our hospital over that period. Quinolone resistance has been noted to be low in NBU compared to other wards due to contra-indication of their use in newborns (n= 25, 38%, p<0.05) (Stephen EM et al., 2013). However, in our study Quinolone resistance in the medical wards was lower than the over-all resistance (36.6% versus 44.4%). Quinolone resistance is plasmid mediated and transferrable from person to person especially amongst patients with long hospital stay. Aminoglycoside exposure has been associated with Quinolone resistance in K. pneumoniae and Pseudomonas aeruginosa, suggesting the need for awareness of the potential cross resistance and thus failure of Quinolones in settings where there is widespread use of Aminoglycosides such as in our hospital (Lautenbach E et al., 2001; Masuda N et al., 1992; Strausbaugh LJ et al., 1996).
The resistance to Carbapenems was lowest at 23.2%. Resistance to Meropenem was 7.0%. Carbapenem resistant K. pneumoniae is considered the most resistant strain. Carbapenem resistance has been shown to result from changes in membrane permeability, high β lactamase and Cephalosporinase levels and production of carbapenemases. Evidence of carbapenemase production from a unit needs to be handled efficiently as they are associated with P lactamase production resulting in penicillin and cephalosporin resistance (Walsh TR et al., 2012). A prospective study of clinical enterobacteriacea isolates in Morocco found a Carbapenemase production rate of 2.8% (Wartiti MA et al., 2012). Data from several European countries record rates of less than 1% except during outbreaks where rates as high as 17-43% are recorded. (Chetcuti Z et al., 2014; Marina K et al., 2014). In Kenya only seven Carbapenem resistant K. pneumoniae were detected in a two years study at the Aga Khan University Hospital in Nairobi (Nordmann P et al., 2011). Most of the isolates were from urine of adult patients in contrast to our study where the isolates were from blood cultures and significantly from neonates. Our results indicate higher rates of Carbapenem resistance and this should be monitored with possible genomic and epidemiologic studies to curb any further rise. There was 100% resistance to Meropenem in the period 2002-2004 (n=8) and all the samples were from the NBU.
Diagnosis and treatment of MDR KP infections and colonization in our hospital and similar hospitals in the developing countries is complicated. A multidisciplinary approach is necessary with teams including clinicians, pharmacists, and microbiologists with support from the hospital management. Prevention of spread of resistance by proper aseptic procedures such as hand washing, disinfection of equipment, reduction in ward congestion and isolation need to be intensified. Awareness on MDR organisms should be raised. Early detection of MDR KP through routine surveillance with profiling of the resistance pattern should be encouraged to avoid ineffective therapy in order curb the spread of the strain responsible for the outbreak. In one incidence in New York, increased use of Ceftazidime in treatment of Acinetobacter infections resulted in an outbreak of Ceftazidime resistant K. pneumoniae, and it was after detection that effective therapy was instituted (Kenneth SM et al., 1993). Attempts to reduce the multi-class resistance should also be made to regain susceptibility to cheaper and more readily available drugs. Antibiotic restriction and rotation are possible options for developing countries where access to newer antibiotics is limited. In the USA, an 80% reduction of hospital consumption of Cephalosporins resulted in a 44.0% reduction in Ceftazidime-resistant K. pneumoniae infection and colonization throughout the medical center (P<.01), a 70.9% reduction of cephalosporin resistance within all intensive care units (P<.001), and an 87.5% reduction within the surgical intensive care unit (P<.001) within 1 year of restriction (Rahal JJ et al., 1998).
Conclusion
There was a high prevalence of MDR KBP isolates in our hospital with most of the isolates in the NBU. The isolates were highly resistant to third generation Cephalosporins and Gentamycin.
Recommendations
Stringent infection prevention control measures need to be instituted especially in the NBU to minimize the nosocomial spread of MDRKP. In case of suspected or confirmed infection with MDRKP; Carbapenems are the drug of choice for treatment. Quinolones and Amikacin may be considered where Carbapenems are unavailable. We also recommend further studies to characterize the molecular genetic composition of MDR and Carbapenem resistant K. pneumoniae in our set up.
Acknowledgements
Mr. Richard Too (Head of the Microbiology Laboratory MTRH) who availed the records of the data and clarifications in an orderly and timely manner. Dr. Wilson Aruasa the Deputy director MTRH, for encouraging and supporting the study. All the people involved in any manner at all stages of this study.
References
- 1.Adamski J, Steggall M, Yeoh KX, Sutton R, Makin GW, Sutton R. Outcome of gram negative infection in immunocompromised children. Pediatric Blood Cancer. 2008;51:499–503. doi: 10.1002/pbc.21587. [DOI] [PubMed] [Google Scholar]
- 2.Bradford PA. Extended-spectrum P-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol Rev. 2001;14:933–951. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2003, August 2003. American Journal of Infection Control. 2003;31:481–498. doi: 10.1016/j.ajic.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Chetcuti Z, Azzopardi N, Sant J. Mortality risk score for Klebsiella pneumonia bacteraemia. European Journal of Internal Medicine. 2014;25:571–576. doi: 10.1016/j.ejim.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Curie K, Speller DCE, Simpson RA. A hospital epidemic caused by gentamycin resistant Klebsiella aurogenes. Journal of Hygiene. 1978;80:115–123. doi: 10.1017/s0022172400053432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George AJ, Luisa SM. The new P-lactamases. New England J Med. 2005;352:380–391. [Google Scholar]
- 7.Hart CA, Gibson MF. Comparative epidemiology of gentamicin-resistant enterobacteriaceae: persistence of carriage and infection. Journal of Clinical Pathology. 1982;35(4):452–457. doi: 10.1136/jcp.35.4.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch EB, VH Tam. Detection and treatment options for Klebsiella pneumonia carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. Journal of Antimicrobial Chemotherapy. 2010;65:1119–1125. doi: 10.1093/jac/dkq108. [DOI] [PubMed] [Google Scholar]
- 9.Kenneth SM, Carl U, Janet AE, Barbara JB, James JR. Nosocomial Outbreak of Klebsiella Infection Resistant to Late-Generation Cephalosporins. Annals of Internal Medicine. 1993;119(5):353–358. doi: 10.7326/0003-4819-119-5-199309010-00001. [DOI] [PubMed] [Google Scholar]
- 10.Lautenbach E, Fishman NO, Bilker WB, Castiglioni A, Metlay JP, Edelstein PH. Epidemiological Investigation of Fluoroquinolone Resistance in Infections Due to Extended-Spectrum P-Lactamase—Producing Escherichia coli and Klebsiella pneumonia. Clin Infect Dis. 2001;33(8):1288–1294. doi: 10.1086/322667. [DOI] [PubMed] [Google Scholar]
- 11.Lee K, Lee MA, Lee CH, Lee J, Roh KH, Kim S. Increase of ceftazidime- and fluoroquinolone-resistant Klebsiella pneumoniae and imipenem-resistant Acinetobacter spp in Korea: analysis of KONSAR study data from 2005 and 2007. YonseiMed J. 2010;51(6):901–911. doi: 10.3349/ymj.2010.51.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marina K, Theodoros R, Nikolaos A, Glykeria V, Ioannis P, Christos N. Bloodstream infections and sepsis in Greece: over-time change of epidemiology and impact of de-escalation on final outcome. BMC Infectious Diseases. 2014;14:272. doi: 10.1186/1471-2334-14-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masuda N, Ohya S. Cross-resistance to meropenem, cephems, and quinolones in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1992;36:1847–1851. doi: 10.1128/aac.36.9.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathure NB, Garg Kumar K. S. Respiratory Distress in Neonates with Special Reference to Pneumonia. Indian Pediatrics. 2002;39:529–537. [PubMed] [Google Scholar]
- 15.Mehrgan H, Rahbar M, Arab-Halvaii Z. High prevalence of extended-spectrum betalactamase-producing Klebsiella pneumoniae in a tertiary care hospital in Tehran, Iran. Journal of Infectious Diseases in Developing Countries. 2010;4:132–138. doi: 10.3855/jidc.488. [DOI] [PubMed] [Google Scholar]
- 16.Nordmann P, Laurent P, Christine L, Sandrine B. Detection of NDM-1-Producing Klebsiella pneumoniae in Kenya. Antimicrobial agents and chemotherapy. 2011;55(2):934. doi: 10.1128/AAC.01247-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patricia AB, Simona B, Carl U, Melissa V, Noriel David M. L. Emergence of carbapenem-resistant Klebsiella species possessing the class A carbapenem-hydrolyzing KPC-2 and inhibitor-resistant TEM-30 b-lactamases in New York City. Clin Infect Dis. 2004;39:55–60. doi: 10.1086/421495. [DOI] [PubMed] [Google Scholar]
- 18.Rahal JJ, Urban C, Horn D, Freeman K, Segal-Maurer S, Maurer J. Class Restriction of Cephalosporin Use to Control Total Cephalosporin Resistance in Nosocomial Klebsiella. JAMA. 1998;280(14):1233–1237. doi: 10.1001/jama.280.14.1233. [DOI] [PubMed] [Google Scholar]
- 19.Rennie RP, Duncan BR. Emergence of gentamycin resistant Klebsiella in a general hospital. Antimicrobial agents and chemotherapy. 1977;11(2):179–184. doi: 10.1128/aac.11.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. Critical Care Medicine. 1999;27:887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Roh KH, Uh Y, Kim JS, Kim HS, Shin DH, Song W. First outbreak of multidrug-resistant Klebsiella pneumoniae producing both SHV-12-type extended-spectrum beta-lactamase and DHA-1-type AmpC beta-lactamase at a Korean hospital. Yonsei Medical J. 2008;49:53–57. doi: 10.3349/ymj.2008.49.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephen EM, Torsten H, Eugen D, Eligius FL, Trinad C, Can I. Predominance of Klebsiella pneumoniae ST14 carrying CTX-M-15 causing neonatal sepsis in Tanzania. BMC Infect Dis. 2013;13:466. doi: 10.1186/1471-2334-13-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strausbaugh LJ, Crossley KB, Nurse BA, Thrupp LD. Antimicrobial resistance in long-term-care facilities. nfect Control Hosp Epidemiol, I. 1996;17:129–140. doi: 10.1086/647257. [DOI] [PubMed] [Google Scholar]
- 24.Tiwari DK, Golia S, Sangeetha KT, Vasudha CL. A Study on the Bacteriological Profile and Antibiogram of Bacteremia in Children below 10 Years in a Tertiary Care Hospital in Bangalore. India J ClinDiagn Res Dec. 2013;7(12):2732–2735. doi: 10.7860/JCDR/2013/6682.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tumaini VM, Francis F, Mecky IM, Augustine M. Neonatal sepsis at Muhimbili National Hospital, Dar es Salaam, Tanzania; aetiology, antimicrobial sensitivity pattern and clinical outcome. BMC Public Health. 2012;12:904. doi: 10.1186/1471-2458-12-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Velasco E, Byington R, Martins CS, Schirmer M, Dias LC, Goncalves VM. Bloodstream infection surveillance in a cancer centre: a prospective look at clinical microbiology aspects. Clinical Microbiology and Infection. 2004;10:542–549. doi: 10.1111/j.1469-0691.2004.00874.x. [DOI] [PubMed] [Google Scholar]
- 27.Walsh TR, Toleman MA. The emergence of pan-resistant gram-negative pathogens merits a rapid global political response. J Antimicrob Chemother. 2012;67 doi: 10.1093/jac/dkr378. [DOI] [PubMed] [Google Scholar]
- 28.Wartiti MA, Bahmani FZ, Elouennass M, Benouda A. Prevalence of Carbapenemase-Producing Enterobacteriaceae in a University Hospital in Rabat, Morocco: A 19-Months Prospective Study. The International Arabic Journal of Antimicrobial Agents. 2012;2:3–4. [Google Scholar]
- 29.Winokur PL, Canton R, Casellas JM, Legakis N. Variations in the prevalence of strains expressing an extended-spectrum P-lactamase phenotype and characterization of isolates from Europe, the Americas, and the Western Pacific region. Clinical Infectious Disease. 2001;32(Suppl 2):S94–S103. doi: 10.1086/320182. [DOI] [PubMed] [Google Scholar]