Abstract
Successful normalization of blood glucose in patients transplanted with pancreatic islets isolated from cadaveric donors established the proof-of-concept that Type 1 Diabetes Mellitus is a curable disease. Nonetheless, major caveats to the widespread use of this cell therapy approach have been the shortage of islets combined with the low viability and functional rates subsequent to transplantation. Gene therapy targeted to enhance survival and performance prior to transplantation could offer a feasible approach to circumvent these issues and sustain a durable functional β-cell mass in vivo. However, efficient and safe delivery of nucleic acids to intact islet remains a challenging task. Here we describe a simple and easy-to-use lentiviral transduction protocol that allows the transduction of approximately 80% of mouse and human islet cells while preserving islet architecture, metabolic function and glucose-dependent stimulation of insulin secretion. Our protocol will facilitate to fully determine the potential of gene expression modulation of therapeutically promising targets in entire pancreatic islets for xenotransplantation purposes.
Keywords: Diabetes Mellitus, Gene transfer, Infection, Lentivirus, Pancreatic islet, Transduction
INTRODUCTION
Type 1 Diabetes Mellitus (T1DM) is one of the most common multifactorial endocrine and metabolic diseases in childhood resulting in persistent hyperglycaemia. Currently, approximately 490,000 children have been diagnosed with T1DM and 78,000 children under the age of 15 are estimated to develop T1DM annually worldwide [1]. More alarmingly, a recent epidemiological study has revealed that the incidence rate of T1DM in children in the United Sates has increased dramatically by 29% between 1985 and 2004 surpassing by 18 times the incidence of Type 2 Diabetes Mellitus (T2DM) in the white population [2]. The most common form of T1DM results from the breakdown of β-cell-specific self-tolerance by T-lymphocytes precipitating an autoimmune attack and eradication of insulin-producing cells [3]. Strong genetic and environmental factors contribute to the onset of T1DM [4]. Existing treatments for T1DM are primarily focused on insulin supplementation. However, despite the beneficial effects of life-long insulin therapy on glucose homeostasis, insulin administration does not eliminate severe diabetic complications such as retinopathy, nephropathy as well as cardiovascular and cerebrovascular diseases [5].
In the past 10 years, clinical islet transplantation has gained much attention as a cell replacement therapy for restoring the functional β-cell mass. Unfortunately, the limited supply of islets from donors has failed to meet demands imposed by the ever-growing number of T1DM patients. An additional major hurdle has been the lack of durability of islet function with insulin independency in less than 10% of patients 5 years after transplantation [6, 7]. Furthermore, several post-transplant events, such as instant blood mediated inflammatory reaction and cytokine cascade, seriously affect the long-term functionality of islets [8-11]. Ex vivo genetic modifications of islets to enhance cell function and survival prior to transplantation have been successfully demonstrated in animal models [12, 13]. This strategy can ultimately increase islet viability and performance providing a tangible approach to improve human islet transplantation and long-term insulin independence. Although protocols designed to modulate gene expression have been extensively used in single cells, the complexity of pancreatic islets has impeded successful gene delivery. Indeed, due to its tridimensional structure, β-cells embedded within the core of islets are sequestered from any significant contact with the remote environment [14-19]. During the last years, several non-viral strategies for genetic modification of islet cells, such as electroporation, microporation, gene gun particle bombardment, cationic liposomes and polymeric particles, have been investigated [15, 19-21]. Unfortunately, in most cases those techniques provided low gene transfer efficiencies and the difficulty of reproducing these protocols have hindered their broad use to allow optimized islet gene transfer. More recently, ex vivo infection of islets was proposed in order to conduct mechanistic studies and also to transfer therapeutically promising genes or alleles prior to islet xenotransplantation [22]. Adenoviral vectors have been used with this purpose since the efficiency of infection in non-dividing cells is greater than other vectors and their epi-chromosomal location reduces the probability of conferring insertional mutations. The efficiency of the majority of adenovial-based infection protocols has been found to be limited to only ~7-30% of islet cells and infected cells were mostly located in the periphery of the islet [14, 15]. Although several studies reported infection of 30-90% of islet cells throughout the whole islet [14, 23, 24] excessive viral dosage were used which may cause cytotoxicity [14, 25, 26]. Alternatively, genetic modifications of adenoviral vectors such as the inclusion of Arg-Gly-Asp motif were attempted to enhance transduction efficiency up to ~80% of islet cells at 10 Plaque Forming Units (PFU) per cell [15]. Unfortunately, the drawback for adenoviral transduction was the methodological difficulties of these experimental protocols and the transient modulation of gene expression [23, 27].
The use of lentiviral vectors in gene therapy has become a powerful tool to safely deliver genetic material with the purpose to rectify molecular defects, enhance functional performance or increase viability of cells. Major advantages of lentiviral vectors include the capacity to infect both dividing and non-dividing cells using repeated dosing, genome integration and long-term expression as well as low immunogenicity [28]. Currently, 89 gene therapy clinical trials using lentiviral vectors are ongoing [29] focusing predominantly on the treatment of primary immunedeficiencies [30]. Transduction protocols using lentiviruses have also been developed for islet infection yielding similar efficiency than adenoviral vectors (~3-50% of β-cells) [14, 16-18, 31-33]. Given the tremendous attributes of lentiviral vectors combined with their current use in clinical trials, we set out to develop a simple and optimal lentiviral transduction protocol for intact human and mouse pancreatic islets with the long-term goal to apply this protocol for gene therapy in islets prior to transplantation without compromising their integrity and functionality.
MATERIALS AND METHODS
Consumables
Reagents and materials used in this study along with reference numbers and companies of purchase are outlined in Table 1.
Table 1.
List of reagents and materials used in this study.
| Product | Vendor | Catalog Number |
|---|---|---|
| 50 x 9 mm Petri dishes | BD Falcon | 351006 |
| Affi-Gel blue beads | Bio-Rad | 153-7301 |
| Bovine Serum Albumin | Sigma-Aldrich | A3294 |
| CalPhos mammalian transfection kit | ClonTech | 631312 |
| CMRL-1066 | Cellgro | 99-663-CV |
| Collagenase | Roche | C9263 |
| DAKO fluorescent mounting medium | Dako | S3023 |
| DAPI | Sigma-Aldrich | 32670 |
| Donkey serum | Sigma-Aldrich | D9663 |
| Fetal Bovine Serum | Sigma-Aldrich | F7524 |
| Formaldehyde | Panreac AppliChem | 252931 |
| Gentamycin | Sigma-Aldrich | G1397 |
| Glutamine | Sigma-Aldrich | G7513 |
| Hanks Balanced Salt Solution 1X | Gibco | 14170088 |
| HEPES | Gibco | 15630-056 |
| HistoGel | Thermo Scientific | R904012 |
| micro-Plate 96 welllibiTreat | IBIDI | 89626 |
| Millex-HV filter 0.45 μm | Merck Millipore | SLHV033RS |
| PBS | Sigma-Aldrich | P5368 |
| Penicillin/Streptomycin | Sigma-Aldrich | P4333 |
| Polystyrene Round-bottom tube | BD Falcon | 352058 |
| RPMI-1640 | Sigma-Aldrich | R0883 |
| Sodium pyruvate | Sigma-Aldrich | S8636 |
| SuperFrost Plus slides | Menzel-Glaser | J1800AMNZ |
| Trypsin-EDTA 10 X | Gibco | 15400054 |
| β-mercaptoethanol | Gibco | 31350-10 |
Animals
Male mice (c57bl/6, 12 week-old) were purchased from Janvier Labs (Le Genest-Saint-Isle, France). Mice experimentations were approved by the CABIMER Animal Committee and performed in accordance with the Spanish law on animal use RD 53/2013.
Islets Procuration and Culture
Mice were sacrificed by cervical dislocation and pancreatic islets were isolated using the collagenase digestion procedure with subsequent handpicking as previously described [34]. Prior to culture islets were washed with Phosphate Buffered Saline (PBS) containing 100 U/ml penicillin and 100 µg/ml streptomycin to minimize post-isolation contaminations. Subsequently islets were cultured in mouse Complete Media (CM) comprised of RPMI 1640 supplemented with 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mM glutamine, 1 mM sodium pyruvate, 50 µM β-mercaptoethanol and 10 mM HEPES. Isolated human islets were either kindly provided by the Cell Isolation and Transplantation Centre (Geneva, Switzerland) or purchased from Tebu-bio (Le Perray En Yvelines, France). Characteristics of human islet preparations are included in Table S1 (802.3KB, pdf) . Islets were cultured in human Complete Media (CM) composed of CMRL-1066 supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine and 100 μg/ml gentamycin.
Lentivirus Generation
We opted for the dual-promoter lentivirus, pHRSIN DUAL-GFP also known as pHRSIN-CSGWdINotIpUbEm (kindly supplied by Dr. Pintor-Toro, CABIMER, Spain) to conduct our studies [35]. This vector allows the cloning and expression of a Gene Of Interest (GOI) under the control of the SFFV promoter while the constitutive Ubiquitin (Ubi) promoter regulates expression of the reporter GFP. Lentivirus amplification and purification was performed by seeding 5 × 106 Hek293T cells into a 100 mm Petri dish and subsequently transfected 24 hours later with: 1) 15 μg of vector, 2) 10 μg the HIV packaging plasmids pCMVDR8.91 and 3) 5 μg of HIV packaging plasmids pVSVG (also known as pMDG). Transient DNA transfection was performed using the CalPhos transfection mammalian kit according to the manufacturer’s recommendations. Viral particles were harvested 72 hours post-transfection, purified using a 0.45 μm Millex-HV filter, and concentrated by ultracentrifugation in an OptimaTM L-100K ultracentrifuge at 87300 x g for 90 minutes at 4º C in a swinging bucket rotor SW-28 (Beckman-Coulter, Spain). Virus particles were resuspended in serum-free DMEM (Invitrogen), distributed in aliquots, snapped frozen in liquid nitrogen, and stored at −80 °C. Viral titer was estimated by transducing Hek293T cells with increasing amounts of pHRSIN DUAL-GFP followed by flow cytometry (FACSCalibur, BD Biosciences, Spain) analysis to determine the PFU/ml based on GFP emission.
Live Imaging and Flow Cytometry
An ImageXpress Micro System (Molecular Devices) was used to monitor GFP fluorescence in living islets. To this end, approximately 20 transduced human or mouse islets were seeded in µ-Plate 96 welllibiTreat plate in a final volume of 200 µl of CM. Islets were cultured for 4 days at 37º C and images (fluorescence or phase contrast) were automatically captured daily and processed using the MetaXpress software. In parallel, islet transduction efficiency was estimated by flow cytometry. In brief, approximately 20 islets were transferred into 5 ml polystyrene Round-bottom tube in a final volume of 50 µl of CM. Islets were disaggregated using 1 X trypsinization for 5 minutes at 37º C and subsequently centrifuged at 200 x g for 5 minutes. Cells were resuspended in 300 μl of PBS and the number of GFP positive cells was estimated as compared to non-infected cells.
Islet Processing and Immunocytochemistry
Islet embedding was performed according to the protocol developed by Cozar-Castellano et al. [36]. In brief, approximately 200 human or murine islets were fixed in 10% formaldehyde at room temperature for 48 hours. Islets were then washed three times in distilled water prior to adding warm (70° C) HistoGel containing 100 µl of 150-300 µm diameter Affi-Gel blue beads. After cooling, Histogel containing the islet-bead mixture was embedded in paraffin following the standard procedures of the CABIMER Histology Core Facility. Paraffin blocks were sectioned (5 μm thickness) using a microtome Leica RM 2255 (Leica Microsystems, Spain). Sections were mounted on SuperFrost Plus slides. After every 10 sections, a slide was stained with hematoxylin-eosin to confirm islet integrity and presence of islets. Sections were deparaffinized/rehydrated at 60˚ C for 20 minutes followed by immersion in decreasing concentrations of ethanol (Xylene 5 minutes/2 x; Ethanol 100% 1 minute/2 x; Ethanol 96% 1 minute; Ethanol 80% 1 minute; Ethanol 70% 1 minute; Distilled water 2 minutes/2 x). After deparaffinization and rehydration, sections were subjected to heat-induced antigen retrieval using 10 mM sodium citrate buffer (pH 6.0) in the microwave in 3 cycles of 3 minutes at 800 W avoiding boiling of the buffer, with 2 minutes at room temperature between heating cycles. Samples were cold down in the same solution for 20 minutes at room temperature. After washing with PBS, samples were incubated in PBS + 0.5% Triton X-100 and then washed again with PBS. Blocking was performed with PBS + 0.2% Triton X-100 containing 1% Bovine Serum Albumin (BSA) and 3% Donkey serum for 1 hour at room temperature. Primary antibodies (Table 2) at the indicated dilutions were added in PBS + 0.1% Triton X-100 containing 1% BSA and 3% Donkey serum and incubated overnight at 4˚ C in a dark humid chamber. Subsequently, sections were washed with PBS for 5 minutes, PBS + 0.2% Triton X-100 for 5 minutes and PBS for 5 minutes. Samples were then incubated with secondary antibodies (Table 2) diluted in PBS + 0.2% Triton X-100 containing 0.1% BSA for 1 hour at room temperature in a dark humid chamber. Nuclear counterstaining was performed by DAPI staining diluted 1:1000 in PBS for 5 minutes at room temperature. Finally, samples were washed three times with PBS for 5 minutes each and sections were mounted using DAKO fluorescent mounting medium. Wide-field immunofluorescence microscopy was performed using a Leica microscope (AF6000) (Leica, England). Images were taken at 40X magnification. Confocal images were acquired using a Leica confocal microscope (TCS SP5). The images were scanned under a HCX PL APO lambda blue 63X/ 1.4 OIL objective. To analyze the whole section, each sample was analyzed using a spatial series through the Z axis. Each spatial series was composed of approximately 5-7 optical sections with a size of 0.8 μm and a 3D projection of each z-stack was performed using three sections.
Table 2.
List of antibodies used in this study.
| Antibody | Dilution | Vendor | Catalog Number |
|---|---|---|---|
| Anti-GFP | 1:200 | Abcam | Ab6673 |
| Anti-insulin (H-86) | 1:500 | Santa Cruz | SC9168 |
| Anti-insulin | 1:500 | Sigma-Aldrich | I2018 |
| Anti-glucagon | 1:150 | Sigma-Aldrich | G2654 |
| Anti-glucagon | 1:200 | Cell Signaling | 2760S |
| Anti-cleaved caspase-3 | 1:150 | Cell Signaling | 9661 |
| Alexa fluor 488 donkey anti-goat | 1:800 | Invitrogen | A11055 |
| Alexa fluor 555 donkey anti-mouse | 1:800 | Life technologies | A31579 |
| Alexa fluor 647 donkey anti-rabbit | 1:800 | Life technologies | A31573 |
Viability and Functional Assay
Islet viability subsequent to transduction was assessed in groups of 35 islets using the Cell Proliferation Kit I (MTT) according to the manufacturer´s recommendations (Roche, Spain). Optical density was determined at 550 nm with a reference wavelength of 650 nm using a Varioskan Flash spectrophotometer (Thermo Scientific, Spain). In parallel, glucose stimulated insulin secretion was performed to assess the functional integrity of islets. Groups of 10 islets were washed in 500 μL of Krebs-Ringer bicarbonate-HEPES buffer (KRBH) (140 mM NaCl, 3.6 mM KCl, 0.5 mM NaH2PO4, 0.5 mM MgSO4, 1.5 mM CaCl2, 2 mM NaHCO3, 10 mM HEPES, 0.1% BSA) and pre-incubated at 37° C for 45 minutes in 300 μl of the same buffer. Islets were then centrifuged and KRBH buffer was discarded. Subsequently, fresh KRBH supplemented with 2.5 mM glucose was added and islets were incubated for 30 minutes. Next, buffer was harvested (basal insulin secretion) and 500 μL of KRBH supplemented with 16.8 mM glucose was added. Islets were incubated for an additional 30 minutes at 37° C and then buffer was harvested (induced insulin secretion). Insulin levels were measured using a mouse or human insulin enzyme immunoassay kit (Mercodia AB, Spain) according to the manufacturer´s instructions. Stimulation index was expressed as the ratio of insulin levels at 16.8 mM glucose divided by insulin levels at 2.5 mM glucose.
Statistical Analysis
Results are expressed as the mean± SEM. Statistical differences were estimated by two-tailed unpaired student’s t-test. *Indicates statistical significance, p value <0.05.
RESULTS
Elaboration of a High Efficiency Transduction Protocol in Mouse Islets
Modulation of gene expression has been particularly challenging in the context of whole pancreatic islets as compared to cell lines due to their three dimensional structure composed of approximately 1000 to 2000 compacted cells. Sophisticated protocols such as in vivo perfusion or microporation using adeno and lenti viruses claim to have successfully and homogenously transduced up to 70% islet cells [24]. As these protocols may be cumbersome to carry out or simply not always feasible (i.e. in vivo perfusion of human islets) we sought to develop a readily accessible and friendly user lentiviral protocol (BOX 1 and Fig. 1). Consistent with previous reports, the mere exposure of islets to increasing PFU/cell of pHRSIN DUAL-GFP resulted in enhanced GFP fluorescence from living islets corroborating with a greater number of islet cells expressing GFP, as assessed by flow cytometry of dispersed islets (Fig. 2A-B). However, the 100 PFU/cell that achieved 80% infection efficiency also considerably reduced islet viability (Fig. 2C) with the appearance of necrotic cells in the islet core (Fig. 2A, arrows). Intriguingly, wide-field and confocal immunocytochemistry revealed that even at high PFU/cell cells at the periphery were preferentially infected (e.g. GFP positive) (Fig. 2D-E). In an attempt to increase accessibility to cells sequestered within the core to viral particles without compromising viability, we mildly loosen up islet cells using either 1X (500 mg/L trypsin; 0.96 mM EDTA) or 0.5X (250 mg/L trypsin; 0.48 mM EDTA) trypsin-EDTA for 3 minutes prior to transduction. Both trypsin concentrations improved the number of GFP-expressing islet cells at either 5 or 20 PFU/cell (Fig. 3A-B). Flow cytometry of disaggregated islets confirmed that the number of GFP-positive cells infected at 5 PFU/cell increased from ~30% in control islets to ~50% in islets pre-treated with trypsin independent of its concentration (Fig. 3B). Similarly, 20 PFU/cell resulted in 80% of cells expressing GFP independent of trypsin concentrations (Fig. 3B). Unexpectedly, 1X trypsin jeopardized viability of cells in all conditions (Fig. 3C). High-resolution confocal microscopy confirmed that 0.5X trypsin-EDTA facilitated infection of cells residing within the islet core (Fig. 3D). We next sought to determine the temporal evolution of GFP expression sub-sequent to transduction using 0.5X trypsin. In order to expose islets to minimal amount of viral particles, we also assessed the transduction efficiency of 7 and 10 PFU/cell. For each viral dosage, the percentage of GFP-positive cells remained relatively constant over the 10 day period (Fig. 4A-B). Of note, at day 10, non-infected (mock) islet cells emit low levels of fluorescent, indicative of auto-fluorescence produced by apoptotic cells [37]. Consistent with this premise, islet architecture was strongly compromised at day 10 with signs of necrosis as compared to islets 4 days post infection (Fig. 4C). In some instances, bacterial contamination was also observed 10 days after transduction (data not shown). Islets transduced at 20 PFU/cell consistently presented ~80% of islet cells at day 4 post-infection, as compared to all other PFU/cell tested (Fig. 4B). GFP immunostaining was detected homogeneously throughout the islet co-localizing with both insulin as well as glucagon-positive cells 4 days post-infections (Fig. 4D-E). More importantly at this time point, neither islet viability (Fig. 4F) nor function, as measured by glucose-induced insulin secretion (Fig. 4G), were altered at 20 PFU/cell as compared to 5, 7, 10 PFU/cell or control non-transduced islets. Furthermore, the apoptotic rate, as assessed by cleaved-caspase 3 immunostaining, was identical in both control and 4 days post-transduction islets indicating, that the protocol is not detrimental for islet health (Fig. 4H-I) In summary, our data indicate that 80% of mouse islet cells express GFP 4 days after exposure to a short and mild trypsin treatment and to a viral dosage of 20 PFU/cell.
Box. (1).
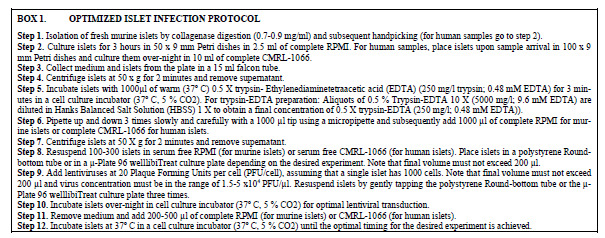
Fig. (1).
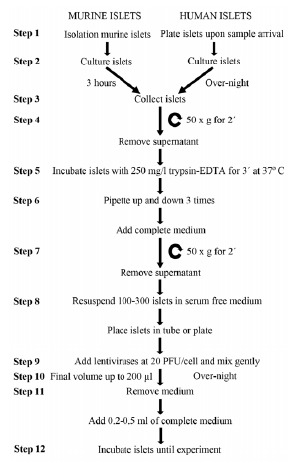
Optimized protocol for lentiviral-mediated islet infection. Summarized scheme of the transduction protocol described in Box 1.
Fig. (2).
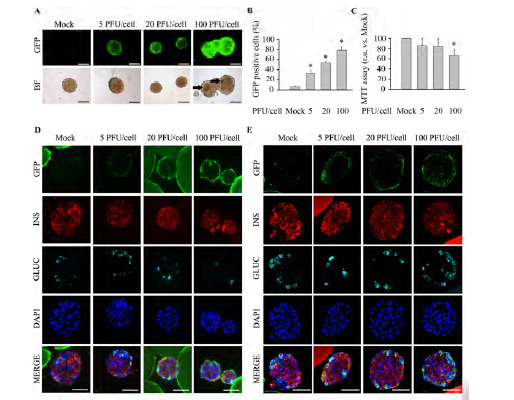
High pHRSIN DUAL-GFP PFU/cell levels compromise islet viability with sub-optimal islet transduction efficiency. Freshly isolated murine islets were exposed to increasing PFU/cell of pHRSIN DUAL-GFP. Non-transduced islets (Mock) were used as control. (A) Representative images of ex vivo cultured entire live transduced islets. Top; GFP expression was assessed by fluorescence acquisition using an ImageXpress Microsystem. Low; Bright field images. Images were captured at 4 days post-infection. Arrows indicate necrotic areas. Scale-bars indicate 100 µm. n=4 experiments per condition. (B) Transduction efficiency, defined as the percentage of islet cells expressing GFP, was determined by flow cytometry in disaggregated islets at 4 days post-infection. n=6 per condition. (C) Determination of islet metabolic activity using the MTT assay at 4 days post-infection n=4-6 per condition. (D-E) Representative immunofluorescence images of Affi-Gel bead-embedded pancreatic islets 4 days post-infection. Antibodies against GFP (green), insulin (red) and glucagon (cyan) were employed. Of note, in some instances the Affi-Gel beads, emitted a non specific fluorescent signal along with GFP (Green) and insulin (red). (D) Wide-field fluorescence microscopy. (E) Confocal microscopy. Scale-bars, 50 µm. n=3 per condition. Data are represented as the mean ± SEM. * p< 0.05 versus control non-transduced islets.
Fig. (3).
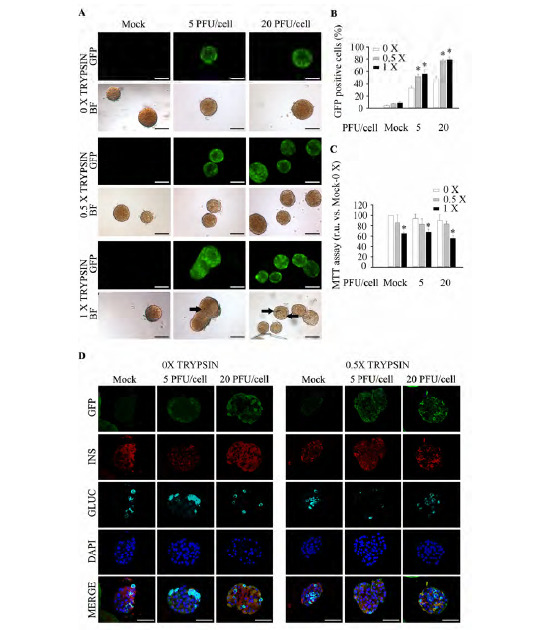
A Mild Trypsin-EDTA treatment increases transduction efficiency in murine islets. Freshly isolated murine islets were treated or not with two concentrations of trypsin-EDTA prior transduction or not with pHRSIN DUAL-GFP. (A) Representative images of live islets exhibiting GFP fluorescence subsequent to treatment: Top; GFP expression was assessed by fluorescence acquisition using an ImageXpress Microsystem. Low; Bright field images. Images were captured at 4 days post-infection. Arrows indicate necrotic areas. Scale-bars 100 µm. n=4 experiments per condition. (B) Transduction efficiency in trypsin-EDTA treated islets was determined by flow cytometry in disaggregated islets at 4 days post-infection. n=3-8 per condition. (C) Determination of islet metabolic activity using the MTT assay 4 days post-infection. n=4 per condition. (D) Representative confocal immunofluorescence images of Affi-Gel bead-embedded pancreatic islets 4 days post-infection with or without 0.5X trypsin-EDTA treatment. Antibodies against GFP (green), insulin (red) and glucagon (cyan) were employed. Of note, in some instances the Affi-Gel beads, emitted a non specific fluorescent signal along with GFP (green). Scale-bars, 50 µm. n=3 per condition. 0 X: Untreated; 0.5 X: 0.5 X trypsin-EDTA treatment (250 mg/l trypsin; 0.48 mM EDTA); 1 X: 1 X trypsin-EDTA treatment (500 mg/l; 0.96 mM EDTA). Data are represented as the mean ± SEM. * p < 0.05 versus control non-transduced trypsin-EDTA untreated islets.
Fig. (4).
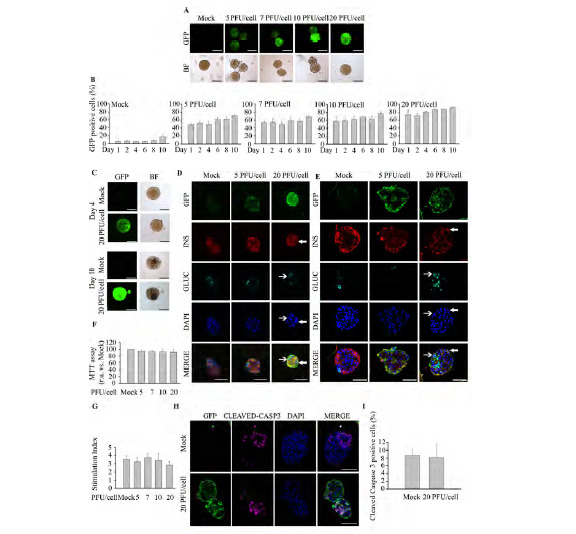
Mild trypsinization combined with 20 PFU/cell represents the optimal infection protocol for murine islets. Freshly isolated murine islets were treated with 0.5 X trypsin-EDTA (250 mg/l; 0.48 mM EDTA) and subsequently exposed to increasing PFU/cell of pHRSIN DUAL-GFP. (A) Representative images of GFP fluorescence emitted from live islets: Top; GFP expression was assessed by fluorescence acquisition using an ImageXpress Microsystem. Low; Bright field images. Images were captured at 4 days post-infection. Scale-bars 100 µm. n=4 experiments per condition. (B) Transduction efficiency in 0.5 X trypsin-EDTA treated islets at different days after transduction was determined by flow cytometry in dispersed islets. n=4 per condition. (C) Representative images of live islets exhibiting GFP fluorescence 4 and 10 days post-treatment: Top; GFP expression was assessed by fluorescence acquisition using an ImageXpress Microsystem. Low; Bright field images. Scale-bars 100 µm. n=4 experiments per condition. (D-E) Representative immunofluorescence images of Affi-Gel bead-embedded pancreatic islets trypsin-treated and transduced or not with pHRSIN DUAL-GFP. Antibodies against GFP (green), insulin (red) and glucagon (cyan) were employed. Images were captured in samples fixed at 4 days post-infection using either wide-field fluorescence microscopy (D) or confocal microscopy (E). Of note, in some instances the Affi-Gel beads, emitted a non specific fluorescent signal along with GFP (green) and insulin (red). Filled arrows indicate transduced cells expressing insulin; Non-filled arrows indicate transduced cells expressing glucagon. Scale-bars 50 µm. n=3 per condition. (F) Determination of islet metabolic activity subsequent to a 0.5 X trypsin-EDTA treatment followed by transduction with 20 PFU/cell. A MTT assay was performed 4 days post-infection. n=3-4 per condition. (G) Glucose-stimulated insulin secretion was assessed in islet treated with 0.5 X trypsin-EDTA followed by transduction with increasing amount of pHRSIN DUAL-GFP. (H) Representative immunofluorescence images of Affi-Gel bead-embedded pancreatic islets 0.5X trypsin-treated and transduced or not with pHRSIN DUAL-GFP. Antibodies against GFP (green) and cleaved Caspase 3 (magenta). Images were captured in samples fixed at 4 days post-infection using confocal fluorescence microscopy. n=3 per condition. (I) Determination of apoptosis rate by quantification of cleaved caspase 3 positive cells in islets 0.5X trypsin-EDTA-treated and transduced or not with pHRSIN DUAL-GFP. n=3 per condition. Data are represented as the mean ± SEM of n=3. * p < 0.05 versus control non-transduced 0.5 X trypsin-EDTA treated islets.
Transduction Protocol Validation in Human Islet
We next validated our transduction protocol in human islets. Live human islets revealed intense GFP expression without apparent ultra-structural abnormalities 4 days post transduction (Fig. 5A). Consistent with mouse islets, approximately 70 to 80% of islet cells were GFP-positive, as determined by flow cytometry of dispersed islets (Fig. 5B). Remarkably, the viability and functionality of transduced islets were not altered (Fig. 5C-D). Finally, GFP immunostaining assessed by wide-field and confocal microscopy was detected homogeneously throughout islets co-localizing with both insulin and glucagon (Fig. 5E-F and Supplemental Fig. S1 (802.3KB, pdf) ). Taken together, our data indicate that the proposed protocol is easy, reliable and allows the transduction of the majority of cells residing in entire islets from murine and human origin.
Fig. (5).
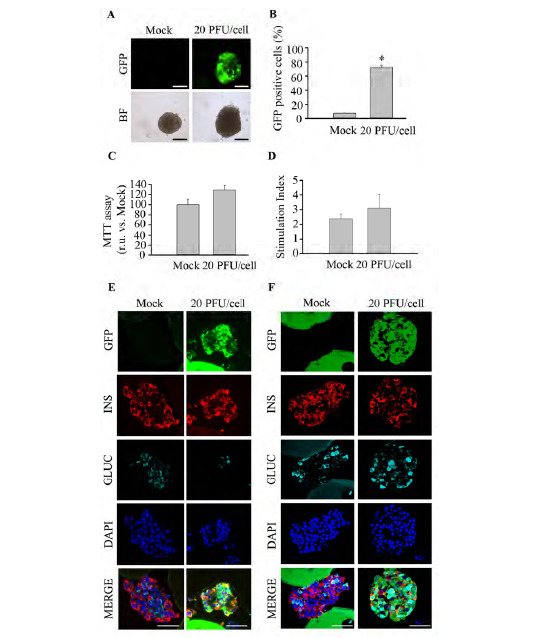
Human islets are efficiently transduced using the optimized protocol. Human islets obtained from cadaveric donors were initially treated with 0.5 X trypsin-EDTA (250 mg/l trypsin; 0.48 mM EDTA) and then transduced with pHRSIN DUAL-GFP at 20 PFU/cell. (A) Live imaging reveals GFP expression in human islets 4 days post-infection: Top; GFP expression, assessed by fluorescence acquisition using an ImageXpress Microsystem, Bottom; Bright field images. Scale-bars 100 µm. n=3 per condition. (B) Transduction efficiency in 0.5 X trypsin-EDTA treated islets was determined by flow cytometry of dispersed islets at 4 days post-transduction with 20 PFU/cell. n=3 per condition. (C) Islet metabolic activity was assessed using the MTT assay. n=3 per condition. (D) Glucose-stimulated insulin secretion was assessed in either control islets or islet treated with 0.5 X trypsin-EDTA followed by transduction with 20 PFU/cell of pHRSIN DUAL-GFP. n=3 per condition. (E-F) Co-immunostaining of GFP (green), insulin (red) and glucagon (cyan) was performed on sections from Affi-Gel bead-embedded human pancreatic islets subsequent to treatment. Images were captured in samples fixed at 4 days post-infection using wide-field fluorescence microscopy (E) or confocal microscopy (F). Scale-bars 50µm. n=3 per condition. Data are represented as the mean ± SEM. * p < 0.05 versus control non-transduced 0.5 X trypsin-EDTA treated islets.
DISCUSSION
Given the indispensable role of pancreatic islets in glucose homeostasis, the modulation of gene expression in transplanted islets could be a promising approach to boost islet performance and durability for the treatment of T1DM [38, 39]. In this context, non-viral strategies, such as electroporation, gene gun particle bombardment, cationic liposomes and polymeric particles, have been developed for genetic modification of islet cells [15, 19-21]. Unfortunately, these techniques provide only low to intermediate gene transfer efficiencies, limiting their applicability. In contrast, published adenovial-based infection protocols claim to have successfully transduced up to 90% of islet cells using high viral doses [14, 15, 23, 25-27]. Although promising, these protocols are technically challenging to perform. Moreover, these protocols result in transient expression and frequently induce cell toxicity. Alternatively, lentiviral vectors have emerged as an alternative strategy to modulate gene expression in intact islets. Up to 50% of β cells in intact islets have been efficiently transduced without adverse viability effects [14, 16-18, 31-33]. Based on these initial successes, we have devised an easy-to-use and reproducible protocol that bestows a significant improvement of murine and human islet transduction efficiency. In summary, our optimized easy-to-use transduction protocol resulted in an infection efficiency of ~70-80% of cells within intact murine and human islets without compromising health. In the optimization of our protocol three non-mutually exclusive parameters were considered: 1) PFU/cell, 2) islet architecture, and 3) time post-transduction. Consistent with other reports, we found that high PFU/cell (e.g. 100 PFU/cell or greater) increased transduction efficiency but to the detriment of islet cell function and survival [14, 25, 26]. The negative impact of high virus dosage has also been substantiated in vivo xenotransplantation studies [15]. We established that a 20 PFU/cell was the optimal viral dosage reaching 50% cell infection in intact islets without jeopardizing either viability or function. This PFU/cell is substantially lower to those (100-1000 PFU/cell) previously utilized in another published protocol [25]. Addition of a mild 0.5X trypsin-EDTA treatment to facilitate core accessibility greatly improved transduction efficiency while preserving islet health and function, reaching approximately 80% of the islet cell population. Interestingly, 1X trypsin-EDTA affected cell viability. Pro-distension agents such as collagenase and triton-X-100 were also found to increase infection efficiency yet compromised islet functionality [14, 23]. Thus, although these treatments seemingly appear to be beneficial, it is of utmost importance to verify that islet function and viability are preserved post-treatment. We also found that time post infection was another critical parameter to the successful outcome of the experiment. Indeed, we established that islet integrity and health is maintained up to 4 days post transduction.
Although islet cell transplantation has demonstrated many clinical successes to date, more work is necessary to further improve its efficacy and universalize this treatment to the vast majority of T1DM patients and to allow long-term insulin independency. From the results shown in this report, we speculate that human islets infected with our protocol may provide a venue to improve health and function prior to transplantation and prevent post-transplantation dismay. Indeed, human islets presented normal metabolic activity and functionality, marked insulin and glucagon expression and normal islet architecture, suggesting that the proposed protocol for islet infection does not compromise human islet health. Therefore, lentiviral-mediated gene expression modulation using this protocol could be therapeutically promising to generate a functional and stable islet transplanted mass in humans.
CONCLUSION
Here we present a protocol that represents a reliable easy-to-use procedure to transduce efficiently human and mouse islets with the dual purpose of studying the impact of therapeutic genes in islet physiology and ultimately facilitating the universalization of islet infection prior transplantation. The stable integrating nature of lentiviral vectors supports the notion that lentiviral-mediated gene transfer might be an optimal method to improve islet function for the treatment of T1DM [40]. In this sense, the value of potential benefits based on the modulation of gene expression in entire islets warrants further experimentation to determine the applicability of our protocol for islet infection prior transplantation.
ACKNOWLEDGEMENTS
This work was funded by grants from the Consejería de Salud, Fundación Pública Andaluza Progreso y Salud, Junta de Andalucía (PI-0727-2010 to B.R.G.), Consejería de Economía, Innovación y Ciencia (P10.CTS.6359 to B.R.G.) and the Ministerio de Economía y Competitividad, Instituto de Salud Carlos III co-funded by Fondos FEDER (PI10/00871 and PI13/00593 to B.R.G). A.M-M. is a recipient of a Miguel Servet grant (CP14/00105) from the Instituto de Salud Carlos III co-funded by Instituto de Salud Carlos III and FEDER. N.C.V is supported by a JDRF subsidy (17-2013-372 to B.R.G.). Human islets were provided through the JDRF award 31-2012-783 (ECIT Islet for Basic Research program). We thank Cindy Cruz-Zambrano at the CABIMER Histology Core Facility for excellent technical assistance in embedding and sectioning of islet samples. We thank I. Cózar for kindly providing the protocol for islet embedding. We thank Dr. Collins and Dr. Pintor-Toro for kindly providing the pHRSIN-DUAL-GFP lentiviral vector.
CM J-M, IG H-G, L L-N, PI L, N C-V, E F-M, JM M-G and A M-M performed the experiments; G P and D B isolated and provided human pancreatic islets. CM J-M, BR G and A M-M designed the study and wrote the manuscript.
LIST OF ABREVIATIONS
- BSA
Bovine Serum Albumin
- CM
Complete Media
- GOI
Gene Of Interest
- KRBH
Krebs-Ringer bicarbonate-HEPES buffer
- PFU/cell
Plaque Forming Units per cell
- PBS
Phosphate Buffered Saline
- T1DM
Type 1 Diabetes Mellitus
- T2DM
Type 2 Diabetes Mellitus
- Ubi
Ubiquitin
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers Web site along with the published article.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Atlas I. IDF Atlas. vol. March12th 2014: http://www.idf.org/diabetesatlas. 2014.
- 2.Lipman T.H., Levitt Katz L.E., Ratcliffe S.J., Murphy K.M., Aguilar A., Rezvani I., Howe C.J., Fadia S., Suarez E. Increasing incidence of type 1 diabetes in youth: twenty years of the Philadelphia Pediatric Diabetes Registry. Diabetes Care. 2013;36(6):1597–1603. doi: 10.2337/dc12-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tisch R., Wang B. Dysrulation of T cell peripheral tolerance in type 1 diabetes. Adv. Immunol. 2008;100:125–149. doi: 10.1016/S0065-2776(08)00805-5. [DOI] [PubMed] [Google Scholar]
- 4.Redondo M.J., Eisenbarth G.S. Genetic control of autoimmunity in Type I diabetes and associated disorders. Diabetologia. 2002;45(5):605–622. doi: 10.1007/s00125-002-0781-1. [DOI] [PubMed] [Google Scholar]
- 5.Roglic G., Unwin N. Mortality attributable to diabetes: estimates for the year 2010. Diabetes Res. Clin. Pract. 2010;87(1):15–19. doi: 10.1016/j.diabres.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Ludwig B, Reichel A, Kruppa A, Ludwig S, et al. Islet transplantation at the Dresden diabetes center: five years' experience. . Horm Metab Res = Horm- und Stoffwechselforschung = Horm et metab . 2015;47:4–8. doi: 10.1055/s-0034-1385876. [DOI] [PubMed] [Google Scholar]
- 7.Ryan E.A., Paty B.W., Senior P.A., Bigam D., Alfadhli E., Kneteman N.M., Lakey J.R., Shapiro A.M. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54(7):2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 8.Johansson H., Lukinius A., Moberg L., Lundgren T., Berne C., Foss A., Felldin M., Källen R., Salmela K., Tibell A., Tufveson G., Ekdahl K.N., Elgue G., Korsgren O., Nilsson B. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes. 2005;54(6):1755–1762. doi: 10.2337/diabetes.54.6.1755. [DOI] [PubMed] [Google Scholar]
- 9.Saito Y., Goto M., Maya K., Ogawa N., Fujimori K., Kurokawa Y., Satomi S. Brain death in combination with warm ischemic stress during isolation procedures induces the expression of crucial inflammatory mediators in the isolated islets. Cell Transplant. 2010;19(6):775–782. doi: 10.3727/096368910X508889. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro A.M., Lakey J.R., Ryan E.A., Korbutt G.S., Toth E., Warnock G.L., Kneteman N.M., Rajotte R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000;343(4):230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 11.Tjernberg J., Ekdahl K.N., Lambris J.D., Korsgren O., Nilsson B. Acute antibody-mediated complement activation mediates lysis of pancreatic islets cells and may cause tissue loss in clinical islet transplantation. Transplantation. 2008;85(8):1193–1199. doi: 10.1097/TP.0b013e31816b22f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang H.J., Lee M., Park J.H., Jung H.S., Kang J.G., Kim C.S., Lee S.J., Ihm S.H. Improved islet transplantation outcome by the co-delivery of siRNAs for iNOS and 17β-estradiol using an R3V6 peptide carrier. Biomaterials. 2015;38:36–42. doi: 10.1016/j.biomaterials.2014.10.060. [DOI] [PubMed] [Google Scholar]
- 13.Wu H., Panakanti R., Li F., Mahato R.I. XIAP gene expression protects β-cells and human islets from apoptotic cell death. Mol. Pharm. 2010;7(5):1655–1666. doi: 10.1021/mp100070j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbu A.R., Bodin B., Welsh M., Jansson L., Welsh N. A perfusion protocol for highly efficient transduction of intact pancreatic islets of Langerhans. Diabetologia. 2006;49(10):2388–2391. doi: 10.1007/s00125-006-0390-5. [DOI] [PubMed] [Google Scholar]
- 15.Bilbao G, Contreras JL, Dmitriev I, Smyth CA. Genetically modified adenovirus vector containing an RGD peptide in the HI loop of the fiber knob improves gene transfer to nonhuman primate isolated pancreatic islets. . American journal of transplantation : off j Am Soc Transplant Am Soc Transplant Surgeons . 2002. [DOI] [PubMed]
- 16.Gallichan W.S., Kafri T., Krahl T., Verma I.M., Sarvetnick N. Lentivirus-mediated transduction of islet grafts with interleukin 4 results in sustained gene expression and protection from insulitis. Hum. Gene Ther. 1998;9(18):2717–2726. doi: 10.1089/hum.1998.9.18-2717. [DOI] [PubMed] [Google Scholar]
- 17.Giannoukakis N., Mi Z., Gambotto A., Eramo A., Ricordi C., Trucco M., Robbins P. Infection of intact human islets by a lentiviral vector. Gene Ther. 1999;6(9):1545–1551. doi: 10.1038/sj.gt.3300996. [DOI] [PubMed] [Google Scholar]
- 18.Leibowitz G., Beattie G.M., Kafri T., Cirulli V., Lopez A.D., Hayek A., Levine F. Gene transfer to human pancreatic endocrine cells using viral vectors. Diabetes. 1999;48(4):745–753. doi: 10.2337/diabetes.48.4.745. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez Rilo H.L., Paljug W.R., Lakey J.R., Taylor M.J., Grayson D. Biolistic bioengineering of pancreatic beta-cells with fluorescent green protein. Transplant. Proc. 1998;30(2):465–468. doi: 10.1016/S0041-1345(97)01358-4. [DOI] [PubMed] [Google Scholar]
- 20.Mahato R.I., Henry J., Narang A.S., Sabek O., et al. Cationic lipid and polymer-based gene delivery to human pancreatic islets. Mol ther: j Am Soc. Gene Ther. 2003;7:89–100. doi: 10.1016/s1525-0016(02)00031-x. [DOI] [PubMed] [Google Scholar]
- 21.Bartlett R., Denis M., Inverardi L., Ricordi C. Introduction of immunomodulatory genes into isolated pancreatic islets via biolistic particle bombardment. Transplant. Proc. 1998;30(2):452. doi: 10.1016/S0041-1345(97)01352-3. [DOI] [PubMed] [Google Scholar]
- 22.Levine F. Gene therapy for diabetes: strategies for beta-cell modification and replacement. Diabetes Metab. Rev. 1997;13(4):209–246. doi: 10.1002/(SICI)1099-0895(199712)13:4<209::AID-DMR198>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi R., Ishihara H., Takahashi K., Tamura A., Yamaguchi S., Yamada T., Katagiri H., Oka Y. Efficient and controlled gene expression in mouse pancreatic islets by arterial delivery of tetracycline-inducible adenoviral vectors. J. Mol. Endocrinol. 2007;38(1-2):127–136. doi: 10.1677/jme.1.02189. [DOI] [PubMed] [Google Scholar]
- 24.Lefebvre B., Vandewalle B., Longue J., Moerman E., Lukowiak B., Gmyr V., Maedler K., Kerr-conte J., Pattou F. Efficient gene delivery and silencing of mouse and human pancreatic islets. BMC Biotechnol. 2010;10:28. doi: 10.1186/1472-6750-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kvell K., Nguyen T.H., Salmon P., Glauser F.M., et al. Transduction of CpG DNA-stimulated primary human B cells with bicistronic lentivectors. Mol ther: j Am Soc. Gene Ther. 2005;12:892–899. doi: 10.1016/j.ymthe.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Rajalingam K., Al-Younes H., Müller A., Meyer T.F., Szczepek A.J., Rudel T. Epithelial cells infected with Chlamydophila pneumoniae (Chlamydia pneumoniae) are resistant to apoptosis. Infect. Immun. 2001;69(12):7880–7888. doi: 10.1128/IAI.69.12.7880-7888.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diraison F., Motakis E., Parton L.E., Nason G.P., Leclerc I., Rutter G.A. Impact of adenoviral transduction with SREBP1c or AMPK on pancreatic islet gene expression profile: analysis with oligonucleotide microarrays. Diabetes. 2004;53(Suppl. 3):S84–S91. doi: 10.2337/diabetes.53.suppl_3.S84. [DOI] [PubMed] [Google Scholar]
- 28.Hughes A., Jessup C., Drogemuller C., Mohanasundaram D., Milner C., Rojas D., Russ G.R., Coates P.T. Gene therapy to improve pancreatic islet transplantation for Type 1 diabetes mellitus. Curr. Diabetes Rev. 2010;6(5):274–284. doi: 10.2174/157339910793360897. [DOI] [PubMed] [Google Scholar]
- 29.Worldwide G.T. Worldwide, GTCT (2014). Gene Therapy Clinical Trials Worldwide. vol. 2014: http://www.abedia.com/wiley/vectors.php. 2014.
- 30.Farinelli G., Capo V., Scaramuzza S., Aiuti A. Lentiviral vectors for the treatment of primary immunodeficiencies. J. Inherit. Metab. Dis. 2014;37(4):525–533. doi: 10.1007/s10545-014-9690-y. [DOI] [PubMed] [Google Scholar]
- 31.Kobinger G.P., Deng S., Louboutin J.P., Vatamaniuk M., Matschinsky F., Markmann J.F., Raper S.E., Wilson J.M. Transduction of human islets with pseudotyped lentiviral vectors. Hum. Gene Ther. 2004;15(2):211–219. doi: 10.1089/104303404772680010. [DOI] [PubMed] [Google Scholar]
- 32.Fenjves E.S., Ochoa M.S., Cechin S., Gay-Rabinstein C., Pérez-Alvarez I., Ichii H., Mendez A., Ricordi C., Curran M.A. Protection of human pancreatic islets using a lentiviral vector expressing two genes: cFLIP and GFP. Cell Transplant. 2008;17(7):793–802. doi: 10.3727/096368908786516828. [DOI] [PubMed] [Google Scholar]
- 33.Chou F.C., Sytwu H.K. Overexpression of thioredoxin in islets transduced by a lentiviral vector prolongs graft survival in autoimmune diabetic NOD mice. J. Biomed. Sci. 2009;16:71. doi: 10.1186/1423-0127-16-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brun T., Franklin I., St-Onge L., Biason-Lauber A., Schoenle E.J., Wollheim C.B., Gauthier B.R. The diabetes-linked transcription factor PAX4 promotes beta-cell proliferation and survival in rat and human islets. J. Cell Biol. 2004;167(6):1123–1135. doi: 10.1083/jcb.200405148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreno-Mateos M.A., Espina A.G., Torres B., Gámez del Estal M.M., Romero-Franco A., Ríos R.M., Pintor-Toro J.A. PTTG1/securin modulates microtubule nucleation and cell migration. Mol. Biol. Cell. 2011;22(22):4302–4311. doi: 10.1091/mbc.E10-10-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cozar-Castellano I., Takane K.K., Bottino R., Balamurugan A.N., Stewart A.F. Induction of beta-cell proliferation and retinoblastoma protein phosphorylation in rat and human islets using adenovirus-mediated transfer of cyclin-dependent kinase-4 and cyclin D1. Diabetes. 2004;53(1):149–159. doi: 10.2337/diabetes.53.1.149. [DOI] [PubMed] [Google Scholar]
- 37.Dittmar R., Potier E., van Zandvoort M., Ito K. Assessment of cell viability in three-dimensional scaffolds using cellular auto-fluorescence. Tissue Eng. Part C Methods. 2012;18(3):198–204. doi: 10.1089/ten.tec.2011.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicolau C., Le Pape A., Soriano P., Fargette F., Juhel M.F. In vivo expression of rat insulin after intravenous administration of the liposome-entrapped gene for rat insulin I. Proc. Natl. Acad. Sci. USA. 1983;80(4):1068–1072. doi: 10.1073/pnas.80.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li W., Wu W., Song H., Wang F., Li H., Chen L., Lai Y., Janicki J.S., Ward K.W., Meyer C.J., Wang X.L., Tang D., Cui T. Targeting Nrf2 by dihydro-CDDO-trifluoroethyl amide enhances autophagic clearance and viability of β-cells in a setting of oxidative stress. FEBS Lett. 2014;588(12):2115–2124. doi: 10.1016/j.febslet.2014.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang H., Hester G., Liao L., Montefiori D.C., Frank M.M. Mechanisms by which HIV envelope minimizes immunogenicity. Immunol. Res. 2011;49(1-3):147–158. doi: 10.1007/s12026-010-8178-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publishers Web site along with the published article.


