Abstract
The endocannabinoid system (ECS) is a group of neuromodulatory lipids and their receptors, which are widely distributed in mammalian tissues. ECS regulates various cardiovascular, nervous, and immune system functions inside cells. In recent years, there has been a growing body of evidence for the use of synthetic and natural cannabinoids as potential anticancer agents. For instance, the CB1 and CB2 receptors are assumed to play an important role inside the endocannabinoid system. These receptors are abundantly expressed in the brain and fatty tissue of the human body. Despite recent developments in molecular biology, there is still a lack of knowledge about the distribution of CB1 and CB2 receptors in the human kidney and their role in kidney cancer. To address this gap, we explore and demonstrate the role of the endocannabinoid system in renal cell carcinoma (RCC). In this brief overview, we elucidate the therapeutic aspects of the endocannabinoid system for various cancers and explain how this system can be used for treating kidney cancer. Overall, this review provides new insights into cannabinoids’ mechanisms of action in both in vivo and in vitro models, and focuses on recent discoveries in the field.
Keywords: CB1 and CB2 receptors, Cannabinoids, Endocannabinoid system, Renal cell carcinoma
INTRODUCTION
Extracts from Cannabis sativa L. have been used for both medicinal and recreational purposes for many centuries. Cannabinoids are involved in a variety of physiological and pathological conditions, including inflammation, immunomodulation, analgesia, and cancer [1]. Nevertheless, the most active component of the plant, Δ9-tetrahydrocannabinol (THC), began to be explored in 1960 [2]. There are approximately 66 unique compounds, known as cannabinoids, derived from Cannabis sativa L. [3]. Cannabinoids are classified into three categories: phytocannabinoids, which occur uniquely in the Cannabis sativa L. plant (i.e., THC, CBD [cannabidiol], and CBN [cannabinol]); endogenous cannabinoids, which are produced in the human body and those of other animals (i.e., anandamide and 2-arachidonoyl-glycerol); and synthetic cannabinoids, which are produced in laboratory conditions (i.e., JWH-133, WIN 55,212-2, and SR141716).
Initially, it was believed that cannabinoids, which are highly hydrophobic, mediate their action by binding directly to biomembrane proteins. However, the first cloning of cannabinoid receptors from the mammalian brain in 1990 led to the discovery of the basic mechanism of cannabinoid action [4]. At this point, cannabinoid research expanded to the study of cannanioids’ therapeutic effects. Currently, two cannabinoid receptor subtypes have been identified: cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2). Both subtypes belong to the G-protein coupled receptor superfamily, which is responsible for the transduction of intercellular signals. The CB1 receptor is abundant in the brain and is also present in other tissues and organs of the body. Therefore, it is believed that the psychoactivity induced by cannabinoids is mostly regulated by CB1 receptors. In contrast to CB1, CB2 receptors are expressed exclusively in immune system cells, including B and T lymphocytes and macrophages [5]. Activation of CB1 receptors stimulates cell signaling through Gi and Go activation, while on the other hand, CB2 increases signaling only with Gi [6]. The activation of these G-protein-coupled and cannabinoid receptors inhibits the enzymatic activity of adenylate cyclase, which is responsible for the production of cyclic adenosine monophosphate (cAMP) inside cells [1]. However, the underlying mechanisms through which cannabinoids and cannabinoid receptors inhibit proliferation and migration, and induce apoptosis of cancer cells, remain obscure.
CANNABINOIDS IN CANCER TREATMENT: CHEMICAL APPROACHES TO THEIR SYNTHESIS
Cannabinoids are a family of compounds belonging to different chemical classes that can act on cannabinoid receptors (CB1 and CB2). They are grouped into three main classes: phytocannabinoids, which are naturally found in Cannabis sativa L. and other plants; endocannabinoids, which are naturally produced by mammalian species as endogenous CB1 and CB2 receptor agonists; and synthetic cannabinoids, which produced under laboratory conditions. Recent studies provide lines of evidence that cannabinoids, in addition to well-known pharmacological activities as an antiemetic and in the treatment of glaucoma, exhibit anticancer and antiangiogenic properties.
Phytocannabinoids
Phytocannabinoids are well-known natural products that are extracted and isolated from the Cannabis sativa L. plant (commonly known as marijuana). Marijuana has long been known for its medicinal and recreational properties. In 1964, Raphael Mechoulam and coworkers isolated one of the most active compounds from Cannabis sativa L., Δ9-tethrahydrocannabinol (Δ9-THC) [2]. They were the first to chemically synthesize this compound, which made possible further biological studies that confirmed Δ9-THC as its major active compound [7]. Since then, 60 unique phytocannabinoids, such as cannabidiol (CBD), cannabinol (CBN), and cannibigerol (CBG), together with endogenous cannabinoids, such as anan-damide (arachidonoylethanolamine or AEA) [8], and 2-Arachido-noylglycerol (2-AG) [9], have been identified by the Mechoulam research group. These seminal studies provided the first complete characterization of the endocannabinoid system (ECS).
Phytocannabinoids are physiologically produced in plant cell substructures called trichomes in the form of resin. From a biochemical point of view, all subclasses of phytocannabinoids originate from cannabigerol-type (CBG) molecules, according to the diverse cyclization products of this precursor Fig. (1). Two possible numberings of the C21 phytocannabinoids have been proposed, depending on either the dibenzopyran or monoterpenoid scaffold. In the present work, we refer to dibenzopyran numbering Fig. (2).
Fig. (1).
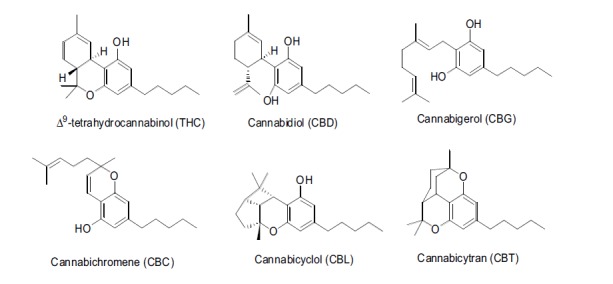
The chemical structures of different classes of phytocannabinoids.
Fig. (2).
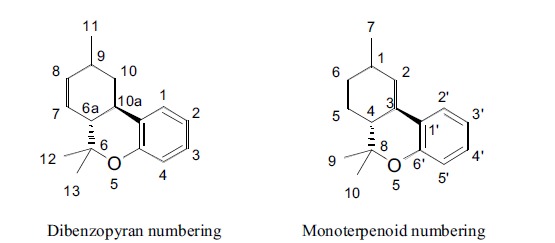
Different numberings of the phytocannabinoid scaffold.
Dibenzopyran numbering Monoterpenoid numbering
Some progenitors of the main subclasses of pytocannabinoids derived from condensation of olivetolic acid and geranyl pyrophosphate are illustrated in Fig. (3).
Fig. (3).
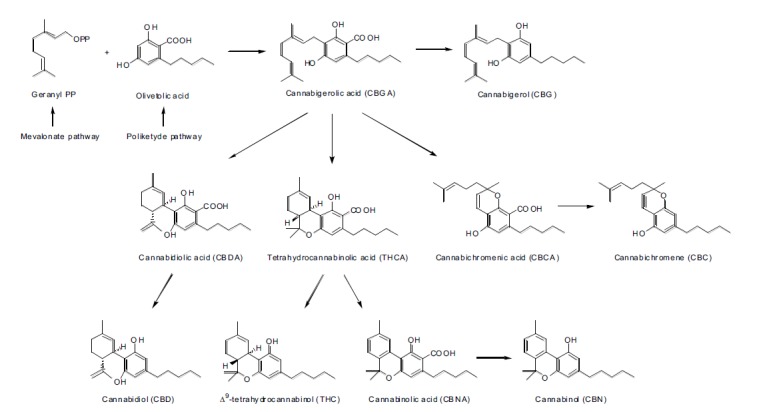
Biosynthetic pathways for some cannabinoids.
Other classes of pythocannabinoids are derived from the condensation of geranyl pyrophosphate (GPP) with divarinolic acid instead of olivetolic acid. In the structure of the latter, propyl side chains replace the pentyl hydrocarbon residue. These chains include tethrahydrocannabivarin (THCV), cannabidivarin (CBDV), cannabivarin (CBV), cannabichromevarin (CBCV), and cannabigerovarin (CBGV) [10, 11].
Δ9-THC
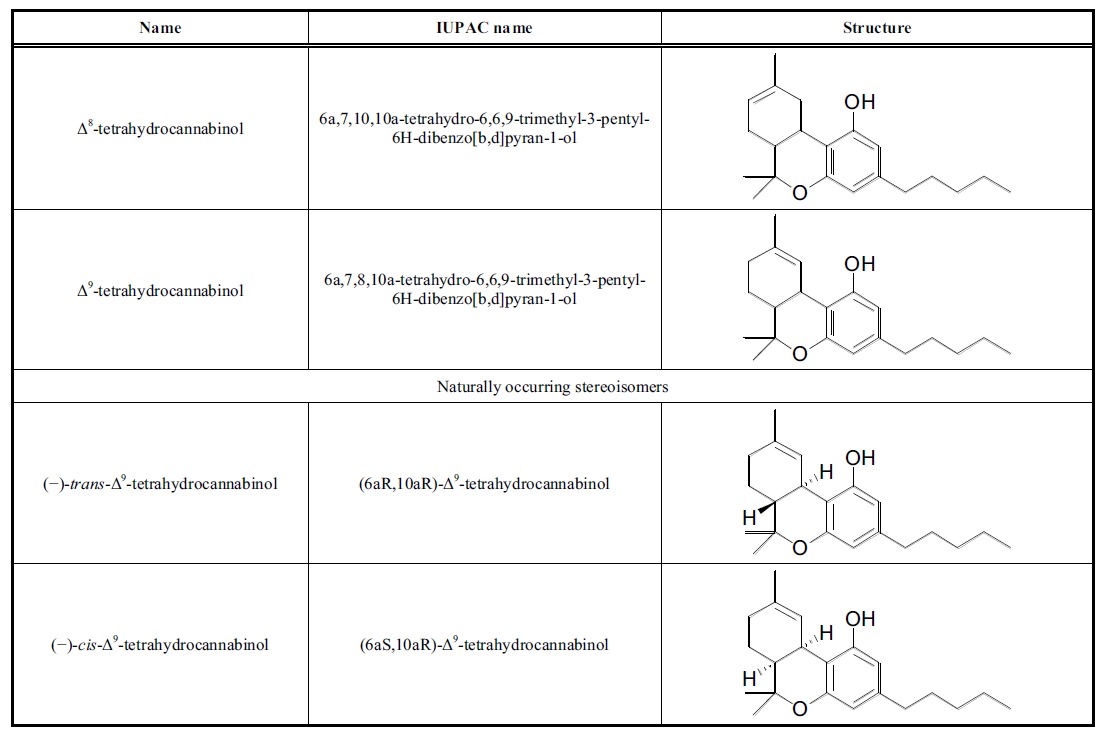
The compound (6aR,10aR)-delta-9-tetrahydrocannabinol is the main psychoactive compound of Cannabis sativa L. and is characterized by a relatively simple structure. Several of this isomer’s compounds are known, which vary in their double-bond positions (in nature, only Δ9 or Δ8 species are found), cis or trans ring junctions, and absolute stereochemical properties.
Four stereoisomers of THC are possible, but only the levorotatory ones occur in nature (Table 1). Accordingly, naturally occurring stereoisomers are energetically favored, due to the trans configuration being less strained than the cis configuration. Because of its double-bond position, Δ9-THC is thermodynamically less stable than Δ8-THC, as indicated by the easy Δ9 – Δ8 conversion under acid treatment. Several efforts have been made to synthesize Δ9-THC, the main isomer found in Cannabis sativa L. In 1967, Mechoulam et al. reported such synthesis from Verbenol and Olivetol using acid catalysis [12]; thereafter, many other synthethic procedures were published, including those that started with different natural products such as crysanthenol, p-menthadienol, p- menthenediol, and carene oxide, resulting in several methods for total synthesis [13-16]. Δ9-THC is currently commercialized with the INN of dronabinol (brand name Marinol) for medical uses, such as stimulating the appetite to prevent anorexia in patients with AIDS and as an antiemetic in patients resistant to conventional antiemetic treatment for cancer chemotherapy. Δ9-THC is an aromatic terpenoid with very low solubility in water but good solubility in polar organic solvents; as such, it is normally accumulated in fatty tissues after prolonged administration.
Cannabinol (CBN)
Cannabinol is a weak psychoactive cannabinoid isolated from Cannabis sativa L. and Cannabis indica [17]. It is structurally related to THC, from which it is obtained biosynthetically by oxidation. CBN differs from THC in that it possesses neither double-bonded isomers nor stereoisomers. It acts mainly as a weak CB1 receptor agonist and possesses a higher affinity than THC for CB2 receptors. It is also the main metabolite of Δ9-THC [18].
Cannabidiol (CBD)
Cannabidiol is one of the most abundant phytocannabinoids isolated from Cannabis sativa L. (up to 40% of the extract) [19]. The biosynthesis of CBD starts from a common precursor of THC — CBG, which undergoes a C–C one ring cyclization catalyzed by cannabidiolic acid (CBDA) synthase — followed by decarboxylation. Multiple CBD double-bonded isomers and their corresponding stereoisomers are possible, but only one isomer is found in nature, the one with a double-bond in the same position as Δ9-THC. Cannabidiol is poorly soluble in water but has good solubility in polar organic solvents. In the presence of a strong base and oxygen, it is oxidized to quinone [20]. It can be converted into THC by acid catalyzed cyclization [21]. The synthesis of CBD has been accomplished by several research groups [13, 22, 23]. In contrast with THC, CBD does not exhibit psychomimetic activities and is capable of antagonizing these effects. For this reason, CBD has aroused increased research interest in its pharmacological properties. Several studies show CBD to have anti-inflammatory, anticonvulsant, antioxidant, antiemetic, anxiolytic, and antipsychotic properties; thus, it may serve as potential drug for the treatment of neuro-inflammation, epilepsy, oxidative injury, vomiting and nausea, and anxiety and schizophrenia, respectively [19]. CBD is commercialized in a 1:1 combination with THC under the trade name Sativex for the relief of neuropathic pain in multiple sclerosis. GW pharmaceuticals received an Orphan Drug Designation from the Food & Drug Administration (FDA) for a purified liquid extract of CBD (brand name Epidiolex) in the treatment of Dravet syndrome. The drug is currently in phases II and III of clinical trials for Dravet syndrome, common epilepsy, schizophrenia, and other rare syndromes. Recently, new evidence of other therapeutic activities of CBD has been found in the treatment of cancer and neurodegenerative disorders [24, 25].
Endocannabinoids
Endogenous cannabinoids, or endocannabinoids, are capable of binding to CB receptors; they are lipid-like structures corresponding to those depicted in Fig. (4). Endocannabinoids are obtained from arachidonic acid, a common precursor in the biosynthesis of prostaglandins, which are key regulators of inflammatory processes. These compounds are derived from the non-oxidative metabolism of arachidonic acid by formal condensation with ethanolamine or glycerol, starting from the precursors eicosanoic phospholipids and diacylglycerols, respectively. They are found primarily in the brain but are present in almost all human tissues [26]. Thus, endocannabinoids play a biological role in inflammation, insulin sensitivity, and fat and energy metabolism. In turn, endocannabinoid inhibitors may be precious tools for reducing the prevalence of metabolic syndrome. Furthermore, the modulation of the endocannabinoid system may display therapeutic potential for diverse chronic neurologic and immune conditions. Anandamide (N-arachidonoyl-ethanolamine) (AEA) and 2-arachidonoylglycerol (2-AG) are by far the most extensively investigated endocannabinoids.
Fig. (4).
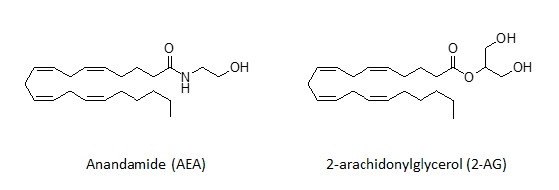
The most common endogenous cannabinoids.
Synthetic Cannabinoids
Since the discovery of natural cannabinoids, novel synthetic analogues with similar structures have been prepared by different research groups, among which, Mechoulam’s and Huffman’s ones are notable examples. Some synthetic THC analogues, such as HU-210 show activity that is 100-fold higher than THC [27]. The pharmacological studies on these artificial cannabinoids have elucidated structure–activity relationships (SARs) and ECS metabolism [28]. Synthetic cannabinoids can be structurally related to THC or can have different scaffolds that do and do not contain heterocycle rings. Most synthetic cannabinoids are lipid-soluble and apolar, consisting of 22–26 carbon atoms. Optimal activity requires more than four and up to nine saturated carbon atoms. Big efforts were made by John W. Huffman et al., who synthesized more than 450 artificial cannabinoids. Some of these compounds, which are known be their acronyms WIN55212-2, JWH-018, JWH-073, JWH-133, and SR141716 (Rimonabant), were shown to be highly bioactive; this discovery paved the way for several pharmaceutical applications as well as illegal use in smoking mixtures called “spice” or “K2.” Synthetic cannabinoids can be classified into seven major structural groups:
Naphthoylindoles (e.g., JWH-018, JWH-073 and JWH-398);
Naphthylmethylindoles;
Naphthoylpyrroles;
Naphthylmethylindenes;
Phenylacetylindoles (i.e., benzoylindoles, e.g., JWH-250);
Cyclohexylphenols (e.g., CP 47,497 and homologues of CP 47,497);
Classical cannabinoids (e.g., HU-210 and JWH-133).
All of these products normally act as agonists or inverse agonists of CB1 and CB2 receptors, and may show alternate selectivity towards one of the receptor types. Currently, some of these compounds (WIN55212-2, JWH-133, and HU-210) are under investigation as potential treatments for Alzheimer’s disease, because they show evidence of counteracting the inflammation caused by amyloid beta proteins [29].
JWH-133
JWH-133 is chemically related to THC and is a potent CB2 selective agonist. This compound was synthesized by John W. Huffman’s research group [28]. Its properties against Alzheimer’s disease and cancer have also been recently investigated [29, 30].
WIN55,212-2
WIN55,212-2 is a naphthoylindole derivative (JWH analogue) possessing THC-like potent, non-selective, CB receptor agonist properties. It has potential therapeutic use as an analgesic and against Alzheimer’s disease [29, 31].
SR141716
Also known as Rimonabant (brand name Acomplia by Sanofi-Aventis), SR141716 is an inverse selective agonist of the CB1 receptor [32]. It is a pyrazole derivative and is remarkably easy to synthesize [33]. SR141716 was approved as an anorectic in Europe for the treatment of obesity, given its efficacy in decreasing appetite. The FDA, however, refused its approval as anti-obesity drug because of the severe depression reported as a side effect.
CANNABINOIDS AND CANCER
The cannabinoid receptors CB1 and CB2 comprise the endocannabinoid system (ECS) inside a cell, and the activation of these receptors is important for a number of physiological processes that regulate nervous, digestive, reproductive, immune, and metabolic functions [34]. There is growing body of evidence that the ECS system and synthetic cannabinoids modulate the activity of enzymes and nuclear factors involved in cancer cell homeostasis, growth, migration, metastasis, and tumor angiogenesis [1, 35-38]. Remarkably, cannabinoids target cancer cells, while non-tumor cells and tissues are avoided. This apparent selectivity for tumor cells makes the ECS system an attractive potential target for cancer therapy. In 1975, the natural cannabinoid THC was recognized as a potential anticancer agent [39], inhibiting lung adenocarcinoma cell growth in vitro and in vivo. Since that time, the therapeutic potential of cannabinoid for various types of cancer have been widely investigated [35]. Today, capsules of THC and its synthetic analogues have been approved for treating nausea and emesis associated with cancer chemotherapy [5].
In 2006, the first human clinical study of THC was successfully performed in patients with recurrent glioblastoma multiforme [40]. The potential palliative effects of cannabinoids as appetite stimulators and pain inhibitors were also confirmed in phase III clinical trials in oncology [5, 41]. Now, several plant-derived, synthetic, and endogenous cannabinoids that exert an antiproliferation effect on various cancers are widely recognized [Table 2]. Cannabinoid administration has been proven to slow tumor growth, as well as to slow lung carcinoma, prostate cancer, glioma, and skin carcinomas in mice [39, 42-46]. Importantly, all of these studies found CB1 receptors, CB2 receptors, or both, which was later confirmed by molecular and pharmacological approaches that examined cannabinoid-receptor expression and used selective cannabinoid receptor agonists (JWH-133 and WIN 55,212-2) and antagonists (such as AM-630 and SR-141716A).
The binding of cannabinoids to their G-protein-coupled cannabinoid receptors affects various cellular pathways, for example, it inhibits adenylate cyclase and activates extracellular-signal-regulated kinase (ERK). This suggests that cannabinoids might also be involved in other pathways important for cell survival, such as MAPK [47]. The activation of MAPK by cannabinoids has been observed in human vascular endothelial cells, neural cell lines, and Chinese hamster ovary cells [5, 48, 49]. Both of these kinases (MAPK and ERK) are stimulated through G-protein-mediated mechanisms [50, 51]. Cannabinoid receptors are also coupled to activation of serine/threonine protein kinase B/Akt (PKB), resulting in increased phosphorylation of glycogen synthase kinase-3 (GSK3) on serine 21 residue [49]. The effect of cannabinoids on sphingomyelin breakdown, resulting in increased intracellular ceramide levels, has been also observed [52]. Finally, the activation of CB1 or CB2 receptors seems to induce apoptosis through de novo synthesis of ceramide in various types of cancer cells, including those associated with glioma, leukemia, colon, and pancreatic cancer [44, 53-55].
CANNABINOIDS AS ANTIANGIOGENESIS FACTORS: MOLECULAR ASPECTS OF CANNABINOIDS IN CANCER PREVENTION
The hallmark of cancer is the ability to proliferate despite the absence of mitogenic signals. Rapid proliferation of cancer cells requires large amounts of energy and oxygen; therefore, hypoxic cells secrete proangiogenic factors (e.g., HIF-1a, VEGF, PIGF, HGF, and Ang-2 [90-92] to induce the formation of new blood vessels to meet their need of increased oxygen. Thus, angiogenesis is one of the most important mechanisms through which solid tumors grow rapidly. Currently, more attention is being paid to the mechanism of angiogenesis, mostly due to its correlation with cancer metastasis, and targeting antiangiogenic molecules has become the most promising therapeutic approach to cancer treatment.
The range of known antiangiogenic factors is still growing; however, most treatment strategies are based on blocking signaling from vascular endothelial grow factors (VEGFs), and especially, using blocking monoclonal antibodies for VEGFR1 and VEGFR2, such as Sunitinib or Bewacizumab [93-95]. Other treatment strategies target various steps in angiogenesis or degrade newly formed blood vessels. However, most of these strategies fail due to the coexistence of strong proangiogenic factors such as VEGFs and EGFs.
Cannabinoids have been proven to inhibit angiogenesis in in vivo and in vitro studies [38]. Blazquez et al. observed that the local administration of JWH-133 altered the blood vessel morphology in C6 glioma and grade IV astrocytoma in rats [38]. All tumors treated with JWH-133 had a paler overall appearance and only very small and narrow capillaries forming differentiated and impermeable vascular networks compared to the controls. Moreover, the authors showed that cannabinoid administration decreased vascular endothelial cell migration, survival, and expression of major proangiogenic factors such as VEGF, Ang-2, and MMP2 proteins [38].
Analysis of microarray data showed that JWH-133 administration in C6 glioma-bearing mice induced altered expression of 10 genes directly or indirectly related to the VEGF pathway. The cannabinoid treatment decreased the expression of VEGF itself, HIF-1a, heme oxygenase-1, Id3, midkine, angiopoietin-2, and Tie-1 [96]. Cannabinoids WIN-55,212-2 and JWH-133 also decreased the expression of phosphorylated VEGFR-2 in the tumors of two glioma patients. The sphingolipid messenger ceramide might be responsible for cannabinoid-induced inhibition of VEGF and VEGFR2 protein expression. The same compounds were also efficient in the treatment of non-melanoma skin cancer by decreasing new blood vessel formation [46, 97].
Matrix metalloproteinases (MMPs) are other possible molecular targets for cannabinoid treatment [98]. MMPs belong to the family of enzymes that participate in the degradation of the vascular basement membrane and remodeling of the extracellular matrix (ECM) [99]. This process sends one of the signals required for endothelial cells to migrate and form new blood vessels. MMPs have been linked to tumor invasion and their increased expression is found in almost every type of cancer [100, 101]. Blazquez et al. investigated the role of cannabinoid agonists (THC, JWH-133, and anadamide) on MMP-2 expression inside glioma cell cultures and glioma-bearing mice [98], and found that THC administration decreased tumor growth and the expression of MMP-2 in C6.9 glioma cells. The MMP-2 level also decreased in biopsies obtained from patients with recurrent glioblastoma multiform after local administration of THC. However, the study also demonstrated the selective nature of THC because the level of other MMP protein family members (MMP-3, MMP-9, and MMP-14) remained unchanged Fig. (5) [98].
Fig. (5).
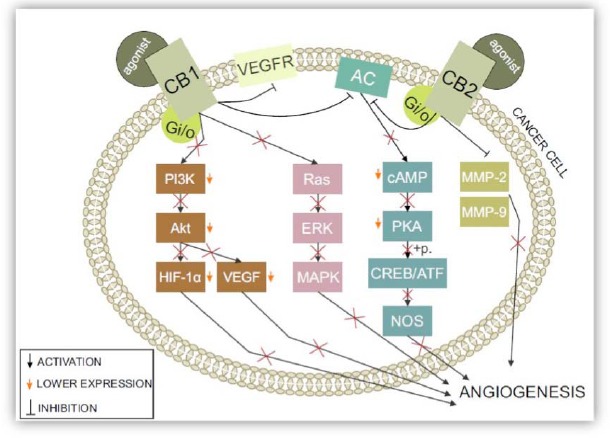
The influence of CB1 and CB2 activity on angiogenesis in cancer: After binding to a specific agonist, both CB1 and CB2 receptors are activated. This leads to direct and indirect changes that have crucial effects on angiogenesis in a cancerous environment. Inactivation of the PI3K/Akt pathway leads to lower HIF-1 and VEGF levels — two of the most known proangiogenic molecules. CB1 receptor activation leads to the direct inhibition of VEGF type 2 receptors, which are major angiogenesis inducers. When activated, CB1 and CB2 receptors strictly inhibit adenylyl cyclase (AC). PKAs, whose activity is strictly dependent on the cellular levels of cAMP, are inactivated and unable to phosphorylate CREB/ATF, decreasing nitric oxide synthase (NOS). Ras inactivation leads to inactivation of the whole pathway, including ERK and MAPK. Metalloproteinases production is stopped by the activation of both cannabinoid type 1 and type 2 receptors. Thus, all of these events have a direct impact on angiogenesis as well as the cell cycle and proliferation.
CANNABINOIDS AND THE TUMOR MICROENVIRONMENT
Interactions between the various cell types observed within a tumor mass influence its development, progression, and metastasis. The composition of the microenvironment and roles of particular cells vary, depending on the tumor type and organ of origin. These cells contribute to tumor development by secreting various pro-tumor factors or by inducing immunosuppression. One of the most important cell types to facilitate tumor development is tumor-associated macrophages (TAMs) [102-104]. TAMs are recruited into a tumor mass by various cytokines and chemokines secreted by cancer cells. Once they migrate to the tumor, they start to secrete various proangiogenic factors or enzymes that facilitate matrix remodeling. On the other hand, cancer cells influenced by macrophages start to express genes and antigens that are atypical (but specific to macrophages, such as chemokines CCL5 and CSF-1 and their receptors CXCR5 and CSF-1R) [105, 106]. Indeed, chemoattractants secreted by cancer cells that increase the migratory abilities of immune cells and their infiltration to a tumor mass can also enhance cancer cell migration in an autocrine manner. Due to the involvement of TAMs in cancer development, inhibition of their migration into a tumor mass has been proposed as one therapeutic approach [102].
There are no published papers about the influence of cannabinoids on the tumor microenvironment and TAMs. However, the expression level of CB2 receptors is considerably upregulated in activated macrophages [107]. CB2 receptors control several functions of macrophages, such as actin polymerization, migration, cell phenotype, and cytokine release from these cells [107]. Cannabinoid ligands share receptors with important chemotactic cytokines that direct the migration of immune cells. These receptors are coupled to heterotrimeric Gi proteins [108]. Ghosh et al. reported that the CB1/CB2 agonist CP55940, and CB2-selective agonist JW-015, caused significant inhibition of chemokine CXCL12-induced chemotaxis of T lymphocytes [109]. Raborn et al. demonstrated that THC and cannabinoids that activate the CB2 inhibit macrophage chemotaxis to CCL5 [108]. Because CCL5 is a very important ligand for G protein-coupled receptors CCR1 and CCR5, activation of the CB2 leads to trans-deactivation of these receptors, which play specialized roles in leukocyte trafficking [110-112].
These results indicate that cannabinoids and chemokines may constitute integrative components of a network of intercommunicating G protein-coupled receptors that not only regulate the immune responses and chemokine/cytokine-regulated chemotaxis of leukocytes but also the migration of cancer cells. Additionally,cannabinoids, as highly lipophilic molecules, can perturb cellular membranes and alter ligand–receptor interaction, disrupt receptor-G protein complexes, and alter the cascade of signal transduction, thus decreasing the migratory abilities of cells [113-115].
The cannabinoid-based inhibition of immune cell migration to a tumor mass (in particular macrophages), or the alteration of chemokine/cytokine signaling in cancer cells, may constitute a beneficial approach in anticancer therapy. Therefore, the regulatory function of cannabinoids on the tumor microenvironment needs further investigation.
CANNABINOIDS IN ANTICANCER THERAPY FOR RENAL CELL CARCINOMA
Renal cell carcinoma (RCC) represents a serious problem in oncology — it is the cause of over 100,000 deaths each year. RCC is the most common type of renal cell tumor, consisting of a substantial number of malignant cancers and benign tumors. This type of cancer has a higher risk of appearance in males [116]. The treatment of choice for renal cancers are nephrectomy combined with immunotherapy [117], due to the high costs of other therapies or late detection. However, novel drugs give hope to cancer patients — for example, therapies concerning the tyrosine kinase receptor inhibitor or multipurpose drugs targeting not only cancer cells but also stimulating the immune response. However, there is no perfect therapy for eliminating tumor cells. Investigation of the anticancer properties of natural compounds, such as cannabinoids, have brought interesting results.
Medical cannabis still remains a prescribed medication in some of therapeutic protocols and Cannabis sativa L. has started to become one of the most commonly used additional medications to treat nausea (caused, for example, by chemotherapy), stimulate the appetite, and deal with chronic pain [5, 118].
Cannabinoids also have an important influence on cancer cells themselves, what was mentioned before in this manuscript. There are many ways they inhibit tumor proliferation and survival. Fig. (6) illustrates a couple of ways CB1 and CB2 receptors are integrated into intracellular pathways. However, all of mechanisms are not identical in every cell and do not occur simultaneously.
Fig. (6).
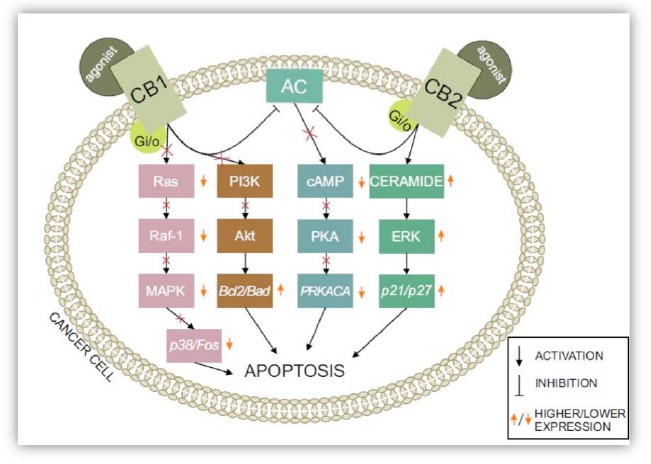
ECS system-related pathways: The activation of CB1/CB2 receptors has an immense effect on the fate of cells, leading them to apoptosis. Adenylyl cyclase is inhibited by the activation of both cannabinoid type 1 and type 2 receptors. Inhibiting various pathways — including the PI3K/Akt pathway and cAMP-dependent PKA and Ras phosphorylation — results in stimulating cell death through numerous secreted molecules, such as Bad, Bcl2, and Fos. Secreting apoptotic proteins, such as p27 and p21 via ceramide synthesis, speeds up the process of apoptosis.
In the case of RCC, research is still lacking on cannabinoids as anticancer compounds. However, the expression of cannabinoid receptors (CB1, CB2, TRPV1, and GPR55) in kidney cells and RCC has been confirmed [119]. The expression of cannabinoid receptors CB1 and CB2 was first confirmed in the human proximal tubule (HK2) cell line, and also lysate from rat kidney [145]. Larrinaga et al. investigated the expression of cannabinoid receptors in human fetal and adult kidneys [120], finding that CB1 (CNR1a) mRNA was present in all samples; however, CB2 (CNR2) mRNA was undetectable in all samples. Further studies are needed to help clarify these differences and the role of ECS in renal function.
Renal cell carcinoma (RCC) is the most common epithelial malignancy of the kidney in adults, accounting for 3% of all neoplasms [121]. Considering the specific cannabinoid receptors in kidneys, as well as the pro-apoptotic, antiproliferative, anti-metas-tatic and antiangiogenic effects of cannabinoids, this field should be explored. So far, there are only a few published papers about the ECS in RCC.
Some results also suggest that patterns of cannabinoid receptor expression could be used in differential diagnosis of kidney cancers. Chromophobe RCC (ChRCC) and renal oncocytoma (RO) are two histologically distinct neoplastic entities of renal tumors. These tumors originate from distinct sites in the kidney and show different molecular abnormalities. In ChRCC samples, the mRNA expression for CB1 receptors was 12-fold lower than in the surrounding normal tissue [86]. However, RO samples showed no difference in the level of CB1 mRNA expression between the tumor and surrounding tissue. The mRNA expression for CB2 receptor was undetectable in both ChRCC and RO. Interestingly, protein expression analysis of these RCC neoplasms failed to provide any evidence for the expression of CB1 [36, 86]. Thus, the CB1 protein may undergo modifications in these tumors at the post-transcriptional level. Therefore, further studies are required to understand the role of cannabinoids and their receptors in RCC.
CONCLUSION
More research is needed to answer all questions about the potential use of cannabinoids to treat cancer. The first question is which type of cannabinoids should be used to treat cancer patients — natural or synthetic? Different cannabinoids seem to have different effects on various cancer types, which may be caused by the different expression of cannabinoid receptors in various cancer types. Similarly, no studies have shown the effects of cannabinoids on the efficacy of chemotherapy. The psychoactive effect of cannabinoids should also be considered before its routine use.
Table 1.
Stereoisomers of Δ9 THC.
|
Table 2.
The cannabinoid modulation of different cancers and pathways.
| Cancer Type | Signaling Pathways Involved | Effects | Experimental System | Receptors Involved | Cannabinoids Tested | References |
|---|---|---|---|---|---|---|
| Glioma | ERK, MAPK, cAMP, LOX, PI3K/Akt/mTOR |
Decrease tumor volume and apoptosis |
In vitro In vivo (rat, mouse) |
CB1, CB2 | THC, WIN55,212-2, JWH-133, AM-1241, CBD, SR144528, SR141716 |
[44, 56-62] |
| Breast | NF-κB, PA-1, cAMP, PKA, MAPK, S1P/ceramide, CXCR4/CXCL12 |
Cell cycle arrest, Apoptosis and inhibit tumor growth |
In vitro In vivo (mouse) |
CB1, CB2 | AEA, THC, CBD, WIN55,212-2, JWH-015, SR 141716A |
[63-68] |
| Lung | MAPKs, PI3K/Akt/mTOR, JNK, ERK | Decrease tumor growth, invade cancer cells, and induce apoptosis |
In vitro In vivo (mouse) |
CB1, CB2 | THC, CBN, CBD, AM-251, AM-630 |
[39, 69-72] |
| Leukemia | cAMP, ECS | Decrease tumor growth, apoptosis |
In vitro In vivo (mouse) |
CB2 | THC, JWH-015, SR144528 | [73-75] |
| Melanoma | COX-2, LOX, Akt | Apoptosis, inhibit cancer cell growth |
In vitro In vivo (mouse) |
CB1, CB2 | JWH-133, WIN55,212-2, AM251, AEA, AM251, SR141716, SR144528 | [46, 76, 77] |
| Prostate | NF-κB/cyclin D/cyclin E, JNK, Akt | Arrest cell cycle, apoptosis, and decrease tumor volume |
In vitro In vivo (mouse) |
CB1, CB2 | AEA, JWH-015, WIN55,212-2, SR 144528, SR141716A |
[37, 78-81] |
| Thyroid | cAMP, ERK, MAPK | Decrease tumor volume, apoptosis |
In vitro In vivo (mouse) |
CB1, CB2 | AEA, JWH133, SR141716A |
[82-84] |
| Pancreatic | (Ca2+) Calcium signaling, p8-ATF-4-TRB3 | Inhibit cancer cells, reduce tumor growth |
In vitro (mouse) |
CB1, CB2 | ACPA, GW, WIN 55,212-2, SR141716, SR144528 |
[55, 85] |
| Kidney | ? | ? | In vitro | CB1? CB2? | ? | [36, 86] |
| Ovary | ? | ? |
In vivo (mouse) |
CB1, CB2 | ? | [87] |
| Bladder | (Ca2+) Calcium signaling | Decrease cancer cell proliferation by delaying the cell cycle progression | In vitro | CB1, CB2 | 2-AG, AEA, AM-281, CP55,940 | [88, 89] |
ACKNOWLEDGMENTS
This work was supported by the National Science Centre (NCN, Poland) under the grant name PRELUDIUM, no. UMO-2013/09/N/NZ5/02809. This work is a result of collaboration within COST Action CM1407 Challenging organic syntheses inspired by nature: from natural products chemistry to drug discovery, www.natchemdrugs.eu. The authors acknowledge the support of the Scribendi, Inc. for professional editing and proofreading of this manuscript.
List of abbreviations
- 2-AG
2-arachidonoylglycerol,
- ACPA
arachidonylcyclopropylamide
- AM-630
6-Iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl)methanone
- Ang2
angiopoietin-2
- cAMP
cyclic adenosine monophosphate
- CB1
Cannabinoid receptor type 1
- CB2
Cannabinoid receptor type 2
- CBD
Cannabidiol
- CBDA
Cannabidiolic-acid
- CBN
Cannabinol
- CCL5
CC chemokine ligand-5
- CCR1
CC chemokine receptor 1
- CCR5
CC chemokine receptor 5
- ChRCC
Chromophobe RCC
- CP55,940
(-)-cis-3-[2-Hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol
- CSF-1
colony stimulating factor-1
- CXCR5
C-X-C chemokine receptor type-5
- ECM
extracellular matrix
- ECS
endocannabinoid system
- ERK
extracellular-signal-regulated kinase
- GSK3
glycogen synthase kinase-3
- GW
1-(2,3-dichlorobenzoyl)-2-methyl-3-(2-(1-morpholine)ethyl)-5-methoxyindole
- HGF
hepatocyte growth factor
- HIF-α
hypoxia-inducible factor-1 alpha
- JNK
c-Jun-NH2-kinase
- JWH-133
(6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetrahydro-6,6,9-trimethyl-6H-dibenzo[b,d]pyran
- MAPKs
mitogen-activated protein kinases
- MMPs
matrix metalloproteinases
- NOS
nitric oxide synthase
- PIGF
placenta growth factor
- PKB
serine/threonine protein kinase B/Akt
- RCC
renal cell carcinoma
- RO
renal oncocytoma
- SAR
Structure-activity relationships
- SR141716
5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-1-piperidinyl-1H-pyrazole-3-carboxamide
- TAM
tumor-associated macrophage
- THC
Δ9tetrahydrocannabinol
- TNF-α
tumor necrosis factor alpha
- VEGF
vascular endothelial growth factor
- VEGFR1
vascular endothelial growth factor receptor 1
- VEGFR2
vascular endothelial growth factor receptor 2
- WIN-55,212-2
(R)-(+)-[2,3-Dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate
CONFLICT OF INTEREST
The authors indicate no potential conflicts of interest.
REFERENCES
- 1.Guindon J., Hohmann A.G. The endocannabinoid system and cancer: therapeutic implication. Br. J. Pharmacol. 2011;163(7):1447–1463. doi: 10.1111/j.1476-5381.2011.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaoni Y., Mechoulam R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964;86(8):1646. [Google Scholar]
- 3.Elsohly M.A., Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005;78(5):539–548. doi: 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Matsuda L.A., Lolait S.J., Brownstein M.J., Young A.C., Bonner T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 5.Guzman M. Cannabinoids: potential anticancer agents. Nat. Rev. Cancer. 2003;3(10):745–755. doi: 10.1038/nrc1188. [DOI] [PubMed] [Google Scholar]
- 6.Glass M., Northup J.K. Agonist selective regulation of G proteins by cannabinoid CB(1) and CB(2) receptors. Mol. Pharmacol. 1999;56(6):1362–1369. doi: 10.1124/mol.56.6.1362. [DOI] [PubMed] [Google Scholar]
- 7.Mechoulam R. Marihuana chemistry. Science. 1970;168(3936):1159–1166. doi: 10.1126/science.168.3936.1159. [DOI] [PubMed] [Google Scholar]
- 8.Devane W.A., Hanus L., Breuer A., et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 9.Mechoulam R., Ben-Shabat S., Hanus L., et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995;50(1):83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 10.Fellermeier M., Eisenreich W., Bacher A., Zenk M.H. Biosynthesis of cannabinoids. Incorporation experiments with (13)C-labeled glucoses. Eur J Biochem FEBS. 2001;268(6):1596–1604. doi: 10.1046/j.1432-1033.2001.02030.x. [DOI] [PubMed] [Google Scholar]
- 11.Dewick P.M. 2009. [Google Scholar]
- 12.Mechoulam R., Braun P., Gaoni Y. A stereospecific synthesis of (-)-delta 1- and (-)-delta 1(6)-tetrahydrocannabinols. J. Am. Chem. Soc. 1967;89(17):4552–4554. doi: 10.1021/ja00993a072. [DOI] [PubMed] [Google Scholar]
- 13.Petrzilk T., Haeflige W., Sikemeie C. Synthesis of Hashish Constituents. 4. Helv. Chim. Acta. 1969;52(4):1102. [Google Scholar]
- 14.Razdn R.K., Puttick A.J., Zitko B.A., Handrick G.R. Hashish VI 1: conversion of (-)- 1(6) -tetrahydrocannabinol to (-)- 1(7) -tetrahydrocannabinol. Stability of (-)- 1 - and (-)- 1(6) -tetrahydrocannabinols. Experientia. 1972;28(2):121–122. doi: 10.1007/BF01935704. [DOI] [PubMed] [Google Scholar]
- 15.Razdan R.K., Handrick G.R. Hashish - a Stereospecific Synthesis of (-)-Delta-1- and (-)-Delta-1(6)-Tetrahydrocannabinols. J. Am. Chem. Soc. 1970;92(20):6061. doi: 10.1021/ja00723a044. [DOI] [PubMed] [Google Scholar]
- 16.Razdan R.K. The Total Synthesis of Cannabinoids. In: Apsimon J., editor. Total Synthesis of Natural Products. 4th ed. USA: John Wiley & Sons, Inc.; 1981. [Google Scholar]
- 17.Karniol I.G., Shirakawa I., Takahashi R.N., Knobel E., Musty R.E. Effects of delta9-tetrahydrocannabinol and cannabinol in man. Pharmacology. 1975;13(6):502–512. doi: 10.1159/000136944. [DOI] [PubMed] [Google Scholar]
- 18.McCallum N.D., Yagen B., Levy S., Mechoulam R. Cannabinol: a rapidly formed metabolite of delta-1- and delta-6-tetrahydrocannabinol. Experientia. 1975;31(5):520–521. doi: 10.1007/BF01932433. [DOI] [PubMed] [Google Scholar]
- 19.Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimaraes FS. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. 1607. [DOI] [PMC free article] [PubMed]
- 20.Mechoulam R., Ben-Zvi Z., Gaoni Y. Hashish--13. On the nature of the Beam test. Tetrahedron. 1968;24(16):5615–5624. doi: 10.1016/0040-4020(68)88159-1. [DOI] [PubMed] [Google Scholar]
- 21.Gaoni Y. Mechoula.R. Hashish. 7. Isomerization of cannabidiol to tetrahydrocannabinols. Tetrahedron. 1966;22(4):1481. [Google Scholar]
- 22.Baek S.H., Srebnik M., Mechoulam R. Boron-Trifluoride etherate on alimina - a modified lewis acid reagent - an improved synthesis of cannabidiol. Tetrahedron Lett. 1985;26(8):1083–1086. [Google Scholar]
- 23.Kobayashi Y., Takeuchi A., Wang Y.G. Synthesis of cannabidiols via alkenylation of cyclohexenyl monoacetate. Org. Lett. 2006;8(13):2699–2702. doi: 10.1021/ol060692h. [DOI] [PubMed] [Google Scholar]
- 24.Massi P., Solinas M., Cinquina V., Parolaro D. Cannabidiol as potential anticancer drug. Br. J. Clin. Pharmacol. 2013;75(2):303–312. doi: 10.1111/j.1365-2125.2012.04298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez-Ruiz J., Sagredo O., Pazos M.R., et al. Cannabidiol for neurodegenerative disorders: important new clinical applications for this phytocannabinoid? Br. J. Clin. Pharmacol. 2013;75(2):323–333. doi: 10.1111/j.1365-2125.2012.04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reisenberg M, Singh PK, Williams G, Doherty P. The diacylglycerol lipases: structure, regulation and roles in and beyond endocannabinoid signalling. 1607. [DOI] [PMC free article] [PubMed]
- 27.Devane W.A., Breuer A., Sheskin T., Jarbe T.U., Eisen M.S., Mechoulam R. A novel probe for the cannabinoid receptor. J. Med. Chem. 1992;35(11):2065–2069. doi: 10.1021/jm00089a018. [DOI] [PubMed] [Google Scholar]
- 28.Huffman J.W. Cannabimimetic indoles, pyrroles, and indenes: Structure-activity relationships and receptor interactions. In: Reggio P.H., editor. The Cannabionoid Receptors. New York: Humana Press; 2009. [Google Scholar]
- 29.Gonzalez-Naranjo P., Campillo N.E., Perez C., Paez J.A. Multitarget cannabinoids as novel strategy for Alzheimer disease. Curr. Alzheimer Res. 2013;10(3):229–239. doi: 10.2174/1567205011310030002. [DOI] [PubMed] [Google Scholar]
- 30.Vidinsky B., Gal P., Pilatova M., et al. Anti-proliferative and anti-angiogenic effects of CB2R agonist (JWH-133) in non-small lung cancer cells (A549) and human umbilical vein endothelial cells: an in vitro investigation. Folia Biol. 2012;58(2):75–80. [PubMed] [Google Scholar]
- 31.Meng I.D., Manning B.H., Martin W.J., Fields H.L. An analgesia circuit activated by cannabinoids. Nature. 1998;395(6700):381–383. doi: 10.1038/26481. [DOI] [PubMed] [Google Scholar]
- 32.Fong T.M., Heymsfield S.B. Cannabinoid-1 receptor inverse agonists: current understanding of mechanism of action and unanswered questions. Int. J. Obes. 2009;33(9):947–955. doi: 10.1038/ijo.2009.132. [DOI] [PubMed] [Google Scholar]
- 33.Yoshioka T., Fujita T., Kanai T., et al. Studies on hindered phenols and analogues. 1. Hypolipidemic and hypoglycemic agents with ability to inhibit lipid peroxidation. J. Med. Chem. 1989;32(2):421–428. doi: 10.1021/jm00122a022. [DOI] [PubMed] [Google Scholar]
- 34.Graham E.S., Ashton J.C., Glass M. Cannabinoid receptors: a brief history and “what's hot”. Front. Biosci. (Landmark Ed.) 2009;14:944–957. doi: 10.2741/3288. [DOI] [PubMed] [Google Scholar]
- 35.Flygare J., Sander B. The endocannabinoid system in cancer-potential therapeutic target? Semin. Cancer Biol. 2008;18(3):176–189. doi: 10.1016/j.semcancer.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Larrinaga G., Sanz B., Perez I., et al. Cannabinoid CB(1) receptor is downregulated in clear cell renal cell carcinoma. J. Histochem. Cytochem. 2010;58(12):1129–1134. doi: 10.1369/jhc.2010.957126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olea-Herrero N., Vara D., Malagarie-Cazenave S., Diaz-Laviada I. Inhibition of human tumour prostate PC-3 cell growth by cannabinoids R(+)-Methanandamide and JWH-015: involvement of CB2. Br. J. Cancer. 2009;101(6):940–950. doi: 10.1038/sj.bjc.6605248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blazquez C., Casanova M.L., Planas A., et al. Inhibition of tumor angiogenesis by cannabinoids. FASEB J. 2003;17(3):529–531. doi: 10.1096/fj.02-0795fje. [DOI] [PubMed] [Google Scholar]
- 39.Munson A.E., Harris L.S., Friedman M.A., Dewey W.L., Carchman R.A. Antineoplastic activity of cannabinoids. J. Natl. Cancer Inst. 1975;55(3):597–602. doi: 10.1093/jnci/55.3.597. [DOI] [PubMed] [Google Scholar]
- 40.Guzman M., Duarte M.J., Blazquez C., et al. A pilot clinical study of Delta9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br. J. Cancer. 2006;95(2):197–203. doi: 10.1038/sj.bjc.6603236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hall W., Christie M., Currow D. Cannabinoids and cancer: causation, remediation, and palliation. Lancet Oncol. 2005;6(1):35–42. doi: 10.1016/S1470-2045(04)01711-5. [DOI] [PubMed] [Google Scholar]
- 42.Mimeault M., Pommery N., Wattez N., Bailly C., Henichart J.P. Anti-proliferative and apoptotic effects of anandamide in human prostatic cancer cell lines: implication of epidermal growth factor receptor down-regulation and ceramide production. Prostate. 2003;56(1):1–12. doi: 10.1002/pros.10190. [DOI] [PubMed] [Google Scholar]
- 43.Ruiz L., Miguel A., Diaz-Laviada I. Delta9-tetrahydrocannabinol induces apoptosis in human prostate PC-3 cells via a receptor-independent mechanism. FEBS Lett. 1999;458(3):400–404. doi: 10.1016/s0014-5793(99)01073-x. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez C., de Ceballos M.L., Gomez del Pulgar T., et al. Inhibition of glioma growth in vivo by selective activation of the CB(2) cannabinoid receptor. Cancer Res. 2001;61(15):5784–5789. [PubMed] [Google Scholar]
- 45.Jacobsson S.O., Rongard E., Stridh M., Tiger G., Fowler C.J. Serum-dependent effects of tamoxifen and cannabinoids upon C6 glioma cell viability. Biochem. Pharmacol. 2000;60(12):1807–1813. doi: 10.1016/s0006-2952(00)00492-5. [DOI] [PubMed] [Google Scholar]
- 46.Casanova M.L., Blazquez C., Martinez-Palacio J., et al. Inhibition of skin tumor growth and angiogenesis in vivo by activation of cannabinoid receptors. J. Clin. Invest. 2003;111(1):43–50. doi: 10.1172/JCI16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guzman M., Sanchez C., Galve-Roperh I. Cannabinoids and cell fate. Pharmacol. Ther. 2002;95(2):175–184. doi: 10.1016/s0163-7258(02)00256-5. [DOI] [PubMed] [Google Scholar]
- 48.Liu J., Gao B., Mirshahi F., et al. Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem. J. 2000;346(Pt 3):835–840. [PMC free article] [PubMed] [Google Scholar]
- 49.Gomez del Pulgar T., Velasco G., Guzman M. The CB1 cannabinoid receptor is coupled to the activation of protein kinase B/Akt. Biochem. J. 2000;347(Pt 2):369–373. doi: 10.1042/0264-6021:3470369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bouaboula M., Poinot-Chazel C., Bourrie B., et al. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem. J. 1995;312(Pt 2):637–641. doi: 10.1042/bj3120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bouaboula M., Poinot-Chazel C., Marchand J., et al. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor. Involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. Eur J Biochem FEBS. 1996;237(3):704–711. doi: 10.1111/j.1432-1033.1996.0704p.x. [DOI] [PubMed] [Google Scholar]
- 52.Sanchez C., Galve-Roperh I., Rueda D., Guzman M. Involvement of sphingomyelin hydrolysis and the mitogen-activated protein kinase cascade in the Delta9-tetrahydrocannabinol-induced stimulation of glucose metabolism in primary astrocytes. Mol. Pharmacol. 1998;54(5):834–843. doi: 10.1124/mol.54.5.834. [DOI] [PubMed] [Google Scholar]
- 53.Cianchi F., Papucci L., Schiavone N., Lulli M., Magnelli L., Vinci M.C., et al. Cannabinoid receptor activation induces apoptosis through tumor necrosis factor alpha-mediated ceramide de novo synthesis in colon cancer cells. Clin. Cancer Res. 2008;14(23):7691–7700. doi: 10.1158/1078-0432.CCR-08-0799. [DOI] [PubMed] [Google Scholar]
- 54.Herrera B., Carracedo A., Diez-Zaera M., Gomez del Pulgar T., Guzman M., Velasco G. The CB2 cannabinoid receptor signals apoptosis via ceramide-dependent activation of the mitochondrial intrinsic pathway. Exp. Cell Res. 2006;312(11):2121–2131. doi: 10.1016/j.yexcr.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Carracedo A., Gironella M., Lorente M., et al. Cannabinoids induce apoptosis of pancreatic tumor cells via endoplasmic reticulum stress-related genes. Cancer Res. 2006;66(13):6748–6755. doi: 10.1158/0008-5472.CAN-06-0169. [DOI] [PubMed] [Google Scholar]
- 56.Malan T.P., Jr, Ibrahim M.M., Lai J., Vanderah T.W., Makriyannis A., Porreca F. CB2 cannabinoid receptor agonists: pain relief without psychoactive effects? Curr. Opin. Pharmacol. 2003;3(1):62–67. doi: 10.1016/s1471-4892(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 57.Torres S., Lorente M., Rodriguez-Fornes F., Hernandez-Tiedra S., Salazar M., Garcia-Taboada E., et al. A combined preclinical therapy of cannabinoids and temozolomide against glioma. Mol. Cancer Ther. 2011;10(1):90–103. doi: 10.1158/1535-7163.MCT-10-0688. [DOI] [PubMed] [Google Scholar]
- 58.Massi P., Valenti M., Vaccani A., et al. 5-Lipoxygenase and anandamide hydrolase (FAAH) mediate the antitumor activity of cannabidiol, a non-psychoactive cannabinoid. J. Neurochem. 2008;104(4):1091–1100. doi: 10.1111/j.1471-4159.2007.05073.x. [DOI] [PubMed] [Google Scholar]
- 59.Scott K.A., Dalgleish A.G., Liu W.M. The combination of cannabidiol and Delta9-tetrahydrocannabinol enhances the anticancer effects of radiation in an orthotopic murine glioma model. Mol. Cancer Ther. 2014;13(12):2955–2967. doi: 10.1158/1535-7163.MCT-14-0402. [DOI] [PubMed] [Google Scholar]
- 60.Solinas M., Massi P., Cinquina V., Valenti M., Bolognini D., Gariboldi M., et al. Cannabidiol, a non-psychoactive cannabinoid compound, inhibits proliferation and invasion in U87-MG and T98G glioma cells through a multitarget effect. PLoS One. 2013;8(10):e76918. doi: 10.1371/journal.pone.0076918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Galve-Roperh I., Sanchez C., Cortes M.L., Gomez del Pulgar T., Izquierdo M., Guzman M. Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat. Med. 2000;6(3):313–319. doi: 10.1038/73171. [DOI] [PubMed] [Google Scholar]
- 62.Sanchez C., Galve-Roperh I., Canova C., Brachet P., Guzman M. Delta9-tetrahydrocannabinol induces apoptosis in C6 glioma cells. FEBS Lett. 1998;436(1):6–10. doi: 10.1016/s0014-5793(98)01085-0. [DOI] [PubMed] [Google Scholar]
- 63.De Petrocellis L., Melck D., Palmisano A., et al. The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation. Proc. Natl. Acad. Sci. USA. 1998;95(14):8375–8380. doi: 10.1073/pnas.95.14.8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takeda S., Ikeda E., Su S., et al. Delta(9)-THC modulation of fatty acid 2-hydroxylase (FA2H) gene expression: possible involvement of induced levels of PPARalpha in MDA-MB-231 breast cancer cells. Toxicology. 2014;326:18–24. doi: 10.1016/j.tox.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murase R., Kawamura R., Singer E., et al. Targeting multiple cannabinoid anti-tumour pathways with a resorcinol derivative leads to inhibition of advanced stages of breast cancer. Br. J. Pharmacol. 2014;171(19):4464–4477. doi: 10.1111/bph.12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Melck D., Rueda D., Galve-Roperh I., De Petrocellis L., Guzman M., Di Marzo V. Involvement of the cAMP/protein kinase A pathway and of mitogen-activated protein kinase in the anti-proliferative effects of anandamide in human breast cancer cells. FEBS Lett. 1999;463(3):235–240. doi: 10.1016/s0014-5793(99)01639-7. [DOI] [PubMed] [Google Scholar]
- 67.Emery S.M., Alotaibi M.R., Tao Q., Selley D.E., Lichtman A.H., Gewirtz D.A. Combined antiproliferative effects of the aminoalkylindole WIN55,212-2 and radiation in breast cancer cells. J. Pharmacol. Exp. Ther. 2014;348(2):293–302. doi: 10.1124/jpet.113.205120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nasser M.W., Qamri Z., Deol Y.S., et al. Crosstalk between chemokine receptor CXCR4 and cannabinoid receptor CB2 in modulating breast cancer growth and invasion. PLoS One. 2011;6(9):e23901. doi: 10.1371/journal.pone.0023901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haustein M., Ramer R., Linnebacher M., Manda K., Hinz B. Cannabinoids increase lung cancer cell lysis by lymphokine-activated killer cells via upregulation of ICAM-1. Biochem. Pharmacol. 2014;92(2):312–325. doi: 10.1016/j.bcp.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 70.Ramer R., Bublitz K., Freimuth N., et al. Cannabidiol inhibits lung cancer cell invasion and metastasis via intercellular adhesion molecule-1. FASEB J. 2012;26(4):1535–1548. doi: 10.1096/fj.11-198184. [DOI] [PubMed] [Google Scholar]
- 71.Ramer R., Heinemann K., Merkord J., et al. COX-2 and PPAR-gamma confer cannabidiol-induced apoptosis of human lung cancer cells. Mol. Cancer Ther. 2013;12(1):69–82. doi: 10.1158/1535-7163.MCT-12-0335. [DOI] [PubMed] [Google Scholar]
- 72.Marshall A.D., Lagutina I., Grosveld G.C. PAX3-FOXO1 induces cannabinoid receptor 1 to enhance cell invasion and metastasis. Cancer Res. 2011;71(24):7471–7480. doi: 10.1158/0008-5472.CAN-11-0924. [DOI] [PubMed] [Google Scholar]
- 73.McKallip R.J., Lombard C., Fisher M., et al. Targeting CB2 cannabinoid receptors as a novel therapy to treat malignant lymphoblastic disease. Blood. 2002;100(2):627–634. doi: 10.1182/blood-2002-01-0098. [DOI] [PubMed] [Google Scholar]
- 74.Wu Z., Shao P., Zhang S., Ling X., Bai M. Molecular imaging of human tumor cells that naturally overexpress type 2 cannabinoid receptors using a quinolone-based near-infrared fluorescent probe. J. Biomed. Opt. 2014;19(7):76016. doi: 10.1117/1.JBO.19.7.076016. [DOI] [PubMed] [Google Scholar]
- 75.Yrjola S., Sarparanta M., Airaksinen A.J., et al. Synthesis, in vitro and in vivo evaluation of 1,3,5-triazines as cannabinoid CB2 receptor agonists. Eur. J. Pharm. Sci. 2015;67:85–96. doi: 10.1016/j.ejps.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 76.Adinolfi B., Romanini A., Vanni A., et al. Anticancer activity of anandamide in human cutaneous melanoma cells. Eur. J. Pharmacol. 2013;718(1-3):154–159. doi: 10.1016/j.ejphar.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 77.Blazquez C., Carracedo A., Barrado L., et al. Cannabinoid receptors as novel targets for the treatment of melanoma. FASEB J. 2006;20(14):2633–2635. doi: 10.1096/fj.06-6638fje. [DOI] [PubMed] [Google Scholar]
- 78.Melck D., De Petrocellis L., Orlando P., et al. Suppression of nerve growth factor Trk receptors and prolactin receptors by endocannabinoids leads to inhibition of human breast and prostate cancer cell proliferation. Endocrinology. 2000;141(1):118–126. doi: 10.1210/endo.141.1.7239. [DOI] [PubMed] [Google Scholar]
- 79.Orellana-Serradell O., Poblete C.E., Sanchez C., et al. Proapoptotic effect of endocannabinoids in prostate cancer cells. Oncol. Rep. 2015;33(4):1599–1608. doi: 10.3892/or.2015.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nithipatikom K., Isbell M.A., Endsley M.P., Woodliff J.E., Campbell W.B. Anti-proliferative effect of a putative endocannabinoid, 2-arachidonylglyceryl ether in prostate carcinoma cells. Prostaglandins Other Lipid Mediat. 2011;94(1-2):34–43. doi: 10.1016/j.prostaglandins.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sarfaraz S., Afaq F., Adhami V.M., Mukhtar H. Cannabinoid receptor as a novel target for the treatment of prostate cancer. Cancer Res. 2005;65(5):1635–1641. doi: 10.1158/0008-5472.CAN-04-3410. [DOI] [PubMed] [Google Scholar]
- 82.Bifulco M., Laezza C., Portella G., et al. Control by the endogenous cannabinoid system of ras oncogene-dependent tumor growth. FASEB J. 2001;15(14):2745–2747. doi: 10.1096/fj.01-0320fje. [DOI] [PubMed] [Google Scholar]
- 83.Cozzolino R., Cali G., Bifulco M., Laccetti P. A metabolically stable analogue of anandamide, Met-F-AEA, inhibits human thyroid carcinoma cell lines by activation of apoptosis. Invest. New Drugs. 2010;28(2):115–123. doi: 10.1007/s10637-009-9221-0. [DOI] [PubMed] [Google Scholar]
- 84.Shi Y., Zou M., Baitei E.Y., et al. Cannabinoid 2 receptor induction by IL-12 and its potential as a therapeutic target for the treatment of anaplastic thyroid carcinoma. Cancer Gene Ther. 2008;15(2):101–107. doi: 10.1038/sj.cgt.7701101. [DOI] [PubMed] [Google Scholar]
- 85.Brandi J., Dando I., Palmieri M., Donadelli M., Cecconi D. Comparative proteomic and phosphoproteomic profiling of pancreatic adenocarcinoma cells treated with CB1 or CB2 agonists. Electrophoresis. 2013;34(9-10):1359–1368. doi: 10.1002/elps.201200402. [DOI] [PubMed] [Google Scholar]
- 86.Larrinaga G., Sanz B., Blanco L., et al. Cannabinoid CB1 receptor is expressed in chromophobe renal cell carcinoma and renal oncocytoma. Clin. Biochem. 2013;46(7-8):638–641. doi: 10.1016/j.clinbiochem.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 87.Prather PL. CB1 and CB2 receptors are novel molecular targets for Tamoxifen and 4OH-Tamoxifen. Biochem. Biophys. Res. Commun. 2013;441(2):339–343. doi: 10.1016/j.bbrc.2013.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jan C.R., Lu Y.C., Jiann B.P., et al. Novel effect of CP55,940, a CB1/CB2 cannabinoid receptor agonist, on intracellular free Ca2+ levels in bladder cancer cells. Chin. J. Physiol. 2002;45(1):33–39. [PubMed] [Google Scholar]
- 89.Gasperi V., Evangelista D., Oddi S., et al. Regulation of inflammation and proliferation of human bladder carcinoma cells by type-1 and type-2 cannabinoid receptors. Life Sci. 2015;138:41–51. doi: 10.1016/j.lfs.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 90.Zhang Y.W., Su Y., Volpert O.V., Vande Woude G.F. Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin 1 regulation. Proc. Natl. Acad. Sci. USA. 2003;100(22):12718–12723. doi: 10.1073/pnas.2135113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang Z., Bao S.D. Roles of main pro- and anti-angiogenic factors in tumor angiogenesis. World J. Gastroenterol. 2004;10(4):463–470. doi: 10.3748/wjg.v10.i4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hirota K., Semenza G.L. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit. Rev. Oncol. Hematol. 2006;59(1):15–26. doi: 10.1016/j.critrevonc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 93.Elaraj D.M., White D.E., Steinberg S.M., Haworth L., Rosenberg S.A., Yang J.C. A pilot study of antiangiogenic therapy with bevacizumab and thalidomide in patients with metastatic renal cell carcinoma. J. Immunother. 2004;27(4):259–264. doi: 10.1097/00002371-200407000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gore M.E., Szczylik C., Porta C., et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol. 2009;10(8):757–763. doi: 10.1016/S1470-2045(09)70162-7. [DOI] [PubMed] [Google Scholar]
- 95.Motzer R.J., Hutson T.E., Tomczak P., et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2009;27(22):3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Blazquez C., Gonzalez-Feria L., Alvarez L., Haro A., Casanova M.L., Guzman M. Cannabinoids inhibit the vascular endothelial growth factor pathway in gliomas. Cancer Res. 2004;64(16):5617–5623. doi: 10.1158/0008-5472.CAN-03-3927. [DOI] [PubMed] [Google Scholar]
- 97.Casanova M.L., Larcher F., Casanova B., et al. A critical role for ras-mediated, epidermal growth factor receptor-dependent angiogenesis in mouse skin carcinogenesis. Cancer Res. 2002;62(12):3402–3407. [PubMed] [Google Scholar]
- 98.Blazquez C., Salazar M., Carracedo A., et al. Cannabinoids inhibit glioma cell invasion by down-regulating matrix metalloproteinase-2 expression. Cancer Res. 2008;68(6):1945–1952. doi: 10.1158/0008-5472.CAN-07-5176. [DOI] [PubMed] [Google Scholar]
- 99.Rundhaug J.E. Matrix metalloproteinases and angiogenesis. J. Cell. Mol. Med. 2005;9(2):267–285. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Deryugina E.I., Quigley J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25(1):9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 101.Overall C.M., Kleifeld O. Tumour microenvironment - opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat. Rev. Cancer. 2006;6(3):227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 102.Balkwill F., Charles K.A., Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7(3):211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 103.Hagemann T., Wilson J., Burke F., et al. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J. Immunol. 2006;176(8):5023–5032. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- 104.Krol M., Mucha J., Majchrzak K., et al. Macrophages mediate a switch between canonical and non-canonical Wnt pathways in canine mammary tumors. PLoS One. 2014;9(1):e83995. doi: 10.1371/journal.pone.0083995. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 105.Qian B.Z., Li J., Zhang H., et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krol M., Pawlowski K.M., Majchrzak K., Gajewska M., Majewska A., Motyl T. Global gene expression profiles of canine macrophages and canine mammary cancer cells grown as a co-culture in vitro. BMC Vet. Res. 2012;8:16. doi: 10.1186/1746-6148-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fung S.S. Targeting endocannabinoid signaling in tumor-associated macrophages as treatment for glioblastoma multiforme. WIREs Membr Transp Signal. 2014;3:39–51. [Google Scholar]
- 108.Raborn E.S., Marciano-Cabral F., Buckley N.E., Martin B.R., Cabral G.A. The cannabinoid delta-9-tetrahydrocannabinol mediates inhibition of macrophage chemotaxis to RANTES/CCL5: linkage to the CB2 receptor. J. Neuroimmune Pharmacol. 2008;3(2):117–129. doi: 10.1007/s11481-007-9077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ghosh S., Preet A., Groopman J.E., Ganju R.K. Cannabinoid receptor CB2 modulates the CXCL12/CXCR4-mediated chemotaxis of T lymphocytes. Mol. Immunol. 2006;43(14):2169–2179. doi: 10.1016/j.molimm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 110.Murdoch C., Finn A. Chemokine receptors and their role in vascular biology. J. Vasc. Res. 2000;37(1):1–7. doi: 10.1159/000025707. [DOI] [PubMed] [Google Scholar]
- 111.Murphy P.M. International Union of Pharmacology. XXX. Update on chemokine receptor nomenclature. Pharmacol. Rev. 2002;54(2):227–229. doi: 10.1124/pr.54.2.227. [DOI] [PubMed] [Google Scholar]
- 112.Charo I.F., Ransohoff R.M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 2006;354(6):610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 113.Martin B.R. Cellular effects of cannabinoids. Pharmacol. Rev. 1986;38(1):45–74. [PubMed] [Google Scholar]
- 114.Makriyannis A., Yang D.P., Griffin R.G., Das Gupta S.K. The perturbation of model membranes by (-)-delta 9-tetrahydrocannabinol. Studies using solid-state 2H- and 13C-NMR. Biochim. Biophys. Acta. 1990;1028(1):31–42. doi: 10.1016/0005-2736(90)90262-m. [DOI] [PubMed] [Google Scholar]
- 115.Cabral G.A., Staab A. Effects on the immune system. Handbook Exp. Pharmacol. 2005;(168):385–423. doi: 10.1007/3-540-26573-2_13. [DOI] [PubMed] [Google Scholar]
- 116.Khan M.I., Czarnecka A.M., Duchnowska R., Kukwa W., Szczylik C. Metastasis-initiating cells in renal cancer. Curr. Signal Transduct. Ther. 2014;8(3):240–246. doi: 10.2174/1574362409666140206222431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Escudier B., Eisen T., Stadler W.M., et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 118.Kirkham T.C. Cannabinoids and appetite: food craving and food pleasure. Int. Rev. Psychiatry. 2009;21(2):163–171. doi: 10.1080/09540260902782810. [DOI] [PubMed] [Google Scholar]
- 119.Hermanson D.J., Marnett L.J. Cannabinoids, endocannabinoids, and cancer. Cancer Metastasis Rev. 2011;30(3-4):599–612. doi: 10.1007/s10555-011-9318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Larrinaga G., Varona A., Perez I., et al. Expression of cannabinoid receptors in human kidney. Histol. Histopathol. 2010;25(9):1133–1138. doi: 10.14670/HH-25.1133. [DOI] [PubMed] [Google Scholar]
- 121.Siegel R., Ma J., Zou Z., Jemal A. Cancer statistics, 2014. CA Cancer J. Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]


