Abstract
Our reported case is a 72 year-old man who presented with hematuria. A transurethral resection of the bladder tumor (TURB-T) has been performed. Histopathological diagnosis was an epithelioid angiosarcoma. CT scan revealed a bladder thickening. The treatment consisted in a complete pelvectomy with urinary and digestive diversion. Following the operation, the patient developed liver and pulmonary metastasis. He died 5 months after the initial diagnosis.
Keywords: Angiosarcoma, Epithelioid cells, Hematuria
Introduction
Angiosarcoma is a vascular malignant tumor developed from blood vessels wall connective tissue. It is a rare neoplasia with bad prognosis and affects any organ. Bladder angiosarcoma is rare and is the subject of very few publications. Information about diagnosis, treatment, evolution and prognosis of this type of tumor are very limited. In this paper we report a 72-year-old patient case, harboring bladder angiosarcoma revealed by macroscopic hematuria.
Case presentation
The patient was 72-year-old man. He was admitted in emergency for a first episode of macroscopic hematuria complicated with acute urinary retention. His medical history was high blood pressure, a weaned smoking addiction, and benign prostatic hyperplasia (BPH). As a formal cinema industry worker, he had undergone chemical products exposure. At the first clinical examination, he presented with no pain, a supple abdomen without palpable mass. The biological checkup was normal. A bladder blood clot was seen on ultrasound. A cystoscopy was performed in emergency revealing a voluminous mass with a wide implantation located in the left side of the bladder. A TURB-T was performed. Pathological analysis reported an epithelioid bladder angiosarcoma (ISUP 2012 classification). The tumor was located in the sub-mucosae bulging the normal urothelium.
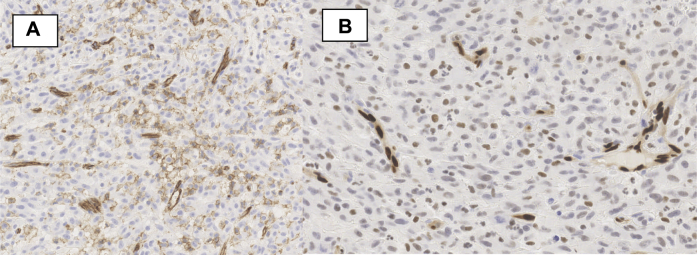
On architectural analysis, tumor cells were undifferentiated; they contained abundant cytoplasm and large, irregular nuclei with many mitosis. Necrosis rate was superior to 50%. There was no vascular embolus (Fig. 1). Immunohistochemical (IHC) staining was positive for CD31 (Fig. 2), FLI-1, ERG (Fig. 2) and vimentin, but negative for CD34 and cytokeratin. Proliferation index with Ki67 staining was of 90%.
Figure 1.
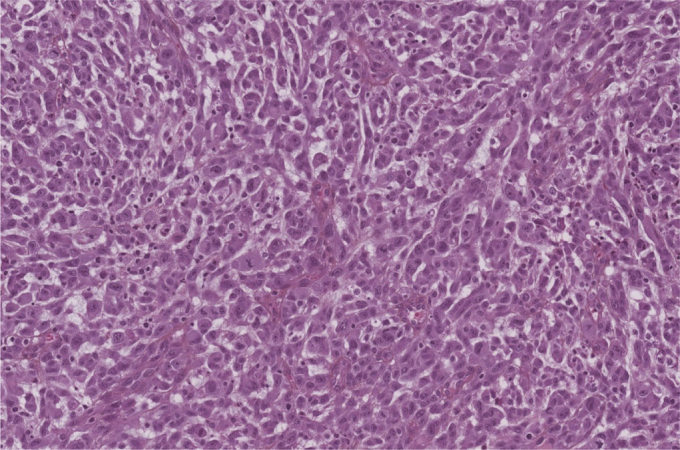
Malignant epithelioid cells, HES stain.
Figure 2.
A: CD31 membranous staining of infiltrating tumor cells. We can observe also the normal vessel wall staining. B: Moderate ERG nuclear staining of tumor cells. We can also observe a stronger staining of normal endothelial capillary cells.
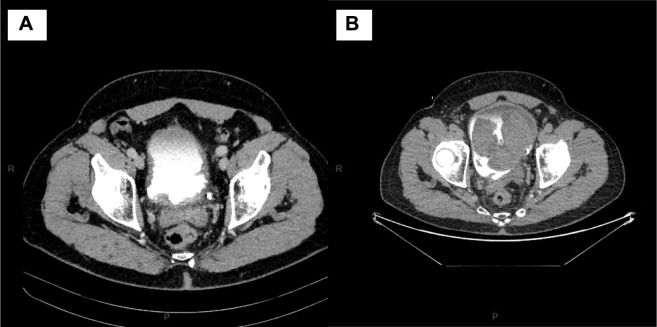
Despite a complete resection, a CT scan performed 3 weeks after TURB-T revealed massive local recurrence with a 42 * 37 mm mass (Fig. 3). No secondary lesion was diagnosed at this time.
Figure 3.
CT images show the rapid local progression of the tumor bladder. A: Day of diagnosis. B: 2 months after diagnosis.
For the treatment, the institutional sarcoma board has decided an initial external radiation therapy followed by surgery if the mass did not increase. After beginning the radiation therapy, the patient degraded. He presented a sepsis. The CT-scan showed an air bubble inside the bladder near the mass evocating tumor necrosis (Fig. 3).
Despite broad-spectrum intra-venous antibiotics, the patient declined indicating a surgical extirpation. A total pelvectomy with urinary and digestive diversion was performed 3 months after initial presentation.
After surgery, the patient presented an atrial fibrillation. One week after surgery, a CT-scan revealed liver and lung metastasis with peritoneal carcinomatosis.
The patient died 5 months after initial diagnosis.
Discussion
Non-urothelial bladder tumors represent less than 5% of bladder tumors. Bladder angiosarcoma was described for the first time in 1907 by Jungano.1 Since that date, thirty-two cases had been reported in literature. Some cases are reported after radiation therapy for prostate or gynecologic cancer. Angiosarcoma following radiation therapy was previously described. Hematuria is always the first symptom (macroscopic or microscopic). Associated symptoms are dysuria, pain, obstruction, vaginal bleeding and weight loss. Men are more affected than women with a ratio of 1/5. Liver and lung are usual metastatic sites.
Pathological diagnosis can be difficult.2 According to Matoso and Epstein, Angiosarcoma is confirmed if at least one endothelial marker including Factor VIII (F VIII), CD31, CD34 or ERG is positive on IHC.3
In our case, epithelioid bladder angiosarcoma diagnosis is founded on CD31, FLI-1 and ERG positive staining. CD34 and Cytokeratin are negative. A second expert opinion concluded in angiosarcoma or undifferentiated sarcomatoid carcinoma. An uropathologist board discussion decided that further molecular analysis would not have any value. In the literature, 27 cases are positive for at least one endothelial marker, including ERG (n = 5), F VIII (n = 12), CD31 (n = 20) and CD34 (n = 12). Five cases report no immunohistochemical analysis. No molecular analysis is reported (Table 1).
Table 1.
Diagnosis, treatment and outcome of bladder angiosarcoma.
| Reference | Age/sex | Presenting symptoms | Immunophenotype | Treatment | Outcome |
|---|---|---|---|---|---|
| Jungano, 19071 | 54/M | Hematuria and obstruction | N/A | Resection of tumor | N/A |
| Casal et al., 1970 | 85/F | Hematuria, dysuria and weight loss | N/A | Partial cystectomy | Died 3 days after diagnosis from myocardial infarction, no autopsy |
| Schwartz et al., 1983 | 46/M | Hematuria and enlarging cutaneous nodules | N/A | 10 courses of cis-platinum, doxorubicin and cyclophosphamide | Died 23 months after diagnosis, angiosarcoma in lung, brain and scrotum |
| Stroup and Chang, 1987 | 68/M | Hematuria | Factor VIII+ Keratin− |
Partial cystectomy | Died 8 month after diagnosis from myocardial infarction; autopsy showed lung and liver metastases |
| Morgan et al., 1989 | 72/F | Vaginal bleeding and hematuria | Factor VIII+ | N/A | N/A |
| Aragona et al., 1991 | 78/M | Dysuria and hematuria | Factor VIII+ ULEX+ Keratin− |
Diverticulectomy | Died 2 months after diagnosis from myocardial infarction, no autopsy |
| Ravi, 1993 | 55/M | Hematuria | N/A | Partial cystectomy and adjuvant radiation therapy (5500 cGy) | Alive 8 months after diagnosis |
| Navon et al., 1997 | 78/M | Hematuria | Factor VIII+ CD34+ ULEX+ |
Radical cytoprostatectomy | Alive 30 months after diagnosis |
| Engel et al., 19984 | 47/M | Hematuria, flank pain and suprapubic pain | N/A | Radical cystoprostatectomy, 5 cycles of chemotherapy and pelvic irradiation. | Alive and well 32 month after diagnosis |
| Schindler et al., 1999 | 47/M | Dysuria, hematuria and suprapubic pain | Vimentin+ CD31+ CD34– Keratin– Factor VIII− |
Radical cystoprostateectomy | N/A |
| Seethala et al., 2006 | 66/M | Hematuria | CD31+ CD34+ Keratin− |
Radical Cystoprostatectomy and 5 cycles of Gemcitabine and Docetaxel | Alive 19 months after diagnosis |
| Kulaga et al., 2006 | 83/F | Microhematuria | Vimentin+ CD31+ Factor VIII– CD34− |
TURB (transurethral bladder resection) | Died 3 months after diagnosis, no autopsy |
| Pazona et al., 20075 | 47/M | Hematuria, suprapubic pain | CD31+ | Radical cystoprostatectomy, 5 cycles ofchemotherapy and pelvic irradiation | Died 6 years after diagnosis, from myocardial infarction. Autopsy: no evidence of recurrent. |
| Williams et al., 2008 | 71/M | Hematuria | Factor VII+ CD31+ CD34+ |
Radical cystoprostatectomy | Died 3 months after diagnosis |
| Tavora et al., 2008 | 73/F | Hematuria | Radical cystectomy | Died 2 months after diagnosis | |
| 77/M | Hematuria | CD31+ and CD34+ for 3 cases | N/A | Died 5 months after diagnosis | |
| 71/M | Hematuria | N/A | Died 4 months after diagnosis | ||
| 63/F | Hematuria | Keratin– for all | N/A | Died 3 months after diagnosis | |
| Warne et al., 2011 | 32/F | Hematuria and left side flank pain | CD31+ Factor VIII+ ULEX+ Keratin+ |
TURB + 6 cycles of ifosfamide, epirubicin + single fraction radiotherapy | Died 19 months after diagnosis, lung metastases. No autopsy |
| Abbasov et al., 2011 | 51/M | Hematuria | Keratin+ Factor VIII+ Vimentin+ CD31+ CD34− |
Radical cystoprostatectomy | Died 5 weeks after diagnosis |
| Beyazal et al., 2014 | 20/M | Hematuria and disseminated pelvic pain | CD34+ | Partial cystectomy | Alive 1 year after diagnosis |
| Bahouth et al., 2015 | 89/M | Hematuria | CD31+ CD34+ Factor VIII+ Keratin− |
Palliative radiotherapy | Died 3 months after diagnosis |
| Matoso et al., 20153 | 73/F | Hematuria | CD31+ Factor VIII+ |
TURB followed by partial cystectomy | Died 6 months after diagnosis |
| 77/M | Hematuria | CD31+ CD34+ Factor VIII− |
TURB | Died 14 months after diagnosis | |
| 71/M | Hematuria | CD31+ Factor VIII+ |
TURB followed by partial cystectomy | Died 7 months after diagnosis | |
| 85/M | Hematuria | CD31+ CD34+ Factor VIII+ |
TURB | Died 6 months after diagnosis | |
| 39/M | Hematuria | CD31+ | TURB followed by partial cystectomy | Died 13 months after diagnosis | |
| 64/M | Hematuria | CD31+ ERG+ |
TURB followed by partial cystectomy | Alive 12 months after diagnosis | |
| 43/M | Hematuria | CD31+ Factor VIII+ ERG+ CD34− |
TURB | Alive 6 months after diagnosis | |
| 73/M | Hematuria | CD31+ ERG+ |
TURB | Died 3 months after diagnosis | |
| 64/M | Hematuria | CD34+ ERG+ |
TURB | Alive 3 months after diagnosis | |
| Ojerholm et al., 2015 | 61/M | Hematuria | N/A | Radical cystoprostatectomy | Alive 4 months after diagnosis |
| Current case | 72/M | Hematuria and urinary retention | ERG+ Vimentin+ CD31+ CD34– Keratin– |
Radiotherapy and radical pelvectomy | Died 5 months after diagnosis |
There is still no consensus on optimal treatment for bladder angiosarcoma. In our case, radiation therapy and radical pelvectomy was performed. Our patient had rapid disease progression after surgery; therefore he did not fit for adjuvant therapy. No significant superiority of any of those strategies has been reported.
Traditionally, sarcomas are treated by chemotherapy, surgery and radiotherapy.2 The triple association has shown promising results in high-grade sarcomas of the head and neck.2 Due to the rarity of angiosarcoma, it is unlikely to reach high level of evidence guidelines. The reported experience suggests that surgery and radiotherapy should be used initially. The role of chemotherapy should be defined.
Suggested treatment option includes chemotherapy, radiotherapy and radical surgery, with some sort of combination. Adjuvant radiotherapy and combined surgical and chemotherapeutic approach are reported with short-term survival.4 Chemotherapy regimens, reported are doxorubicin and ifosfamide.2, 4 These drugs are reported as active in sarcoma treatment. In unextirpable or metastatic disease, chemotherapy and/or radiotherapy could be an option.
Bladder angiosarcoma has a poor prognosis. In our reported case, the patient died 5 months after diagnosis. In the literature, ten patients survived more than 1 year and 3 more than 2 years. Mean age at diagnosis is 63.5 (range 20–89). Mean overall survival is 10.6 months (range 3 days to 6 years). Pazona5 and Engel4 report long-term survival after multimodal treatments. Pazona5 reports a 6 years survival with no evidence of disease on autopsy after radical cystectomy with adjuvant chemotherapy followed by pelvic irradiation. Engel4 reports on another combined cystectomy, chemotherapy and external beam radiation with no evidence of recurrence after 32 months of follow-up.
Conclusion
We report one of the very few cases of bladder angiosarcoma. It remains an aggressive tumor with poor prognosis. Optimal treatment for bladder angiosarcoma remains unclear, a multimodal aggressive approach combining radical surgery, radiotherapy and chemotherapy might be the key strategy.
Conflict of interest
Authors declare no conflict of interest.
Footnotes
Organizations that supported the research: None.
Sources of financial grants: None.
Author's industrial links and affiliations: None.
References
- 1.Jungano F. Sur un cas d'angiosarcome de la vessie. Ann Mal Organes Genitourinares. 1907;25:1451–1464. [Google Scholar]
- 2.Mark R.J., Poen J.C., Tran L.M. Angiosarcoma. A report of 67 patients and a review of the literature. Cancer. 1996 Jun 1;77(11):2400–2406. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2400::AID-CNCR32>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 3.Matoso A., Epstein J.I. Epithelioid angiosarcoma of the bladder: a series of 9 cases. Am J Surg Pathol. 2015 Oct;39(10):1377–1382. doi: 10.1097/PAS.0000000000000444. [DOI] [PubMed] [Google Scholar]
- 4.Engel J.D., Kuzel T.M., Moceanu M.C. Angiosarcoma of the bladder: a review. Urology. 1998 Nov;52(5):778–784. doi: 10.1016/s0090-4295(98)00286-6. [DOI] [PubMed] [Google Scholar]
- 5.Pazona J.F., Gupta R., Wysock J. Angiosarcoma of bladder: long-term survival after multimodal therapy. Urology. 2007 Mar;69(3) doi: 10.1016/j.urology.2007.01.021. [DOI] [PubMed] [Google Scholar]