Abstract
The legalization of marijuana in the USA for both medicinal and recreational use has increased in the past few years. Currently, 24 states have legalized marijuana for medicinal use. The US Drug Enforcement Administration has classified marijuana as a Schedule I substance. The US Food and Drug Administration does not regulate formulations or packages of marijuana that are currently marketed in states that have legalized marijuana. Marijuana edibles or “medibles” are typically packages of candies and baked goods consumed for medicinal as well as recreational marijuana use. They contain major psychoactive drug in marijuana, delta-9-tetrahydrocannabinol (THC) and/or cannabidiol (CBD), which has reputed medical properties. Presented is a method for the preparation and application of THC and CBD containing brownies used as quality control (QC) material for the analysis of marijuana or cannabinoid baked medibles. The performance parameters of the assay including possible matrix effects and cannabinoid stability in the brownie QC over time are presented. It was determined that the process used to prepare and bake the brownie control material did not degrade the THC or CBD. The brownie matrix was found not to interfere with the analysis of a THC or a CBD. Ten commercially available brownie matrixes were evaluated for potential interferences; none of them were found to interfere with the analysis of THC or CBD. The laboratory baked medible QC material was found to be stable at room temperature for at least 3 months.
Introduction
The legalization of marijuana in the USA for both medicinal and recreational use has increased in the past few years. Currently, marijuana is legal for medicinal use in 24 states. Marijuana is classified as a Schedule 1 substance by the US Drug Enforcement Administration (1). The US Food and Drug Administration (FDA) does not regulate or enforce manufacturing restrictions on formulations of marijuana or marijuana constituents other than Marinol®, the only federally legal formulation of delta-9-tetrahydrocannabinol (THC). Therefore, the FDA does not regulate formulations or packages of marijuana that are currently marketed in states that have legalized marijuana. Marijuana is increasingly sold in candies or baked goods, known as marijuana edibles or “medibles”. Medibles contain psychoactive THC and cannabidiol (CBD), a cannabinoid whose reported medical properties include analgesic, anticonvulsant and anti-psychotic activity (2–4). The State of Colorado classifies a medible serving of THC as 10 mg, with a maximum of 100 mg per retail package (5). The State of Oregon classifies a medible serving of THC as 5 mg, with a maximum of 50 mg per retail package (6).
Currently, there are no published methods that evaluate medible product matrix effects or the effects of preparation and/or baking of medibles on the stability of THC and/or CBD. Such data are essential to the use of quality control (QC) materials in the analysis of medibles. The Scientific Working Group for Forensic Toxicology (SWGTOX) and the FDA require the use of matrix-matched calibrators and controls for instrumental analysis of drug products and formulations (7, 8).
The Washington State Police Laboratory has a procedure for the analysis of cannabinoids in baked goods (9). The procedure involves a 1:50 dilution of the extracted baked goods before instrumental analysis. This dilution brings the cannabinoid concentrations into the linearity range of the instrument; it also effectively eliminates any matrix effect from the baked goods. In this study, baked QC material extracts were not diluted further before analysis. Duncan Hines® states 1 box of brownie mix prepares 20 brownie servings or ~48 brownie bite servings. Therefore, a 5-mg THC brownie serving would equate to a 2-mg THC bite serving, and a 10-mg THC brownie serving would equate to a 4-mg THC bite serving. To determine the baked brownie matrix effect, QC materials were prepared at 1/50th of the medicinal THC serving, thereby determining the effect of the brownie matrix on the analysis. Thus, baked brownie QC materials at a 5- or 10-mg THC and CBD serving would contain 40 or 82 ng of both THC and CBD, respectively.
We present a method for the preparation and application of marijuana brownies as matrix-matched calibration and QC material for use in the analysis of marijuana or cannabinoid baked medibles. The performance parameters of the assay including possible matrix effects and cannabinoid stability in the brownie QC material are presented.
Experimental
Materials
The primary reference material for THC, THC-d3 and CBD was obtained from Cerilliant Corporation (Round Rock, TX). Duncan Hines® Dark Chocolate Fudge Brownies Extra Thick & Fudgy, Betty Crocker™ Favorites Fudge Brownie Mix, Pillsbury™ Chocolate Fudge Brownie mix, Entenmann's® Little Bites® Fudge Brownies, Food Lion Two-Bite® Brownies, Kroger® Bakery Fresh Goodness Chocolate Brownies with walnuts, Little Debbie® Cosmic Brownies with Chocolate Chip Candy, Mrs. Thinster's™ Cookie Thins Brownie Batter, Pepperidge Farm® Chocolate Brownie, Sheila G's™ Brownie Brittle Peanut Butter Chip, canola oil and eggs were purchased from local grocery stores. Water used in the preparation of the brownie mix was tap water. Ammonium formate, 99% (Acros Organics), methanol (Fluka™ LC-MS grade) and water (Fluka™ LC/MS grade) were purchased.
Control and matrix preparation
Baked brownie matrix materials were prepared using the manufacture's recipe for “cake like” brownies printed on the mix's packaging. Backed QC materials were prepared at 5 and 10 mg equivalent servings or 40 and 80 ng of THC and CBD per brownie bite, respectively. The brownie batter was prepared according to the manufacture's recommendations and weighed. Five and ten milligram equivalent servings were prepared by weighing a 1/10 aliquot of the batter and fortifying the respective aliquot with either 200 or 400 ng of THC and CBD. The cannabinoids were mixed well into each aliquot. Each fortified aliquot was portioned into five wells of a dark-coated bite size brownie baking pan. Then baked at 300°F in a laboratory oven, until an inserted toothpick came out with no uncooked batter attached. The remaining non-fortified batter was baked in the same manner and use as drug-free matrix for calibration and method validation control material.
Instrumental analysis
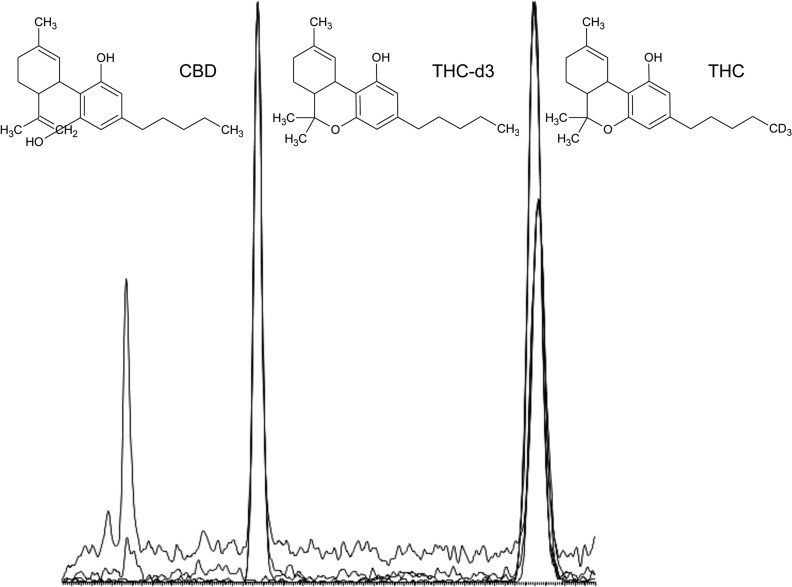
The UPLC–MS-MS analysis was performed on a Waters AcQuity Xevo TQD system (Milford, MA) controlled by MassLynx 4.5 software using a modified cannabinoid method (10). Chromatographic separation was performed on a Zorbax Eclipse XDB-C18, 3.5 μm, 4.6 × 75 mm (Santa Clara, CA), Figure 1. The mobile phase was 20 mM ammonium formate in water (A) and 20 mM ammonium formate in methanol (B) isocratic at 10:90 for 6 min followed by a gradient to 0:100 over 1 min. The column flow rate was 0.5 mL/min. Under these conditions, the retention times of CBD, THC-d3 and THC were 3.2, 5.5 and 5.5 min, respectively. The source and desolvation temperatures were 150 and 600°C, respectively. Desolvation and cone gas flows were 600 and 100 L/h, respectively. The capillary and cone voltages were 3.5 kV and 34 V, respectively. The acquisition mode was multiple reaction monitoring (MRM). The following transition ions (m/z) were monitored in MRM mode with their corresponding collision energies (eV) in parentheses: CBD: 315 > 43 (35), 93 (23) and 193 (21); THC: 315 > 43 (35), 122 (29) and 193 (29); and THC-d3; 318 > 93 (25), 123 (35) and 196 (21). The total run time for the analytical method was 8.0 min.
Figure 1.
Chromatogram of a 5-mg equivalent baked brownie.
Sample preparation
Sample preparation was by a modification of the Washington State Police – Crime Laboratory Divisions method for identifying cannabinoids in baked goods (9). Ten nanograms of THC-d3 were added to 25 mg of baked brownie sample (calibration or QC), which was then allowed to equilibrate for 30 min. Two milliliters of methanol was then added to the sample. The sample was vortexed on medium speed for 1 min, allowed to sit for 2 min, centrifuged at 10,000 g for 5 min, and then the supernatant was transferred to an autosampler for analysis by UPLC–MS-MS.
Method validation
The evaluation of the baked brownie QC material was conducted over five separate days (n = 3). Sample runs were analyzed as recommended for bioassay validation (9, 10) for linearity, lower limit of quantification (LOQ), accuracy/bias, precision, carryover, selectivity, absolute recovery, matrix effect and stability. Validation sample runs contained calibrators in duplicate, drug-free control (negative control) with ISTD added and a double negative QC brownie sample. Fortified brownie QC samples were analyzed in triplicate on 4 days and; for the inter-day precision study, in replicates of six on a single day yielding a total of 18 data points for each of the following QC samples containing CBD and THC: limit of quantification quality control (LOQC), target concentration of 0.8 mcg/g; low control (LQC), target concentration of 2.4 mcg/g; medium control (MQC), target concentration of 24 mcg/g; high control (HQC), target concentration of 60 mcg/g. Fortified brownie QC samples were prepared by adding the appropriate amount of THC, CBD and THC-d3 to backed drug-free brownie matrix each day of analysis.
Linearity, limit of quantification and limit of detection
Linearity was verified from seven non-zero fortified drug-free baked brownie matrix materials., These were prepared fresh in duplicate with concentrations of 0.8, 1.6, 4, 8, 16, 40 and 80 mcg/g, each day for five separate days (n = 10). A linear regression of the ratio of the peak area of THC/THC-d3 and CBD/THC-d3 versus concentration for each fortified sample of each separate set was used to construct the linear regressions to assess linearity, limit of quantitation and limit of detection.
Accuracy/bias and precision
Accuracy/bias and precision were determined from the fortified brownie QC samples (0.8, 2.4, 24 and 60 mcg/g). Intra-run precision was the run with the largest calculated intra-run % coefficient of variation (CV) (n = 3) for each concentration over the five validation runs. Inter-run precision was calculated for each concentration over the five validation runs by using the combined QC values (n = 3) over the 5 days for a total of 15 replicates at each concentration.
Carryover
Sample carryover was evaluated in each of the five validation batches using two different procedures. First, immediately following the injection of the 80 mcg/g linearity material from each linearity set, either a drug-free control with internal standard (negative control) or a drug-free control without internal standard (double negative control) was injected. No carryover above the LOD was observed in either control. Second, an injection of the HQC was immediately followed by injection of the LQC. This procedure was routinely applied each time the 80 mcg/g linearity material, HQC and LQC were analyzed with the determined concentration of the LQC at 85–115% of the mean.
Selectivity
The selectivity of the assay was determined using 10 different brands of brownies. Each brand was analyzed with and without ISTD. No peaks that co-eluted with the THC, CBD or THC-d3 were detected. This ensured that compounds in the brownies did not interfere with the assay. Possible inter-brownie matrix effects were determined by fortifying the 10 brands of commercially available brownies with 5 mcg/g of THC and CBD. These samples were then analyzed in triplicate.
Process efficiency
The process efficiency of the assay for THC and CBD was determined at 8 mcg/g (n = 3) and for THC-d3, at 8 mcg/g (n = 9). The process efficiency was determined by first preparing THC, CBD and THC-d3 at 8 mcg/g in methanol without brownie matrix. Then, brownie matrix samples were prepared at 8 mcg/g. The prepared brownie samples were then extracted with methanol. The process efficiency of the assay was determined by comparing the absolute peak area of extracted samples to the absolute peak area of the drugs in methanol.
Interferences
Other cannabinoids commonly encountered in elevated concentrations were analyzed as part of the interference study. Cannabinol (CBN), cannabichromene (CBC), cannabigerol (CBG), 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THCA), Δ9-tetrahydrocannabinolic acid (THCA-A) and tetrahydrocannabivarin (THCV) were analyzed.
Post-preparative stability
The post-preparative stability of THC, CBD and THC-d3 was evaluated by having extracts of the LQC, MQC and HQC sit in the UPLC–MS-MS's autosampler. A batch (n = 3) of the extracted QCs was quantified against a freshly prepared calibration. The extracted controls were then allowed to sit in the autosampler for 72 h at 10°C. They were re-injected and quantified using the initial calibration after 24, 48 and 72 h. The results of the initial analysis were compared with those of the re-injected samples. In the post-preparative study, THC, CBD and THC-d3 were considered stable if the concentrations of the re-injected QC samples were within ±20% of their concentration determined by their initial injection.
Baked controls stability
Stability of the THC, CBD and THC-d3 in brownie matrix was determined under specific conditions and at different time intervals. The experiments were performed using the baked brownie QC (5 and 10 mg control samples). All studies included three replicate analyses of each QC sample. Because brownies are not usually stored frozen, freeze-thaw stability was not evaluated. The QC the stability of THC, CBD and THC-d3 in baked brownie matrix was evaluated at 24 h, 48 h, 1 week, 1 month, 2 months and 3 months. These QC samples were stored at room temperature in reclosable plastic bags. The QC samples were extracted and quantified against freshly prepared calibrators. THC and CBD were considered stable if the concentrations of the QC samples were within +20% of the target concentration samples.
Results and Discussion
The presented method demonstrates a procedure for the preparation of THC and CBD baked matrix-matched QC materials. The method demonstrated acceptable accuracy and reproducibility for the detection and quantification of THC and CBD in baked brownies. The method was linear from 0.8 to 80 mcg/g with the coefficients of determination (r2) in the 10 linearity sets yielding r2 = 0.9989–1.0000 for THC and CBD. The lower limit of detection and quantitation of THC and CBD were administratively set at 0.8 mcg/g. LOQC samples were used to verify that the LOQ was within ±20% of the target value and had a response at least 10 times greater than the signal-to-noise ratio of negative QC. Intra-run bias for QC materials ranged from −18% (0.8 mcg/g THC) to 9% (0.8 mcg/g CBD) (n = 3). Inter-run bias for QC materials ranged from −6% (0.8 mcg/g THC) to 5% (24 mcg/g THC) (n = 12). Accuracy as well as intra-day and inter-day precision of the assay were determined not to exceed CVs of ±15% over the dynamic range of the assay (Table I). No analytical carryover was detected in any of the five analytical runs. Process efficiency, determined at 8 mcg/g, was THC 92 ± 4% (n = 3), CBD 95 ± 5% (n = 3) and THC-d3 94 ± 9% (n = 9). No interferences were detected from other cannabinoids (CBN, CBC, CBG, THCA, THCA-A or THCV). No interferences were detected from the 10 brownie matrixes. Fortification of the 10 brownie matrixes at 5 mcg/g ranged from 4.9 to 5.8 mcg/g (<0.7 SD). Sample extracts stored on the UPLC–MS-MS were stable up to 72 h after preparation. The laboratory baked brownie QC materials were stable up to 3 months when stored at room temperature (Table II).
Table I.
Intra-run and inter-run bias and precision
| Bias% (Precision %CV) | Intra-run (n = 3) | Inter-run (n = 15, 5 days) | ||
|---|---|---|---|---|
| QC samples | THC | CBD | THC | CBD |
| LOD (0.8 mcg/g) | −18 (13) | 9 (8) | −6 (12) | −1 (11) |
| Low (2.4 mcg/g) | −10 (6) | −7 (15) | 1 (10) | −1 (11) |
| Mid (24 mcg/g) | 7 (3) | 3 (4) | 5 (2) | 4 (4) |
| High (60 mcg/g) | 1 (3) | 9 (13) | 1 (4) | 5 (7) |
Table II.
Bench-top brownie prepared controls stability
| Baked controls (n = 3), mean ± SD | THC | CBD | THC | CBD |
|---|---|---|---|---|
| 24 h | 4.8 ± 0.6 | 4.6 ± 0.2 | 9.0 ± 1.3 | 9.5 ± 1.2 |
| 72 h | 5.2 ± 0.5 | 5.1 ± 0.1 | 11.9 ± 0.5 | 11.9 ± 0.5 |
| 1 Week | 5.2 ± 0.3 | 5.6 ± 0.2 | 11.4 ± 0.9 | 12.0 ± 0.9 |
| 1 Month | 5.6 ± 0.5 | 5.7 ± 0.7 | 10.3 ± 0.9 | 10.9 ± 1.1 |
| 2 Months | 5.6 ± 0.7 | 6.0 ± 0.3 | 10.3 ± 0.9 | 10.8 ± 0.3 |
| 3 Months | 5.9 ± 0.5 | 5.8 ± 0.2 | 12.0 ± 0.1 | 11.9 ± 1.1 |
The Washington State method involves a 50:1 dilution of the methanol extract before analysis. This essentially removes any matrix effect from the baked good. To better evaluate the matrix effect in the analysis scheme, the analytes of interest should be present in a low concentration in relation to the matrix. This study did not further dilute the methanol extract of the baked goods. Five and ten milligram equivalent baked QC materials were prepared at 1/50th of the Oregon and Colorado THC servings to evaluate the effect of matrix in baked goods.
Conclusion
A method for the determination of THC and CBD in brownie matrix was developed. The assay uses a simple dilute and shoot sample preparation. This method was used to analyze baked matrix-matched brownie medible QC material. Preparation of matrix-matched QC material is an important part of analyzing medibles to conform to SWGTOX and FDA guidelines. The process of preparing and baking brownie control material did not degrade the THC or CBD added to the brownie mix. The brownie matrix did not interfere with the analysis of added THC or CBD. The 10 commercially obtained brownie matrixes tested did not interfere with THC or CBD analysis. The medible QC material was determined to be stable for at least 3 months.
Funding
This work was supported by the National Institutes of Health (NIH) grant: P30DA033934.
References
- 1.Title 21, United States Code, Controlled Substances Act, Chapter 13, Subchapter 1, Part B, Section 812. Schedule of Controlled Substances, 2012.
- 2.Friedman D., Devinsky O. (2015) Cannabinoids in the treatment of epilepsy. The New England Journal of Medicine, 373, 1048–1058. doi:10.1056/NEJMra1407304. [DOI] [PubMed] [Google Scholar]
- 3.Johnson J.R., Burnell-Nugent M., Lossignol D., Ganae-Motan E.D., Potts R., Fallon M.T. (2010) Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. Journal of Pain and Symptom Management, 39, 167–179. doi:10.1016/j.jpainsymman.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Manseau M.W., Goff D.C. (2015) Cannabinoids and schizophrenia: risks and therapeutic potential. Neurotherapeutics, 12, 816–824. doi:10.1007/s13311-015-0382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orens A., Light M., Rowberry J., Matsen J., Lewandowski B. Marijuana Equivalency in Portion and Dosage: An assessment of physical and pharmacokinetic relationships in marijuana production and consumption in Colorado. Colorado Department of Revenue, August 10, 2015.
- 6.Oregon Administrative Rules, Oregon Health Authority, Public Health Division, Chapter 333, Division 7, Marijuana labeling, concentration Limits and Testing 333-007-0210: Table 1 Retail Marijuana Item Concentration Limits, 2016.
- 7.Scientific Working Group for Forensic Toxicology. (2008) Report from the Scientific Working Group for Forensic Toxicology: Scientific Working Group for Forensic Toxicology (SWGTOX) standard practices for method validation in forensic toxicology. Journal of Analytical Toxicology, 37, 452–474. [DOI] [PubMed] [Google Scholar]
- 8.US Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation. Rockville, MD: US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. 2001.
- 9.Stenzel J.R., Jiang G. Identification of Cannabinoids in Baked Goods by UHPLC/MS. Application Note: 433, Thermo Fisher Scientific, Inc. 2008.
- 10.Poklis J.L., Thompson C.C., Long K.A., Lichtman A.H., Poklis A. (2010) Disposition of cannabichrome, cannabidiol, and 9-tetrahydrocannabinol and its metabolites in mouse brain following marijuana inhalation determined by high-performance liquid chromatography – tandem mass spectrometry. Journal of Analytical Toxicology, 34, 516–520. [DOI] [PMC free article] [PubMed] [Google Scholar]