Abstract
Aims
Peripartum cardiomyopathy (PPCM) is a systolic left ventricular dysfunction developing in the peripartum phase in previously healthy women. Relaxin-2 is a pregnancy hormone with potential beneficial effects in heart failure patients. We evaluated Relaxin-2 as a potential diagnostic marker and/or a therapeutic agent in PPCM.
Methods and results
In healthy peripartum women, serum Relaxin-2 levels (measured by ELISA in the second half of pregnancy) were elevated showing a decreasing trend in the first postpartum week and returned to non-pregnant levels thereafter. In PPCM patients diagnosed in the first postpartum week, serum Relaxin-2 levels were lower compared to healthy postpartum stage-matched controls. In PPCM patients diagnosed later (0.5–10 months postpartum) Relaxin-2 levels were in the range of non-pregnant controls and not different from healthy postpartum stage-matched controls. In mice, serum Relaxin-1 (functional equivalent of human Relaxin-2) was increased late in pregnancy and rapidly cleared in the first postpartum week. In mice with PPCM due to a cardiomyocyte-specific knockout of STAT3 (CKO) neither low nor high dose of recombinant Relaxin-2 (serelaxin, sRlx-LD: 30 µg/kg/day; sRlx-HD: 300 µg/kg/day) affected cardiac fibrosis, inflammation and heart failure but sRlx-HD increased capillary/cardiomyocyte ratio. sRlx-HD significantly increased heart/body weight ratio and cardiomyocyte cross-sectional area in postpartum CKO and wild-type mice without changing the foetal gene expression program (ANP or β-MHC). sRlx-HD augmented plasma Prolactin levels in both genotypes, which induced cardiac activation of STAT5. In vitro analyses showed that Prolactin induces cardiomyocyte hypertrophy via activation of STAT5.
Conclusion
Although Relaxin-2 levels seemed lower in PPCM patients diagnosed early postpartum, we observed a high pregnancy-related variance of serum Relaxin-2 levels peripartum making it unsuitable as a biomarker for this condition. Supplementation with sRlx may contribute to angiogenesis and compensatory hypertrophy in the diseased heart, but the effects are not sufficient to prevent heart failure in an experimental PPCM model.
Keywords: Relaxin, Serelaxin, Heart failure, Peripartum cardiomyopathy , Biomarker, STAT3, Hypertrophy , STAT5
1. Introduction
Last decades’ research on Relaxin-2, a 6kDa peptide hormone structurally related to the insulin superfamily, has revealed a broad range of mechanisms and effects underlying its role for adaptations of the cardiovascular system during pregnancy. Relaxin-2 is primarily produced in the corpus luteum, mammary gland, endometrium, and during pregnancy also in the placenta.1 Serum levels of Relaxin-2 are substantially increased in the first trimester and remain high until the end of pregnancy. Relaxin-2 attenuates the vascular tone both rapidly and persistently through different mechanisms including activation of a NO-cGMP-pathway in the endothelium and/or antagonism to endothelin-1, a potent vasoconstrictor and hereby markedly decreases systemic vascular resistance (SVR) during pregnancy.2,3 In fact, it can act as a potent vasodilator and a pro-angiogenic factor.4 Additionally, Relaxin-2 reduces oxidative stress, lowers levels of the endogenous endothelial NOS inhibitor asymmetric dimethylarginine (ADMA) and increases NO bioavailability during angiotensin II induced hypertension.5 Relaxin-2 has also been shown to increase myofilament activity in cardiac cells in mice via a PKC-mediated pathway6 and to attenuate cardiac fibrosis,7,8 all of which may play an additional role in the changes of cardiac output throughout pregnancy. Potential beneficial effects of Relaxin-2 have been shown for treatment of experimental renal disease in rats.9 Moreover, first clinical studies show positive effects of recombinant Relaxin-2 (serelaxin, sRlx) in acute heart failure (RELAX-AHF-trial).10
Based on the above described essential role of Relaxin-2 in pregnancy-related physiological adaptation and protection of the vasculature and its promising therapeutic potential for acute heart failure,10 we hypothesized that disturbed Relaxin-2 pathways play a role in the pathophysiology of pregnancy-induced cardiovascular diseases, such as peripartum cardiomyopathy (PPCM). PPCM is a life-threatening heart disease in women defined by the Working Group on PPCM of the European Society of Cardiology (ESC)11 as ‘an idiopathic cardiomyopathy presenting with heart failure secondary to left ventricular (LV) systolic dysfunction mostly with an ejection fraction (EF) <45%, towards the end of pregnancy or in the months following delivery, where no other cause of heart failure is found.’ The reported incidence of PPCM varies among different geographic regions with high rates in certain areas in Africa (1 per 100-1:1000) and Haiti (1:299).12 In the USA, a rising incidence rate was observed from 1 in 4350 pregnancies in 1993 to 1 in 2229 pregnancies in 200212. No precise numbers exist for Europe but estimated incidence rates are between 1 in 1500 to 2000 pregnancies.12 Meanwhile large prospective international registries, i.e. the ESC EURObservational Research Programme (http://www.eorp.org, last accessed 8 December 2016) are initiated that will provide further insights in the disease. Albeit the aetiology of PPCM is still largely unknown, recent research identified an impairment of the vasculature, especially endothelial damage and dysfunction as a major factor inducing and driving PPCM.13,14 In this regard, increased oxidative stress and the subsequent generation of the anti-angiogenic N-terminal 16kDa Prolactin, increased levels of ADMA as well as an up-regulation of the anti-angiogenic soluble receptor sFlt-1 emerged as disease causing factors.15,16 Based on the beneficial effect of Relaxin-2 in patients with heart failure10 and based on experimental data showing that chronic application of sRlx protects the vasculature and has anti-fibrotic properties,17,18 we further hypothesized that serum levels of Relaxin-2 may be decreased in PPCM and that, as a consequence, administration of exogenous Relaxin-2, i.e. sRlx, may have therapeutic effects in PPCM. This assumption was also supported by a recent study reporting that higher Relaxin-2 levels early postpartum are associated with faster recovery in PPCM patients.19
Here, we show that Relaxin-2 serum levels tended to be lower in PPCM patients diagnosed in the first week after delivery compared to healthy postpartum stage-matched control women, but returned rapidly to normal levels in both PPCM patients and postpartum stage-matched controls. Treatment of the experimental PPCM mouse model (mice with cardiomyocyte-restricted deletion of STAT3 αMHC-Cretg/-; STAT3fl/fl mice: CKO)15,20 with sRlx revealed no beneficial effect on heart failure. Interestingly, high dose sRlx treatment increased capillary density in postpartum CKO mice and promoted cardiac hypertrophy in postpartum CKO and wild-type (WT) mice possibly via upregulation of Prolactin secretion and subsequent activation of STAT5.
2. Methods
Cell culture media were obtained from Biochrom. Recombinant human Relaxin-2 (serelaxin, sRlx) was received from Novartis. STAT5 inhibitor was obtained from Calbiochem. All other chemicals were purchased from Sigma-Aldrich.
2.1 Patients
This study was approved by the local ethics committee of Hannover Medical School, Germany. All patients provided written informed consent. The study conforms with the declaration of Helsinki. All 55 patients from the German PPCM registry described in13 were diagnosed with PPCM according to the position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy.11 All PPCM patients were diagnosed postpartum and all blood samples analysed in the present study were collected at the time of diagnosis. The control collective consisted of healthy postpartum women without echocardiographic abnormalities of cardiac structure or function [left ventricular ejection fraction (LVEF) >55%, n = 47], from whom blood samples were obtained within the first week after delivery and/or between 2 weeks and 6 months postpartum, healthy non-pregnant (n = 9) and healthy pregnant women (2nd half of pregnancy, n = 10). The control collective was also selected from the German PPCM registry.13
2.2 Blood tests
Blood samples were collected in S-Monovette® tubes containing ethylenediaminetetraacetic acid or clot activator at the time point of first diagnosis in PPCM patients and from pregnancy stage-matched healthy controls (pre- and post-partum, time points of blood sampling as indicated above) and age matched non-pregnant women. Laboratory workup was performed as routine investigation by hospital laboratories for N-terminal pro-brain natriuretic peptide (NT-proBNP). For analyses of relaxin levels, serum was separated by centrifugation at 500 × g for 10 min and aliquots were stored at −80 °C. Relaxin-2 was measured using the Human Relaxin-2 Quantikine ELISA Kit (R&D Systems) strictly according to the manufacturer’s protocol. Mouse serum Relaxin-1 levels were quantified using the mouse Relaxin-1 ELISA Kit purchased from ORIGENE following the provided protocol. Mouse Prolactin was quantified from plasma samples using the mouse Prolactin ELISA Kit from Abcam according to the manufacturer’s protocol.
2.3 Animal experiments
The generation of mice with cardiomyocyte-restricted deletion of STAT3 αMHC-Cretg/-; STAT3fl/fl mice (CKO) was described previously.15,20 In brief, CKO mice develop a heart failure phenotype after two pregnancies, which is comparable to the human PPCM. In the present study, sibling CKO and wild-type (WT: STAT3fl/fl) mice were used. To determine the kinetics of Relaxin-1 in mice, serum was obtained from the same mice late in pregnancy (3 days before delivery), in the first week postpartum and at the end of the nursing period (3 weeks postpartum). Serelaxin, a recombinant form of human Relaxin-2 (sRlx-LD 30 µg/kg/d or sRlx-HD 300 µg/kg/d, Novartis), was applied by subcutaneous bolus injection into CKO and WT females daily during two consecutive pregnancies and nursing periods starting 3–4 days before delivery of the first pregnancy and ending at the end of the second nursing period (21d after the second delivery). Echocardiography was performed in sedated mice before the first pregnancy and after ending of the second nursing period (2% isoflurane inhalation, connected to a rodent ventilator) using a Vevo 770 (Visual Sonics) as described.15,20,21 For measuring acute effects of sRlx-HD, it was applied by subcutaneous bolus injection in nulli pari (NP) non-pregnant female mice for 2h (for measuring plasma Prolactin levels and STAT5 activation) or 6h (for measuring serum Relaxin-2 levels). Prolactin (400 IU/kg) was applied by acute i.p. injection for 30 min in NP female mice. Mice were euthanized (cervical dislocation) and plasma was taken. Hearts were harvested, weighed, and halves were either directly frozen or embedded in Tissue-Tek OCT compound for subsequent analyses and all was stored at −80 °C. All animal studies were in accordance with the German animal protection law and with the European Communities Council Directive 86/609/EEC and 2010/63/EU for the protection of animals used for experimental purposes. All experiments were approved by the Local Institutional Animal Care and Research Advisory Committee and permitted by the local authority.
2.4 qRT-PCR, Western blot
Total RNA from adult murine hearts was isolated with Trizol (Invitrogen) and cDNA synthesis was performed as described previously.21,22 Real-time PCR with SYBR green dye method (Brilliant SYBR Green Mastermix-Kit, Thermo Fisher) was performed with the AriaMx Real-Time PCR System (Agilent Technologies) as described.21,22 List of qRT-PCR primers used in this study is provided below. Expression of mature miR-146a was determined using miR-qRT-PCR on an ABI7500 cycler (Applied Biosystems, Foster City, USA) and was normalized using the 2-ΔΔCT method relative to U6 as described.23
Sequences of qRT-PCR primers
| mRNA | Sense primers (5’ to 3’) | Antisense primers (5’ to 3’) |
|---|---|---|
| mmu 18S | GTAACCCGTTGAA CCCCATT | CCATCCAATCGGTA GTAGCG |
| mmu ANP | GCCGGTAGAAGA TGAGGTCA | GGGCTCCAATCCT GTCAATC |
| mmu α-MHC | GGAAGAGCGAGC GGCGCATCAAGG | GTCTGCTGGAGAGGT TATTCCTCG |
| mmu β-MHC | CAAGTTCCGCA AGGTGC | AAATTGCTTTATTCTG CTTCCAC |
| mmu Col1a1 | ACAGACGAACAAC CCAAACT | GGTTTTTGGTCACG TTCAGT |
Protein expression levels were determined by Western blotting, using SDS-PAGE as previously described.20 The following antibodies were used: Anti-ErbB2, anti-ErbB4, anti-MMP2, anti-phospho-Tyrosine 705-STAT3, anti-STAT3, anti-phospho-Tyrosine 694 STAT5 and anti-STAT5 (Cell Signalling Technology), anti-MMP3 and anti-MMP9 (Abcam).
2.5 Histology and immunostaining
For cardiac morphological analyses, hearts were embedded in Tissue Tek OCT and frozen at −80 °C. Subsequently, cryosections were stained with H&E as described previously.20 Interstitial collagen was analysed in picro-sirius red F3BA-stained sections using polarized light.22 Inflammation was determined in LV cryosections with an antibody recognizing CD45 (BD Pharmingen).
Mean cardiomyocyte cross-sectional area (CSA) was determined in LV cryosections stained with wheat germ agglutinin (WGA, Vector) and capillary density was determined by Isolectin B4 (Vector) staining.21,22 DAPI was used for nuclear stain. Size of neonatal rat cardiomyocytes (NRCM) was analysed by phase contrast microscopy and/or after staining of NRCM was performed with α-actinin (Sigma-Aldrich).
2.6 Cell culture experiments with cardiomyocytes
Isolation and cultivation of neonatal rat cardiomyocytes (NRCM) were performed as described previously.20,21 In brief, neonatal rats were sacrificed by decapitation and hearts were harvested. Cardiomyocytes were isolated by collagenase digestion followed by purification by Percoll gradient centrifugation, then seeded in petri dish in DMEM/M199 (4:1) supplemented with 10% FCS overnight. Subsequently, they were washed and set serum free in DMEM/M199 (4:1) overnight prior to stimulation. Stimulation with Prolactin (0.2 iU/ml, Sigma-Aldrich) or recombinant Relaxin-2 (rRlx, 100 ng/ml, Sigma-Aldrich) was carried out on NRCM under serum-free conditions in DMEM/M199 (4:1) for 48h. The STAT5 inhibitor (100 µM, Calbiochem) was applied 1 h prior to stimulation.
2.7 Statistical analyses
Database management and statistical analyses were performed with PRISM software, version 6.0 statistical program (GraphPad Software Inc., La Jolla, CA, USA). Data are presented as mean ± SD or median with range. Differences between groups were analysed by unpaired, two-tailed t-test or analysis of variance (ANOVA). Nonparametric Mann–Whitney test was performed on data not fitting a Gaussian distribution (as analysed by D’Agostino and Pearson omnibus normality test). When comparing more than two groups, we used Bonferroni's Multicomparison Test after ANOVA testing and Bartlett's testing for equal variances. A two-tailed P-value of <0.05 was considered statistically significant.
3. Results
3.1 Patient characteristics
In order to evaluate serum Relaxin-2 levels, n = 55 PPCM patients from the German PPCM registry were analysed.13 The mean LVEF in these patients at time of diagnosis was 26 ± 9% (mean±SD). For comparison, n = 47 age and postpartum stage-matched healthy control women from the German PPCM registry13 were analysed with a mean LVEF of 63 ± 4% (mean±SD, P < 0.0001 vs. PPCM). All other clinical baseline characteristics are provided in Table 1.
Table 1.
Clinical parameters, electrocardiographic findings, and laboratory test results
| Parameters | Post-partum controls, 1–7 days after delivery (n = 36) | PPCM, 1–7 days after delivery (n = 15) | Post-partum controls 0.5–10 months after delivery (n = 11) | PPCM 0.5–10 months after delivery (n = 40) |
|---|---|---|---|---|
| Age (years) Mean ± SD | 30 ± 5 | 34 ± 8 | 34 ± 3 | 34 ± 3 |
| Gravida Median (range) | 1 (1–4) | 1 (1–7) | 1 (1–2) | 2 (1–5) |
| Parity Median (range) | 1 (1–4) | 1 (1–4) | 1 (1–2) | 2 (1–4) |
| Gestational age at partum (weeks) Mean ± SD | 38 ± 3 | 37 ± 4 | 39 ± 2 | 38 ± 2 |
| %EF Mean ± SD | 63 ± 4 | 30 ± 9** | 64 ± 6 | 27 ± 9## |
| Heart rate (bpm) Mean ± SD | – | 99 ± 14 | – | 88 ± 20 |
| Systolic BP (mmHg) Mean ± SD | – | 128 ± 19 | – | 111 ± 16 |
| Diastolic BP (mmHg) Mean ± SD | – | 86 ± 13 | – | 68 ± 12 |
| Relaxin-2 (pg/ml) Median (range) | 55 (1–1075) | 2 (1–276)** | 0.6 (0–7) | 0.5 (0–609) |
PPCM, peripartum cardiomyopathy; BP, blood pressure; SD, standard deviation.
P < 0.01 early PPCM vs. early controls,
##P < 0.01 late PPCM vs. late controls, two-way ANOVA, Bonferroni’s Multiple Comparison Test.
3.2 In humans, serum Relaxin-2 is elevated prior to delivery and rapidly cleared in the first postpartum days
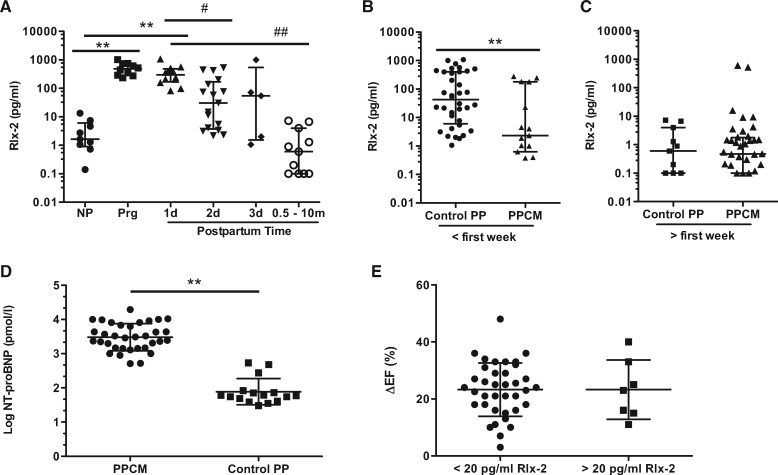
It has been shown that serum Relaxin-2 levels are substantially increased in pregnancy and rapidly decline after delivery24,25 as also confirmed in the present study where Relaxin-2 was highly elevated in women at the second half of pregnancy, still elevated in the first days after delivery with a decreasing trend but was in the range of non-pregnant controls in women analysed 0.5–10 months postpartum (Figure 1A).
Figure 1.
Serum Relaxin levels in healthy peripartum women and in PPCM. (A) Serum Relaxin-2 levels in healthy non-pregnant (NP, n = 9) women, pregnant women (Prg, n = 10) and postpartum women (1 day n = 12, 2d n = 17, 3d n = 5, 0.5–10 months n = 11), **P < 0.01 vs. NP; #P < 0.05, ##P < 0.01 PP vs. Prg), data are median with interquartile range, one-way ANOVA, Bonferroni’s Multiple Comparison Test. (B) Serum Relaxin-2 levels in PPCM patients vs. healthy postpartum controls with serum collected in the first postpartum week (PPCM: n = 15 vs. Control PP: n = 36, **P < 0.01) and (C) measured in later diagnosed patients and postpartum stage-matched controls where serum was obtained after the first postpartum week (PPCM: n = 40 and Control PP: n = 11). Data are shown as median with interquartile range, please note that patients and controls with Relaxin-2 levels below the limit of detection are not included within the scatter plot due to logarithmical scaling, but were used for median and statistical calculation, normality test by D’Agostino–Pearson, Mann-Whitney test for PPCM vs. controls. (D) Serum NT-proBNP levels in PPCM patients vs. healthy control postpartum women (PPCM: n = 34 vs. Control PP: n = 16, **P < 0.01, data are mean ± SD, unpaired, two-tailed t-test. (E) Improvement of left ventricular ejection fraction (ΔEF: LVEF at follow-up–LVEF at diagnosis) of PPCM patients with serum Relaxin-2 levels at diagnosis <20 pg/ml (n = 37) or >20 pg/ml (n = 7), data are mean ± SD.
3.3 In PPCM patients diagnosed within the first postpartum week, serum Relaxin-2 levels were lower while they were similar in later diagnosed patients compared to postpartum stage-matched healthy controls
All PPCM patients were diagnosed postpartum. Full-term pregnancy (delivery after gestational week 37) was present in 95% of PPCM patients diagnosed in the first postpartum week and in 87% of patients diagnosed later and in 92% and 91% of respective postpartum controls (Table 1). In PPCM patients, where the diagnosis and the blood sampling were made within the first postpartum week (blood collection: 5 ± 2 days postpartum), serum Relaxin-2 levels were slightly but significantly lower compared to healthy postpartum stage-matched women (blood collection: 3 ± 2 days postpartum) (Figure 1A, Table 1), although still above the NP controls (vs. NP: P < 0.001). In PPCM patients of whom blood sampling was obtained after the first postpartum week, serum Relaxin-2 levels had decreased to the level of NP controls and were with this similar to the post-partum healthy controls with blood sampling taken after 1 week postpartum (Figure 1C, Table 1). For comparison we also measured NT-proBNP, which was matching previous reports,13 elevated in all PPCM patients at diagnosis of disease independent of the postpartum stage (Figure 1D).
Serum Relaxin-2 levels did not correlate with cardiac improvement in PPCM patients since similar difference in LVEF from baseline to follow up (5.4 ± 2 months) was observed in patients with baseline serum Relaxin-2 levels below or above 20 pg/ml (Figure 1E).
3.4 Pregnancy-related kinetics of Relaxin-1 in mice
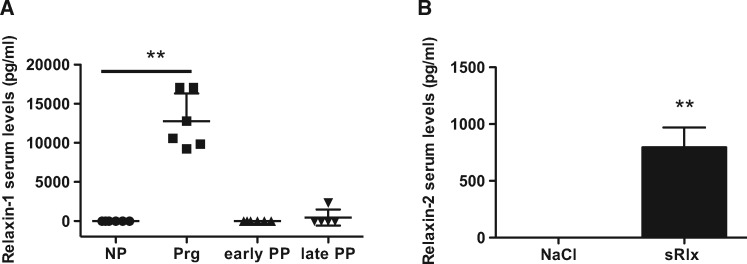
Next, we analysed the kinetics of Relaxin blood levels with regards to the pregnancy stage in mice by analysing blood levels of Relaxin-1, the mouse equivalent to Relaxin-2 in humans,26 late in pregnancy (3 days before delivery), in the first week postpartum and at the end of the nursing period (3 weeks postpartum). We observed that in WT mice Relaxin-1 serum levels were highly elevated at the end of pregnancy but were in the range of non-pregnant controls in the first week after delivery and at the end of the nursing period (Figure 2A).
Figure 2.
Kinetics of mouse serum Relaxin-1 and serum levels of Relaxin-2 reached by sRlx injection. (A) Relaxin-1 levels in WT female mice, nulli pari (NP), pregnant (Prg), early (early PP) and late (late PP) postpartum mice (**P < 0.01 vs. NP, n = 5–6), one-way ANOVA, Bonferroni’s Multiple Comparison Test. (B) Serum Relaxin-2 levels in WT-NP mice after daily subcutaneous injection of sRlx-HD and NaCl for 2 days, 6h after the last injection (**P < 0.01 vs. NaCl, n = 3–4 each), unpaired, two-tailed t-test. All data are presented as mean ± SD.
3.5 Injection of serelaxin augmented serum Relaxin-2 blood levels in mice
Subcutaneous bolus injection of the recombinant Relaxin-2, serelaxin (sRlx,) similar to the dose used in other mouse studies (sRlx-HD, 300 µg/kg/d) ,18 significantly elevated serum levels of Relaxin-2 in non-pregnant WT (WT-NP) females with around 800-fold increased levels present still 6h after bolus injection. However, these levels were markedly below serum levels of endogenous Relaxin-1 in pregnant mice (Figure 2Aand B).
3.6 sRlx applied during pregnancy and postpartum had no influence on foetal survival and raising offspring in CKO mice
In order to analyse whether sRlx treatment in pregnancy and postpartum is safe for the offspring, we applied sRlx in daily bolus injections for two subsequent pregnancies and nursing periods either at low doses (sRlx-LD, 30 µg/kg/d), which is comparable to the dose used in the RELAX-AHF study10 or at high doses (sRlx-HD, 300 µg/kg/d). The number of offspring was similar between postpartum WT (WT-PP) and CKO (CKO-PP) mice that had been injected with NaCl, sRlx-LD, or sRlx-HD (Table 2). Further analyses showed that the average weight of pups after weaning was also similar (data not shown).
Table 2.
Cardiac function and morphometry in mice after two pregnancies and nursing periods
| NaCl WT-PP | sRlx-HD WT-PP | NaCl CKO-PP | sRlx-LD CKO-PP | sRlx-HD CKO-PP | |
|---|---|---|---|---|---|
| %FS | 33 ± 3 (n = 6) | 33 ± 4 (n = 7) | 22 ± 10** (n = 15) | 25 ± 9* (n = 12) | 21 ± 9** (n = 12) |
| LVEDD (mm) | 4.2 ± 0.45 (n = 6) | 3.77 ± 0.3 (n = 7) | 4.29 ± 0.55 (n = 15) | 4.31 ± 0.64 (n = 12) | 4.74 ± 0.53*,# (n = 12) |
| LVESD (mm) | 2.78 ± 0.32 (n = 6) | 2.53 ± 0.31 (n = 7) | 3.37 ± 0.80* (n = 15) | 3.26 ± 0.83 (n = 12) | 3.78 ± 0.78** (n = 12) |
| HR (bpm) | 503 ± 22 (n = 6) | 529 ± 43 (n = 7) | 492 ± 38 (n = 15) | 480 ± 66 (n = 12) | 465 ± 83 (n = 12) |
| HW (g) | 0.14 ± 0.01 (n = 7) | 0.15 ± 0.02 (n = 7) | 0.14 ± 0.01 (n = 15) | 0.13 ± 0.02 (n = 12) | 0.16 ± 0.02**,## (n = 12) |
| BW (g) | 33 ± 3 (n = 7) | 32 ± 4 (n = 7) | 30 ± 2** (n = 15) | 30 ± 3* (n = 12) | 31 ± 1* (n = 12) |
| HW/BW | 4.1 ± 0.2 (n = 7) | 4.5 ± 0.4$ (n = 7) | 4.6 ± 0.4** (n = 15) | 4.4 ± 0.5 (n = 12) | 5.1 ± 0.5**,## (n = 12) |
| Litter size | 7.9 ± 2.0 (n = 7) | 8 ± 1.3 (n = 7) | 7.1 ± 1.3 (n = 15) | 7.5 ± 1.9 (n = 12) | 7.5 ± 1.9 (n = 12) |
Data from mice treated during pregnancy or only postpartum were similar therefore both groups were pooled. Number of mice analysed is indicated below each parameter.
FS, fractional shortening; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; heart rate (beats per minute, bpm); HW, heart weight; BW, body weight determined two weeks after the second pregnancy.
Data are means ± SD,
P < 0.05,
P < 0.01 vs. NaCl WT-PP,
#P < 0.05 sRlx-HD CKO-PP vs NaCl CKO-PP,
##P < 0.01 sRlx-HD CKO-PP vs. NaCl CKO-PP, $P < 0.05 sRlx-HD WT-PP vs. NaCl WT-PP, two-way ANOVA, Bonferroni’s Multiple Comparison Test.
3.7 Neither treatment with low nor high doses of sRlx prevented heart failure in CKO mice
Echocardiographic analyses after two pregnancies and nursing periods confirmed that CKO-PP mice developed PPCM with marked systolic LV dysfunction compared to pregnancy matched postpartum WT mice (WT-PP, Table 2).15,27 Daily injections over two pregnancies and nursing periods with either sRlx-LD or sRlx-HD did not attenuate LV dysfunction in CKO-PP mice (Table 2). In addition, end-diastolic (LVEDD) and end-systolic (LVESD) diameters were similarly enlarged in control (NaCl) and sRlx-LD-treated CKO-PP mice, but increased in sRlx-HD treated CKO mice (Table 2). In WT-PP mice, sRlx treatment had no effect on cardiac function or dimension (Table 2).
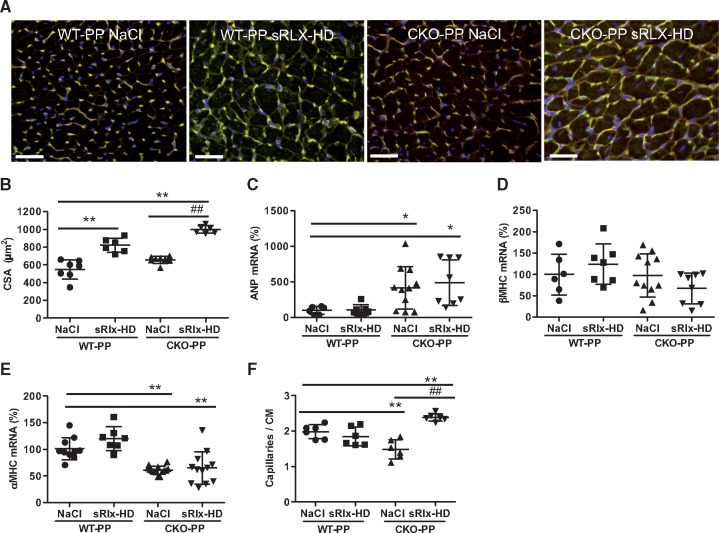
3.8 High dose sRlx treatment promoted cardiac hypertrophy in postpartum CKO-PP and WT-PP mice
Further analyses showed that sRlx-HD treatment increased heart weight (HW) and caused a higher HW/body weight (BW) ratio compared to NaCl treatment in WT-PP and in CKO-PP mice (Table 2). The increased HW and HW/BW ratio in sRlx-HD treated WT-PP and CKO-PP mice was associated with an increase in cardiomyocyte CSA compared to NaCl treated postpartum mice of either genotype (Figure 3Aand B). sRlx-treatment neither changed atrial natriuretic peptide (ANP), β-myosin heavy chain (β-MHC) and α-MHC expression in WT mice nor did it change the altered expression of ANP (upregulated, compared to WT) and α-MHC (down-regulated, compared to WT) or affect β-MHC expression (unchanged compared to WT) in CKO-PP mice (Figure 3C–E). In contrast, sRlx-LD treatment did not alter HW and HW/BW ratio in CKO-PP mice (Table 2). It did also not change ANP, α-MHC, or β-MHC expression compared to NaCl-treated CKO-PP mice (data not shown).
Figure 3.
Effect of sRlx treatment on WT and CKO mice after two subsequent pregnancies with regards to cardiac hypertrophy and capillary density. (A) LV cryosections stained with isolectin B4 (blood vessels, green), WGA (cell membranes, red) and nuclei (DAPI, blue) scale bar: 50 μm. (B) Bar graph summarizing cardiomyocyte cross-sectional area (CSA) (**P < 0.01 vs. NaCl-treated WT-PP, ##P < 0.01 sRlx vs. NaCl-treated CKO-PP n = 6–7), two-way ANOVA, Bonferroni’s Multiple Comparison Test. (C–E) Bar graphs summarizing qRT-PCR results for (C) ANP, (D) β-MHC, and (E) α-MHC mRNA expression in WT-PP and CKO-PP mice injected with NaCl or sRlx-HD (mean of NaCl WT-PP was set at 100%, *P < 0.05, **P < 0.01 vs. NaCl WT-PP, n = 6–14), two-way ANOVA, Bonferroni’s Multiple Comparison Test. (F) Bar graph summarizing capillaries to cardiomyocyte (CAP/CM) ratio (**P < 0.01 vs. NaCl-treated WT-PP, ##P < 0.01 vs. NaCl-treated CKO-PP, n = 6), two-way ANOVA, Bonferroni’s Multiple Comparison Test. All data are presented as mean ± SD.
3.9 Capillary to cardiomyocyte ratio is increased by sRlx in CKO-PP mice
We showed previously, that LV capillary density is diminished in CKO-PP mice with PPCM.15 Chronic treatment with sRlx-HD significantly increased the capillary to cardiomyocyte ratio in CKO-PP mice (Figure 3Aand F) while capillary density per area was not changed (data not shown). No significant change in capillary density per cardiomyocyte was observed in WT-PP mice (Figure 3Aand F).
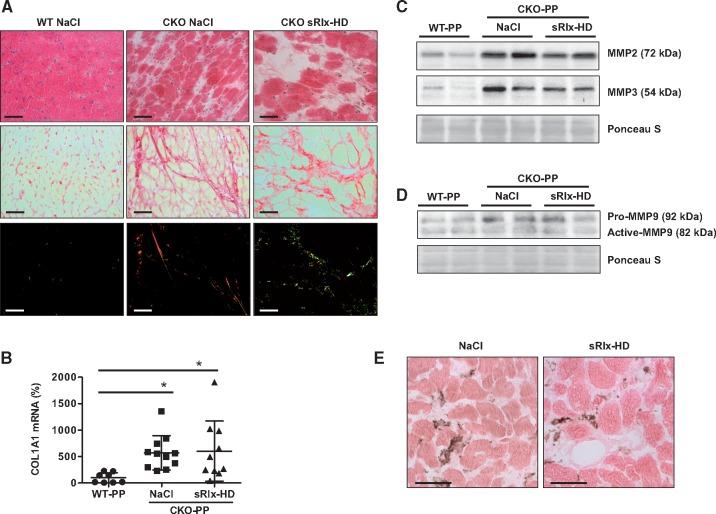
3.10 sRlx does not change cardiac inflammation but alters collagen fibre quality in CKO-PP mice
It has been shown that relaxin inhibits cardiac fibrosis.18,28 The degree of fibrosis and the expression of collagen type I alpha 1 (COL1A1) was similar in NaCl- and sRlx-HD-treated CKO-PP mice (Figure 4Aand B). However, polarized light microscopy revealed that collagen in fibrotic areas in sRlx-HD-treated CKO-PP mice appeared less dense with a higher degree of collagen I and III fragmentation (Figure 4A). Relaxin exerts its anti-fibrotic effect mainly by inducing the expression of matrix metalloproteinase (MMP)-2, -3, and -9.7,29 As shown in Figure 4C, the expression of MMP2 (MMP2: CKO-PP NaCl: 3-fold, CKO-PP sRlx-HD: 2,57-fold vs. WT-PP, *P < 0.05, n = 6) and -3 (MMP3: CKO-PP NaCl: 3,2-fold, CKO-PP sRlx-HD: 2,5-fold vs. WT-PP, *P < 0.05, n = 6) was substantially higher in CKO-PP mice compared to WT-PP but did not differ between NaCl or sRlx-HD-treated CKO-PP mice (Figure 4C). MMP9 expression was slightly higher in NaCl and sRlx-HD-treated CKO-PP mice compared to WT-PP (Pro-MMP9: CKO-PP NaCl: 1,4-fold, CKO-PP sRlx-HD: 1,5-fold vs. WT-PP, *P < 0.05, n = 6) although no signs of increased accumulation of active MMP9 were observed (Figure 4D). Likewise, expression of TIMP Metallopeptidase Inhibitor 1 (TIMP1) was not different between NaCl and sRlx-HD treated CKO-PP mice (data not shown). One-way ANOVA, Bonferroni’s Multiple Comparison Test was used for MMP2, 3, 9 and TIMP1 analyses.
Figure 4.
Effect of sRlx treatment of CKO-PP mice with regards to cardiac fibrosis and inflammation (A) Representative sections with haematoxylin and eosin staining (upper panels), Sirius red staining in bright field (middle panels) and in polarized light (bottom panels) depicting fibrosis and collagen fibres in WT-PP (n = 6), CKO-PP treated with NaCl (n = 6) or sRlx-HD (n = 6) with at least four sections per mouse analysed, scale bars: 50 μm. (B) Bar graph summarizing qRT-PCR results of mRNA for COL1A1 (mean of WT-PP was set at 100%, *P < 0.05 vs. WT-PP (n = 8), CKO-PP n = 11 (NaCl), and n = 10 (sRlx-HD), data are means±SD, one-way ANOVA, Bonferroni’s Multiple Comparison Test. (C) Western blot showing protein levels of MMP2 and -3 and membrane stained with Ponceau S (PS) for loading control, (D) Western blot showing protein levels of pro- and active MMP9, membrane stained with Ponceau S (PS) for loading control. Western blots in C-D are representative for data from CKO-PP treated with NaCl or sRlx-HD with n = 6 for each condition. (E) Representative immunohistochemical staining with the pan-inflammatory marker CD45 (brown staining; counterstained with eosin, red), in CKO-PP treated with NaCl (n = 6) or sRlx-HD (n = 6), at least four sections per mouse were analysed, scale bars: 50 μm.
Since more inflammation could also be responsible for more fragmented collagen, we evaluated the degree of inflammatory infiltrates using the pan-inflammatory marker CD45. As shown in Figure 4E, the degree of CD45 positive inflammatory cells in cardiac tissue was not different (n.s., unpaired, two-tailed t-test) between NaCl- and sRlx-HD-treated CKO-PP mice.
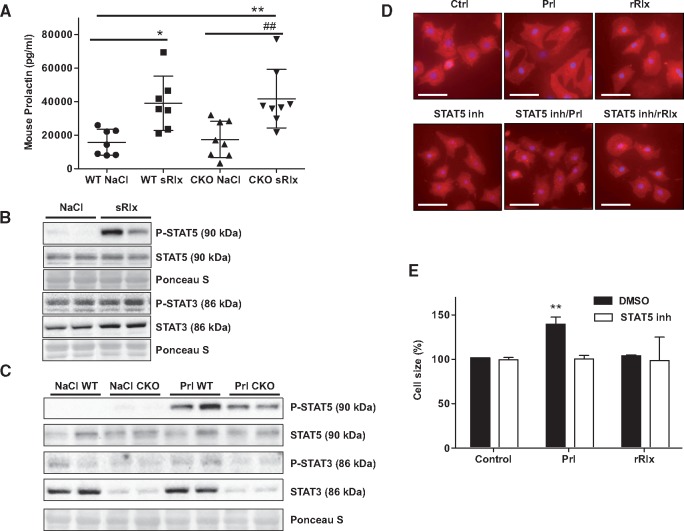
3.11 sRlx augments plasma levels of Prolactin, which is responsible for activation of STAT5 in the mouse heart
It has been hypothesized that Relaxin may augment Prolactin.30,31 Moreover, we previously showed that Prolactin promotes cardiac STAT5 activation.15 Indeed, we observed that acute injection with sRlx-HD increased plasma levels of Prolactin to a similar degree in WT-NP and CKO-NP mice (Figure 5A). sRlx-HD increased activation of STAT5 in LV tissue from WT-NP mice (STAT5 phosphorylation after sRlx-HD injection: 5-fold vs. NaCl injection, **P < 0.01, n = 6 each, Figure 5B). In contrast, cardiac STAT3 activation was not different in NaCl and sRlx-HD injected mice (NaCl vs. sRlx-HD, n.s. n = 6 each, Figure 5B). Acute injection of Prolactin (400 IU/kg) strongly induced activation of STAT5 in WT-NP mice and in CKO-NP mice (STAT5 activation after Prl treatment: WT Prl: 5,5-fold, CKO Prl: 4-fold vs. WT NaCl, **P < 0.01, n = 6). Prolactin injection induced only a slightly increase in STAT3 activation in WT-NP mice (STAT3 activation after Prl treatment: WT Prl: 1.6-fold vs. WT NaCl, *P < 0.05, n = 6) and no activation in CKO-NP mice (Figure 5C). One-way ANOVA, Bonferroni’s Multiple Comparison Test was used for STAT3 and 5 analyses in Figure 5B.
Figure 5.
sRlx upregulated Prolactin in WT and CKO mice and Prolactin promotes via STAT5 cardiomyocyte hypertrophy. (A) Plasma Prolactin measured in WT and CKO female mice 2h after sRlx-HD injection, n = 7–8 each genotype and condition, *P < 0.05, **P < 0.01 vs. NaCl-treated WT mice, ##P < 0.01 vs. NaCl-treated CKO mice; data are means±SD, two-way ANOVA, Bonferroni’s Multiple Comparison Test (B) Western Blot showing STAT5 (phospho-Tyrosine 694) activation in LV tissue from WT mice 2 h after sRlx-HD injection and (C) Western Blot depicting activation state of STAT3 (phospho-Tyrosine 705) and STAT5 in WT and CKO LV tissue 30 min after injection with Prolactin (Prl). Western blots in B and C are representative for n = 4–6 mice per condition and time point. (D) Fluorescence microscopy with α-actinin staining (red) showing cell surface area in NRCM stimulated with or without Prl (0.2 iU/ml) or rRelaxin-2 (100 ng/ml) for 48h, STAT5 inhibitor (100 μM) were added 1h prior stimulation and (E) bar graphs summarizing cardiomyocyte surface area in these NRCM after 48h (**P < 0.01, vs. control) All experiments are representative for three different cell isolations and are performed in duplicates or triplicates, data are means±SD, two-way ANOVA, Bonferroni’s Multiple Comparison Test.
3.12 Prolactin but not Relaxin promotes cardiomyocyte hypertrophy via activation of STAT5
Next, we analysed whether Prolactin may exert pro-hypertrophic effects via STAT5 in isolated NRCM. Indeed, Prolactin stimulation over 48h increased size of NRCM, which could be attenuated by pharmacological inhibition of STAT5 (STAT5 inhibitor, 100 μM, Figure 5Dand E). Addition of relaxin to NRCM had no effect on cardiomyocyte size (Figure 5D and E).
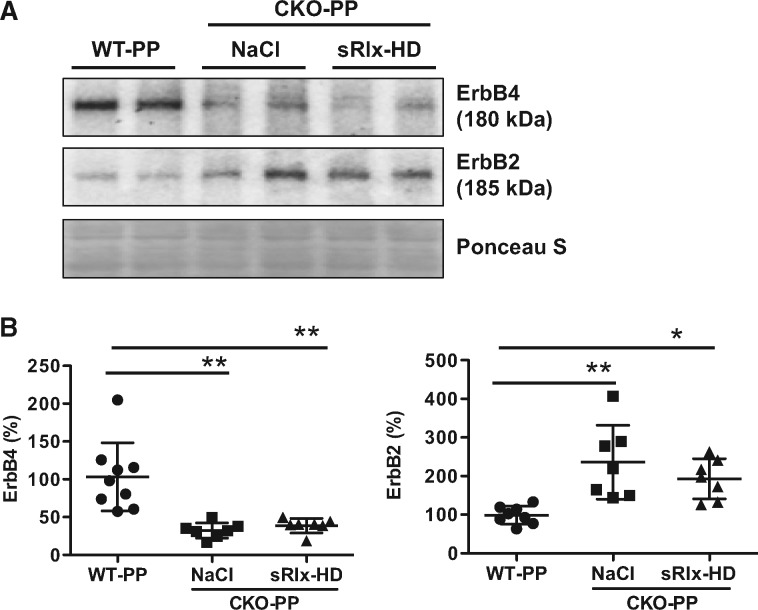
3.13 Relaxin does not enhance 16kDa Prolactin-mediated pathophysiology in postpartum CKO mice
We reported previously that in postpartum CKO mice Prolactin is cleaved into its anti-angiogenic 16kDa Prolactin form.15 Since it is technically not possible to detect the 16kDa Prolactin in mice directly and since 16kDa Prolactin mediates most of its adverse effects in CKO-PP mice via upregulation of microRNA-146a (miR-146a),23 we evaluated the expression of miR-146a in LV tissue of CKO-PP mice. We observed no difference in miR-146a levels between CKO-PP with or without sRlx-HD treatment (sRlx: 1.05-fold vs. NaCl, n.s.). This finding is in line with a similar decrease of the miR-146a cardioprotective target gene ErbB4 in control and sRlx-HD treated CKO-PP compared to WT-PP mice (Figure 6A and B). Expression of ErbB2 was increased to a similar degree (2.5-fold, compared to WT-PP P < 0.05, n = 7-9 each) in NaCl- and sRlx-HD-treated CKO-PP mice (Figure 6A and B).
Figure 6.
sRlx does not enhance 16kDa Prolactin-mediated pathophysiology in post-partum CKO mice (A) Western blots of ErbB4 and ErbB2 and (B) bar graphs summarizing protein levels of ErbB4 and ErbB2 in WT-PP, and CKO-PP with NaCl or sRlx-HD treatment. Membranes were stained with Ponceau S (PS) for loading control. All data are mean±SD. Mean of WT-PP was set at 100%. *P < 0.05, **P < 0.01 vs. WT-PP, n = 7–9 each, one-way ANOVA, Bonferroni’s Multiple Comparison Test.
4. Discussion
The maternal heart needs strong protection against stress factors induced by physiological and pathophysiological stimuli during pregnancy, delivery, and the early postpartum phase.32 In pregnancy, Relaxin-2 appears to be important for adaption of the cardio-reno-vascular system to pregnancy-related changes as it exerts vascular protective effects thereby counteracting pregnancy-induced endothelial damage.33,34 Here, we confirmed previous data showing that serum levels of Relaxin-2 are high in pregnancy with a decreasing trend in the first postpartum days rapidly returning to non-pregnant levels in healthy women. We observed slightly lower Relaxin-2 levels in PPCM patients diagnosed in the first postpartum week compared to postpartum-matched healthy controls while in PPCM patients diagnosed and measured later than the first week, Relaxin-2 levels were similar to postpartum-matched healthy controls. This observation suggests that lower Relaxin-2 levels may be associated with onset of PPCM early after delivery. In contrast to a previous study suggesting that higher Relaxin-2 levels may correlate with faster improvement in PPCM patients19 no such correlation was observed in our collective. In our opinion, the highly pregnancy-specific variability of serum levels of Relaxin-2 makes it ineligible as a diagnostic or prognostic marker in the postpartum period. Even if Relaxin-2 may indeed be lower in early onset PPCM, due to the rapid postpartum kinetics, it would be too difficult to define clear threshold values.
We previously showed that a circuit involving unbalanced oxidative stress and subsequent cleavage of Prolactin in an anti-angiogenic 16kDa Prolactin form is responsible for the development of PPCM in mice harbouring a cardiomyocyte-specific deletion of STAT3 and possibly also in patients with PPCM who frequently display reduced cardiac STAT3 levels.15 Further evidence in the past years supported the idea that an angiogenic imbalance is causal for PPCM.16 Beside impaired angiogenesis, endothelial dysfunction is a key feature of PPCM as indicated by increased levels of asymmetric dimethlaginine (ADMA) in PPCM patients.13
Recent findings from the RELAX-AHF study showing that sRlx relieved dyspnoea and reduced 180-day mortality in patients with acute heart failure10 and several experimental studies describing beneficial effects of Relaxin-2 against adverse cardiac remodelling after myocardial infarction (reviewed by Teichmann et al.17) suggest that Relaxin-2 supplementation might be beneficial in PPCM patients. This idea was further supported by the observation that Relaxin-2 levels are lower in early-diagnosed PPCM patients and by a recent observation of showing that higher Relaxin-2 levels soon after delivery were associated with faster recovery.19 Our data showed that sRlx-HD treatment increased serum Relaxin markedly in NP mice, which however were still substantially below endogenous levels of Relaxin-1, the mouse equivalent to Relaxin-2, in late pregnancy. sRlx-HD and -LD treatment during pregnancy and postpartum is safe for the mouse offspring since we did not observe differences with regards to offspring number and weight in sRlx-LD, sRlx-HD, or NaCl treated CKO-PP females. However, in contrast to beneficial effects of Relaxin in several cardiac diseases including ischemia/reperfusion and myocardial infarction, diabetic cardiomyopathy and catecholamine-induced cardiomyopathies,18,28 neither sRlx-LD nor sRlx-HD could attenuate heart failure associated with PPCM in CKO mice. Along the same line, it has been shown that Relaxin treatment did not improve preeclampsia.35
PPCM appears as a disease mainly targeting the vasculature and especially the cardiac capillary system.15,16 Although we did not find improved cardiac function, the sRlx-HD treatment significantly increased the capillaries to cardiomyocyte ratio in CKO-PP mice suggesting that it may have beneficial effects on the vasculature. This effect however was not observed in WT-PP mice.
In addition, the sRlx-HD treatment caused increased cardiac hypertrophy, cardiomyocyte enlargement in WT-PP and CKO-PP mice, which however, was not associated with changes in the foetal gene expression programme normally associated with pathological hypertrophy, i.e. ANP and β-MHC expressions were not changed by sRlx-HD in both genotypes when compared to respective NaCl-treated controls. Since we did not observe a pro-hypertrophic effect when Relaxin was directly applied to cultured cardiomyocytes (NRCM), we suspected that Relaxin may promote cardiac hypertrophy in postpartum mice indirectly. In this respect, it has been hypothesized that Relaxin may augment Prolactin.30,31 Indeed, acute injection of sRlx-HD augmented plasma levels of Prolactin significantly in WT-NP and CKO-NP mice. Moreover, we could show that sRlx-HD via Prolactin induces activation of the Prolactin major target signalling molecule STAT536 in LV tissue. Direct injection of Prolactin activated STAT5 in both genotypes further explaining how sRlx via Prolactin and STAT5 activation could induce cardiomyocyte growth in both genotypes. This notion is further supported by the observation that Prolactin-mediated increase in cardiomyocyte size could be reduced by STAT5 blockade in cultured cardiomyocytes.
The Relaxin-induced increase in Prolactin production may also promote the generation of the anti-angiogenic factor 16kDa Prolactin, a major factor driving PPCM.15,20 However, the expression of the 16kDa direct target miR-146a23 was not different in LV tissue from CKO-PP treated with or without sRlx-HD. Moreover, protein levels of the miR-146a target ErbB423 were also not different in NaCl- or sRlx-HD-treated CKO-PP mice suggesting that Relaxin-induced Prolactin levels are not enhancing Prolactin-mediated cardiac damage, which is also supported by the observation that ANP and β-MHC were not further increased in the sRlx-HD treated CKO-PP mice. This observation suggests that sRlx treatment may also protect from postpartum oxidative stress, a major factor for the generation of 16kDa Prolactin in PPCM.15,20 In fact, the increased Prolactin levels induced by sRlx could even explain the beneficial effect of sRlx treatment on the capillarization in CKO-PP hearts since it has been shown that full-length Prolactin via STAT5 activation promotes angiogenesis.37
Part of the beneficial role of Relaxin in acute heart failure has been attributed to its positive effects on cardiac fibrosis. Relaxin interferes with fibroblast proliferation and differentiation, attenuates endothelial-mesenchymal transition, and increases expression of the collagen degrading enzymes MMP2, 3, and 97,8,29 Indeed, we observed that sRlx-HD treatment caused a substantially higher degradation of cardiac collagen fibres in CKO-PP mice. Although expression of MMP2, 3, and 9 was higher in NaCl-treated CKO-PP mice compared to WT-PP, sRlx treatment did not further increase the expression and/or activation of these MMPs in whole LV tissue extracts. It has been shown that Relaxin increases the expression of MMP2 and MMP9 in human endothelial cells38 and therefore, higher fragmentation of collagen fibres in sRlx-HD-treated CKO-PP mice may be caused via upregulation/activation of MMPs in cardiac endothelial cell, which however could not be detected in the whole tissue homogenates.
In summary, our data show that early after delivery serum Relaxin-2 was lower in PPCM patients while no difference compared to healthy postpartum women was noted in later diagnosed patients. No correlation of Relaxin-2 levels with outcome of PPCM patients was observed in the present PPCM collective. This feature and its rapid clearance after delivery make it difficult to use Relaxin-2 blood levels as a diagnostic or prognostic marker for PPCM. Our experimental data show that sRlx supplementation induced a slight hypertrophic growth in postpartum WT mice with no signs of pathophysiological alterations. In postpartum CKO mice sRlx seemed to promote adaptive cardiac hypertrophy with increased numbers of capillary per cardiomyocyte in postpartum CKO mice with PPCM albeit with no functional improvement. Our data suggest that improved cardiac angiogenesis and hypertrophy seem rather indirect effects of sRlx caused by Prolactin, which is upregulated by sRlx. Interestingly, sRlx-induced upregulation of Prolactin was not associated with enhanced Prolactin cleavage and generation of the anti-agniogenic 16kDa Prolactin in CKO-PP mice suggesting that sRlx may protect from postpartum oxidative stress. In conclusion, sRlx treatment seems not to generate additional adverse effects in experimental PPCM and may even have positive effects on the cardiac vasculature. Future investigations will be needed to further explore potential therapeutic effects of sRlx in PPCM.
4.1 Limitation of the present study
The average day of blood sampling in the PPCM patients diagnosed in the first week after delivery was 2 days later that in the postpartum-matched controls, which could also be responsible for the slightly lower Relaxin-2 blood levels in these PPCM patients. Because of pregnancy, it was not possible to use osmotic minipumps for constant sRlx application as it was used in other studies but instead subcutaneous bolus injections were used. Furthermore, potential differences between murine Relaxin-1 and human Relaxin-2 administrated in CKO mice could account for the observed lack of beneficial effects in terms of cardiac function.
Acknowledgement
We thank Birgit Brandt, Sergej Erschow, Martina Kasten and Silvia Gutzke for technical assistance.
Funding
Arash Haghikia received a scholarship from the ‘‘Junge Akademie’’ program of Hannover Medical School. The study was supported by Novartis (Project-No. MRLX030_FVMS010) and Fondation Leducq: Transaltlantic Network of Excellence (n° 05 CVD 02) and Rebirth II.
Conflict of interest: The study was in part funded by Novartis (Project-No. MRLX030_FVMS010).
References
- 1. Bathgate RA, Halls ML, van der Westhuizen ET, Callander GE, Kocan M, Summers RJ.. Relaxin family peptides and their receptors. Physiol Rev 2013;93:405–480. [DOI] [PubMed] [Google Scholar]
- 2. Conrad KP, Shroff SG.. Effects of relaxin on arterial dilation, remodeling, and mechanical properties. Curr Hypertens Rep 2011;13:409–420. [DOI] [PubMed] [Google Scholar]
- 3. McGuane JT, Danielson LA, Debrah JE, Rubin JP, Novak J, Conrad KP.. Angiogenic growth factors are new and essential players in the sustained relaxin vasodilatory pathway in rodents and humans. Hypertension 2011;57:1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Samuel CS, Hewitson TD, Unemori EN, Tang ML.. Drugs of the future: the hormone relaxin. Cell Mol Life Sci 2007;64:1539–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sasser JM, Cunningham MW Jr, Baylis C.. Serelaxin reduces oxidative stress and asymmetric dimethylarginine in angiotensin II-induced hypertension. Am J Physiol Renal Physiol 2014;307:F1355–F1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shaw EE, Wood P, Kulpa J, Yang FH, Summerlee AJ, Pyle WG.. Relaxin alters cardiac myofilament function through a PKC-dependent pathway. Am J Physiol Heart Circ Physiol 2009;297:H29–H36. [DOI] [PubMed] [Google Scholar]
- 7. Lekgabe ED, Kiriazis H, Zhao C, Xu Q, Moore XL, Su Y, Bathgate RA, Du XJ, Samuel CS.. Relaxin reverses cardiac and renal fibrosis in spontaneously hypertensive rats. Hypertension 2005;46:412–418. [DOI] [PubMed] [Google Scholar]
- 8. Sassoli C, Chellini F, Pini A, Tani A, Nistri S, Nosi D, Zecchi-Orlandini S, Bani D, Formigli L.. Relaxin prevents cardiac fibroblast-myofibroblast transition via notch-1-mediated inhibition of TGF-beta/Smad3 signaling. PLoS One 2013;8:e63896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoshida T, Kumagai H, Kohsaka T, Ikegaya N.. Protective effects of relaxin against cisplatin-induced nephrotoxicity in rats. Nephron Exp Nephrol 2014;128:9–20. [DOI] [PubMed] [Google Scholar]
- 10. Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF Jr, Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M; Investigators REiAHF. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet 2013;381:29–39. [DOI] [PubMed] [Google Scholar]
- 11. Sliwa K, Hilfiker-Kleiner D, Petrie MC, Mebazaa A, Pieske B, Buchmann E, Regitz-Zagrosek V, Schaufelberger M, Tavazzi L, van Veldhuisen DJ, Watkins H, Shah AJ, Seferovic PM, Elkayam U, Pankuweit S, Papp Z, Mouquet F, McMurray JJ.. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail 2010;12:767–778. [DOI] [PubMed] [Google Scholar]
- 12. Hilfiker-Kleiner D, Haghikia A, Nonhoff J, Bauersachs J.. Peripartum cardiomyopathy: current management and future perspectives. Eur Heart J 2015;36:1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haghikia A, Podewski E, Libhaber E, Labidi S, Fischer D, Roentgen P, Tsikas D, Jordan J, Lichtinghagen R, von Kaisenberg CS, Struman I, Bovy N, Sliwa K, Bauersachs J, Hilfiker-Kleiner D.. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol 2013;108:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hilfiker-Kleiner D, Sliwa K.. Pathophysiology and epidemiology of peripartum cardiomyopathy. Nat Rev Cardiol 2014;11:364–370. [DOI] [PubMed] [Google Scholar]
- 15. Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, Luchtefeld M, Poli V, Schneider MD, Balligand JL, Desjardins F, Ansari A, Struman I, Nguyen NQ, Zschemisch NH, Klein G, Heusch G, Schulz R, Hilfiker A, Drexler H.. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 2007;128:589–600. [DOI] [PubMed] [Google Scholar]
- 16. Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, Hacker MR, Rhee JS, Mitchell J, Mahmood F, Hess P, Farrell C, Koulisis N, Khankin EV, Burke SD, Tudorache I, Bauersachs J, del Monte F, Hilfiker-Kleiner D, Karumanchi SA, Arany Z.. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 2012;485:333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teichman SL, Unemori E, Teerlink JR, Cotter G, Metra M.. Relaxin: review of biology and potential role in treating heart failure. Curr Heart Fail Rep 2010;7:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Samuel CS, Bodaragama H, Chew JY, Widdop RE, Royce SG, Hewitson TD.. Serelaxin is a more efficacious antifibrotic than enalapril in an experimental model of heart disease. Hypertension 2014;64:315–322. [DOI] [PubMed] [Google Scholar]
- 19. Damp J, Givertz MM, Semigran M, Alharethi R, Ewald G, Felker GM, Bozkurt B, Boehmer J, Haythe J, Skopicki H, Hanley-Yanez K, Pisarcik J, Halder I, Gorcsan J III, Rana S, Arany Z, Fett JD, McNamara DM, Investigators I.. Relaxin-2 and soluble Flt1 levels in peripartum cardiomyopathy: results of the multicenter IPAC study. JACC Heart Fail 2016;4:380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hilfiker-Kleiner D, Hilfiker A, Fuchs M, Kaminski K, Schaefer A, Schieffer B, Hillmer A, Schmiedl A, Ding Z, Podewski E, Poli V, Schneider MD, Schulz R, Park JK, Wollert KC, Drexler H.. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ Res 2004;95:187–195. [DOI] [PubMed] [Google Scholar]
- 21. Hoch M, Fischer P, Stapel B, Missol-Kolka E, Sekkali B, Scherr M, Favret F, Braun T, Eder M, Schuster-Gossler K, Gossler A, Hilfiker A, Balligand JL, Drexler H, Hilfiker-Kleiner D.. Erythropoietin preserves the endothelial differentiation capacity of cardiac progenitor cells and reduces heart failure during anticancer therapies. Cell Stem Cell 2011;9:131–143. [DOI] [PubMed] [Google Scholar]
- 22. Hilfiker-Kleiner D, Shukla P, Klein G, Schaefer A, Stapel B, Hoch M, Muller W, Scherr M, Theilmeier G, Ernst M, Hilfiker A, Drexler H.. Continuous glycoprotein-130-mediated signal transducer and activator of transcription-3 activation promotes inflammation, left ventricular rupture, and adverse outcome in subacute myocardial infarction. Circulation 2010;122:145–155. [DOI] [PubMed] [Google Scholar]
- 23. Halkein J, Tabruyn SP, Ricke-Hoch M, Haghikia A, Nguyen NQ, Scherr M, Castermans K, Malvaux L, Lambert V, Thiry M, Sliwa K, Noel A, Martial JA, Hilfiker-Kleiner D, Struman I.. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest 2013;123:2143–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McGuane JT, Debrah JE, Debrah DO, Rubin JP, Segal M, Shroff SG, Conrad KP.. Role of relaxin in maternal systemic and renal vascular adaptations during gestation. Ann N Y Acad Sci 2009;1160:304–312. [DOI] [PubMed] [Google Scholar]
- 25. Lafayette RA, Hladunewich MA, Derby G, Blouch K, Druzin ML, Myers BD.. Serum relaxin levels and kidney function in late pregnancy with or without preeclampsia. Clin Nephrol 2011;75:226–232. [DOI] [PubMed] [Google Scholar]
- 26. Samuel CS, Zhao C, Bathgate RA, Du XJ, Summers RJ, Amento EP, Walker LL, McBurnie M, Zhao L, Tregear GW.. The relaxin gene-knockout mouse: a model of progressive fibrosis. Ann N Y Acad Sci 2005;1041:173–181. [DOI] [PubMed] [Google Scholar]
- 27. Ricke-Hoch M, Bultmann I, Stapel B, Condorelli G, Rinas U, Sliwa K, Scherr M, Hilfiker-Kleiner D.. Opposing roles of Akt and STAT3 in the protection of the maternal heart from peripartum stress. Cardiovasc Res 2014;101:587–596. [DOI] [PubMed] [Google Scholar]
- 28. Bennett RG. Relaxin and its role in the development and treatment of fibrosis. Transl Res 2009;154:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bennett RG, Heimann DG, Singh S, Simpson RL, Tuma DJ.. Relaxin decreases the severity of established hepatic fibrosis in mice. Liver Int 2014;34:416–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li Y, Huang C, Klindt J, Anderson LL.. Stimulation of prolactin secretion in the pig: central effects of relaxin and the antiprogesterone RU 486. Endocrinology 1993;133:1205–1212. [DOI] [PubMed] [Google Scholar]
- 31. Tang M, Mazella J, Zhu HH, Tseng L.. Ligand activated relaxin receptor increases the transcription of IGFBP-1 and prolactin in human decidual and endometrial stromal cells. Mol Hum Reprod 2005;11:237–243. [DOI] [PubMed] [Google Scholar]
- 32. Hilfiker-Kleiner D, Arany Z.. Focus on pregnancy-mediated heart and vascular disease. Cardiovasc Res 2014;101:543–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Conrad KP, Baker VL.. Corpus luteal contribution to maternal pregnancy physiology and outcomes in assisted reproductive technologies. Am J Physiol Regul Integr Comp Physiol 2013;304:R69–R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tkachenko O, Shchekochikhin D, Schrier RW.. Hormones and hemodynamics in pregnancy. Int J Endocrinol Metab 2014;12:e14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Haase N, Golic M, Herse F, Rugor J, Linz D, Solano ME, Muller DN, Dechend R.. Relaxin treatment in an Ang-II-based transgenic preeclamptic-rat model. PLoS One 2016;11:e0150743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang X, Friedl A.. A positive feedback loop between prolactin and STAT5 promotes angiogenesis. Adv Exp Med Biol 2015;846:265–280. [DOI] [PubMed] [Google Scholar]
- 37. Yang X, Meyer K, Friedl A.. STAT5 and prolactin participate in a positive autocrine feedback loop that promotes angiogenesis. J Biol Chem 2013;288:21184–21196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sarwar M, Samuel CS, Bathgate RA, Stewart DR, Summers RJ.. Serelaxin-mediated signal transduction in human vascular cells: bell-shaped concentration-response curves reflect differential coupling to G proteins. Br J Pharmacol 2015;172:1005–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]