Abstract
Diabetes is a risk factor for heart failure and cardiovascular mortality with specific changes to myocardial metabolism, energetics, structure, and function. The gradual impairment of insulin production and signalling in diabetes is associated with elevated plasma fatty acids and increased myocardial free fatty acid uptake and activation of the transcription factor PPARα. The increased free fatty acid uptake results in accumulation of toxic metabolites, such as ceramide and diacylglycerol, activation of protein kinase C, and elevation of uncoupling protein-3. Insulin signalling and glucose uptake/oxidation become further impaired, and mitochondrial function and ATP production become compromised. Increased oxidative stress also impairs mitochondrial function and disrupts metabolic pathways. The diabetic heart relies on free fatty acids (FFA) as the major substrate for oxidative phosphorylation and is unable to increase glucose oxidation during ischaemia or hypoxia, thereby increasing myocardial injury, especially in ageing female diabetic animals. Pharmacological activation of PPARγ in adipose tissue may lower plasma FFA and improve recovery from myocardial ischaemic injury in diabetes. Not only is the diabetic heart energetically-impaired, it also has early diastolic dysfunction and concentric remodelling. The contractile function of the diabetic myocardium negatively correlates with epicardial adipose tissue, which secretes proinflammatory cytokines, resulting in interstitial fibrosis. Novel pharmacological strategies targeting oxidative stress seem promising in preventing progression of diabetic cardiomyopathy, although clinical evidence is lacking. Metabolic agents that lower plasma FFA or glucose, including PPARγ agonism and SGLT2 inhibition, may therefore be promising options.
Keywords: Diabetes, Diabetic cardiomyopathy, Diabetic heart, Metabolism, Metabolic remodelling
1. Diabetic cardiomyopathy: an individual entity
Although heart failure and diabetes were thought to co-exist as a single entity as early as in 1881,1 it was only in 1972 that Rubler and colleagues provided the first evidence in four diabetic patients with overt heart failure: they described ventricular hypertrophy and diffuse myocardial fibrosis, independent of alcohol consumption, structural, vascular, and coronary disease.2 The term ‘diabetic cardiomyopathy’ (DCM) was coined from then and is commonly used to describe myocardial structural and functional changes that occur in patients with diabetes. Although it is known that diabetes increases the risk of developing heart failure by two- to three-fold after adjustment for other cardiovascular (CV) risk factors,3 a clear diagnostic algorithm for DCM is lacking. Clinical distinction between DCM and cardiomyopathies of other aetiologies is limited, making it diagnostically impractical. Nevertheless, there are pathophysiological differences (discussed further in this Spotlight issue) and metabolic remodelling (discussed in this review) characteristic of DCM, suggesting that it is indeed a unique entity requiring further investigation and early intervention to halt disease progression.
2. Effects of diabetes on myocardial metabolism
2.1 The healthy heart
The heart, predominantly an aerobic organ, relies heavily on the oxidation of substrates, such as free fatty acids (FFA), glucose, lactate, ketone bodies, and some amino acids, to generate adenosine triphosphate (ATP), the major source of energy. The process of substrate selection is dynamic and depends largely on substrate availability, oxygen concentration, and myocardial workload. Such dynamic processes are integrated to ensure that myocardial contractile performance and housekeeping functions are maintained. Under normal physiological conditions, more than 90% of ATP is generated in mitochondria, and 60–70% from the oxidation of FFA.4 Interaction between substrate utilization is governed by the ‘Randle cycle’, the reciprocal and dependent metabolic relationship between FFA and glucose oxidation (see Figure 1).
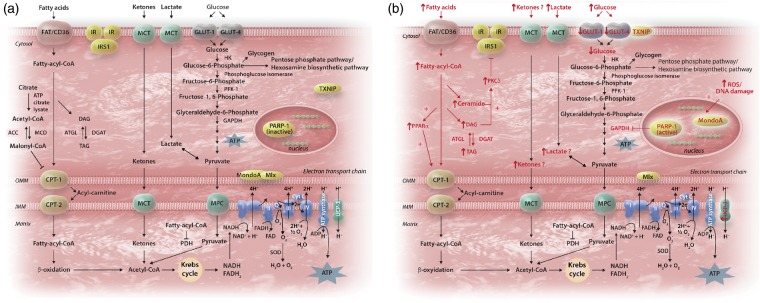
Figure 1.
(A) The healthy heart. The reciprocal relationship between myocardial substrate oxidation is governed by Randle cycle for mitochondrial generation of ATP: oxidation of fatty acid leads to increased fatty acyl-CoA which inhibits pyruvate dehydrogenase; whereas glucose oxidation increases cytosolic citrate, a precursor of malonyl-CoA which inhibits CPT-1. In healthy heart, the predominant substrate used is fatty acid and glucose, and occasionally lactate, pyruvate or ketone bodies. (B) The diabetic heart. Hyperglycaemia increases ROS and activates PARP-1, which then inhibits GAPDH and increases glycolytic intermediates. Under the transcription factor MondoA, TXNIP also shuttles from cytosol to plasma membrane and inhibits GLUT-1, reducing further uptake of glucose. Furthermore, as diabetes progresses, fatty acids (and potentially ketone bodies and other substrates) become increasingly relied on as the substrate for oxidation; parallels the upregulation of UCP-3. However, the uncoupling between uptake and oxidation of fatty acids leads to accumulation of toxic metabolites, which activates protein kinase C and further impairs insulin signalling. ACC, acyl-coA carboxylase; ATGL, adipose triglyceride lipase; CPT-1/2, carnitine palmitoyl transferase-1/2; DAG, diacyglycerol; DGAT, diacylglycerol transferase; IR, insulin receptor; IRS1, insulin receptor substrate-1; FAT, fatty acid transporter; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GLUT, glucose transporter; HK, hexokinase; MCD, malonyl-CoA decarboxylase; MCT, monocarboxylase transporter; MPC, mitochondrial pyruvate carrier; O/IMM, outer/inner mitochondrial membrane; PDH, pyruvate dehydrogenase; PFK-1, phosphofructose kinase-1; PKC, protein kinase C; PPAR, peroxisome proliferator activated receptor; ROS, reactive oxygen species; SOD, superoxide dismutase; UCP, uncoupling protein; TAG, triacylglycerol; TXNIP, Thioredoxin interacting protein.
Free fatty acids enter the cytosolic compartment via transporters, such as FA translocase/CD36 (FAT/CD36), plasma membrane FA binding protein (FABPpm), and FA transport proteins (FATP1 and 6).5 In response to certain stimuli, such as increased insulin or activation of AMP-activated protein kinase (AMPK), FAT/CD36 ‘shuttles’ from intracellular vesicles to the sarcolemma to increase the uptake of FA.6 Upon entry into the cytosol, the non-esterified FA are esterified to fatty acyl-CoA. Depending on myocardial demand, fatty acyl-CoA is either stored in the myocardial lipid pool or enters the mitochondria for β-oxidation via the carnitine shuttle: carnitine palmitoyl transferase-1 (CPT-1) being the rate-limiting enzyme for mitochondrial uptake of FA. Oxidation of FFA triggers an increase in mitochondrial ratios of [acetyl-CoA]/[CoA] and [NADH]/[NAD+], both of which inhibit the activity of PDH complex. Ketone bodies, produced from FFA in the liver, can also be metabolised to acetyl-CoA for entry into Krebs cycle.5
Glucose uptake is facilitated by transporters, most notably the insulin-independent GLUT1 and insulin-dependent GLUT4 in the heart. Similar to FAT/CD36, glucose transporters also ‘shuttle’ between intracellular vesicles and the sarcolemma in response to stimuli. After entering the cytosol, glucose is phosphorylated by hexokinase to glucose-6-phosphate, which enters glycolysis, glycogenesis, the pentose phosphate pathway, or the hexosamine biosynthetic pathway. Glycolysis generates a small amount of ATP independent of oxygen availability, and is regulated mainly by phosphofructokinase, which is inhibited by cytosolic citrate from the Krebs cycle. Cytosolic citrate is also the major precursor of malonyl-CoA, which inhibits CPT-1. The end product of glycolysis is pyruvate, which enters mitochondria for oxidation in normoxia or is reduced to lactate in the cytosol under hypoxia. Mitochondrial pyruvate dehydrogenase (PDH) is the key enzyme governing the oxidative decarboxylation of pyruvate to acetyl-CoA. Lactate, readily extracted from the bloodstream, can be converted to pyruvate in the cytosol and further metabolized to acetyl-CoA for ATP generation.5
Arising from the oxidation of a variety of substrates, acetyl-CoA enters the Krebs cycle to produce NADH and FADH2, which donate electrons to the electron transport chain thereby creating the proton electrochemical gradient needed to generate ATP. Oxidation of FFAs generates more ATP compared to glucose, but at the expense of greater oxygen consumption. Therefore, when oxygen availability is low, glucose oxidation is more ‘metabolically efficient’.
2.2 The diabetic heart
Normally, with post-prandial elevated blood glucose, pancreatic β-cells take up glucose leading to increased generation of mitochondrial ATP, which closes the ATP-sensitive KATP channel, with accumulation of K+ ions that depolarises the plasma membrane.7 Depolarisation activates calcium channels, causing an influx of Ca2+ and the eventual exocytosis of insulin.7 However, this process is defective in diabetes for various reasons, which may include decreased glucokinase activity8,9 and reduction in mitochondrial mass or in the ability to generate ATP.7 Metabolically, it is characterised by rapid defective (type 1 diabetes, T1D) or gradual diminution (type 2 diabetes, T2D) of insulin secretion, leading to increased extracellular glucose and greater reliance on fatty acid oxidation. Early in T2D, the lack of response to insulin in peripheral organs is over-compensated by increased insulin secretion, resulting in hyperinsulinaemia. Hyperinsulinaemia may be sustained for a long time, or may cause a gradual loss of pancreatic function, resulting in hypoinsulinaemia and hyperglycaemia.5 In both T1D and T2D, failure of insulin to suppress hormone sensitive lipase in adipose tissue and very low-density lipoprotein secretion in the liver increases circulating FFAs. This, in turn activates peroxisome proliferator activated receptor-α (PPARα), a transcription factor that upregulates myocardial FFA uptake and metabolism while decreasing GLUT4,10,11 leading to systemic hyperglycaemia. Consequently, therapeutic strategies should perhaps focus on minimizing the delivery or type of substrates supplied.12
In the T2D male GK rat hearts, despite normal basal glucose uptake, insulin-stimulated glucose uptake was 50% lower in diabetic rat hearts than controls.13 Initial decreases occurred in the GLUT-4, phosphorylated insulin receptor β-subunit, insulin receptor substrate-1, and PI3-kinase,13 suggesting that these pre-receptor changes are responsible for early insulin resistance.
2.2.1 Impact of substrate overload on myocardial mitochondria
The overabundance of food, as occurs in certain western cultures, is thought to cause metabolic dysfunction, obesity, and diabetes, and is a risk factor for developing DCM. Used in many sweeteners, high dietary fructose induced cardiac dysfunction in diabetes via post-translational modifications, including the formation of advanced glycation end products and O-GlcNAcylation (for a comprehensive review, see ref.14). On the other hand, 3 weeks of high fat diet in male Wistar rat led to decreased myocardial efficiency (hydraulic power divided by oxygen consumption) despite normal glucose oxidation. This was driven by elevated FFA oxidation and mitochondrial uncoupling with increased expression of mitochondrial uncoupling protein-3 (discussed later).15 Nine-week-old wild-type mice (C57BL/6NJ) provided with 4 months of high fat diet exhibited diastolic dysfunction associated with impaired mitochondrial respiration and ATP production.16 However, in different animal models of heart failure a high fat diet did not further impair cardiac and mitochondrial function,17–19 suggesting that the originating stimulus may be important.
2.2.2 Myocardial steatosis
Diabetic myocardium has an increased triacylglycerol (TAG) content, largely owing to greater FA availability than oxidation. In several clinical studies, proton (1H)-MRS has revealed that diabetic patients have between 1.5- and 2.3-fold higher myocardial TAG levels compared to non-diabetic controls,20–22 the levels predicting concentric left ventricular (LV) remodelling and subclinical contractile dysfunction.20 However, substrate oxidation and metabolic flexibility were not assessed in humans, making it difficult to determine whether it was the overabundance of substrate, or excessive substrate oxidation, leading to oxidative stress that leads to the cardiac dysfunction.23–25
Increased plasma FFA concentrations increase the flux through myocardial FFA oxidation via activation of the PPARα transcription factor,15,26 leading to the upregulation of enzymes involved in FFA oxidation, such as the acyl-CoA dehydrogenases. However, because cardiomyocytes are not specialised to store lipids, increased long-chain fatty acyl-CoA is diverted towards the production of diacylglycerol and ceramide (Figure 1). Such intermediates are thought to be toxic, compromising ATP production and overall cell viability, via the activation of several stress kinases, including protein kinase C (PKC; for review, see ref.27). PKC inhibits the metabolic action of insulin by phosphorylating the serine/threonine residues on the insulin receptor and/or its substrates,28 disrupting insulin signalling, inhibiting insulin-stimulated translocation of GLUT4, inducing apoptosis, and leading to lower basal expression of hypoxia inducible factor-1α and vascular endothelial growth factor.27 Importantly, pharmacological inhibition of PKC ameliorates FFA-mediated inhibition of basal and insulin-stimulated glucose oxidation and normalizes diastolic function in the STZ-treated T1D heart without altering the circulating metabolites.29
2.2.3 Ketosis: friend or foe for the diabetic heart?
The ketone bodies, acetoacetate and β-hydroxybutyrate (β-OHB), are generated by the liver from non-esterified FAs in response to hypoinsulinaemia and hypoglycaemia, and are oxidized by most body tissues to form acetyl-CoA. Ketosis has always been feared in patients with diabetes, being associated with life-threatening acidosis. However, a recent study showed that, amongst patients with type 2 diabetes who presented with hyperglycaemic crisis, those with ketosis (but not acidosis) had lower all-cause mortality than those without,30 suggesting that ketosis may potentially be protective in diabetes. Two recent independent studies also showed that ketone body metabolism is elevated in the failing (albeit non-diabetic) myocardium.31,32 Given that exogenous d-β-hydroxybutyrate, consumed as a ketone ester drink, was metabolised by exercising skeletal muscle to increase endurance performance in athletes and healthy rats,33,34 it may be that increased ketone metabolism in the diabetic heart is compensating for defects in mitochondrial energy transduction associated with acute insulin deficiency.35
2.2.4 Uncoupling proteins
Uncoupling proteins (UCPs) are mitochondrial anion carriers that dissipate the proton electrochemical gradient by transferring protons, generated during oxidative phosphorylation, back into the mitochondrial matrix without the concomitant synthesis of ATP (Figure 1). In patients undergoing coronary bypass surgery, upregulation of cardiac UCP-3 correlated positively with plasma concentrations of FFA.36 In mice, elevation of UCP-3 expression is mediated via increased FFA stimulation of nuclear transcription factor, PPARα.26 In chronically infarcted or high-fat diet induced rat hearts, increased UCP-3 concentrations are associated with mitochondrial uncoupling and decreased cardiac efficiency.37,15 The db/db mice has increased myocardial UCP3 that increased mitochondrial inefficiency following ischaemia.38 Activation of UCPs may be controlled by reactive oxygen species (ROS), potentially via glutathionylation.39
3. Oxidative stress and metabolic dysfunction in diabetic cardiomyopathy
Diabetes is often linked to inflammation and is associated with increased levels of C-reactive protein and interleukin-6.40 Although there is a long-standing idea that insulin resistance and ectopic adiposity confer an increased risk of CV events, a new school of thought is that myocardial insulin resistance maybe a defence against glucotoxicity and oxidative stress.12 This is based on pre-clinical evidence that impaired mitochondrial oxidative capacity is not an early event in the development of insulin resistance, but follows increased ROS production with inhibition of mitochondrial ROS production reversing insulin resistance.41
Mitochondrial respiration is the major source of ROS, central to a number of biological processes, including cell proliferation, differentiation, adaptation to hypoxia, autophagy, immune function, hormone signalling, and cell survival. ROS production is usually counterbalanced by clearance via cellular antioxidant defence systems, such as superoxide dismutase, glutathione peroxidase, catalase, the thioredoxin system, and antioxidant molecules, such as vitamin E. However, in diabetes, ROS accumulates and causes non-specific oxidative damage to DNA, proteins, lipids, or other macromolecules.42
Hyperglycaemia also induces cellular damage via four major pathways: activation of the PKC pathway via diacylglycerol, increased hexosamine pathway flux, increased advanced glycation end products, and increased polyol pathway flux.43,44 All pathways increase ROS production and activated nuclear poly-(ADP-ribose)-polymerase (PARP), which cleaves NAD+ into nicotinamide and ADP-ribose.44 Overactivation of PARP in hyperglycaemia forces the cell to synthesize NAD+ via the salvage pathway which consumes ATP.45 The process also leads to the ribosylation and inactivation of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which in turn increases glycolytic intermediates and activates the proinflammatory transcription factor NF-κB.44 Although pharmacological inhibition of PARP abolishes hyperglycaemia-induced cardiac structural dysfunction in T1D models of female NOD mice and STZ-induced male Wistar rats,46 to date there has been no evidence that PARP inhibition improves the systemic metabolic profile in diabetes.
Catalase plays an important role in catabolizing hydrogen peroxide, and cardiac catalase activity is elevated in diabetes potentially as an early defence against reactive oxidants produced during aerobic metabolism.47–49 Inhibition of cardiac catalase (by 3-amino-1,2,4-triazole) reduced the antioxidant transcription factor, nuclear factor erythroid-factor-2 (Nrf2), elevating PARP-1 and lipid peroxidation in STZ-induced T1D animals.50 Importantly, both direct and indirect activation of catalase in STZ-induced T1D and KK T2D rats prevented protein nitration, inflammation, and cardiomyopathy.48,50,51 However, clinical evidence in this area is lacking and it remains unknown if targeting inflammation or oxidative stress in DCM confers benefit.
In 2002, thioredoxin interacting protein (TXNIP) was reportedly the gene most upregulated by high glucose concentrations in a human islet oligonucleotide gene expression microarray;52 and one of the most responsive genes to blood glucose levels and insulin signalling in T2D patients.53 Ubiquitously expressed and pro-apoptotic, TXNIP exerts its effect via inhibition of the antioxidant thioredoxin, but also has some thioredoxin-independent effects,54 including direct inhibition of glucose uptake by GLUT155,56 through the transcriptional complex, MondoA:Mlx.57 In both high dose STZ-induced T1D and ob/ob T2D mice, administration of a calcium channel blocker reduced the cardiac expression of TXNIP and cleaved caspases in vivo,58 but it is not known if cardiac function was preserved. Although TXNIP may reduce hypertrophy, making its reduction undesirable in DCM, the reported effects of TXNIP on cardiac hypertrophy are conflicting and inconclusive.59,60 Stimuli of TXNIP, such as shear stress, commonly cause hypertrophy and agents that reduced TXNIP are anti-hypertrophic.58,61 Interestingly, patients with diabetes who use calcium channel blockers, in particular verapamil, have lower serum glucose levels than non-users,62 suggesting a potential protective role in pancreatic islet cells and DCM.
4. How does the diabetic heart cope with hypoxia or ischaemia?
Even in the normal heart, hypoxia or ischaemia cause profound changes in metabolic substrate utilization and oxidation. In particular, myocardial FFA oxidation, PPARα expression (together with its downstream targets, such as UCP3), and mitochondrial oxygen consumption are decreased in chronic hypoxia, whereas glycolysis is enhanced.63–65 In mice with activated PPARα, myocardial FFA oxidation is increased and associated with a reduction in cardiac efficiency and decreased recovery of contractile function post low-flow ischaemia,66,67 suggesting that mechanical dysfunction occurs as a result of the inability to increase glycolysis during a decrease in oxygen availability.
It is increasingly recognised that it is the lack of metabolic flexibility, rather than specific substrate preference that predisposes the diabetic heart to injury. Abnormal myocardial substrate metabolism was attenuated when high-fat/low dose STZ-induced T2D rats were subjected to chronic hypoxia; suggesting that the diabetic heart retained sufficient metabolic plasticity to adapt to hypoxia.68 Additionally, during low-flow ischaemia, the isolated T1D rat heart used FFA oxidation for oxidative phosphorylation and production of ATP, suggesting a protective non-deleterious role of FFAs when glucose metabolism was down-regulated.69 Myocardial TAG may be a dynamic, instead of inert, reservoir for FFAs23–25 (for review, see ref.70). Diabetic hearts contain a high TAG content, which contributes significantly to overall oxidative metabolism.71 While some studies suggested that accumulation of TAG is cardioprotective by virtue of channeling FFA oxidation away from toxic metabolites72 and improves cardiac function from ischaemia,73 others argue that lower TAG protects against DCM (in Akita and STZ-T1D mice and T2D patients).74,75 Overall, TAG contribution to ischaemic recovery has not been explored in diabetes.
Activation of AMPK by metformin, a metabolic-sensing ‘master switch’ that promotes both cellular uptake of glucose and β-oxidation of FFAs, not only reduces ischaemic-reperfusion injury and limits myocardial infarct size, but also attenuates remodelling and heart failure in diabetes. However, animal experiments involving pharmacological activation of PPAR in diabetic hearts are inconclusive; potentially due to the specificity of the agent for the various PPAR isoforms. Whilst all, except for tetradecythioacetic acid, TTA, a PPARα agonist that also has potent antioxidant properties76 demonstrated reduction in circulating FFA and increased glucose oxidation, overall cardiac effects were inconsistent: those employing a PPARγ agonist rosiglitazone and TTA demonstrated improved ischaemic tolerance;76–78 whereas others using BM17.0744 or 2-(2-(4-phenoxy-2-propylphenoxy)ethyl)indole-5-acetic acid (PPARα and PPARγ agonists, respectively) showed no difference.79,80 It has been suggested that there may be an interaction between substrate availability, PPARα activation and ceramide formation,70 in which rats treated with a PPARα agonist and fed with high fat diet (34% fat) have increased myocardial ceramide, when the effect was attenuated in rats fed with normal chow diet (3% fat).81 Although ceramide formation was not assessed, rats fed a high fat diet had increased PPARα expression, elevated FA oxidation, increased UCP3 expression, reduced glycolysis and consequent contractile dysfunction when subjected to hypoxia.65
4.1 Age and gender
Age-dependent studies in animals (db/db mice, Zucker fa/fa, and Goto-Kakizaki rats) reveal that diabetic hearts rely increasingly on FFA oxidation and less on glucose oxidation for the formation of acetyl-CoA with increasing age, potentially due to substrate availability.78,82 Age was associated with increased FFA oxidation, reduced glucose oxidation, worsened contractility and decreased recovery from ischaemic insult.78,82,83 Compared to age-matched, non-diabetic counterparts, both the young and ageing diabetic rats had increased FFA oxidation.78 However, glucose uptake and lactate production were unchanged regardless of diabetes in the younger rats during ischaemia. On the other hand, the ageing fa/fa rats had lower glucose uptake and lactate production than the age-matched controls, suggesting an overreliance of ageing diabetic hearts on FFA oxidation.78
With respect to gender, female diabetic animals typically display greater myocardial abnormalities than those of the male, including increased cardiac hypertrophy and lower insulin-stimulated glucose uptake,82,84 mimicking clinical observations in diabetic patients.85 Female STZ-induced T1D animals developed diastolic and systolic dysfunction much earlier than their male counterparts, with earlier ventricular remodelling, including increased LV dilation and reduced ejection fraction.86 These changes were associated with down regulation of pro-survival Pim-1, and upregulation of proapoptotic signalling caspases, microRNA-1, and microRNA-208a86 (see ref.87 for comprehensive review).
5. Energetic changes in diabetic heart: evidence from magnetic resonance imaging studies
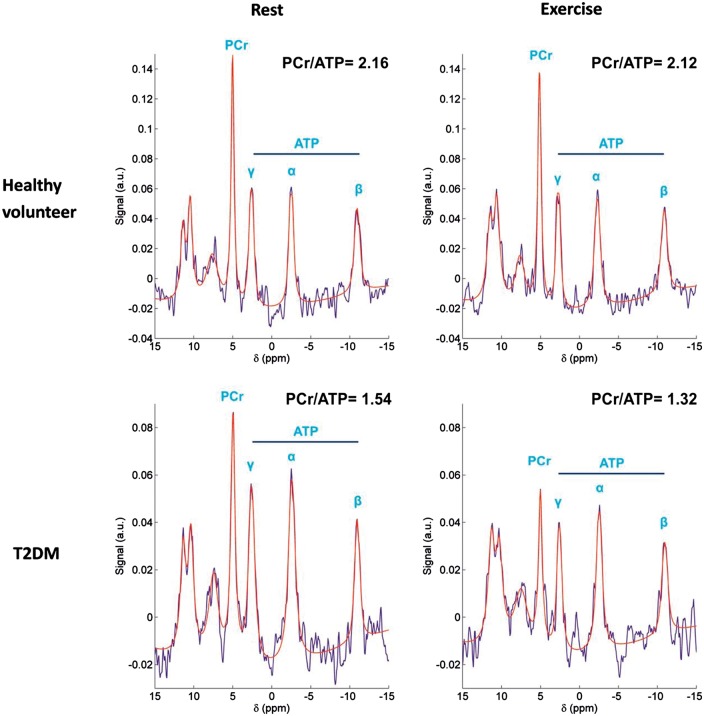
The clinical assessment of myocardial energetic status can be determined using the ratios of PCr to ATP (PCr/ATP) non-invasively via phosphorus-31 cardiac magnetic resonance spectroscopy (31P-MRS). 31P-MRS yields peaks for PCr and the three phosphorus atoms of ATP, all of which are proportional to the cellular concentration of the metabolites. The myocardial PCr/ATP ratio also correlates well with the New York Heart Association functional status, indices of systolic or diastolic function and survival rate in heart failure patients.4 Despite ‘normal’ cardiac function measured using echocardiography and the lack of known coronary artery disease or ECG detectable ischaemic changes, diabetic patients have a lower myocardial PCr/ATP than the matched healthy controls, suggesting that diabetic patients are ‘cardiac energy-deficient’.88 The PCr/ATP ratios also correlated negatively with fasting plasma FFA concentrations.88 Additionally, the pre-existing energetic deficit in DCM was exacerbated by exercise (Figure 2),89 supporting the notion that the cardiac metabolic reserve is impaired in T2D.
Figure 2.
Rest and exercise myocardial 31P-MR spectra in a healthy volunteer (top row) and a T2DM patient (bottom row). T2DM was associated with significantly lower myocardial PCr/ATP than control at rest, and the decrease was exacerbated during exercise, suggesting a pre-existing myocardial energy deficit in type 2 diabetes mellitus. (Reprinted with permission).
In 1999, Cline and colleagues used 13C- and 31P-MRS to measure intracellular concentrations of glucose, glucose-6-phosphate and glycogen in gastrocnemius muscle of T2D patients, to demonstrate that insulin-stimulated glycogen synthesis is impaired.90 Additionally, studies using 18F-fluorodeoxyglucose positron emission tomography showed that T2D patients had lower insulin-stimulated glucose uptake in the skeletal muscle,91 with either normal91 or lower92 glucose uptake in the myocardium. The disparities in the glucose findings may be due to difference in the severity of diabetes.
Multidetector-computed tomography, MRI, ultrasonography, and 1H-MRS have been used to quantify lipid content within an organ, and to examine the association of fat depots with both systemic and local manifestations of disease as the distribution of excess fat may be an important determinant of CV risk.93 As compared to subcutaneous adiposity, ectopic, and visceral adiposity or ‘acquired lipodystrophy’ is linked to insulin resistance and diabetes.94 Epicardial adipose tissue (EAT), a form of visceral fat, has no anatomical barriers with the myocardium and by secreting proinflammatory adipokines and cytokines may play a significant role in diabetic heart. Supporting this, there is a negative correlation between EAT volumes and cardiac contractile function in obese T2D patients.95
6. Myocardial structural and functional changes in diabetes
Although an increased LV mass is independently associated with diabetes,96 often the increase in patients with diabetes is modest. Frequently reported in patients with T2D, LV concentric remodelling represents the main structural characteristic and is more predictive of CV mortality than eccentric remodelling.20,97,98 Importantly, a stepwise multivariable regression study revealed myocardial steatosis to be the only independent predictor of concentric remodelling in patients with T2D.20 Although it is tempting to suggest that myocardial steatosis represents a major link between T2D and the development of LV concentric remodelling, a cause-effect relationship has yet to be established.
Interstitial fibrosis has also been implicated in the pathogenesis of LV hypertrophy and occurs in the more advanced stages of DCM.2 In stable/early DCM the role of interstitial fibrosis is much less clear, as abnormal myocyte hypertrophy rather than fibrosis appears to predominate.99 Cardiac magnetic resonance (CMR) T1 mapping for extracellular volume (ECV) quantification allows the non-invasive measurement of fibrosis100 that correlates closely with collagen area in histology.101 Using this technique, two studies have demonstrated no significant increase in ECV and native (pre-contrast) T1 mapping in young patients with well-controlled T2D, suggesting the absence of significant fibrosis in the presence of LV concentric remodelling and diastolic dysfunction.20
Diastolic abnormalities are an early functional defect in the diabetic heart, with the prevalence rates in asymptomatic, normotensive diabetic patients ranging from 15 to 75%.95,102,103 Yet, there is mild to little systolic dysfunction,95 which may depend on the severity or duration of disease. Detection of subclinical dysfunction is made available via the use of echocardiographic strain imaging or CMR, with reduced longitudinal contractility and impaired systolic circumferential strain.
7. Rescuing diabetic cardiomyopathy: clinical perspectives
Over the past 10–20 years, the therapeutic approach to the prevention and/or treatment of DCM has largely been aimed at both reducing the incidence of CV events associated with diabetes and halting the progression of diabetic heart towards heart failure. After concerns of cardiac adverse events related to the use of the PPARγ activator, Rosiglitazone, were raised, it has become mandatory for national drug regulatory bodies to enforce the evaluation of CV safety of new anti-diabetic medications. One such medication is Empagliflozin, a renal sodium-glucose cotransporter-2 (SGLT2) inhibitor. Initially designed to evaluate CV safety, the EMPA-REG OUTCOME trial104 showed significant reduction of CV or all-cause mortality and the incidence of new heart failure, even though the impact on glucose concentrations was modest (about 0.4% reduction of HbA1c over 94 weeks in the empaglifozin arm compared to control).104 The mortality benefits were remarkable, as the beneficial effect was almost immediate (< 3 months), suggesting that the benefits may well extend beyond SGLT2 inhibition. Explanations for the effects include the inhibition of renin-angiotensin-aldosterone system beyond ACE-inhibition,105 activation of AMPK,106 and improved energetics via induction of mild ketosis.107,108 Plasma concentrations of β-OHB rose from 0.25 to 0.56 mM after 4 weeks of treatment;107 ketone body oxidation yields more ATP per oxygen consumption than palmitate, so being more more ‘energy-efficient’.109
A number of other metabolic agents have also been proposed to benefit the diabetic heart. Theoretically PPARγ agonism may be beneficial, but the clinical utility is limited by the associated sodium/water retention properties. Agents that lower substrate availability by means of slowing gastric emptying (such as a glucagon-like-peptide110), inhibiting hepatic gluconeogenesis (such as glucagon antagonist and metformin111,112), or inhibiting renal reabsorption of glucose (such as SGLT2 inhibitors) have yielded relatively positive CV outcomes.
Newer therapeutic approaches towards DCM have focused on reducing oxidative stress associated with diabetes (for comprehensive review, see ref.113). Epidemiological studies show that inhibition of the renin-angiotensin-aldosterone-system reduces CV adverse events in T2D. The mechanism(s) of benefit are not completely understood, although prevention of mitochondrial dysfunction and oxidative stress are commonly postulated. By virtue of reducing cellular oxidative activity, several other agents, including calcium channel antagonists and statins may also limit DCM. Pre-clinical therapies of DCM targeting oxidative stress include upregulation of enzymes such as catalase, superoxide dismutase, glutathione peroxidase or thioredoxin, and antioxidants such as vitamin C, vitamin E, zinc, resveratrol or coenzyme Q10. Depending on the disease severity, the timing of an intervention, especially on antioxidant enzymes, may be critical, as it is likely that excessive production of free radicals in chronic disease may render such interventions impractical.47 With the exception of vitamin C and E (which showed no evidence for prevention or reversal of CV events in patients with diabetes),114,115 other approaches have not yet been examined clinically. Similarly, ruboxistaurin, a protein kinase C-β inhibitor, limits DCM,116 nephropathy,117 and retinopathy118 in animal studies. Although effects on limiting other complications of diabetes have been promising in phase 2-3 clinical trials,119,120 an effect of ruboxistaurin on DCM has not yet been demonstrated in humans.
8. Conclusions
Metabolic changes in the diabetic heart are complicated. In general, it is characterised by failure of insulin to promote glucose uptake, substrate overload and increased reliance on fatty acid oxidation. The initial adaptation and subsequent maladaptation of the diabetic heart reflects not only a loss of metabolic flexibility, but also abnormal molecular signalling cascades including accumulation of toxic metabolites, upregulation of UCPs, and activation of stress kinases. Significant advances have been made in characterising the myocardial metabolic changes during the development and progression of DCM, including the myocardial metabolic response to ischaemia or hypoxia, and the impact of ageing and gender on the challenges. Although clinical evidence is still lacking, novel therapies targeting oxidative stress and downstream signalling seem promising. Therefore anti-diabetic therapies with pleiotropic actions may remain a mainstream strategy to treat DCM.
Conflict of interest: none declared.
Funding
Cher-Rin Chong thanks the Nuffield Medical Dominion Trust Fund, University of Oxford for her fellowship funding.
References
- 1.Leyden E. Asthma and diabetes mellitus. Zeutschr Klin Med 1881;3:358–364. [Google Scholar]
- 2.Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A.. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 1972;30:595–602. [DOI] [PubMed] [Google Scholar]
- 3.Aksnes TA, Kjeldsen SE, Rostrup M, Omvik P, Hua TA, Julius S.. Impact of new-onset diabetes mellitus on cardiac outcomes in the Valsartan Antihypertensive Long-term Use Evaluation (VALUE) trial population. Hypertension 2007;50:467–473. [DOI] [PubMed] [Google Scholar]
- 4.Neubauer S. The failing heart—an engine out of fuel. N Engl J Med 2007;356:1140–1151. [DOI] [PubMed] [Google Scholar]
- 5.Heather LC, Clarke K.. Metabolism, hypoxia and the diabetic heart. J Mol Cell Cardiol 2011;50:598–605. [DOI] [PubMed] [Google Scholar]
- 6.Heather LC, Cole MA, Atherton HJ, Coumans WA, Evans RD, Tyler DJ, Glatz JF, Luiken JJ, Clarke K.. Adenosine monophosphate-activated protein kinase activation, substrate transporter translocation, and metabolism in the contracting hyperthyroid rat heart. Endocrinology 2010;151:422–431. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman BA, Li C, Soleimanpour SA.. Mitochondrial regulation of beta-cell function: maintaining the momentum for insulin release. Mol Aspects Med 2015;42:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doliba NM, Qin W, Najafi H, Liu C, Buettger CW, Sotiris J, Collins HW, Li C, Stanley CA, Wilson DF, Grimsby J, Sarabu R, Naji A, Matschinsky FM.. Glucokinase activation repairs defective bioenergetics of islets of Langerhans isolated from type 2 diabetics. Am J Physiol Endocrinol Metab 2012;302:E87–E102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matschinsky FM. Banting Lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes 1996;45:223–241. [DOI] [PubMed] [Google Scholar]
- 10.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, Kelly DP.. The cardiac phenotype induced by PPARα overexpression mimics that caused by diabetes mellitus. J Clin Invest 2002;109:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gulick T, Cresci S, Caira T, Moore DD, Kelly DP.. The peroxisome proliferator-activated receptor regulates mitochondrial fatty acid oxidative enzyme gene expression. Proc Natl Acad Sci USA 1994;91:11012–11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taegtmeyer H, Beauloye C, Harmancey R, Hue L.. Insulin resistance protects the heart from fuel overload in dysregulated metabolic states. Am J Physiol Heart Circ Physiol 2013;305:H1693–H1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desrois M, Sidell RJ, Gauguier D, King LM, Radda GK, Clarke K.. Initial steps of insulin signaling and glucose transport are defective in the type 2 diabetic rat heart. Cardiovasc Res 2004;61:288–296. [DOI] [PubMed] [Google Scholar]
- 14.Delbridge LM, Benson VL, Ritchie RH, Mellor KM.. Diabetic cardiomyopathy: the case for a role of fructose in disease etiology. Diabetes 2016;65:3521–3528. [DOI] [PubMed] [Google Scholar]
- 15.Cole MA, Murray AJ, Cochlin LE, Heather LC, McAleese S, Knight NS, Sutton E, Jamil AA, Parassol N, Clarke K.. A high fat diet increases mitochondrial fatty acid oxidation and uncoupling to decrease efficiency in rat heart. Basic Res Cardiol 2011;106:447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sverdlov AL, Elezaby A, Qin F, Behring JB, Luptak I, Calamaras TD, Siwik DA, Miller EJ, Liesa M, Shirihai OS, Pimentel DR, Cohen RA, Bachschmid MM, Colucci WS.. Mitochondrial reactive oxygen species mediate cardiac structural, functional, and mitochondrial consequences of diet-induced metabolic heart disease. J Am Heart Assoc 2016;5:e002555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chess DJ, Khairallah RJ, O'Shea KM, Xu W, Stanley WC.. A high-fat diet increases adiposity but maintains mitochondrial oxidative enzymes without affecting development of heart failure with pressure overload. Am J Physiol Heart Circ Physiol 2009;297:H1585–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okere IC, Chandler MP, McElfresh TA, Rennison JH, Sharov V, Sabbah HN, Tserng KY, Hoit BD, Ernsberger P, Young ME, Stanley WC.. Differential effects of saturated and unsaturated fatty acid diets on cardiomyocyte apoptosis, adipose distribution, and serum leptin. Am J Physiol Heart Circ Physiol 2006;291:H38–H44. [DOI] [PubMed] [Google Scholar]
- 19.Rennison JH, McElfresh TA, Chen X, Anand VR, Hoit BD, Hoppel CL, Chandler MP.. Prolonged exposure to high dietary lipids is not associated with lipotoxicity in heart failure. J Mol Cell Cardiol 2009;46:883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levelt E, Mahmod M, Piechnik SK, Ariga R, Francis JM, Rodgers CT, Clarke WT, Sabharwal N, Schneider JE, Karamitsos TD, Clarke K, Rider OJ, Neubauer S.. Relationship between left ventricular structural and metabolic remodelling in type 2 diabetes mellitus. Diabetes 2015;65:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGavock JM, Lingvay I, Zib I, Tillery T, Salas N, Unger R, Levine BD, Raskin P, Victor RG, Szczepaniak LS.. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation 2007;116:1170–1175. [DOI] [PubMed] [Google Scholar]
- 22.Rijzewijk LJ, van der Meer RW, Smit JWA, Diamant M, Bax JJ, Hammer S, Romijn JA, de Roos A, Lamb HJ.. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol 2008;52:1793–1799. [DOI] [PubMed] [Google Scholar]
- 23.Saddik M, Lopaschuk GD.. Myocardial triglyceride turnover and contribution to energy substrate utilization in isolated working rat hearts. J Biol Chem 1991;266:8162–8170. [PubMed] [Google Scholar]
- 24.Saddik M, Lopaschuk GD.. Triacylglycerol turnover in isolated working hearts of acutely diabetic rats. Can J Physiol Pharmacol 1994;72:1110–1119. [DOI] [PubMed] [Google Scholar]
- 25.O'Donnell JM, Fields AD, Sorokina N, Lewandowski ED.. The absence of endogenous lipid oxidation in early stage heart failure exposes limits in lipid storage and turnover. J Mol Cell Cardiol 2008;44:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray AJ, Panagia M, Hauton D, Gibbons GF, Clarke K.. Plasma free fatty acids and peroxisome proliferator-activated receptor alpha in the control of myocardial uncoupling protein levels. Diabetes 2005;54:3496–3502. [DOI] [PubMed] [Google Scholar]
- 27.Geraldes P, King GL.. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res 2010;106:1319–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL.. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest 1999;104:447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arikawa E, Ma RC, Isshiki K, Luptak I, He Z, Yasuda Y, Maeno Y, Patti ME, Weir GC, Harris RA, Zammit VA, Tian R, King GL.. Effects of insulin replacements, inhibitors of angiotensin, and PKCbeta's actions to normalize cardiac gene expression and fuel metabolism in diabetic rats. Diabetes 2007;56:1410–1420. [DOI] [PubMed] [Google Scholar]
- 30.Kruljac I, Cacic M, Cacic P, Ostojic V, Stefanovic M, Sikic A, Vrkljan M. . Diabetic ketosis during hyperglycemic crisis is associated with decreased all-cause mortality in patients with type 2 diabetes mellitus. Endocrine 2016;55(1):139–143. [DOI] [PubMed] [Google Scholar]
- 31.Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Kruger M, Hoppel CL, Lewandowski ED, Crawford PA, Muoio DM, Kelly DP.. The failing heart relies on ketone bodies as a fuel. Circulation 2016;133:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bedi KC Jr, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, Rame JE.. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation 2016;133:706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox PJ, Kirk T, Ashmore T, Willerton K, Evans R, Smith A, Murray AJ, Stubbs B, West J, McLure SW, King MT, Dodd MS, Holloway C, Neubauer S, Drawer S, Veech RL, Griffin JL, Clarke K.. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab 2016;24:256–268. [DOI] [PubMed] [Google Scholar]
- 34.Murray AJ, Knight NS, Cole MA, Cochlin LE, Carter E, Tchabanenko K, Pichulik T, Gulston MK, Atherton HJ, Schroeder MA, Deacon RM, Kashiwaya Y, King MT, Pawlosky R, Rawlins JN, Tyler DJ, Griffin JL, Robertson J, Veech RL, Clarke K.. Novel ketone diet enhances physical and cognitive performance. FASEB J 2016;30:4021–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato K, Kashiwaya Y, Keon CA, Tsuchiya N, King MT, Radda GK, Chance B, Clarke K, Veech RL.. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J 1995;9:651–658. [DOI] [PubMed] [Google Scholar]
- 36.Murray AJ, Anderson RE, Watson GC, Radda GK, Clarke K.. Uncoupling proteins in human heart. Lancet 2004;364:1786–1788. [DOI] [PubMed] [Google Scholar]
- 37.Murray AJ, Cole MA, Lygate CA, Carr CA, Stuckey DJ, Little SE, Neubauer S, Clarke K.. Increased mitochondrial uncoupling proteins, respiratory uncoupling and decreased efficiency in the chronically infarcted rat heart. J Mol Cell Cardiol 2008;44:694–700. [DOI] [PubMed] [Google Scholar]
- 38.Banke NH, Lewandowski ED.. Impaired cytosolic NADH shuttling and elevated UCP3 contribute to inefficient citric acid cycle flux support of postischemic cardiac work in diabetic hearts. J Mol Cell Cardiol 2015;79:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mailloux RJ, Seifert EL, Bouillaud F, Aguer C, Collins S, Harper ME.. Glutathionylation acts as a control switch for uncoupling proteins UCP2 and UCP3. J Biol Chem 2011;286:21865–21875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM.. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001;286:327–334. [DOI] [PubMed] [Google Scholar]
- 41.Sakai K, Matsumoto K, Nishikawa T, Suefuji M, Nakamaru K, Hirashima Y, Kawashima J, Shirotani T, Ichinose K, Brownlee M, Araki E.. Mitochondrial reactive oxygen species reduce insulin secretion by pancreatic beta-cells. Biochem Biophys Res Commun 2003;300:216–222. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Tocchetti CG, Krieg T, Moens AL.. Oxidative and nitrosative stress in the maintenance of myocardial function. Free Radic Biol Med 2012;53:1531–1540. [DOI] [PubMed] [Google Scholar]
- 43.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M.. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000;404:787–790. [DOI] [PubMed] [Google Scholar]
- 44.Du X, Matsumura T, Edelstein D, Rossetti L, Zsengeller Z, Szabo C, Brownlee M.. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest 2003;112:1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szabo C. Poly(ADP-ribose) polymerase activation by reactive nitrogen species: relevance for the pathogenesis of inflammation. Nitric Oxide 2006;14:169–179. [DOI] [PubMed] [Google Scholar]
- 46.Pacher P, Liaudet L, Soriano FG, Mabley JG, Szabo E, Szabo C.. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes 2002;51:514–521. [DOI] [PubMed] [Google Scholar]
- 47.Szaleczky E, Prechl J, Feher J, Somogyi A.. Alterations in enzymatic antioxidant defence in diabetes mellitus: a rational approach. Postgrad Med J 1999;75:13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye G, Metreveli NS, Donthi RV, Xia S, Xu M, Carlson EC, Epstein PN.. Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes 2004;53:1336–1343. [DOI] [PubMed] [Google Scholar]
- 49.Turdi S, Li Q, Lopez FL, Ren J.. Catalase alleviates cardiomyocyte dysfunction in diabetes: role of Akt, Forkhead transcriptional factor and silent information regulator 2. Life Sci 2007;81:895–905. [DOI] [PubMed] [Google Scholar]
- 50.Ivanovic-Matic S, Bogojevic D, Martinovic V, Petrovic A, Jovanovic-Stojanov S, Poznanovic G, Grigorov I.. Catalase inhibition in diabetic rats potentiates DNA damage and apoptotic cell death setting the stage for cardiomyopathy. J Physiol Biochem 2014;70:947–959. [DOI] [PubMed] [Google Scholar]
- 51.Cong W, Ruan D, Xuan Y, Niu C, Tao Y, Wang Y, Zhan K, Cai L, Jin L, Tan Y.. Cardiac-specific overexpression of catalase prevents diabetes-induced pathological changes by inhibiting NF-kappaB signaling activation in the heart. J Mol Cell Cardiol 2015;89:314–325. [DOI] [PubMed] [Google Scholar]
- 52.Shalev A, Pise-Masison CA, Radonovich M, Hoffmann SC, Hirshberg B, Brady JN, Harlan DM.. Oligonucleotide microarray analysis of intact human pancreatic islets: identification of glucose-responsive genes and a highly regulated TGFbeta signaling pathway. Endocrinology 2002;143:3695–3698. [DOI] [PubMed] [Google Scholar]
- 53.Parikh H, Carlsson E, Chutkow WA, Johansson LE, Storgaard H, Poulsen P, Saxena R, Ladd C, Schulze PC, Mazzini MJ, Jensen CB, Krook A, Bjornholm M, Tornqvist H, Zierath JR, Ridderstrale M, Altshuler D, Lee RT, Vaag A, Groop LC, Mootha VK.. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med 2007;4:e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chong CR, Chan WP, Nguyen TH, Liu S, Procter NE, Ngo DT, Sverdlov AL, Chirkov YY, Horowitz JD.. Thioredoxin-interacting protein: pathophysiology and emerging pharmacotherapeutics in cardiovascular disease and diabetes. Cardiovasc Drugs Ther 2014;28:347–360. [DOI] [PubMed] [Google Scholar]
- 55.Myers RB, Fomovsky GM, Lee S, Tan M, Wang BF, Patwari P, Yoshioka J.. Deletion of thioredoxin-interacting protein improves cardiac inotropic reserve in the streptozotocin-induced diabetic heart. Am J Physiol Heart Circ Physiol 2016;310:H1748–H1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patwari P, Chutkow WA, Cummings K, Verstraeten VL, Lammerding J, Schreiter ER, Lee RT.. Thioredoxin-independent regulation of metabolism by the alpha-arrestin proteins. J Biol Chem 2009;284:24996–25003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stoltzman CA, Peterson CW, Breen KT, Muoio DM, Billin AN, Ayer DE.. Glucose sensing by MondoA:Mlx complexes: a role for hexokinases and direct regulation of thioredoxin-interacting protein expression. Proc Natl Acad Sci USA 2008;105:6912–6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen J, Cha-Molstad H, Szabo A, Shalev A.. Diabetes induces and calcium channel blockers prevent cardiac expression of proapoptotic thioredoxin-interacting protein. Am J Physiol Endocrinol Metab 2009;296:E1133–E1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshioka J, Imahashi K, Gabel SA, Chutkow WA, Burds AA, Gannon J, Schulze PC, MacGillivray C, London RE, Murphy E, Lee RT.. Targeted deletion of thioredoxin-interacting protein regulates cardiac dysfunction in response to pressure overload. Circ Res 2007;101:1328–1338. [DOI] [PubMed] [Google Scholar]
- 60.Yoshioka J, Schulze PC, Cupesi M, Sylvan JD, MacGillivray C, Gannon J, Huang H, Lee RT.. Thioredoxin-interacting protein controls cardiac hypertrophy through regulation of thioredoxin activity. Circulation 2004;109:2581–2586. [DOI] [PubMed] [Google Scholar]
- 61.Sverdlov AL, Chan WP, Procter NE, Chirkov YY, Ngo DT, Horowitz JD.. Reciprocal regulation of NO signaling and TXNIP expression in humans: impact of aging and ramipril therapy. Int J Cardiol 2013;168:4624–4630. [DOI] [PubMed] [Google Scholar]
- 62.Khodneva Y, Shalev A, Frank SJ, Carson AP, Safford MM.. Calcium channel blocker use is associated with lower fasting serum glucose among adults with diabetes from the REGARDS study. Diabetes Res Clin Pract 2016;115:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heather LC, Cole MA, Tan JJ, Ambrose LJ, Pope S, Abd-Jamil AH, Carter EE, Dodd MS, Yeoh KK, Schofield CJ, Clarke K.. Metabolic adaptation to chronic hypoxia in cardiac mitochondria. Basic Res Cardiol 2012;107:268. [DOI] [PubMed] [Google Scholar]
- 64.Ambrose LJ, Abd-Jamil AH, Gomes RS, Carter EE, Carr CA, Clarke K, Heather LC.. Investigating mitochondrial metabolism in contracting HL-1 cardiomyocytes following hypoxia and pharmacological HIF activation identifies HIF-dependent and independent mechanisms of regulation. J Cardiovasc Pharmacol Ther 2014;19:574–585. [DOI] [PubMed] [Google Scholar]
- 65.Cole MA, Abd Jamil AH, Heather LC, Murray AJ, Sutton ER, Slingo M, Sebag-Montefiore L, Tan SC, Aksentijevic D, Gildea OS, Stuckey DJ, Yeoh KK, Carr CA, Evans RD, Aasum E, Schofield CJ, Ratcliffe PJ, Neubauer S, Robbins PA, Clarke K.. On the pivotal role of PPARalpha in adaptation of the heart to hypoxia and why fat in the diet increases hypoxic injury. FASEB J 2016;30:2684–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hafstad AD, Khalid AM, Hagve M, Lund T, Larsen TS, Severson DL, Clarke K, Berge RK, Aasum E.. Cardiac peroxisome proliferator-activated receptor-alpha activation causes increased fatty acid oxidation, reducing efficiency and post-ischaemic functional loss. Cardiovasc Res 2009;83:519–526. [DOI] [PubMed] [Google Scholar]
- 67.Sambandam N, Morabito D, Wagg C, Finck BN, Kelly DP, Lopaschuk GD.. Chronic activation of PPARalpha is detrimental to cardiac recovery after ischemia. Am J Physiol Heart Circ Physiol 2006;290:H87–H95. [DOI] [PubMed] [Google Scholar]
- 68.Mansor LS, Mehta K, Aksentijevic D, Carr CA, Lund T, Cole MA, Le Page L, Sousa Fialho Mda L, Shattock MJ, Aasum E, Clarke K, Tyler DJ, Heather LC.. Increased oxidative metabolism following hypoxia in the type 2 diabetic heart, despite normal hypoxia signalling and metabolic adaptation. J Physiol 2016;594:307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.King LMS, R.J., Jones B.E., Radda G.K., Clarke K.. Fatty acids, ischaemic damage and the diabetic heart. MAGMA 1998;6:173–174. [DOI] [PubMed] [Google Scholar]
- 70.Carley AN, Lewandowski ED.. Triacylglycerol turnover in the failing heart. Biochim Biophys Acta 2016;1860:1492–1499. [DOI] [PubMed] [Google Scholar]
- 71.O'Donnell JM, Zampino M, Alpert NM, Fasano MJ, Geenen DL, Lewandowski ED.. Accelerated triacylglycerol turnover kinetics in hearts of diabetic rats include evidence for compartmented lipid storage. Am J Physiol Endocrinol Metab 2006;290:E448–E455. [DOI] [PubMed] [Google Scholar]
- 72.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV Jr, Ory DS, Schaffer JE.. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA 2003;100:3077–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kolwicz SC Jr, Liu L, Goldberg IJ, Tian R.. Enhancing cardiac triacylglycerol metabolism improves recovery from ischemic stress. Diabetes 2015;64:2817–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pulinilkunnil T, Kienesberger PC, Nagendran J, Waller TJ, Young ME, Kershaw EE, Korbutt G, Haemmerle G, Zechner R, Dyck JR.. Myocardial adipose triglyceride lipase overexpression protects diabetic mice from the development of lipotoxic cardiomyopathy. Diabetes 2013;62:1464–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hammer S, Snel M, Lamb HJ, Jazet IM, van der Meer RW, Pijl H, Meinders EA, Romijn JA, de Roos A, Smit JW.. Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J Am Coll Cardiol 2008;52:1006–1012. [DOI] [PubMed] [Google Scholar]
- 76.Khalid AM, Hafstad AD, Larsen TS, Severson DL, Boardman N, Hagve M, Berge RK, Aasum E.. Cardioprotective effect of the PPAR ligand tetradecylthioacetic acid in type 2 diabetic mice. Am J Physiol Heart Circ Physiol 2011;300:H2116–H2122. [DOI] [PubMed] [Google Scholar]
- 77.How OJ, Larsen TS, Hafstad AD, Khalid A, Myhre ES, Murray AJ, Boardman NT, Cole M, Clarke K, Severson DL, Aasum E.. Rosiglitazone treatment improves cardiac efficiency in hearts from diabetic mice. Arch Physiol Biochem 2007;113:211–220. [DOI] [PubMed] [Google Scholar]
- 78.Sidell RJ, Cole MA, Draper NJ, Desrois M, Buckingham RE, Clarke K.. Thiazolidinedione treatment normalizes insulin resistance and ischemic injury in the zucker Fatty rat heart. Diabetes 2002;51:1110–1117. [DOI] [PubMed] [Google Scholar]
- 79.Aasum E, Belke DD, Severson DL, Riemersma RA, Cooper M, Andreassen M, Larsen TS.. Cardiac function and metabolism in Type 2 diabetic mice after treatment with BM 17.0744, a novel PPAR-alpha activator. Am J Physiol Heart Circ Physiol 2002;283:H949–H957. [DOI] [PubMed] [Google Scholar]
- 80.Carley AN, Semeniuk LM, Shimoni Y, Aasum E, Larsen TS, Berger JP, Severson DL.. Treatment of type 2 diabetic db/db mice with a novel PPARgamma agonist improves cardiac metabolism but not contractile function. Am J Physiol Endocrinol Metab 2004;286:E449–E455. [DOI] [PubMed] [Google Scholar]
- 81.Baranowski M, Blachnio A, Zabielski P, Gorski J.. PPARalpha agonist induces the accumulation of ceramide in the heart of rats fed high-fat diet. J Physiol Pharmacol 2007;58:57–72. [PubMed] [Google Scholar]
- 82.Aasum E, Hafstad AD, Severson DL, Larsen TS.. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes 2003;52:434–441. [DOI] [PubMed] [Google Scholar]
- 83.Desrois M, Clarke K, Lan C, Dalmasso C, Cole M, Portha B, Cozzone PJ, Bernard M.. Upregulation of eNOS and unchanged energy metabolism in increased susceptibility of the aging type 2 diabetic GK rat heart to ischemic injury. Am J Physiol Heart Circ Physiol 2010;299:H1679–H1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Desrois M, Sidell RJ, Gauguier D, Davey CL, Radda GK, Clarke K.. Gender differences in hypertrophy, insulin resistance and ischemic injury in the aging type 2 diabetic rat heart. J Mol Cell Cardiol 2004;37:547–555. [DOI] [PubMed] [Google Scholar]
- 85.Galderisi M, Anderson KM, Wilson PWF, Levy D.. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (The Framingham Heart Study). Am J Cardiol 1991;68:85–89. [DOI] [PubMed] [Google Scholar]
- 86.Moore A, Shindikar A, Fomison-Nurse I, Riu F, Munasinghe PE, Ram TP, Saxena P, Coffey S, Bunton RW, Galvin IF, Williams MJ, Emanueli C, Madeddu P, Katare R.. Rapid onset of cardiomyopathy in STZ-induced female diabetic mice involves the downregulation of pro-survival Pim-1. Cardiovasc Diabetol 2014;13:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reichelt ME, Mellor KM, Bell JR, Chandramouli C, Headrick JP, Delbridge LM.. Sex, sex steroids, and diabetic cardiomyopathy: making the case for experimental focus. Am J Physiol Heart Circ Physiol 2013;305:H779–H792. [DOI] [PubMed] [Google Scholar]
- 88.Scheuermann-Freestone M MP, Manners D, Blamire AM, Buckingham RE, Styles P, Radda GK, Neubauer S, Clarke K.. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation 2003;107:3040–3046. [DOI] [PubMed] [Google Scholar]
- 89.Levelt E, Rodgers CT, Clarke WT, Mahmod M, Ariga R, Francis JM, Liu A, Wijesurendra RS, Dass S, Sabharwal N, Robson MD, Holloway CJ, Rider OJ, Clarke K, Karamitsos TD, Neubauer S.. Cardiac energetics, oxygenation, and perfusion during increased workload in patients with type 2 diabetes mellitus. Eur Heart J 2016;37(46):3461–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cline GW, Petersen KF, Krssak M, Shen J, Hundal RS, Trajanoski Z, Inzucchi S, Dresner A, Rothman DL, Shulman GI.. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N Engl J Med 1999;341:240–246. [DOI] [PubMed] [Google Scholar]
- 91.Utriainen T, Takala T, Luotolahti M, Rönnemaa T, Laine H, Ruotsalainen U, Haaparanta M, Nuutila P, Yki-Järvinen H.. Insulin resistance characterizes glucose uptake in skeletal muscle but not in the heart in NIDDM. Diabetologia 1998;41:555–559. [DOI] [PubMed] [Google Scholar]
- 92.Ohtake T, Yokoyama I, Watanabe T, Momose T, Serezawa T, Nishikawa J, Sasaki Y.. Myocardial glucose metabolism in noninsulin-dependent diabetes mellitus patients evaluated by FDG-PET. J Nucl Med 1995;36:456–463. [PubMed] [Google Scholar]
- 93.Fox CS, Gona P, Hoffmann U, Porter SA, Salton CJ, Massaro JM, Levy D, Larson MG, D'Agostino RB, O'Donnell CJ, Manning WJ.. Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: the Framingham heart study. Circulation 2009;119:1586–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fantuzzi G, Mazzone T.. Adipose tissue and atherosclerosis: exploring the connection. Arterioscler Thromb Vasc Biol 2007;27:996–1003. [DOI] [PubMed] [Google Scholar]
- 95.Levelt E, Pavlides M, Banerjee R, Mahmod M, Kelly C, Sellwood J, Ariga R, Thomas S, Francis J, Rodgers C, Clarke W, Sabharwal N, Antoniades C, Schneider J, Robson M, Clarke K, Karamitsos T, Rider O, Neubauer S.. Ectopic and visceral fat deposition in lean and obese patients with type 2 diabetes. J Am Coll Cardiol 2016;68:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Devereux RB, Roman MJ, Paranicas M, O'Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Howard BV.. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation 2000;101:2271–2276. [DOI] [PubMed] [Google Scholar]
- 97.Eguchi K, Kario K, Hoshide S, Ishikawa J, Morinari M, Shimada K.. Type 2 diabetes is associated with left ventricular concentric remodeling in hypertensive patients. Am J Hypertens 2005;18:23–29. [DOI] [PubMed] [Google Scholar]
- 98.Krumholz HM, Larson M, Levy D.. Prognosis of left ventricular geometric patterns in the Framingham heart study. J Am Coll Cardiol 1995;25:879–884. [DOI] [PubMed] [Google Scholar]
- 99.van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJJ, Schalkwijk CG, Bronzwaer JGF, Diamant M, Borbély A, van der Velden J, Stienen GJM, Laarman GJ, Niessen HWM, Paulus WJ.. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation 2008;117:43–51. [DOI] [PubMed] [Google Scholar]
- 100.White SK, Sado DM, Fontana M, Banypersad SM, Maestrini V, Flett AS, Piechnik SK, Robson MD, Hausenloy DJ, Sheikh AM, Hawkins PN, Moon JC.. T1 mapping for myocardial extracellular volume measurement by cmr: bolus only versus primed infusion technique. JACC: Cardiovasc Imaging 2013;6:955–962. [DOI] [PubMed] [Google Scholar]
- 101.Liu S, Han J, Nacif M, Jones J, Kawel N, Kellman P, Sibley C, Bluemke D.. Diffuse myocardial fibrosis evaluation using cardiac magnetic resonance T1 mapping: sample size considerations for clinical trials. J Cardiovasc Magn Reson 2012;14:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boyer JK, Thanigaraj S, Schechtman KB, Pérez JE.. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol 2004;93:870–875. [DOI] [PubMed] [Google Scholar]
- 103.Schannwell CM, Schneppenheim M, Perings S, Plehn G, Strauer BE.. Left ventricular diastolic dysfunction as an early manifestation of diabetic cardiomyopathy. Cardiology 2002;98:33–39. [DOI] [PubMed] [Google Scholar]
- 104.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, Investigators E-RO.. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 105.Ceriello A, Genovese S, Mannucci E, Gronda E.. Understanding EMPA-REG OUTCOME. Lancet Diabetes Endocrinol 2015;3:929–930. [DOI] [PubMed] [Google Scholar]
- 106.Hawley SA, Ford RJ, Smith BK, Gowans GJ, Mancini SJ, Pitt RD, Day EA, Salt IP, Steinberg GR, Hardie DG.. The Na+/glucose cotransporter inhibitor canagliflozin activates AMPK by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes 2016;65:2784–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, Mari A, Pieber TR, Muscelli E.. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes 2016;65:1190–1195. [DOI] [PubMed] [Google Scholar]
- 108.Mudaliar S, Alloju S, Henry RR.. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? a unifying hypothesis. Diabetes Care 2016;39:1115–1122. [DOI] [PubMed] [Google Scholar]
- 109.Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids 2004;70:309–319. [DOI] [PubMed] [Google Scholar]
- 110.Inoue T, Inoguchi T, Sonoda N, Hendarto H, Makimura H, Sasaki S, Yokomizo H, Fujimura Y, Miura D, Takayanagi R.. GLP-1 analog liraglutide protects against cardiac steatosis, oxidative stress and apoptosis in streptozotocin-induced diabetic rats. Atherosclerosis 2015;240:250–259. [DOI] [PubMed] [Google Scholar]
- 111.Inzucchi SE, Masoudi FA, Wang Y, Kosiborod M, Foody JM, Setaro JF, Havranek EP, Krumholz HM.. Insulin-sensitizing antihyperglycemic drugs and mortality after acute myocardial infarction: insights from the National Heart Care Project. Diabetes Care 2005;28:1680–1689. [DOI] [PubMed] [Google Scholar]
- 112.McAlister FA, Eurich DT, Majumdar SR, Johnson JA.. The risk of heart failure in patients with type 2 diabetes treated with oral agent monotherapy. Eur J Heart Fail 2008;10:703–708. [DOI] [PubMed] [Google Scholar]
- 113.Huynh K, Bernardo BC, McMullen JR, Ritchie RH.. Diabetic cardiomyopathy: mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol Ther 2014;142:375–415. [DOI] [PubMed] [Google Scholar]
- 114.Lonn E, Yusuf S, Hoogwerf B, Pogue J, Yi Q, Zinman B, Bosch J, Dagenais G, Mann JF, Gerstein HC.. Effects of vitamin E on cardiovascular and microvascular outcomes in high-risk patients with diabetes: results of the HOPE study and MICRO-HOPE substudy. Diabetes Care 2002;25:1919–1927. [DOI] [PubMed] [Google Scholar]
- 115.Sahyoun NR, Jacques PF, Russell RM.. Carotenoids, vitamins C and E, and mortality in an elderly population. Am J Epidemiol 1996;144:501–511. [DOI] [PubMed] [Google Scholar]
- 116.Liu Y, Lei S, Gao X, Mao X, Wang T, Wong GT, Vanhoutte PM, Irwin MG, Xia Z.. PKCbeta inhibition with ruboxistaurin reduces oxidative stress and attenuates left ventricular hypertrophy and dysfunction in rats with streptozotocin-induced diabetes. Clin Sci (Lond) 2012;122:161–173. [DOI] [PubMed] [Google Scholar]
- 117.Al-Onazi AS, Al-Rasheed NM, Attia HA, Al-Rasheed NM, Ahmed RM, Al-Amin MA, Poizat C.. Ruboxistaurin attenuates diabetic nephropathy via modulation of TGF-beta1/Smad and GRAP pathways. J Pharm Pharmacol 2016;68:219–232. [DOI] [PubMed] [Google Scholar]
- 118.Yokota T, Ma RC, Park JY, Isshiki K, Sotiropoulos KB, Rauniyar RK, Bornfeldt KE, King GL.. Role of protein kinase C on the expression of platelet-derived growth factor and endothelin-1 in the retina of diabetic rats and cultured retinal capillary pericytes. Diabetes 2003;52:838–845. [DOI] [PubMed] [Google Scholar]
- 119.Sheetz MJ, Aiello LP, Shahri N, Davis MD, Kles KA, Danis RP, Mbdv Study G.. Effect of ruboxistaurin (RBX) On visual acuity decline over a 6-year period with cessation and reinstitution of therapy: results of an open-label extension of the Protein Kinase C Diabetic Retinopathy Study 2 (PKC-DRS2). Retina 2011;31:1053–1059. [DOI] [PubMed] [Google Scholar]
- 120.Cherney DZ, Reich HN, Scholey JW, Lai V, Slorach C, Zinman B, Bradley TJ.. Systemic hemodynamic function in humans with type 1 diabetes treated with protein kinase Cbeta inhibition and renin-angiotensin system blockade: a pilot study. Can J Physiol Pharmacol 2012;90:113–121. [DOI] [PubMed] [Google Scholar]