Abstract
An oral dose study with perfluorooctanesulfonate (PFOS) was undertaken to identify potential associations between serum PFOS and changes in serum clinical chemistry parameters in purpose-bred young adult cynomolgus monkeys (Macaca fascicularis). In this study, control group (n = 6/sex) was sham-dosed with vehicle (0.5% Tween 20 and 5% ethanol in water), low-dose group (n = 6/sex) received 1 single K+PFOS dose (9 mg/kg), and high-dose group (n = 4–6/sex) received 3 separate K+ PFOS doses (11–17.2 mg/kg). Monkeys were given routine checkups and observed carefully for health problems on a daily basis. Scheduled blood samples were drawn from all monkeys prior to, during, and after K+PFOS administration for up to 1 year and they were analyzed for PFOS concentrations and clinical chemistry markers for coagulation, lipids, hepatic, renal, electrolytes, and thyroid-related hormones. No mortality occurred during the study. All the monkeys were healthy, gained weight, and were released back to the colony at the end of the study. The highest serum PFOS achieved was approximately 165 μg/ml. When compared with time-matched controls, administration of K+PFOS to monkeys did not result in any toxicologically meaningful or clinically relevant changes in serum clinical measurements for coagulation, lipids, hepatic, renal, electrolytes, and thyroid-related hormones. A slight reduction in serum cholesterol (primarily the high-density lipoprotein fraction), although not toxicologically significant, was observed. The corresponding lower-bound fifth percentile benchmark concentrations (BMCL1sd) were 74 and 76 μg/ml for male and female monkeys, respectively. Compared to the 2013–2014 geometric mean serum PFOS level of 4.99 ng/ml (0.00499 μg/ml) in US general population reported by CDC NHANES, this represents 4 orders of magnitude for margin of exposure.
Keywords: perfluorooctanesulfonate, PFOS, monkeys, cholesterol, HDL, thyroid.
Perfluorooctanesulfonyl fluoride (POSF)-based compounds are a class of surfactant chemicals that have been used in a wide variety of industrial and consumer applications (Kissa, 2001). Perfluorooctanesulfonate (PFOS, C8F17SO3−) is the ultimate metabolite for POSF-based chemistry. It is bioaccumulative and resistant to further degradation (OECD, 2002). The exceptional stability has attributed to its observed slow elimination in most species once absorbed (Chang et al., 2012; Olsen et al., 2007), contributing to its bioaccumulation potential (OECD, 2002). With the initial identification of PFOS for its widespread occurrence in the environment in the late 1990s–early 2000s (Giesy and Kannan, 2001), the potential consequences of prolonged exposure to environmental levels of PFOS have drawn increased attention from international regulatory agencies (Brooke et al., 2004; Canadian Government Department of the Environment, 2008; OECD, 2002; Stockholm Convention, 2009).
In several PFOS risk assessments, changes in serum clinical measurement related to lipid and thyroid from a previous 6-month oral gavage capsule dosing toxicity study of potassium PFOS (K+PFOS) in cynomolgus monkeys (Seacat et al., 2002) have been used for establishing safe exposure levels for PFOS in food and water (Butenhoff and Rodricks, 2015; European Food Safety Authority, 2012; Minnesota Department of Health, 2009; UKDWI, 2009; USEPA, 2009). Specifically, changes in 3 clinical parameters have been used to derive drinking water advisory guidance values for the public: decreased serum high-density lipoprotein (HDL), increased serum thyrotropin (thyroid-stimulating hormone [TSH]), and decreased serum total triiodotyronine (TT3). There are uncertainties and limitations associated with interpretation of these endpoints in the aforementioned study. The serum HDL values were determined only during the last 2 months of dosing. Thus, the interpretation of treatment-related changes in HDL was confounded by the lack of baseline and other HDL measurements before the fifth month. With respect to thyroid-related hormones, the initial method used to determine TSH was not optimized for cynomolgus monkeys and it was deemed unreliable after a subset of samples was cross-checked by a clinical reference laboratory, which showed normal TSH levels. Furthermore, unlike TSH, TT3 measurement is not a useful primary clinical diagnostic tool for thyroid function because it reflects >99% of inactive (protein-bound) T3 (Molina, 2013; Oppenheimer et al., 1995). Therefore, the interpretations from these selected endpoints based on data provided by Seacat et al. (2002) are problematic. Public health risk assessment would therefore benefit from a re-evaluation of these clinical parameters.
Because key toxicological and histological (pathological) endpoints in monkeys associated with exposure to PFOS had been previously identified by Seacat et al. (2002), this study reported herein was not entirely designed to be a (replicated) toxicology study. Instead, the objective of this study centered on clinical chemistry and the scope was to undertake a rigorous investigation of serum lipid, thyroid, and hepatic clinical chemistries in association with measured serum PFOS concentrations in cynomolgus monkeys (Macacafascicularis). Extended baselines were established for each monkey (including control) followed by oral dosing with K+PFOS, with multiple samples collected for up to 1-year post dosing.
MATERIALS AND METHODS
Materials
All chemicals used in this study were reagent-grade. Potassium perfluorooctanesulfonate (K+PFOS, FC-95, lot 217) was supplied by 3M Company (St Paul, Minnesota, USA). This is the same lot of K+PFOS that was used in the 6-month gavage capsule dosing study reported by Seacat et al. (2002). The purity reported in the Seacat et al. (2002) study was 86.9%. Re-analysis of this material in 2012 using improved instrumentation and analytical technique gave a purity of 88.9%. Lesser homologs constituted the majority of the impurities, they were similar to that reported by Seacat et al., with perfluorohexanesulfonate (C6 homolog, PFHxS) present in highest proportion in the sample at 3.2%, followed by 1.2% of perfluoroheptanesulfonate (C7 homolog, PFHpS), 1.1% of perfluoropentanesulfonate (C5 homolog, PFPeS), 0.97% perfluorobutanesulfonate (C4 homolog, PFBS), and 0.74% perfluoropropanesulfonate (C3 homolog, PFPS). Stable-isotope-labeled 18O2-PFOS (CF3(CF2)7S(18O2)O−) was used as an internal standard (Research Triangle Institute, Research Triangle Park, North Carolina, USA). For this study, dosing solution concentration was targeted at 2 mg/ml for the PFOS anion. K+PFOS was solubilized in vehicle (0.5% Tween 20 + 5% absolute ethanol in reagent grade water) with adjustment for purity and potassium salt content. The corresponding concentration was verified by LC-MS/MS prior to each dose administration.
Animal Selection and Ethical Consideration
The cynomolgus monkey (M.fascicularis) was chosen for this study because it was the species used by Seacat et al. (2002) in their evaluation of PFOS toxicity. Furthermore, use of the cynomolgus monkey maximized the likelihood of identifying responses that are similar to those which may be expected in humans (WHO, 2004). As stated earlier, because key toxicological and histological (pathological) endpoints in monkeys had been previously identified by Seacat et al. (2002), the study reported herein concentrated only on the evaluation of clinical chemistry outcomes and serum PFOS concentrations.
Animal Usage Guideline and Husbandry
The treatment of the monkeys was conducted entirely at Charles River Laboratories Montreal ULC, Senneville Site (Senneville, QC, Canada). The facility is, accredited by the Association for Assessment and for the Accreditation of Laboratory Animal Care International. All procedures involving animals were reviewed and approved by its Institutional Animal Care and Use Committee and conformed to Guide for the Care and Use of Laboratory Animals (ILAR, 2011).
Eighteen male and eighteen female purpose-bred cynomolgus monkeys (M.fascicularis) were used in this study. They were purchased from Primus Bio-Resources (Laval, Qubec, Canada) and an acclimation period of 6 weeks was allowed at the testing facility between animal receipt and the start of treatment in order to accustom the animals to the laboratory environment. These monkeys were young adults and sexually-matured.
Before dosing initiation, all monkeys were weighed and assigned to treatment groups using a computerized randomization procedure. Sex-specific randomization was done by stratification using body weight as the parameter. Monkeys of same sex and same dosing group were socially housed together (for psychological/environmental enrichment) in stainless steel cages equipped with a stainless steel mesh floor and an automatic watering valve. Each cage was labeled with a color-coded cage card indicating study, group, animal and tattoo number, and sex. Veterinary care was provided throughout the course of the study. Each cage was supplied with perches, floor toys, foraging devices and/or hanging devices. Additional enrichment, such as music, natural sounds or color video films were also provided.
Certified commercial dry monkey chow no. 5048 (PMI Nutrition International, St Louis, Missouri) was fed to the monkeys twice daily in amounts appropriate for the size and age of the animals, except during designated procedures in which fasting was required. The diet was supplemented daily with certified treats, fresh fruit and/or Prima Foraging Crumbles. Municipal tap water supplied to the testing facility was treated by reverse osmosis and ultraviolet irradiation and was available to the monkeys ad libitum. The monkeys were housed separately by sex in different rooms, and the rooms were maintained at a temperature of 20–26°C and a relative humidity of 30–70%. An automatic timer was set to control the room lights which provided 12-h of light/dark cycle per day.
Study Design—Animal Experimental Phase
The monkeys were acclimated to the laboratory environment in the testing facility for 6 weeks prior to the start of treatment. They were approximately 4–6-years old, and, at the start of treatment, weighed between 5.4 and 8.2 kg for males and 3.0 and 4.3 kg for females. Monkeys also were acclimated to the oral gavage procedure for at least 3 days prior to the commencement of each dose administration with water via disposable catheter attached to a plastic syringe.
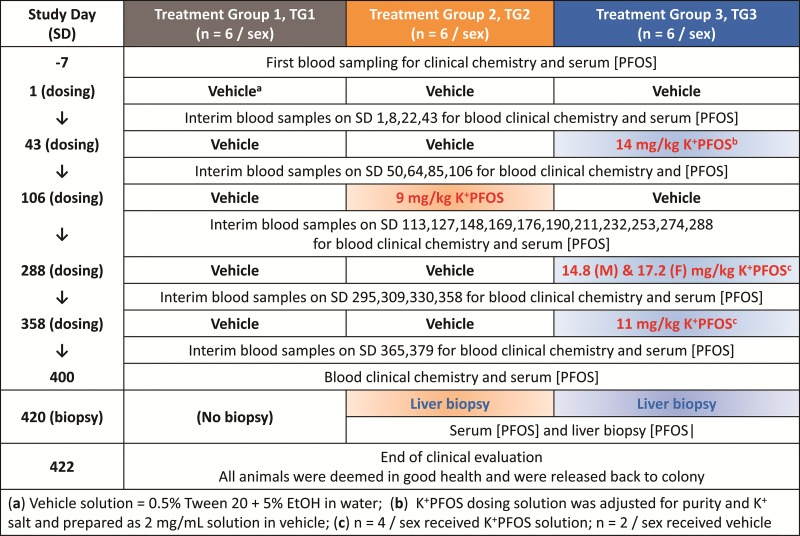
In this study, there were 3 treatment groups assigned (n = 6/sex/treatment group) designated hereafter as Treatment Group 1 (TG1), Treatment Group 2 (TG2), and Treatment Group (TG3). The overall study design is illustrated in Figure 1. Briefly, the first day of the study was designated as Study Day 1. TG1 served as the control group and was sham-dosed with vehicle (0.5% Tween 20 + 5% absolute ethanol in water) on Study Day 1, 43, 106, 288, and 358. TG2 received only 1 K+PFOS dose (9 mg/kg) during the study on Study Day 106. Based on the known pharmacokinetics of PFOS in cynomolgus monkeys (Chang et al., 2012), this dose was aimed to achieve an internal serum PFOS concentration around 70 μg/ml that was observed in the mid-dose (0.15 mg/kg–d) group monkeys from Seacat et al. (2002) study after 182 days of repeated dosing. TG3 received 3 doses of K+PFOS (in vehicle) as follows: 14 mg/kg (both sexes) on Study Day 43; 14.8, and 17.2 mg/kg for males and females, respectively, on Study Day 288; and 11 mg/kg (both sexes) on Study Day 358. These doses were aimed to achieve serum PFOS concentrations around 100 μg/ml (on Study Day 43), 150 μg/ml (on Study Day 288), and 170 μg/ml (on Study Day 358), which were observed in the high-dose (0.75 mg/kg−d) group monkeys from Seacat et al. (2002) study. In that study, total cholesterol decreased significantly compared with the control when the serum PFOS concentrations reached approximately 100 μg/ml. Also, in that study, serum total cholesterol continued to decrease in the high-dose group monkeys throughout the remaining dosing period where the final serum PFOS concentration was approximately 170 μg/ml after 182 days of repeated dosing. The K+PFOS doses given on Study Days 288 and 358 were adjusted based on the last serum PFOS concentrations measured. The goal was to achieve similar target PFOS concentration in males and females after dosing.
FIG. 1.
Study design with respect to time (study day, SD) and treatment groups (TG1–TG3). Schedules for dosing, blood sampling, and liver needle biopsy are depicted in this illustration.
In designing the study, the intention was to avoid toxicity. Considering the previously observed reduction in body weight and mortality in the high dose group monkeys (0.75 mg/kg−d) in the Seacat et al. (2002) study occurred at serum PFOS concentration at approximating 150 μg/ml and above, in this study, only 4 monkeys per sex in TG3 received additional K+PFOS doses on Study Days 288 and 358. When not scheduled to receive K+PFOS, monkeys in TG2 and TG3 were sham-dosed with vehicle. The dose volume for each monkey was based on the most recent weekly body weight measurement. To ensure dose delivery, 6 ml of water was administered as a flush immediately following each dose.
Interim venipuncture blood samples were obtained from all the monkeys (fasted overnight) throughout the study. The blood samples were taken from the femoral vein on Study Days −7, 1, 8, 22, 43, 50, 64, 85, 106, 113, 127, 148, 169, 176, 190, 211, 232, 253, 274, 288, 295, 309, 330, 358, 365, 379, and 400. An aliquot of blood was processed as plasma for coagulation parameter evaluation, and the remaining sample was processed to serum for various clinical chemistry and PFOS concentration determinations.
In laboratory studies with rodents and monkeys, it has been shown that PFOS preferentially distributes to serum and liver (Seacat et al., 2002, 2003). Therefore, on Study Day 420, ultrasound-guided liver needle biopsy was performed for TG2 and TG3 monkeys under anesthesia. Time-matched blood samples were obtained from these monkeys and both liver and blood samples were analyzed for PFOS concentrations (no clinical chemistry or histological evaluation was performed). Following the completion of the liver needle biopsy procedure, all monkeys were evaluated by a clinical veterinarian for their health. On Study Day 422, they were released to the colony (Charles River Laboratories—Preclinical Services, Senneville, QC, Canada).
Clinical Observations
Throughout the study, all monkeys were observed for daily clinical evaluation of wellness, evidence and extent of daily food consumption, and weekly clinical observations including measurement of body weight.
Clinical Parameters
The following coagulation and clinical chemistry parameters were analyzed by Charles River Laboratories using Roche Modular Analytics P-800 analyzer from the serum/plasma samples collected: alanine aminotransferase (ALT); aspartate aminotransferase (AST); alkaline phosphatase (AP); gamma-glutamyltransferase (GGT); creatinine kinase (CK); total bilirubin (TBIL); blood urea nitrogen (BUN); creatinine (CREAT); total protein (TPROT); total albumin (ALB); globulin (GLOB); albumin/globulin ratio (A/G); glucose (GLU); cholesterol (CHOL); low-density lipoprotein (LDL); high-density lipoprotein (HDL); triglycerides (TG); Sodium (Na); Potassium (K); Chloride (Cl); Calcium (Ca); and Phosphorus (PHOS). Beckman Coulter ACL Advance analyzer was used to determine prothrombin time (PT), activated partial thromboplastin time (APTT), and fibrinogen (FIB). The following thyroid hormone parameters were analyzed by Antech Diagnostics (Mississauga, Ontario, Canada): primate-specific TSH by chemiluminescence method (Syntar XT); free thyroxine (FT4) by equilibrium dialysis followed by radioimmunoassay (Nichols FT4 kit); total thyroxine (TT4) by enzymatic immunoassay (Beckman Coulter AU5400); and total triiodothyronine (TT3) by chemiluminescence method (Siemens Immulite 2000).
PFOS Concentration Determinations by LC-MS/MS
All dosing solutions as well as the biological specimens collected during the study (serum and liver biopsies) were analyzed for PFOS concentrations by LC-MS/MS at 3M Medical Department. In addition, 2 lots of certified monkey chow (PMI 5048) also were analyzed for background PFOS concentration.
Dosing Solution
After dilution with vehicle (0.5% Tween 20 + 5% absolute ethanol in water), K+PFOS dosing solutions were analyzed as-is without further extraction.
Monkey Chow
One gram of monkey chow (sampled from top, middle, and bottom of the bag) were pulverized and followed by addition of 50% methanol in water (9 ml). After overnight incubation at room temperature on a roller shaker, samples were then sonicated for 15 min followed by centrifugation (2500 × g, 5 min). One hundred μl of the top layer was transferred to a new tube which contained a fixed amount of the stable isotope-labeled internal standard (18O2-PFOS), followed by the addition of 1 N formic acid (1 ml) and 100 μl saturated ammonium sulfate. The solution was mixed by vortexing and then subjected to solid phase extraction (SPE) with Phenomenex Strata-X 3-ml SPE columns according to method described in Ehresman et al. (2007).
Serum
Newborn calf serum (Invitrogen Corporation, Carlsbad, California) was spiked with known amounts of PFOS for the matrix-matched standard curve. To all standard and test samples, a fixed amount of the stable isotope-labeled internal standard (18O2-PFOS) was added. Similar to chow samples, 100 μl of serum samples were subject to further sample preparation and SPE with Phenomenex Strata-X 3-ml SPE columns according to method described in Ehresman et al. (2007).
Liver
Liver needle biopsy samples were subjected to acid digestion (rather than homogenization) given that limited amounts of tissues (2–8 mg) were obtained. Naïve rat liver tissues were spiked with known amounts of PFOS for the matrix-matched standard curve. Each standard and test sample was digested overnight at 50 °C with 3N KOH (prepared in 60% EtOH) at 1:4 ratio (w/v) followed by addition of a fixed amount of the stable isotope-labeled internal standard (18O2-PFOS) and a series of liquid-liquid extractions. One hundred μl of tissue digest was further treated with 2N HCl (100 μl), 1N formic acid (500 μl), saturated ammonium sulfate (500 μl), and acetonitrile (7 ml). The solution was mixed on a shaker (30 min at room temperature) followed by centrifugation (2500 × g, 5 min). The top organic layer was subsequently transferred to a new tube and evaporated under vacuum and heat (120 mbar, 47 °C, 45 min). After evaporation, 1N KOH (500 μL) and MTBE (7 ml) were added and the tube was mixed using a shaker (30 min at room temperature). Again, after centrifuge (2500 × g, 5 min), the top organic layer was transferred to a new tube and let evaporate under vacuum and heat (120 mbar, 35 °C, 20 min). The resulting sample was reconstituted with 1 ml buffer that consisted of 0.1M ammonium acetate, acetonitrile, and water (1:2:1) prior to LC-MS/MS analysis.
LC-MS/MS
The instrument used for all the LC-MS/MS analyses was API 6500 mass spectrometer (AB SCIEX, Redwood City, California) with Turbo Ion Spray (pneumatically assisted electrospray ionization source) that operated in a negative ion mode. Mac-Mod ACE C-18 HPLC column (2.1 mm i.d. × 75 mm, 5 µm particle size) was used with a flow rate of 0.25 ml/min for the chromatography with gradient applied using 95%/5% 2 mM ammonium acetate/acetonitrile and acetonitrile. All source parameters were optimized under the conditions according to manufacturer’s guidelines. Transition ions monitored were: (1) PFOS: 499 → 80 m/z; and (2) Internal Standard 18O2-PFOS: 503 → 84 m/z.
Statistical and Pharamacokinetic Analysis
The Student’s t-test was used to compare data resulting from K+ PFOS treatment to control values using SAS Software (SAS Institute, Inc., Cary, North Carolina). All data are expressed as means ± standard deviation. In addition when applicable, pharmacokinetic parameters were calculated from the serum PFOS concentration versus time data using WinNonlin (Professional Version 5.1; Pharsight Corporation, Mountain View, California) with a noncompartmental analysis. Half-life (T1/2) values were calculated from the first-order rate constant associated with the observed terminal (loglinear) portion of the serum concentration versus time curve.
Benchmark Concentration Analysis
A Bayesian analysis was performed for HDL, TT3, and TSH followed by benchmark concentration (BMC) estimation.
(1) For Bayesian analysis, the observed background values (and the model parameters corresponding to them) were assumed to vary across individual animals according to a normal distribution with a mean and standard deviation to be estimated. This analysis was hierarchical with respect to background responses (ie, a random effect analysis), where the background parameter is the random effect.
(2) Linear and baseline models used are shown below as Equation (1), linear model, and Equation (2), baseline model.
| (1) |
| (2) |
This form of the linear model shows the hierarchical structure for the background parameter, a, indicating that that parameter depends on the individual, indexed by i, and potentially varies among individuals. The distributions of the a[i] values were assumed to be normal across the population of Cynomolgus monkeys, with mean = “a_mean” and standard deviation = “a_sigma”. Both of the hyperparameters, “a_mean” and “a_sigma”, were estimated separately for each sex. Similarly, different endpoints were analyzed separately (eg, one set of hyperparameters was estimated for HDL and another set was estimated for TSH). A hyperparameter is a parameter that defines the distribution of other parameters in the model; in this case, the values of a[i] depend on “a_mean” and “a_sigma”.
The index i in Equation (1) corresponds to particular measurements within each individual monkey. There were 27 measurements of serum PFOS concentration per individual monkey. The term e[i,j] represents the random (or unexplained) variation in these measurements. For these analyses, we have assumed that e[i, j] was distributed normally with mean = 0 and standard deviation = sigma (constant for all measurements). The baseline model shown in Equation (2) is Equation (1) where slope b = 0. Both models were fit to the data and compared within each sex; so, for example, there were a total of four models run for HDL (linear and baseline models for each sex).
(3) The Bayesian approach was implemented using Monte Carlo Markov Chain (MCMC) techniques as implemented in OpenBUGS (version 3.2.3, rev 1012, March 3, 2014). MCMC simulation was carried out using 3 chains per endpoint and sex combination, burn-in was set at 1000 iterations, and then 100 000 additional iterations per chain were computed. Those iterations were thinned by 10, resulting in 30 000 samples from the posterior distributions (10 000 from each of the 3 chains) for each endpoint and sex combination. The Gelman-Rubin statistic, as modified by Brooks and Gelman (1998) was used to determine convergence (a value of the statistic less than 1.05 indicated convergence). The Bayesian approach entails that prior distributions for the parameters (discussed below) are specified. The study results are then expressed in terms of posterior distributions for the parameters analyzed and the results of the analyses are represented in terms of distributions rather than point estimates. For model comparison (linear vs baseline), the Watanabe-Akaike Information Criterion (WAIC) was used to assess model choice. The WAIC is discussed and justified for use in the Bayesian context by Vehtari and Gelman (2014).
A key aspect of a Bayesian analysis is the definition of prior distributions for the parameters in the model. The priors used for these analyses are shown in Table 1. The priors for HDL were based on the clinical reference range data used by Seacat et al. (2002). For that endpoint, there was an obvious sex difference in background levels; hence, the males and females were assigned different a_mean values (defining the center of the normal distribution of background HDL values in the population). Moreover, the Seacat et al. (2002) results, though limited with respect to HDL, suggested that there might be a slight downward trend for HDL in relation to serum PFOS. The −0.06 value for the mean of the prior for the slope, b, was estimated from the change in HDL relative to the change in PFOS observed in the male and female monkeys from day 153 to 182 in Seacat et al., (2002). Each scale parameter for the half-Cauchy distribution was set equal to a value somewhat greater than a reasonable upper limit on the standard deviation term for which it is defined (Gelman, 2005). Because the data available for defining priors was limited and it was difficult to separate out inter- and intra-individual variability, the scales for “a_sigma” and “sigma” were set to the same value.
TABLE 1.
Prior Distributions
| Parameter | Distribution | Endpoint: HDL |
|---|---|---|
| a[i] | Normal(a_mean, a_sigma) | – |
| a_mean | Normal | males: μ = 76; σ = 5 females: μ = 63; σ = 5 |
| a_sigma | Half-Cauchy | scale = 15 |
| b | Normal | μ = −0.06; σ = 0.61 |
| sigma | Half-Cauchy | scale = 15 |
Note: The prior for b is ignored in the baseline model. All other priors remained the same for the baseline model runs.
(4) The estimates of BMC presented for the linear model corresponded to the standard deviation-based definition of the benchmark response (BMR), as implemented in EPA’s BMDS software (see http://www2.epa.gov/benchmark-dose-software, accessed January 15, 2016). In this particular study, it is the BMC for which the predicted mean response is 1 standard deviation different from the predicted background mean response. These calculations are population-level predictions. Equation (3) shown below is for the derivation of BMC while taking these considerations into account:
| (3) |
Each of the 30 000 MCMC iterations (per endpoint/sex combination) yielded an estimate of the BMC. The summary statistics for the posterior BMC distribution were the mean and the 5th and 95th percentiles, which together represent a 90% CI for the BMC. Note that the choice of an one standard deviation difference is based on the observation that, if one considers that 1% of the population is in the “abnormal” range in the absence of exposure (values in the lower tail of HDL even when the population is unexposed), then changing the mean by approximately 1 standard deviation will entail that 10% more individuals will fall in the tail. Thus, this definition of BMR is conceptually the same as increasing the risk of “abnormality” by 10% (additional risk of 10%). It is independent of the units and the background level of the response measure, making it a good choice for endpoints for which a clearly defined cut-point separating “normal” from “abnormal” is absent or difficult to define (perhaps because of inter-laboratory differences in normal ranges).
RESULTS
Animal Observations and Body Weight
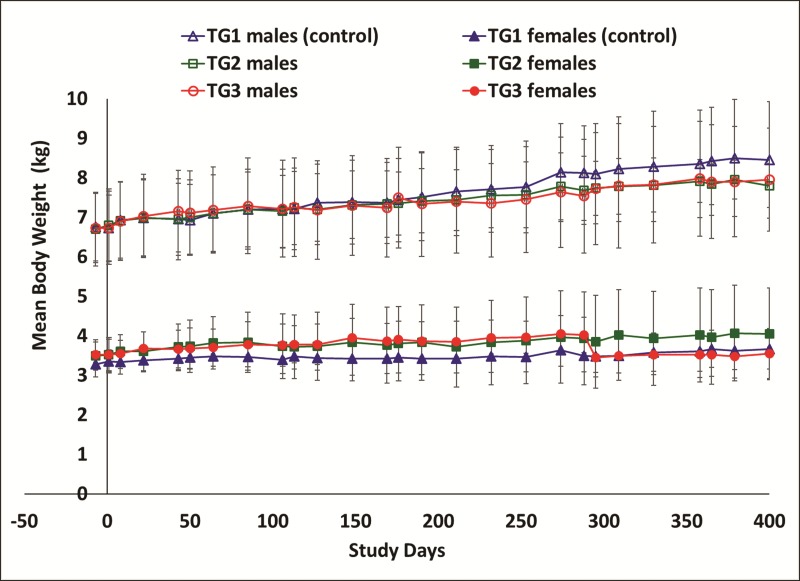
During the conduct of the study, there were no mortalities related to K+PFOS treatments. All monkeys (control and K+PFOS-treated) appeared normal. When compared with time-matched controls, K+PFOS-treated monkeys had similar body weight and body weight-gains without any statistical significance (Figure 2). At the end of the study, all the monkeys were diagnosed to be in good health by the study facility’s veterinarian and all monkeys were released back to the colony at Charles River Laboratories Montreal ULC, Senneville Site (Senneville, QC, Canada).
FIG. 2.
Mean group body weights in male and female monkeys throughout study. For male monkeys, TG1 (control), TG2, and TG3 are represented in open triangle (Δ), open square (□), and open circle (○), respectively. For female monkeys, TG1 (control), TG2, and TG3 are represented in solid triangle (▲), solid square (▪), and solid circle (●), respectively. Error bars shown are standard deviation.
Dosing Solutions and Monkey Chow Analyses
For this study, mean PFOS dosing solution concentrations ranged from 1.89 ± 0.04 to 2.00 ± 0.04 mg/ml, representing 94.3 to 100.2% of target concentration. Concentrations of PFOS in 2 different lots of certified commercial dry monkey chow (PMI 5048) were measured and all were below the lower limit of quantification of 1 ng/g.
Serum and Liver PFOS Concentrations and Pharmacokinetic Analysis
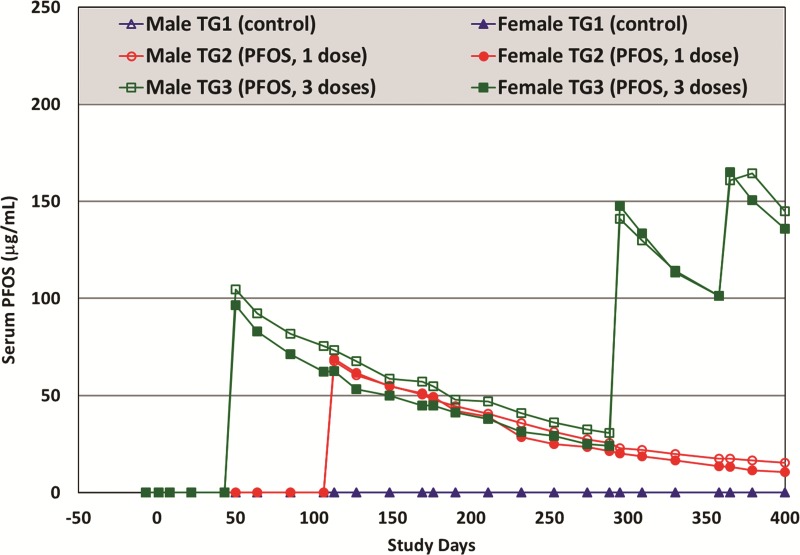
Over the entire study period of more than 400 days, there were 27 serum PFOS determinations for each monkey from all treatment groups (including controls). The LC-MS/MS percent recovery for serum samles ranged between 90 and 105% based on spiked QC samples. Mean serum PFOS concentrations (by treatment group and sex) are illustrated in Figure 3 (also see Supplemental Material for group means by Study Days). Presented in Table 2 are the mean serum PFOS concentrations measured after each dosing (from blood samples collected at 7 days post dose) and the mean serum and liver PFOS concentrations (from needle biopsy) obtained on Study Day 420 (for TG2 and TG3 monkeys only). The highest serum PFOS concentration achieved was 165 μg/ml for TG3 monkeys which was similar to the mean value (approximately 170 μg/ml) at the termination of the 6-month study reported by Seacat et al. (2002). At the end of this study, the liver-to-serum PFOS concentration ratio was approximately 1:1, which was lower than the approximate 2:1 value reported by Seacat et al.
FIG. 3.
Mean group serum PFOS concentrations (μg/ml), determined by LC-MS/MS, in male and female monkeys throughout study. For male monkeys, TG1 (control), TG2, and TG3 are represented in open triangle (Δ), open circle (○), and open square (□), respectively. For female monkeys, TG1 (control), TG2, and TG3 are represented in solid triangle (▲), solid circle (●), and solid square (▪), respectively.
TABLE 2.
Mean Serum PFOS Concentrations (μg/ml) for TG1–TG3 Monkeys at 7 Days Post Gavage Dose. Also Included are serum (μg/ml) and liver (μg/g) PFOS concentrations obtained during liver biopsy on Study Day 420. All values reported are with ± standard deviation.
| Group | n | Treatments | Study Day (SD) | [PFOS] in μg/ml (serum) or μg/g (liver) |
|
|---|---|---|---|---|---|
| Male | Female | ||||
| TG1 | 6 | 0 mg/kg (SD 1) | Serum [PFOS] on SD 8 | 0.002 ± 0.000 | 0.001 ± 0.001 |
| 6 | 0 mg/kg (SD 43) | Serum [PFOS] on SD 50 | 0.003 ± 0.000 | 0.003 ± 0.001 | |
| 6 | 0 mg/kg (SD 106) | Serum [PFOS] on SD 113 | 0.005 ± 0.001 | 0.005 ± 0.001 | |
| 6 | 0 mg/kg (SD 288) | Serum [PFOS] on SD 295 | 0.012 ± 0.005 | 0.006 ± 0.002 | |
| 6 | 0 mg/kg (SD 358) | Serum [PFOS] on SD 365 | 0.013 ± 0.004 | 0.007 ± 0.002 | |
| 6 | Liver needle biopsy (SD 420) | No needle biopsy was performed on TG1 monkeys | |||
| TG2 | 6 | 0 mg/kg (SD 1) | Serum [PFOS] on SD 8 | 0.002 ± 0.000 | 0.002 ± 0.000 |
| 6 | 9 mg/kg (SD 106) | Serum [PFOS] on SD 113 | 67.7 ± 7.5 | 68.8 ± 2.5 | |
| 6 | Liver needle biopsy (SD 420) | Serum [PFOS] on SD 420 | 14.1 ± 2.0 | 9.5 ± 4.4 | |
| Liver [PFOS] on SD 420 | 7.8 ± 5.5 | 8.3 ± 3.3 | |||
| TG3 | 6 | 0 mg/kg (SD 1) | Serum [PFOS] on SD 8 | 0.002 ± 0.001 | 0.002 ± 0.001 |
| 6 | 14 mg/kg (SD 43) | Serum [PFOS] on SD 50 | 104.8 ± 5.2 | 96.5 ± 6.2 | |
| 4a | 14.8 mg/kg (M) and 17.2 mg/kg (F) (SD 288) | Serum [PFOS] on SD 295 | 141.0 ± 13.1 | 147.6 ± 17.5 | |
| 4a | 11 mg/kg (SD 358) | Serum [PFOS] on SD 365 | 160.8 ± 14.2 | 165.0 ± 6.7 | |
| 4a | Liver needle biopsy (SD 420) | Serum [PFOS] on SD 420 | 130.5 ± 14.7 | 127.0 ± 4.1 | |
| Liver [PFOS] on SD 420 | 94.5 ± 33.9 | 112.0 ± 18.7 | |||
For TG3, only 4 monkeys per sex were dosed with K+ PFOS on Study Days 288 and 358.
Several pharmacokinetic parameters were modeled using WinNonlin based on measured serum PFOS concentrations over time for TG2 and TG3 monkeys after K+PFOS was given. As shown in Table 3, the estimated mean serum elimination half-life of PFOS in cynomolgus monkeys ranged from 102 to 124 days. These half-life estimations are similar to those reported by Chang et al. (2012) after single IV dose, which were 110–132 days. There did not appear to be a sex-difference in terms of serum PFOS uptake or elimination in these monkeys, an observation that is also consistent with our previous work (Chang et al., 2012; Seacat et al., 2002).
TABLE 3.
Mean ± Standard Deviation Values for Pharmacokinetic Parameters in Cynomolgus Monkeys Given Oral Dose of K+PFOS
| K+PFOS Dose (mg/kg Body Weight) |
|||
|---|---|---|---|
| Parameter | Sex | 9 mg/kg | 14 mg/kg |
| T1/2 (day) | Male | 124 ± 3.89a | 117 ± 17.2a |
| Female | 102 ± 29.2b | 102 ± 45.6a | |
| Kel (1/day) | Male | 0.00559 ± 0.000175 | 0.00605 ± 0.000951 |
| Female | 0.00729 ± 0.00223 | 0.00757 ± 0.00270 | |
| CL (ml/day/kg) | Male | 0.712 ± 0.0812 | 0.816 ± 0.111 |
| Female | 0.897 ± 0.196 | 1.06 ± 0.510 | |
| Vd (ml/kg) | Male | 127 ± 10.9 | 135 ± 6.69 |
| Female | 127 ± 18.9 | 141 ± 38.5 | |
| AUC/dose (ng.day/ml/ mg/kg) | Male | 271 333 ± 21 733 | 249 667 ± 14 468 |
| Female | 265 200 ± 15 057 | 220 333 ± 9019 | |
n = 3 monkeys only. The other 3 monkeys had R2 < 0.800.
n = 5 monkeys only. One monkey had R2 < 0.800.
Coagulation Parameters
There were no K+PFOS treatment-related changes in the coagulations parameters (PT, APTT, and FIB).
Serum Clinical Chemistry
Throughout the entire study, all parameters were within normal biological variation and were not considered K+PFOS treatment-related when compared with either time-matched control values, or monkey’s own baseline values, or clinical laboratories historical data for cynomolgus monkeys. However, decreases of 2 parameters, HDL and TT4, while remaining within the normal range, appeared to be associated with K+PFOS treatment.
Liver
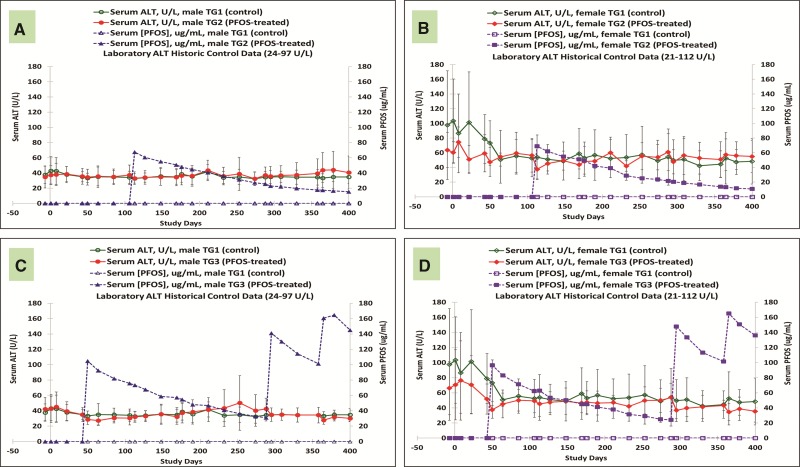
Throughout the entire study, there were no K+PFOS treatment-related changes in serum liver enzymes. For example, Figures 4A–D are the mean serum ALT values for control and K+PFOS-treated monkeys, plotted with their respective serum PFOS concentration data. For TG2 monkeys where the highest mean serum PFOS concentration was 70 μg/ml, there were no K+PFOS treatment-related changes in ALT in either TG2 males (Figure 4A) or TG2 females (Figure 4B) when compared with TG1 controls. For TG3 monkeys where the highest mean serum PFOS concentration was 165 μg/ml, there were no K+PFOS treatment-related changes in ALT in either TG3 males (Figure 4C) or TG3 females (Figure 4D) when compared with TG1 controls (also see Supplemental Material for ALT group means by Study Days).
FIG. 4.
Mean group serum ALT concentrations (U/L) in male and female monkeys throughout study. Each panel represents mean serum ALT values (±standard deviation) for sex-matched control and K+ PFOS-treated monkeys, plotted with their respective serum PFOS concentration data (μg/ml). For Panel (A), open circle (○), solid circle (●), open triangle (Δ), and solid triangle (▲) represent serum ALT for male TG1 control group, serum ALT for male TG2 K+PFOS-treated group, serum PFOS concentration for male TG1 control group, and serum PFOS concentration for male TG2 K+PFOS-treated group monkeys, respectively. For Panel (B), open diamond (◇), solid diamond (◆), open square (□), and solid square (▪) represent serum ALT for female TG1 control group, serum ALT for female TG2 K+PFOS-treated group, serum PFOS concentration for female TG1 control group, and serum PFOS concentration for female TG2 K+PFOS-treated group monkeys, respectively. For Panel (C), open circle (○), solid circle (●), open triangle (Δ), and solid triangle (▲) represent serum ALT for male TG1 control group, serum ALT for male TG3 K+PFOS-treated group, mean serum PFOS concentration for male TG1 control group, and mean serum PFOS concentration for male TG3 K+PFOS-treated group monkeys, respectively. For Panel (D), open diamond (◇), solid diamond (◆), open square (□), and solid square (▪) represent serum ALT for female TG1 control group, serum ALT for female TG3 K+PFOS-treated group, mean serum PFOS concentration for female TG3 control group, and mean serum PFOS concentration for female TG3 K+PFOS-treated group monkeys, respectively.
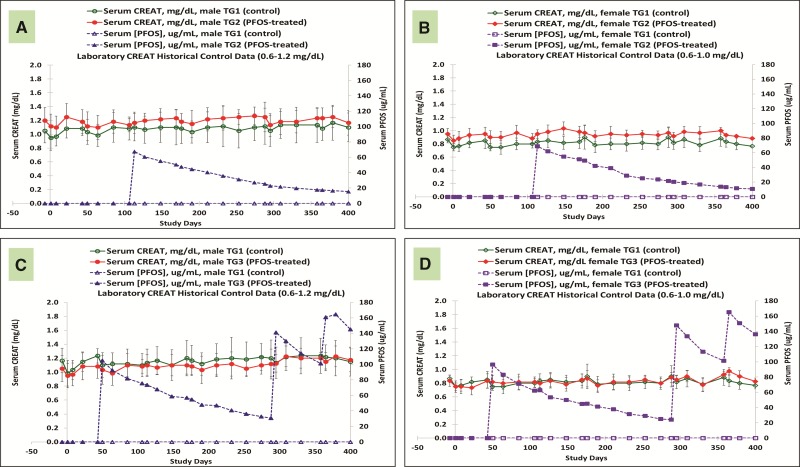
Kidney
Throughout the entire study, there were no K+PFOS treatment-related changes in serum BUN or CREAT. There were no K+PFOS treatment-related changes in the serum electrolyte levels. Figures 5A–D are the mean serum CREAT values for control and K+PFOS-treated monkeys, plotted with their respective serum PFOS concentration data. For TG2 monkeys where the highest mean serum PFOS concentration was 70 μg/ml, there were no K+PFOS treatment-related changes in CREAT in either TG2 males (Figure 5A) or TG2 females (Figure 5B) when compared with TG1 controls. For TG3 monkeys where the highest mean serum PFOS concentration was 165 μg/ml, there were no K+PFOS treatment-related changes in CREAT in either TG3 males (Figure 5C) or TG3 females (Figure 5D) when compared with TG1 controls (also see Supplemental Material for CREAT group means by Study Days).
FIG. 5.
Mean group serum CREAT concentrations (mg/dl) in male and female monkeys throughout study. Each panel represents mean serum CREAT values (±standard deviation) for sex-matched control and K+ PFOS-treated monkeys, plotted with their respective mean serum PFOS concentration data (μg/ml). For Panel (A), open circle (○), solid circle (●), open triangle (Δ), and solid triangle (▲) represent serum CREAT for male TG1 control group, serum CREAT for male TG2 K+PFOS-treated group, mean serum PFOS concentration for male TG1 control group, and mean serum PFOS concentration for male TG2 K+PFOS-treated group monkeys, respectively. For Panel (B), open diamond (◇), solid diamond (◆), open square (□), and solid square (▪) represent serum CREAT for female TG1 control group, serum CREAT for female TG2 K+PFOS-treated group, mean serum PFOS concentration for female TG1 control group, and mean serum PFOS concentration for female TG2 K+PFOS-treated group monkeys, respectively. For Panel (C), open circle (○), solid circle (●), open triangle (Δ), and solid triangle (▲) represent serum CREAT for male TG1 control group, serum CREAT for male TG3 K+PFOS-treated group, mean serum PFOS concentration for male TG1 control group, and mean serum PFOS concentration for male TG3 K+PFOS-treated group monkeys, respectively. For Panel (D), open diamond (◇), solid diamond (◇), open square (□), and solid square (▪) represent serum CREAT for female TG1 control group, serum CREAT for female TG3 K+PFOS-treated group, mean serum PFOS concentration for female TG1 control group, and mean serum PFOS concentration for female TG3 K+PFOS-treated group monkeys, respectively.
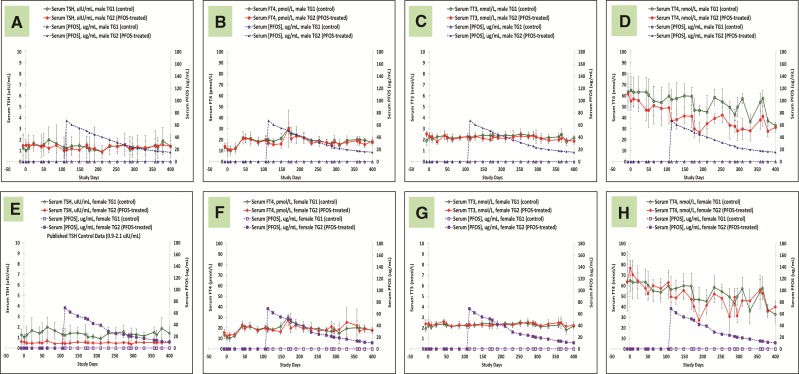
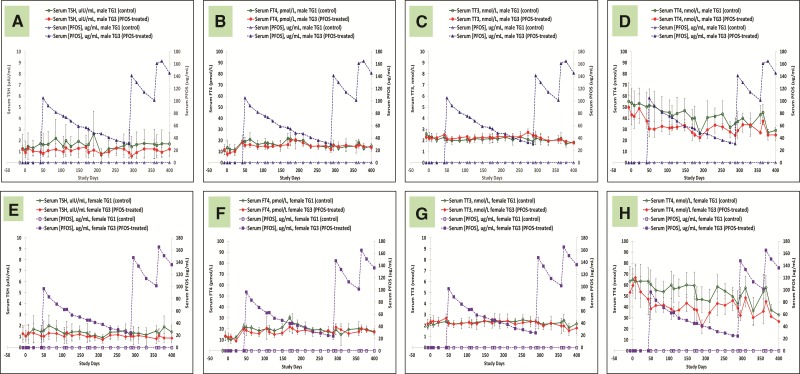
Thyroid Hormones
Throughout the entire 400 days of study, serum thyroid hormones were followed extensively for all the monkeys. Figures 6A–H are serum thyroid hormone data for control and K+PFOS-treated monkeys in TG2, and Figures 7A–H are serum thyroid hormone data for control and K+ PFOS-treated monkeys in TG3. For each figure subset, A, B, C, and D represent the mean serum TSH, FT4, TT3, and TT4, respectively, for male monkeys from control and K+PFOS-treated group plotted with their respective serum PFOS concentration data. For each figure subset, E, F, G, and H represent the mean serum TSH, FT4, TT3, and TT4, respectively, for female monkeys from control and K+PFOS-treated group plotted with their respective serum PFOS concentration data (also see Supplemental Material for group means for these thyroid-related hormones by Study Days). There were no significant changes in the clinically-relevant thyroid hormone diagnostic parameters (TSH and FT4) that would suggest altered thyroid functions. Although PFOS treatment did not affect TT3 levels, it did lead to decreased serum TT4 without concomitant change in TSH or FT4 among TG2 and TG3 monkeys (Figs. 6D and H and 7D and H). Because measurement of TT4 reflected >99% of the biologically inactive thyroxine, it is, therefore, not considered a clinically relevant endpoint for interpreting thyroid function (see Discussion).
FIG. 6.
Mean group serum thyroid hormone concentrations for TG1 and TG2 monkeys throughout study. Each panel represents group mean thyroid hormone (±standard deviation) for sex-matched control and K+ PFOS-treated monkeys, plotted with their respective serum PFOS concentration data (μg/ml). Panels (A–D) illustrate mean serum TSH, FT4, TT3, and TT4 data for TG1 and TG2 male monkeys. In that, open circle (○), solid circle (●), open triangle (Δ), and solid triangle (▲) represent mean serum thyroid hormone for male TG1 control group, mean serum thyroid hormone for male TG2 K+PFOS-treated group, mean serum PFOS concentration for male TG1 control group, and mean serum PFOS concentration for male TG2 K+PFOS-treated group monkeys, respectively. Panels (E–H) illustrate mean serum TSH, FT4, TT3, and TT4 data for TG1 and TG2 female monkeys. In that, open diamond (◇), solid diamond (◆), open square (□), and solid square (▪) represent mean serum thyroid hormone for female TG1 control group, mean serum thyroid hormone for female TG2 K+PFOS-treated group, mean serum PFOS concentration for female TG1 control group, and mean serum PFOS concentration for female TG2 K+PFOS-treated group monkeys, respectively.
FIG. 7.
Mean group serum thyroid hormone concentrations for TG1 and TG3 monkeys throughout study. Each panel represents group mean thyroid hormone (±standard deviation) for sex-matched control and K+ PFOS-treated monkeys, plotted with their respective serum PFOS concentration data (μg/ml). Panels (A–D) illustrate mean serum TSH, FT4, TT3, and TT4 data for TG1 and TG3 male monkeys. In that, open circle (○), solid circle (●), open triangle (Δ), and solid triangle (▲) represent mean serum thyroid hormone for male TG1 control group, mean serum thyroid hormone for male TG3 K+PFOS-treated group, mean serum PFOS concentration for male TG1 control group, and mean serum PFOS concentration for male TG3 K+PFOS-treated group monkeys, respectively. Panels (E–H) illustrate mean serum TSH, FT4, TT3, and TT4 data for TG1 and TG3 female monkeys. In that, open diamond (◇), solid diamond (◆), open square (□), and solid square (▪) represent mean serum thyroid hormone for female TG1 control group, mean serum thyroid hormone for female TG3 K+PFOS-treated group, mean serum PFOS concentration for female TG1 control group, and mean serum PFOS concentration for female TG3 K+PFOS-treated group monkeys, respectively.
Regardless of treatment or dose or sex, there was no K+PFOS treatment-related effect on TSH in any monkeys. However, an interesting observation was the consistent lower TSH levels in TG2 females (approximately 50% lower) compared with controls (Figure 6E). Clearly this was not a treatment-related effect because the same observation was noticed pre- and post K+PFOS administration that began on Study Day 106. Several areas of inquiries were investigated to possibly explain this observation between TG2 females and controls, including: possible inbreeding of the TG2 females; appropriate randomization; animal transportation history from breeding colony in Marituis Island to the acclimation at the study site in Montreal; animal husbandry conditions throughout the study (which did not change, same cage mates); compliance of serum sample handling procedures per SOP; and verification of the analytical data. Amongst these factors, no explanatory lines of evidence were established. The one factor not evaluated in this study was estrous cycles. Even though the animal husbandry condition did not change throughout the course of this study, there were some research data that suggested that housing arrangement of cynomolgus monkeys may influence the variability for serum thyroid hormone measurement onto itself (Flow and Jaques, 1997).
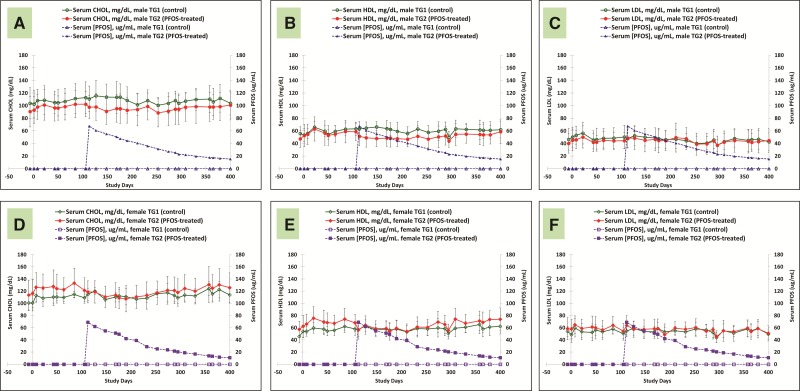
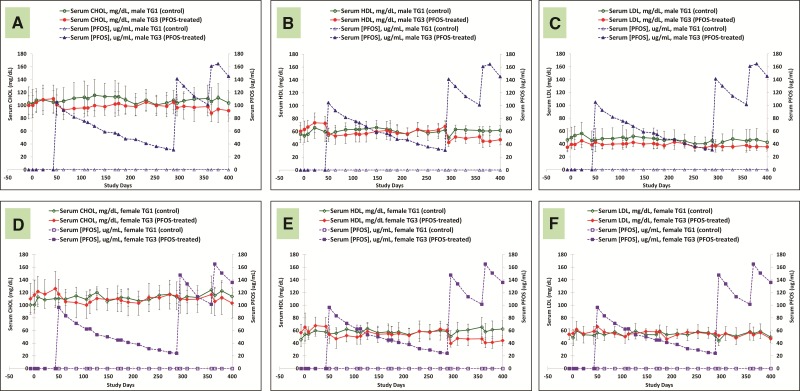
Lipid
Figures 8A–F are serum lipid data for control and K+PFOS-treated monkeys in TG2, and Figures 9A–F are serum lipid data for control and K+PFOS-treated monkeys in TG3. For each figure subset, A, B, and C represent the mean serum total cholesterol, HDL, and LDL, respectively, for male monkeys from control and K+PFOS-treated group plotted with their respective serum PFOS concentration data. For each figure subset, D, E, and F represent the mean serum total cholesterol, HDL, and LDL, respectively, for female monkeys from control and K+PFOS-treated group plotted with their respective serum PFOS concentration data (also see Supplemental Material for lipid group means by Study Days). In this study, the only notable change related to K+PFOS treatments was the observation of an approximately 5–10% decrease in serum total cholesterol that was primarily due to lower HDL fraction. For TG3 monkeys after the first K+PFOS dose administration on Study Day 43, regardless of sex, the mean serum total cholesterol (primarily in HDL fraction) was decreased by approximately 4 and 12% at 1 and 3 weeks postdose, respectively, when compared with mean time-matched control or baseline values. As total cholesterol and HDL appeared to rebound around Study Day 211 and on, similar reductions were also observed after the second and third K+PFOS dosing on Study Days 288 and 358. Similar magnitude in declining trends for mean total cholesterol and HDL was also observed for TG2 monkeys.
FIG. 8.
Mean group serum lipid concentrations for TG1 and TG2 monkeys throughout study. Each panel represents group mean lipid concentration (±standard deviation) for sex-matched control and K+ PFOS-treated monkeys, plotted with their respective serum PFOS concentration data (μg/ml). Panels (A–C) illustrate mean serum total cholesterol, HDL, and LDL data for TG1 and TG2 male monkeys. In that, open circle (○), solid circle (●), open triangle (Δ), and solid triangle (▲) represent mean serum lipid for male TG1 control group, mean serum lipid for male TG2 K+PFOS-treated group, mean serum PFOS concentration for male TG1 control group, and mean serum PFOS concentration for male TG2 K+PFOS-treated group monkeys, respectively. Panels (D–F) illustrate mean serum total cholesterol, HDL, and LDL data for TG1 and TG2 female monkeys. In that, open diamond (◇), solid diamond (◆), open square (□), and solid square (▪) represent mean serum lipid for female TG1 control group, mean serum lipid for female TG2 K+PFOS-treated group, mean serum PFOS concentration for female TG1 control group, and mean serum PFOS concentration for female TG2 K+PFOS-treated group monkeys, respectively.
FIG. 9.
Mean group serum lipid concentrations for TG1 and TG3 monkeys throughout study. Each panel represents group mean lipid concentration (±standard deviation) for sex-matched control and K+PFOS-treated monkeys, plotted with their respective serum PFOS concentration data (μg/ml). Panels (A–C) illustrate mean serum total cholesterol, HDL, and LDL data for TG1 and TG3 male monkeys. In that, open circle (○), solid circle (●), open triangle (Δ), and solid triangle (▲) represent mean serum lipid for male TG1 control group, mean serum lipid for male TG3 K+PFOS-treated group, mean serum PFOS concentration for male TG1 control group, and mean serum PFOS concentration for male TG3 K+PFOS-treated group monkeys, respectively. Panels (D–F) illustrate mean serum total cholesterol, HDL, and LDL data for TG1 and TG3 female monkeys. In that, open diamond (◇), solid diamond (25C6), open square (□), and solid square (▪) represent mean serum lipid for female TG1 control group, mean serum lipid for female TG3 K+PFOS-treated group, mean serum PFOS concentration for female TG1 control group, and mean serum PFOS concentration for female TG3 K+PFOS-treated group monkeys, respectively.
BMC Data
BMC modeling results for the HDL analyses are summarized in Tables 4 (males) and 5 (females). All parameters of all models reached convergence according to the Gelman-Rubin statistic. The WAIC metric of relative fit indicated a slight preference for the linear model over the baseline (i.e., intercept-only) model for the males; the opposite is the case for females. In both sexes, the difference in the WAICs for the 2 models was within the variation of the WAIC estimates themselves (assessed by reference to the corresponding expected log probability density [ELPD] estimates and its SE). The BMC1sd estimates for HDL are shown in Table 6. The lower-bound (fifth percentile) BMC for HDL (BMCL1sd) was 74 and 76 μg/ml for male and female monkeys, respectively.
TABLE 5.
Analysis Results for HDL; Females
| WAIC | ELPD | SE of ELPD | Model Parameter Est’s |
2.5th %tile | Mean | 97.5th %tile | ||
|---|---|---|---|---|---|---|---|---|
| Name in Model | Description | |||||||
| Baseline model | 456.1623 | −228.081 | 9.084566 | a_mean | Average background value | 55.29 | 60.45 | 65.85 |
| a_sigma | Var’n of background values | 9.521 | 13.34 | 19.06 | ||||
| sigma | Intra-individual var’n | 6.755 | 7.2 | 7.683 | ||||
| Linear model | 458.774 | −229.387 | 9.668431 | a_mean | Average background value | 57.49 | 62.6 | 67.78 |
| a_sigma | Var’n of background values | 9.164 | 12.86 | 18.35 | ||||
| sigma | Intra-individual var’n | 5.913 | 6.302 | 6.726 | ||||
| b | PFOS slope | −1.42E-4 | −1.22E-4 | −1.022E-4 | ||||
TABLE 4.
Analysis Results for HDL; Males
| WAIC | ELPD | SE of ELPD | Model Parameter Est’s |
2.5th %tile | Mean | 97.5th %tile | ||
|---|---|---|---|---|---|---|---|---|
| Name in Model | Description | |||||||
| Baseline model | 458.4667 | −229.233 | 9.67548 | a_mean | Average background value | 56.85 | 61.81 | 67.82 |
| a_sigma | Var’n of background values | 8.128 | 11.8 | 17.56 | ||||
| sigma | Intra-individual var’n | 5.847 | 6.24 | 6.661 | ||||
| Linear model | 455.2345 | −227.617 | 10.20133 | a_mean | Average background value | 59.17 | 63.86 | 69.37 |
| a_sigma | Var’n of background values | 7.926 | 11.33 | 16.7 | ||||
| sigma | Intra-individual var’n | 5.078 | 5.404 | 5.767 | ||||
| b | PFOS slope | −1.261E-4 | −1.092E-4 | −9.218E-5 | ||||
TABLE 6.
Serum PFOS Concentration BMC (μg/ml) for Possible Effect on Lowering HDL
| Sex | BMCL1sd | BMC | BMCU1sd |
|---|---|---|---|
| Male | 74.259 | 104.409 | 145.736 |
| Female | 76.373 | 106.148 | 145.869 |
Reported are the 5th percentile, the mean, and the 95th percentile from the 30 000 samples from the posterior distribution of BMC estimates obtained in the MCMC implementation, as the BMCL, BMC and BMCU, respectively. A typical choice for the BMCL corresponds to a 5% 1-sided bound; hence the choice of the fifth percentile from the distribution of BMC estimates. Together, the BMCL1sd and BMCU1sd represent a 90% credible interval for the BMC.
There was no K+PFOS treatment-related effect on TSH or TT3. However, because TSH is the primary diagnostic parameter for human thyroid disease, and TT3 had been used as an endpoint for deriving risk-based values, BMC analyses were attempted for both TSH and TT3. In both cases, the linear model was not a superior fit over the baseline model and therefore, there was no evidence to further support that dosing with K+PFOS had an effect on either serum TSH or TT3 in this study.
DISCUSSION
This study undertook a rigorous investigation to determine the treatment-effect of PFOS associated with changes in selected clinical chemistry endpoints in cynomolgus monkeys. Of particular interest were changes in lipid chemistries and thyroid-related hormones, because HDL, TSH, and TT3 had been used in deriving risk-based values for exposure in water and food, as reviewed by Butenhoff and Rodricks (2015).
In our study, K+PFOS treatment did not result in any mortality or decreased body weight, and all of the monkeys remained healthy throughout the entire study with maximum serum PFOS concentrations approaching approximately 165 μg/ml. In contrast, monkeys from the high dose group (0.75 mg/kg−d) in a previous 6-month gavage capsule dosing study with K+PFOS (Seacat et al., 2002) experienced declines in group mean body weight, and one male died on Day 155 and another one was humanely sacrificed due to illness on Day 179. In those male and female monkeys, terminal serum PFOS concentrations approximated 170 μg/ml. We are uncertain why such disparate health outcomes arose between the 2 studies with similar maximal serum PFOS concentrations. In the Seacat et al. study, daily gastric intubation was used to deliver daily dose in the form of a gelatin capsule containing K+ PFOS, which is a strong anionic surfactant. In this study, up to 3 doses total were given at fixed intervals spread out over time by delivering a solution of 0.2% (w/v) in 0.5% Tween 20 with 5% absolute ethanol in water through intragastric tube. The treatment regimen in this study may have reduced the stress associated with the dosing.
At serum PFOS concentrations up to 165 μg/ml, we did not observe clinically relevant changes with active thyroid hormones, liver enzymes, kidney function, coagulation, and electrolytes for all monkeys after PFOS administration. The only exception was slightly reduced serum total cholesterol driven by a concurrent reduction in HDL. Given all the clinical observations that were evaluated were normal and there was no effect on body weight or body weight-gain in these monkeys, the magnitude of the changes in serum lipid was not considered adverse from a clinical perspective.
Seacat et al. (2002) reported 20–30% decline in serum total cholesterol when serum PFOS concentration reached 100 μg/ml during second and third month of dosing at 0.75 mg/kg−d. At study termination (182 days) when mean group serum PFOS concentration reached 170 μg/ml, Seacat et al. observed an even greater reduction of total cholesterol, 50–70% relative to time-matched control as well as predose baseline values. When compared with concurrent control values, both male and female high dose groups in the Seacat et al. study had clearly reduced serum HDL. Because Seacat et al. did not have either baseline or earlier HDL measures during dosing, they acknowledged that a statistically significant decrease (relative to concurrent control) in HDL in the mid-dose females (0.15 mg/kg−d) with mean terminal serum PFOS concentration approximately 67 μg/ml was difficult to interpret as a treatment-related effect. In addition, the values for the males at the mid-dose were not statistically significantly different compared with concurrent control with mean terminal serum PFOS concentration approximately 83 μg/ml. Because total cholesterol and HDL levels did return to normal during recovery, Seacat et al. concluded that the mid-dose (0.15 mg/kg−d) represented the NOAEL for their study. BMD modeling based on serum PFOS and HDL concentrations in this study resulted in a lower-bound (fifth percentile) BMC values for HDL (BMCL1sd) of 74 and 76 μg/ml for male and female monkeys, respectively. This is consistent with the serum concentrations associated with an equivocal reduction in HDL in female monkeys reported by Seacat et al. Therefore, based on the extensive data collected in this study, the BMCL1sd of approximately 75 μg/ml serum PFOS for a clinically insignificant reduction in HDL would be a conservative point of departure for use in risk assessment.
In this study, we observed a smaller effect on the reduction of total cholesterol than that observed by Seacat et al. (2002), even though serum PFOS concentrations reached were of similar magnitude. The reason for this apparent difference is not entirely clear. Because studies on the mechanism of PFOS-induced cholesterol reduction have identified modulation by PFOS of hepatic processes associated with the development of hypolipidemia (infra vide), therefore it is reasonable to speculate that the concentration of PFOS in liver is an important factor in the determining the magnitude of hypolipidemic effect, with increasing concentrations causing greater effect. There is limited data on liver PFOS concentrations attained in the study by Seacat et al. and in this study. For Seacat et al., liver PFOS concentration was obtained for 4 female and 2 male monkeys at the highest study dose after 6 months of dosing. The mean (±SD) values, in μg PFOS/g liver, for these males and females were 395 (±24) and 273 (±14), respectively. However, a reduction in total cholesterol became apparent after 2 (females) and 3 (males) months of dosing, a time period for which liver PFOS concentration is not available. In the TG3 monkeys with the highest PFOS exposure from this study (n = 4/sex), end of study liver PFOS concentrations were 94.5 (±33.9) and 112.0 (±18.7) μg/g, for males and females, respectively. These liver biopsy samples were obtained 62 days after the last dose; therefore, liver PFOS concentration soon after the last dose is not known. In the Seacat et al. study, total cholesterol had recovered to normal values within 36 days in recovery after termination of dosing, and HDL returned to normal by 61 days in recovery. However, liver PFOS concentration data were not obtained from high-dose recovery monkeys until 211 days (approximately 7 months) after cessation of dosing. Those liver concentration data, obtained by partial hepatectomy from 2 male and 2 female monkeys who were not sampled at the end of dosing, were 138 and 142 μg/g for the males and 175 and 421 μg/g for the females. The 421 μg/g value for 1 female is suspicious, given the small coefficient of variation among the 4 females for which liver was analyzed at the end of the 6-month dosing period (±5%). Although the liver-to-serum concentration ratio associated with a hypolipidemic response in monkeys may be of value, such information is not available for the time period in which reduction of cholesterol is first observed. These facts, coupled with the fact that analytical methodology has progressed significantly since the time frame in which the Seacat et al. study data were obtained, make it difficult to draw conclusions and comparisons between the Seacat et al. study and this study with respect to liver concentration associated with the PFOS-induced hypolipidemic effect in cynomolgus monkeys.
In this study, the decrease in the serum lipids was driven by reduction in serum HDL. PFOS has been established as a hypolipidemic agent in mechanistic studies and reduction in serum cholesterol has been shown to be an early effect related to dosing with PFOS in toxicological studies with rodents and primates (Bijland et al., 2011; Elcombe et al., 2012a; Seacat et al., 2002, 2003), where measured serum PFOS concentrations ranged from 100 to 150 μg/ml when the reductions of total cholesterol were observed in these studies. The hypolipidemic activity of PFOS occurs via the activation of xenosensor nuclear receptors peroxisome proliferator-activated receptor alpha (PPARα) and pregnane X receptor, which can influence fatty acid β-oxidation and lipid synthesis (Bijland et al., 2011; Bjork et al., 2011; Elcombe et al., 2012a,b). Although it did not exceed 2-fold increase over control, Seacat et al. (2002) showed that hepatic PPARα activities (measured as palmitoyl CoA oxidase activity) were statistically significantly increased in the high dose group monkeys (both males and females). Using ApoE*3.Leiden.CETP mice, a humanized model having attenuated clearance of ApoB-containing lipoprotein and exhibiting human-like lipoprotein metabolism on a Western-type diet (ApoE*3 model paper), Bijland et al. (2011) demonstrated that high dietary doses of PFOS resulted in lower serum cholesterol by reducing VLDL production with enhanced triglyceride clearance (mediated by lipoprotein lipase) as well as decreased production of apolipoprotein B. PFOS also affected the rate of apolipoprotein A1 synthesis which ultimately resulted in the reduction of circulating HDL. Thus, the observed reduction in HDL after administration of K+ PFOS in this study is likely the result of this mechanism and represents an early biological response to PFOS exposure.
As stated earlier, thyroid hormones have been used to establish the point of departure for PFOS-related health risk assessments, and as such, serum thyroid hormones were evaluated extensively in the study reported herein. When compared with time-matched control or each monkey’s own baseline value, repeated measurements of TSH and FT4 throughout 400+ days did not demonstrate a PFOS-related effect, indicating normal thyroid hormone function. While PFOS had no effect on TT3 (an endpoint selected by several health risk assessments), it did result in decreased TT4.
In serum, 99% of the thyroid hormones are T4 and <1% is T3; and among these, 99.9% of T4 and 99.5% of T3 are protein-bound and metabolically inactive. Even though FT4 and FT3 are very small fractions compared with their bound counterparts (<0.5%), they are the metabolically active moiety of the thyroid hormones (Refetoff et al., 1970). Both bound and unbound thyroxines exist in a state equilibrium and the association and dissociation between the two are within seconds (Mendel et al., 1988). Under the condition when there is reduced TT4 (which is the measurement of both bound and unbound thyroxine), such as attribution from competitive protein binding between thyroxine and naturally occurring free fatty acids or even with pharmaceutical drugs like heparin or aspirin (Faber et al., 1993), an individual is likely able to maintain his thyroid hormone homeostasis because of the large reservoir of bound (inactive) thyroxine (Mendel et al., 1988), thus the reason why TT4 is not considered clinically diagnostic. Rather, with its essential regulatory functions in the hypothalamus-pituitary-thyroid (HPT) axis, the primary clinical indicator for diagnosing thyroid function is TSH followed by FT4 because it inversely correlates with the TSH levels as part of the HPT feedback mechanism (Molina, 2013; Oppenheimer et al., 1995).
We had previously reported that PFOS can result in lowering of TT4 in Sprague Dawley rats by displacing thyroxine from the binding proteins which resulted in a net loss of TT4 but still maintaining thyroid hormone homeostasis (Chang et al., 2007, 2008). Therefore, decreased TT4 observed in the current monkey study upon dosing with PFOS is also most likely due to competitive displacement of thyroxine and its increased metabolism and elimination. This does not suggest an abnormal thyroid function in these monkeys given there were no concomitant changes in TSH and FT4, and as such, administration of K+ PFOS did not affect thyroid hormone homeostasis in these monkeys and should not be used as a health-related endpoint.
Overall, administration of K+PFOS to monkeys did not result in any toxicologically meaningful or clinically relevant changes in serum clinical measurements for coagulation, lipids, hepatic, renal, electrolytes, and thyroid-related hormones. A slight reduction in serum cholesterol (primarily the HDL fraction), although not toxicologically significant, was observed and the corresponding lower-bound fifth percentile benchmark concentrations (BMCL1sd) were 74 and 76 μg/ml for male and female monkeys, respectively. Compared to the 2013–2014 geometric mean serum PFOS level of 4.99 ng/ml (0.00499 μg/ml) in the U.S. general population (CDC NHANES, 2017), this represents 4 orders of magnitude for the margin of exposure.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to acknowledge Dr Carol A. Ley, Jay F. Schulz, and Thomas A. Kestner for their expert advices. The authors would also like to acknowledge the technical contributions of staff at Charles River Laboratories Montreal ULC in the conduct of this study.
CONFLICT OF INTEREST
The study was supported by 3M Company, former manufacturer of PFOS and related materials. Sue Chang, Kara Andres, David Ehresman, Geary Olsen, and John Butenhoff are employees or former employees of the 3M Company. Bruce Allen and John Butenhoff are consultants to 3M Company. Ria Falvo and Anne Provencher are employees of Charles River Laboratories that was contracted by 3M Company to conduct the study in monkeys.
REFERENCES
- Bijland S., Rensen P. C. N., Pieterman E. J., Maas A. C. E., van der Hoorn J. W., Van Erk M. J., Havekes L. M., van Dijk K. W., Chang S. C., Ehresman D. J., et al. (2011). Perfluoroalkyl sulfonates cause alkyl chain length-dependent hepatic steatosis and hypolipidemia mainly by impairing lipoprotein production in APOE*3-leiden CETP Mice. Toxicol. Sci. 123, 290–303. [DOI] [PubMed] [Google Scholar]
- Bjork J. A., Butenhoff J. L., Wallace K. B. (2011). Multiplicity of nuclear receptor activation by PFOA and PFOS in primary human and rodent hepatocytes. Toxicology 288, 8–17. [DOI] [PubMed] [Google Scholar]
- Brooke D., Footitt A., Nwaogu T. A. (2004). Environmental Risk Evaluation Report: Perfluorooctanesulfonate (PFOS). Environment Agency. Available at: http://www.pops.int/documents/meetings/poprc/submissions/Comments_2006/sia/pfos.uk.risk.eval.report.2004.pdf. Accessed December 4, 2016.
- Brooks S. P., Gelman A. (1998). General methods for monitoring convergence of iterative simulations. J. Comput. Graph. Stat. 7, 434–455. [Google Scholar]
- Butenhoff J. L., Rodricks J. V. (2015). Human Health Risk Assessment of Perfluoroalkyl Acids. In Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances (DeWitt J., Eds.), pp. 363–418. Springer International Publishing, Switzerland. [Google Scholar]
- Canadian Government Department of the Environment. (2008). Pefluorooctanesulfonate and its salts and certain other compounds regulations. Canada Gazette Part II, 1322–1325. [Google Scholar]
- CDC NHANES. (2017). Fourth National Report on Human Exposure to Environmental Chemicals. Available at: https://www.cdc.gov/biomonitoring/pdf/FourthReport_UpdatedTables_Volume1_Jan2017.pdf. Accessed February 22, 2017.
- Chang S. C., Thibodeaux J. R., Eastvold M. L., Ehresman D. J., Bjork J. A., Froehlich J. W., Lau C. S., Singh R. J., Wallace K. B., Butenhoff J. L. (2007). Negative bias from analog methods used in the analysis of free thyroxine in rat serum containing perfluorooctanesulfonate (PFOS). Toxicology 234, 21–33. [DOI] [PubMed] [Google Scholar]
- Chang S. C., Thibodeaux J. R., Eastvold M. L., Ehresman D. J., Bjork J. A., Froehlich J. W., Lau C., Singh R. J., Wallace K. B., Butenhoff J. L. (2008). Thyroid hormone status and pituitary function in adult rats given oral doses of perfluorooctanesulfonate (PFOS). Toxicology 243, 330–339. [DOI] [PubMed] [Google Scholar]
- Chang S. C., Noker P. E., Gorman G. S., Gibson S. J., Hart J. A., Ehresman D. J., Butenhoff J. L. (2012). Comparative pharmacokinetics of perfluorooctanesulfonate (PFOS) in rats, mice, and monkeys. Reprod. Toxicol. 33, 428–440. [DOI] [PubMed] [Google Scholar]
- Ehresman D. J., Froehlich J. W., Olsen G. W., Chang S. C., Butenhoff J. L. (2007). Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environ. Res. 103, 176–184. [DOI] [PubMed] [Google Scholar]
- Elcombe C. R., Elcombe B. M., Foster J. R., Chang S. C., Ehresman D. J., Butenhoff J. L. (2012a). Hepatocellular Hypertrophy and Cell Proliferation in Sprague-Dawley Rats from Dietary Exposure to Potassium Perfluorooctanesulfonate Results from Increased Expression of Xenosensor Nuclear Receptors PPARα and CAR/PXR. Toxicology 293, 16–29. [DOI] [PubMed] [Google Scholar]
- Elcombe C. R., Elcombe B. M., Foster J. R., Chang S. C., Ehresman D. J., Noker P. E., Butenhoff J. L. (2012b). Evaluation of hepatic and thyroid responses in male sprague dawley rats for up to eighty-four days following seven days of dietary exposure to potassium perfluorooctanesulfonate. Toxicology 293, 30–40. [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority (2012). Perfluoroalkylated substances in food: Occurrence and dietary exposure. EFSA J. 10, 2743. [Google Scholar]
- Faber J., Waetjen I., Siersbaek-Nielsen K. (1993). Free thyroxine measured in undiluted serum by dialysis and ultrafiltration: Effects of non-thyroidal illness, and an acute load of salicylate or heparin. Clin. Chim. Acta 223, 159–167. [DOI] [PubMed] [Google Scholar]
- Flow B. L., Jaques J. T. (1997). Effect of Room Arrangement and Blood Sample Collection Sequence on Serum Thyroid Hormone and Cortisol Concentrations in Cynomolgus Macaques (Macaca fascicularis). Contemp. Top. Lab. Anim. Sci. 36, 65–68. [PubMed] [Google Scholar]
- Gelman A. (2005). Prior distributions for variance parameters in hierarchical models. Bayesian Anal. 1, 1–19. [Google Scholar]
- Giesy J. P., Kannan K. (2001). Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 35, 1339–1342. [DOI] [PubMed] [Google Scholar]
- ILAR. (2011). Guide for the Care and Use of Laboratory Animals (8th Edition) National Research Council, Institute of Laboratory Animal Resources. National Academy Press, Washington, DC. [Google Scholar]
- Kissa E. (2001). Fluorinated Surfactants and Repellents. Marcel Dekker, New York. [Google Scholar]
- Mendel C. M., Cavalieri R. R., Weisiger R. A. (1988). Uptake of thyroxine by the perfused rat liver: Implications for the free hormone hypothesis. Am. J. Physiol. 255, E110–E119. [DOI] [PubMed] [Google Scholar]
- Minnesota Department of Health. (2009). Toxicological Summary for Perfluorooctane Sulfonate (PFOS) and Salts - Health Risk Limit (HRL) for Groundwater Availabe at: http://www.health.state.mn.us/divs/eh/risk/guidance/gw/pfos.pdf. Accessed December 4, 2016.
- Molina P. (2013). Thyroid Gland, In Endocrine Physiology (M. Weitz and C. Naglieri Eds.), 4th ed., pp. 73–98. McGraw-Hill Education. New York, NY, USA. [Google Scholar]
- OECD. (2002). Hazard Assessment of Perfluorooctane Sulfonate (PFOS) and Its Salts, pp. 1–362, Paris, France.
- Olsen G. W., Burris J. M., Ehresman D. J., Froehlich J. W., Seacat A. M., Butenhoff J. L., Zobel L. R. (2007). Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 115, 1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz A. L., Strait K. A. (1995). An Integrated View of Thyroid Hormone Actions in vivo (Weintraub B. D. E., Eds.), pp. 249–65. Raven Press, Ltd, New York. [Google Scholar]
- Refetoff S., Robin N. I., Fang V. S. (1970). Parameters of thyroid function in serum of 16 selected vertebrate species: A study of PBI, serum T4, free T4, and the pattern of T4 and T3 binding to serum proteins. Endocrinology 86, 793–805. [DOI] [PubMed] [Google Scholar]
- Seacat A. M., Thomford P. J., Hansen K. J., Olsen G. W., Case M. T., Butenhoff J. L. (2002). Subchronic toxicity studies on perfluorooctanesulfonate potassium salt in cynomolgus monkeys. Toxicol. Sci. 68, 249–264. [DOI] [PubMed] [Google Scholar]
- Seacat A. M., Thomford P. J., Hansen K. J., Clemen L. A., Eldridge S. R., Elcombe C. R., Butenhoff J. L. (2003). Sub-chronic dietary toxicity of potassium perfluorooctanesulfonate in rats. Toxicology 183, 117–131. [DOI] [PubMed] [Google Scholar]
- Stockholm Convention. (2009). Available at: http://chm.pops.int/TheConvention/ThePOPs/ListingofPOPs. Accessed January 4, 2017.
- UKDWI. (2009). Guidance on the water supply (water quality) regulations 2000 specific to PFOS (perfluorooctane sulphonate) and PFOA (perfluorooctanoic acid) concentrations in drinking water. Available at: http://dwi.defra.gov.uk/stakeholders/information-letters/2009/10_2009annex.pdf. Accessed December 4, 2016.
- USEPA. (2009). Provisional Health Advisories for Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS). Available at: https://www.epa.gov/sites/production/files/2015-09/documents/pfoa-pfos-provisional.pdf. Accessed November 30, 2016.
- Vehtari A., Gelman A. (2014). WAIC and cross-validation in Stan (submitted). Available at: http://www.stat.columbia.edu/∼ gelman/research/unpublished/waic_stan.pdf. Accessed November 30, 2016.
- WHO. (2004). Handbook: Non-clinical safety testing (WHO Reference Number: TDR/PRD/NCT/04.1). Available at: http://www.who.int/tdr/publications/documents/safety_handbook.pdf?ua=1. Accessed November 30, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.