Abstract
Assessing intra-specific variation in drought stress response is required to mitigate the consequences of climate change on forest ecosystems. Previous studies suggest that European larch (Larix decidua Mill.), an important European conifer in mountainous and alpine forests, is highly vulnerable to drought. In light of this, we estimated the genetic variation in drought sensitivity and its degree of genetic determination in a 50-year-old common garden experiment in the drought-prone northeastern Austria. Tree ring data from larch provenances originating from across the species' natural range were used to estimate the drought reaction in four consecutive drought events (1977, 1981, 1990–1994, and 2003) with extremely low standardized precipitation- and evapotranspiration-index values that affected growth in all provenances. We found significant differences among provenances across the four drought periods for the trees’ capacity to withstand drought (resistance) and for their capacity to reach pre-drought growth levels after drought (resilience). Provenances from the species' northern distribution limit in the Polish lowlands were found to be more drought resistant and showed higher stability across all drought periods than provenances from mountainous habitats at the southern fringe. The degree of genetic determination, as estimated by the repeatability, ranged up to 0.39, but significantly differed among provenances, indicating varying degrees of natural selection at the provenance origin. Generally, the relationship between the provenances’ source climate and drought behavior was weak, suggesting that the contrasting patterns of drought response are a result of both genetic divergence out of different refugial lineages and local adaptation to summer or winter drought conditions. Our analysis suggests that European larch posseses high genetic variation among and within provenances that can be used for assisted migration and breeding programs.
Keywords: common garden experiment, degree of genetic determination, drought response, European larch, Larix decidua, repeatability
Introduction
The behavior of plants under environmental stress has always been an object of interest for plant ecologists, physiologists and geneticists (e.g., Hsiao 1973, Seki et al. 2002, Farooq et al. 2009). Amongst others, drought represents one of the most important stress factors for terrestrial plants, since their whole carbon assimilation mechanism is triggered by water supply from the soil via the roots to the leaves (Smirnoff 1993, Gleason et al. 2016). Due to the predicted increasing frequency and severity of future global change type droughts (Calanca 2007; Sheffield and Wood 2008), they are at present amongst the most discussed topics in plant and animal ecology with the objective to gain a better understanding on whether and how single individuals, populations and whole ecological communities will adapt and evolve if one of these scenarios is becoming true (McCarty 2001, Svenning and Skov 2004, Park Williams et al. 2012, Cook et al. 2015). For trees, forest canopies as well as whole forest communities, the occurrence of long periods of water shortage, probably accompanied by high air temperature, had already resulted in notable dimensions of growth decline, mortality as well as in subsequent damages like wildfire and insect outbreaks (Martínez-Vilalta and Pinol 2002, Breshears et al. 2005, Allen et al. 2010, Peng et al. 2011, Barbeta et al. 2013, Anderegg et al. 2015).
While annual plants are likely to experience only a limited number of drought events during their life-cycle, trees often undergo several droughts and are therefore frequently used as a tool in dendrochronology for reconstructing and detecting past extreme climate events that had left their specific marks in tree rings (e.g., Schweingruber et al. 1990, Neuwirth et al. 2004).
Intra-specific (i.e., genetic) variation in growth traits, phenology and stress response has become an integral part of adaptive forest management and its understanding is required for estimating the consequences of climate change on prospective tree species’ range shifts, local adaptation of tree populations and suitability of forest reproductive material that might be planted in the future (e.g., Anekonda et al. 2002, Wang et al. 2006, Aitken et al. 2008, Arend et al. 2011, Alberto et al. 2013). Most of the studies that assessed intra-specific variation in stress response of forest trees made use of common garden experiments, where trees from different geographical origins are reciprocally planted in different environments to estimate the intra-specific variation in phenotypic plasticity for growth traits and mortality (i.e., genotype-by-environment interactions) (e.g., Schmidtling, 1994, Mátyás 1996, Alia et al. 1997, Taeger et al. 2013). However, only little attention has been paid to the question whether the drought response of a given genotype or provenance is stable across several repeatedly occurring drought events during their life-time or at least over a certain episode of their life-time (i.e., a genotype-by-time interaction). Such an experimental design would also allow accounting for another important source of variation: the random character of meteorological drought occurrence in space and time and the variation in its duration and intensity (e.g., Estrela et al. 2000, Lloyd-Hughes and Saunders 2002, Corzo Perez et al. 2011).
The tree species investigated in the present study is European larch (Larix decidua Mill.), a deciduous conifer growing mainly at high altitudes of 1500–2000 m a.s.l. and occurring in the Alps, Sudetes, and Carpathians. The species also has a limited distribution in the lowlands of southern Poland and the eastern Alps (Matras and Pâques 2008). Its pioneer character and the ability to colonize mountainous habitats which are inaccessible for most other conifers makes it an essential forest tree species in Europe for soil protection and the mitigation of avalanche risks in areas with steep and actively eroding habitats. Due to its fast juvenile growth and superior wood quality, European larch has also become subject of intensive breeding programs and is widely used for afforestations outside its natural range (Pâques et al. 2013). However, recent studies suggest that European larch in the Alpine region is highly vulnerable to soil water deficit and drought events (Eilmann and Rigling 2012, Levesque et al. 2013) and might have only limited potential under future climate. On the other hand, new phylogeographic analysis revealed high differentiation and strong genetic structure probably due to the survival of European larch in up to six glacial refugia (Wagner et al. 2015a , 2015b ), suggesting that the species may encompass also wide adaptive genetic variation that might help to increase the species' prospects in a changing climate. Such genetic variation can be expressed as differences in drought response between provenances of the species due to local adaptation to regional drought regimes. Moreover, drought stress is likely to affect survival and fitness of trees (e.g., White 1987, Vranckx et al. 2014) and hence causes varying degrees of genetic determination of drought sensitivity depending on the local environment. We claim that such hypotheses need to be addressed before any kind of forest management or conservation actions are undertaken that are aiming at safeguarding European larch populations within a warmer future (e.g., assisted population migration or breeding).
Therefore, the objectives of the present study are: first, to analyze the genetic variation in drought response among provenances of European larch; second, to investigate the stability of drought response of these provenances across repeated drought periods with varying degrees in intensity, duration as well as time of drought occurrence, and third, to estimate the degree of genetic determination of drought response as given by the trait resemblance between relatives (Falconer and Mackay 1996).
Material and methods
Plant material, sampling and trial site
The analyzed trees and provenances are part of the 2nd international IUFRO Larch provenance trial that was established in 1958 with 1-year old seedlings in a 2 × 2 m2 plant spacing (Schober 1977). Seven provenances are originating from the core distribution of the species from the central Alps (Bruneck, IT; Schönwies, AT), the Western Alps (Embrun, F) and the Southern Alps (Cavalese, IT; Cavedine, IT; Pergine, IT), whereas provenance Pergine is represented twice, once from a moderate (600–800 m) and once from a high altitude site (1300–1400 m; see Figure 1 and Table 1). Additionally, two provenances are from lowlands in Southern Poland (Blyzin and Gora Chelmowa) and one from low elevations of the northeastern Alpine foothills (Wienerwald, AT). Although the occurrence of the latter is far away from the high mountainous areas of the Central Alps, its occurrence is supposed to be native (Tschermak 1935). These 10 provenances were sampled in summer 2013 by taking two increment cores per tree at breast height with a minimum of 11–15 trees per provenance. In addition to the core samples, diameter at breast height (DBH) and height growth were measured in 2013 with a diameter measuring tape and a vertex ultrasonic hypsometer (Haglöf, Sweden), respectively.
Figure 1.
Gray shading and gray dots indicate the core distribution and fragmented occurrence of Larix decidua, respectively (according to the EUFORGEN-network). Black dots indicate origins of analyzed plant material. Black triangle marks the location of the trial site. Mapdata were taken from the rworldmap package in R.
Table 1.
Analyzed provenances of Larix decidua Mill. MAT, mean annual temperature; MAP, mean annual precipitation sum; AHM, annual heat-moisture index; N, number of analyzed trees; roman numerals refer to provenances occurring outside of the core distribution.
| Provenance | Name | Country | Area | Latitude | Longitude | Altitude [m a.s.l.] | MAT [°C] | MAP [mm] | AHM | N |
|---|---|---|---|---|---|---|---|---|---|---|
| I | Blyzin | Poland | Polish lowlands | 51°00′ | 21°00′ | 320 | 7.0 | 615 | 27.60 | 15 |
| II | Gora Chelmowa | Poland | Polish lowlands | 51°10′ | 20°45′ | 320–340 | 6.7 | 622 | 26.75 | 13 |
| III | Wienerwald | Austria | Eastern Alps | 48°10′ | 16°10′ | 400 | 8.7 | 684 | 27.39 | 13 |
| 2 | Schönwies | Austria | Central Alps | 47°12′ | 10°40′ | 1100 | 4.5 | 985 | 14.71 | 14 |
| 15 | Bruneck | Italy | Central Alps | 47°00′ | 12°00′ | 1200 | 1.8 | 1078 | 10.99 | 14 |
| 16 | Cavalese | Italy | Southern Alps | 46°19′ | 11°27′ | 1200 | 4.9 | 792 | 18.84 | 14 |
| 17 | Pergine | Italy | Southern Alps | 46°00′ | 11°00′ | 600–800 | 8.0 | 794 | 22.69 | 13 |
| 19 | Pergine | Italy | Southern Alps | 46°06′ | 11°23′ | 1300–1400 | 3.5 | 817 | 16.52 | 13 |
| 20 | Cavedine | Italy | Southern Alps | 45°59′ | 11°04′ | 600–700 | 8.3 | 795 | 22.99 | 14 |
| 22 | Embrun | France | Western Alps | 44°47′ | 06°57′ | 1600 | 3.0 | 1312 | 9.90 | 11 |
| Total | 134 | |||||||||
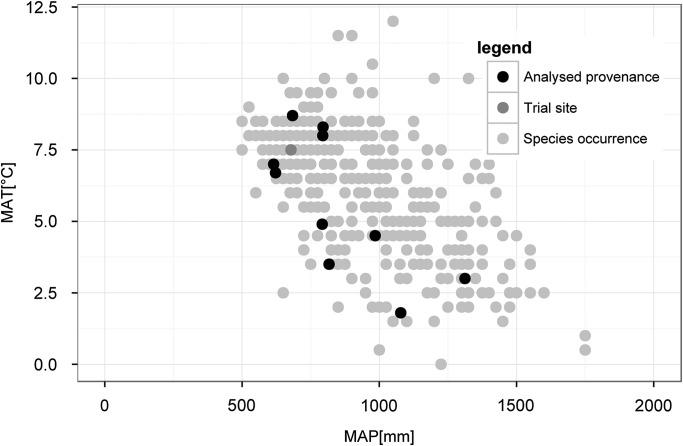
The climate conditions at the places of seed origin represent a cross-section through the species range of occurrence from warm–dry habitats (provenances I, II, III) towards cold and wet growth conditions (provenances 15 and 22, Figure 2). The trial site Ottenstein is located in northern lower Austria on 540 m a.s.l. on a deep soil of moderately fresh sandy loam on granite with average nutrition. Mean annual temperature is 7.5 °C and mean annual precipitation 680 mm with 300 mm falling from April to September (long-term mean from 1961 to 2012, Austrian Central Institute for Meteorology and Geodynamics (ZAMG)).
Figure 2.
Species occurrence data were taken from the ICP Forest Program ‘Large-scale forest condition monitoring Level I’ from the period 1987–2007 (ICP Forests 2010).
Core sample preparation and measurements of annual increment
Core samples were cut into approx. 1.4 mm thick cross sections with a double-blade circular saw, placed on microfilms and exposed to a 10 kV (24 mA) X-ray source for 25 min. After exposure to the X-ray source, the obtained microfilms were analyzed with WinDENDRO 2009 (Regent Instrument, Quebec, CAN) and ring-width for each year was measured to the nearest 0.001 mm. Values from the two cores of the same tree were averaged in order to retain only the climatic and genetic variance and to reduce non-climatic noise such as reaction wood. For further analysis, the data were converted into single-tree time-series and mean chronologies using the dplR package in R (R Development Core Team 2008, Bunn 2010).
Climate data, drought years and drought response measurement
For the characterization of climate conditions at seed origin (Table 1), we used data from the WorldClim database (Hijmans et al. 2005) as well as the extract function implemented in the raster package in R (R Development Core Team 2008). To assess whether seed originated from a rather dry or moist climate, we calculated the annual heat-moisture index (AHM) according to Wang et al. (2006) for each location of origin. The AHM is given as (MAT + 10)/(MAP/1000), where MAT is the mean annual temperature in degrees centigrade and MAP the mean annual precipitation in mm (Table 1). Climate data of the trial site were interpolated from the four nearest weather stations of the Austrian Central Institute for Meteorology and Geodynamics (ZAMG) by inverse distance weighted interpolation. To identify past drought years we used the standardized precipitation index (SPI) developed by McKee et al. (1993) and the standardized precipitation evapotranspiration index (SPEI) according to Vicente-Serrano et al. (2010). While the SPI calculates actual deficits/surpluses of rainfall in relation to the standardized long-term mean, the SPEI also integrates temperature and hence is based on a climatic water balance. The fundamental advantage of using SPI and SPEI instead of other drought indices is their ability to work on different time-scales which makes it possible to distinguish between short-, moderate and long-term water-supply deficits (Mishra and Singh 2010). SPI and SPEI were calculated using the command line program Spisl 6 (NDMC 2014) and the R-package SPEI (R Development Core Team 2008), respectively. Both indices were calculated for time-scales of one and three months to uncover biologically meaningful drought events for forest trees under consideration of soil water deficit and evapotranspiration (e.g., Pasho et al. 2011, George et al. 2015). Years were considered as drought years, when at least one month within the vegetation period (April–September) showed either ‘severe’ or ‘extreme’ deviations from the standardized long-term mean (equivalent to −1.50 and −2.00 standard deviations following the classification of McKee et al. 1993).
To quantify a tree's response to drought stress we made use of four different response indices developed by Lloret et al. (2011): we calculated resistance (Res), which characterizes the ability of a tree to withstand a period of low water supply as the ratio between ring-width during (Dr) and before the drought event (preDr). Recovery (Rec), in contrast, is defined as the ability to recruit after drought and is calculated as the ratio of annual increment after drought (postDr) and during drought (Dr). Resilience (Rsl) indicates if trees are able to reach their pre-drought increment levels immediately after a drought and is calculated as postDr/preDr. The latter index can be extended to (postDr-Dr)/preDr and describes how fast a tree is able to recover to pre-drought level by accounting for the experienced damage during drought and is called relative resilience (rRsl). Even though the relative resilience is not unequivocal at individual level (Lloret et al. 2011), because a high value for resistance can automatically determine a low relative resilience and vice versa, it was included in the analysis, since it was rather our aim to assess the intra-specific norm of reaction. For resistance and resilience, values of 1 indicate no decline in increment or an immediate return to pre-drought level, respectively. For recovery and relative resilience, values <1 indicate the persistence of negative effects of the drought event, whereas values >1 suggest an immediate regeneration from a drought event. The first three indices (Res, Rec, Rsl) can take exclusively positive values, but rRsl can also take negative ones in cases where post-drought performance is lower than performance during the drought. Calculations of indices were applied to the raw and untransformed ring-width series in order to retain the intra-specific variation in drought response among the different provenances based on sudden changes in ring-width within relatively short timeframes (see for example Schweingruber et al. 1990, Desplanque et al. 1999).
Statistical analysis
To test for the stability of drought response through time and for differences among provenances and drought years, respectively, we used a mixed model design, where the repeated drought performance of the same tree was treated as a random block effect in a repeated measure ANOVA and ‘provenance’ (i.e., genotype) as a between-group variable in a one-way ANOVA. Since the four analyzed drought events varied in intensity, time of occurrence and duration (see results below), they were translated into factors and treated as nested variables within the repeated measure ANOVA as an additional source of variance. The model can be formulated in a multivariate way as
| (1) |
where yi are the four repeated measurements made on the same tree, µ is a vector of means for all drought years grouped by provenance, and εi is a vector of random errors associated with the repeated measurements. Under the assumption that drought response on intra-specific level is stable through time, we do not expect an interaction between drought events and genotype. The within-subject and between-group variables and their interaction were included hierarchically and compared to a baseline model including no predictors other than the intercept. We used the Akaike information criterion (AIC) as well as the log likelihood and likelihood ratio for model evaluation. These steps were performed using the lme function in the nlme package in R (R Development Core Team 2008) for linear mixed-effects models, which explicitly allows for the analysis of variance for dependent residuals as described in Laird and Ware (1982).
To analyze the variation within provenances and to estimate the degree of genetic determination, we calculated the repeatability of drought response. The repeatability gives the proportion of total variation of a certain trait – here, the four drought response measures – that is due to differences between individuals. Repeatability (further abbreviated as ‘Rep’) is calculated as
| (2) |
with VG being the genotypic variance, VEG the general environmental variance and VP the total phenotypic variance, where ‘phenotype’ refers to the respective drought response of an individual tree. Repeatability is related to the heritability in the narrow sense, as the additive genetic variance VA, the numerator of heritability, can never be larger than VG+ VEG (Falconer and MacKay 1996). Thus, Rep gives the upper limit of heritability of the given trait (Boake 1989, Merilä and Sheldon 2000, but see Dohm 2002). For the calculation of ‘Rep’, drought response indices were standardized and log transformed to achieve adjusted repeatabilities, which are corrected for differences in means and variances between drought years (Nakagawa and Schielzeth 2010). Variance components as described in (2) were estimated with mixed-effects models employing restricted maximum likelihood estimation (REML) in the software ASReml (Gilmour et al. 2009). First, a mixed-effect model with individual trees and provenances as random effects was used in order to estimate repeatability across all provenances and assuming equal selection pressures throughout all larch populations. Second, an univariate mixed-effect model with individual trees as single random effect was fitted individually for each provenance. These models allowed for the calculation of provenance specific repeatability by assuming that the environmental origin of the provenances resulted in different selection pressures on drought response. Repeatability and its standard errors were calculated with the post-processing modul of ASReml (Gilmour et al. 2009).
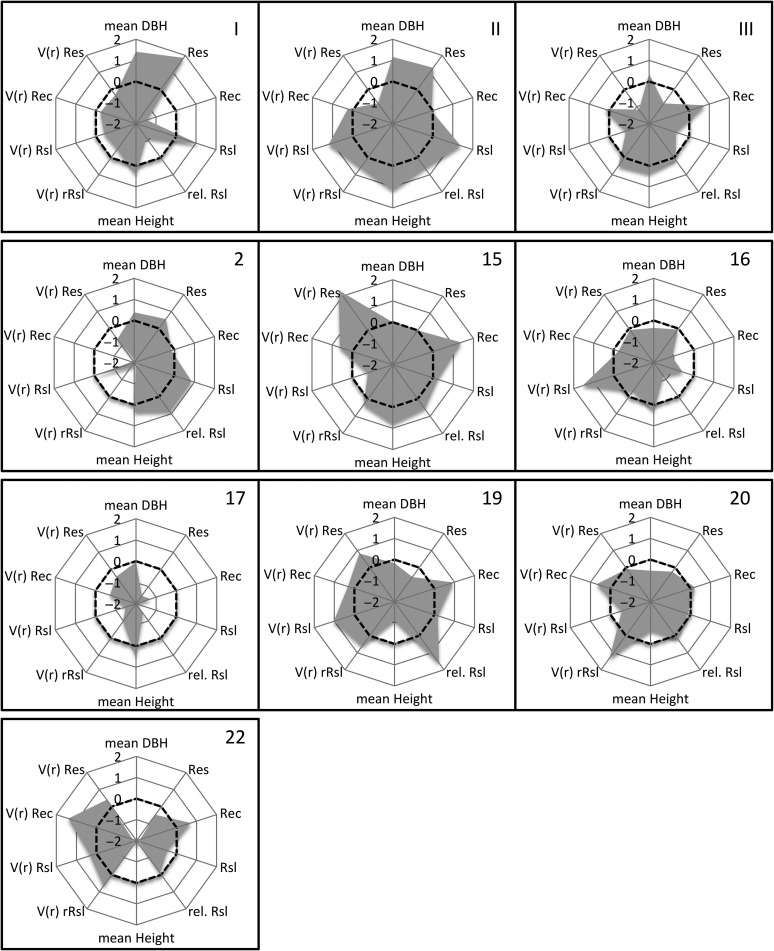
Finally, we characterized the ten provenances in respect to three important features: (i) their general overall performance in drought response across all drought periods, (ii) their stability of mean drought response through time (Vr) expressed as the variance of the ranks across all drought events (rank = 1 for the best performing provenance and rank = 10 for the worst performing provenance) as
| (3) |
with rij being the rank of the provenance i in the drought year j and with being the average rank of each provenance over N drought periods (Huehn 1990) and (iii) their competitive force expressed as mean DBH and mean height growth. For this final evaluation, the data were normalized and transformed into z-scores and illustrated in radar charts.
Results
Identification of past drought events
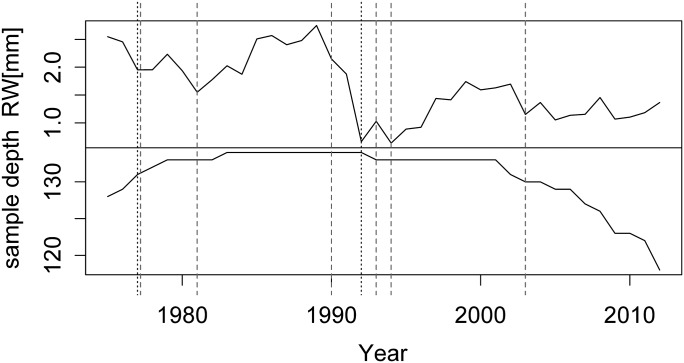
Within the period from 1960 to 2012 we found 9 years indicating either a severe or extreme water deficit as revealed by the two standardized drought indices. These drought events occurred in 1974, 1977, 1981, 1990, 1992, 1993, 1994, 2003, and in 2007 with the majority of drought events being detected on a timescale of 3 months (Table 2). Since ring width data for the very early and late years were missing for some trees, drought events that occurred at the terminal ends of the time series (1974 and 2007) were not considered for the analysis. From 1990 to 1994, a cascade of drought events occurred at the trial site, resulting in a rigorous decline in ring width from 1990 to 1992 with a remarkable lack of recovery in the following 2 years (Figure 3). This observation did not allow unbiased calculations of drought response measures and prompted us to pool these 5 years into one drought period, where the average ring width from 1990 to 1994 was used as increment during drought to avoid overlapping of closely spaced reference periods. In summary, four major drought events (1977, 1981, 1990–1994 and 2003) were the basis for our statistical analysis corresponding to tree ages of 19, 23, 32–36, and 45 years, respectively.
Table 2.
Analyzed drought years. SPI, standardized precipitation index; SPEI, standardized precipitation-evapotranspiration index; numbers in the column ‘timescale’ refer to one-month and three-month averaging periods, respectively. If both numbers are present, the drought appeared statistically on both time-scales.
| Drought year | SPI value | SPEI value | Month | Timescale | Pre-drought period | Post-drought period |
|---|---|---|---|---|---|---|
| 1977 | −2.96 | −2.32 | June | 1/3 | 1974–1976 | 1978–1980 |
| 1981 | −2.31 | −1.54 | June | 3 | 1978–1980 | 1982–1984 |
| 1990 | −2.09 | −2.13 | August | 1/3 | 1987–1989 | 1995–1997 |
| 1992 | −2.77 | −2.06 | May | 1 | ||
| 1993 | −2.57 | −2.12 | May | 3 | ||
| 1994 | −2.54 | −2.34 | July | 3 | ||
| 2003 | −2.81 | −1.89 | April | 3 | 2000–2002 | 2004–2006 |
Figure 3.
The upper panel represents the bi-weight robust-mean chronology of all 134 larch trees. Vertical lines indicate the presence of a drought year. Dotted line: drought occurred on a 1-month-interval scale; dashed line: drought occurred on a 3-month-interval scale (see Table 2).
Impacts of drought events on tree growth and stability through time
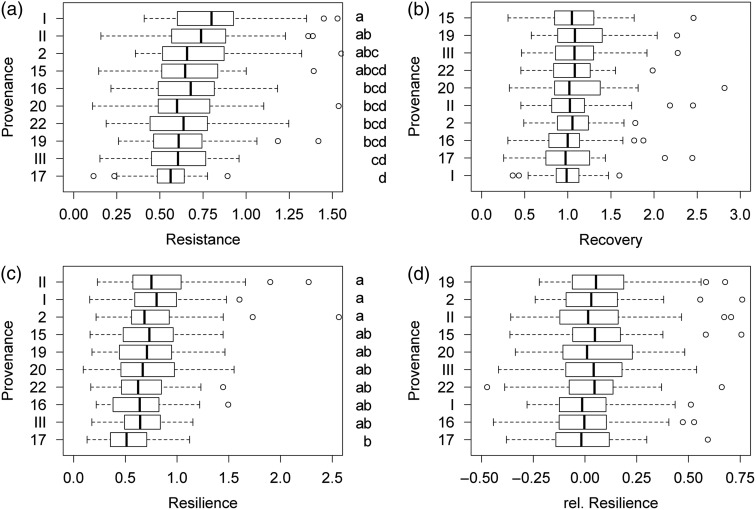
All four drought events significantly affected tree growth, with the strongest reductions in annual increment during the major drought event from 1990 to 1994 (see Figure S1 and Table S1 available as Supplementary Data at Tree Physiology Online). The calculation of overall drought response (across all four events) revealed that provenances from Poland (I and II) were the most resistant ones (mean values: 0.807 and 0.763, respectively) as well as the most resilient ones (0.805 and 0.822, respectively, Table 3). Provenance #15 showed the best performance in recovery (1.175) and provenance #19 the highest mean value in relative resilience (0.093). In contrast, provenance #17 was found to be highly drought sensitive as it had the lowest mean values for three out of the four applied drought response measures (Res, Rsl and rRsl) and the second lowest for Rec (Table 3 and Figure 4). Mean values of drought response were not significantly related to relative dryness of seed origin (expressed as AHM) for any of the four applied response measures (see Figure S2 available as Supplementary Data at Tree Physiology Online).
Table 3.
Mean and standard error for the analyzed response measures and rank stability across the four drought periods. rmean: mean rank across the four drought periods (r = 1 for best performing provenance); Vr: common variance of the ranks according to Huehn (1990).
| Provenance | Resistance | Recovery | Resilience | rel. Resilience | ||||
|---|---|---|---|---|---|---|---|---|
| mean ± s.e. | rmean (Vr) | mean ± s.e. | rmean (Vr) | mean ± s.e. | rmean (Vr) | mean ± s.e. | rmean (Vr) | |
| I | 0.8065 ± 0.032 | 2.00 (4.00) | 0.9860 ± 0.029 | 6.75 (9.75) | 0.8045 ± 0.040 | 3.75 (4.25) | (−)0.0021 ± 0.021 | 7.25 (7.58) |
| II | 0.7630 ± 0.036 | 3.25 (2.25) | 1.0782 ± 0.057 | 5.75 (11.75) | 0.8224 ± 0.059 | 3.75 (10.25) | 0.0593 ± 0.039 | 6.00 (12.00) |
| III | 0.6066 ± 0.029 | 7.75 (0.92) | 1.1352 ± 0.084 | 5.75 (11.75) | 0.6499 ± 0.037 | 8.00 (2.67) | 0.0433 ± 0.031 | 5.25 (11.58) |
| 2 | 0.7094 ± 0.035 | 3.25 (2.92) | 1.0725 ± 0.036 | 4.75 (0.75) | 0.7776 ± 0.055 | 4.00 (6.00) | 0.0681 ± 0.032 | 4.25 (0.92) |
| 15 | 0.6670 ± 0.033 | 4.50 (13.67) | 1.1745 ± 0.112 | 5.00 (13.33) | 0.7243 ± 0.046 | 5.25 (2.92) | 0.0573 ± 0.033 | 4.25 (10.92) |
| 16 | 0.6634 ± 0.033 | 5.25 (4.92) | 1.0099 ± 0.048 | 5.00 (9.67) | 0.6590 ± 0.043 | 6.25 (11.58) | (−)0.0044 ± 0.032 | 6.25 (8.25) |
| 17 | 0.5525 ± 0.019 | 8.50 (3.67) | 0.9970 ± 0.055 | 7.50 (7.67) | 0.5411 ± 0.033 | 9.75 (0.25) | (−)0.0114 ± 0.026 | 6.50 (5.67) |
| 19 | 0.6262 ± 0.033 | 7.25 (8.25) | 1.1438 ± 0.055 | 5.25 (12.08) | 0.7195 ± 0.046 | 3.50 (9.67) | 0.0933 ± 0.034 | 4.00 (12.00) |
| 20 | 0.6498 ± 0.035 | 6.25 (4.92) | 1.0960 ± 0.062 | 4.50 (13.67) | 0.6946 ± 0.046 | 4.50 (3.67) | 0.0448 ± 0.040 | 5.75 (14.92) |
| 22 | 0.6311 ± 0.038 | 7.00 (6.67) | 1.1293 ± 0.092 | 4.75 (16.75) | 0.6656 ± 0.0433 | 6.25 (6.92) | 0.03452 ± 0.033 | 5.50 (12.33) |
| Overall | 0.6706 ± 0.011 | 1.0797 ± 0.021 | 0.7083 ± 0.015 | 0.0376 ± 0.01 | ||||
Figure 4.
Provenance-specific drought response evaluated after four consecutive drought events and averaged over interaction terms for the four response measures (a–d). Provenances are sorted towards descending mean values. Letters (if available) indicate significant pairwise differences and homogenous groups after applying Tukey's HSD. Plots were produced with the help of the R package multcompView.
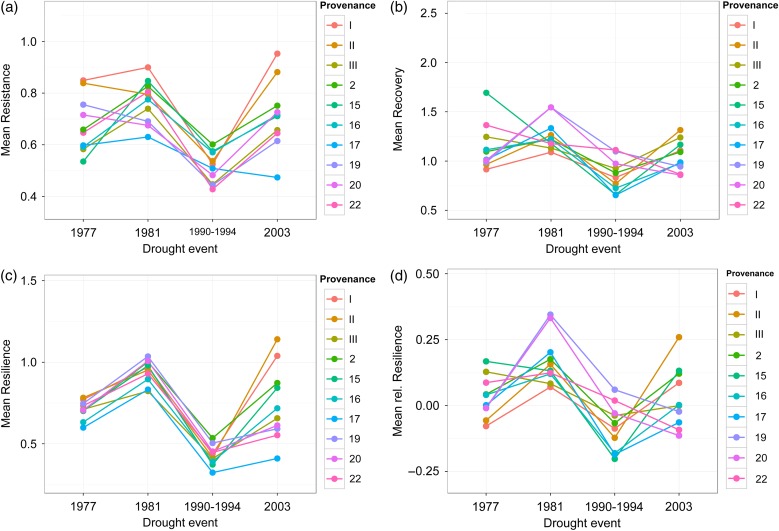
Results from the mixed-model ANOVA strongly indicated that there is significant variation in drought sensitivity among drought years and provenances, respectively. While ‘drought year’ was a highly significant factor for all four response measures, provenance (i.e., ‘genotype’) played a significant role only for resistance and resilience, but not for recovery and relative resilience (Table 4). Moreover, interactions between both factors were highly significant for each of the four response measures. These interactions resulted in visible shifts of mean values for drought response measures between single drought years (Figure 5). To make sure that these significant interactions as well as the significant differences among drought years and provenances were not solely caused by the major drought event of 1990–1994, all calculations were also done without this multiyear event. Hereupon, results remained largely the same with only slight changes in significance levels for Res (see Table S2 and Figure S3 available as Supplementary Data at Tree Physiology Online).
Table 4.
Results from the mixed-model ANOVA for intra-specific drought response. AIC, Akaike information criterion; LogLike: log likelihood; Like.ratio, likelihood ratio; df, degrees of freedom; significance levels: * significant on α < 0.05; ** significant on α < 0.01; *** significant on α < 0.001; ‘×’ indicates interaction between two variables.
| Response measure | Model | Variable | df | AIC | logLike | Like.ratio | P-value |
|---|---|---|---|---|---|---|---|
| Resistance | 1 | Intercept | 3 | −11.17567 | 8.58783 | ||
| 2 | Drought Year | 6 | −108.05061 | 60.0253 | 102.87494 | <0.001*** | |
| 3 | Provenance | 15 | −131.53454 | 80.76727 | 41.48393 | <0.001*** | |
| 4 | Drought Year × Provenance | 42 | −135.77673 | 109.88836 | 58.24219 | <0.001*** | |
| Recovery | 1 | Intercept | 3 | 671.263 | −332.6315 | ||
| 2 | Drought Year | 6 | 619.9467 | −303.9734 | 57.31629 | <0.001*** | |
| 3 | Provenance | 15 | 628.6928 | −299.3464 | 9.25386 | 0.4142 | |
| 4 | Drought Year × Provenance | 42 | 603.276 | −259.638 | 79.41687 | <0.001*** | |
| Resilience | 1 | Intercept | 3 | 309.07012 | −151.53506 | ||
| 2 | Drought Year | 6 | 95.82482 | −41.91241 | 219.2453 | <0.001*** | |
| 3 | Provenance | 15 | 84.7049 | −27.35245 | 29.11992 | <0.001*** | |
| 4 | Drought Year × Provenance | 42 | 57.79621 | 13.1019 | 80.90869 | <0.001*** | |
| rel. Resilience | 1 | Intercept | 3 | −54.68064 | 30.34032 | ||
| 2 | Drought Year | 6 | −142.23319 | 77.1166 | 93.55255 | <0.001*** | |
| 3 | Provenance | 15 | −135.06735 | 82.53367 | 10.83416 | 0.2872 | |
| 4 | Drought Year × Provenance | 42 | −183.47621 | 133.73811 | 102.40886 | <0.001*** |
Figure 5.
Drought events on the x-axis are equally spaced to indicate that ‘drought event’ is a plasticity variable and not a time-series. Lines between events are simply drawn for illustration and traceability. Error bars were omitted for achieving a better visibility.
Phenotypic stability, expressed as the common variance of the ranks (Vr), confirmed the results above and varied considerably among provenances. The range of Vr was 0.92–13.67 for Res, 0.75–16.75 for Rec, 0.25–11.58 for Rsl, and 0.92–14.92 for rRsl (Table 3).
Repeatability of drought response
Treating larch as a single homogenous group resulted in low, but significant repeatability for resistance (Repres = 0.074) and resilience (Reprsl = 0.209), but not for recovery and relative resilience (Table 5). However, when we calculated repeatability values for individual provenances, significant differences among provenances were found, where three provenances (II, 2 and 19) showed significant values for resistance and seven for resilience (I, II, III, 2, 15, 19, 20). These Rep-values ranged up to values of Repres = 0.38 and Reprsl = 0.39 (Table 5) and were much higher than across all provenances, suggesting a high degree of genetic determination and varying degrees of drought selection for individual provenances.
Table 5.
Repeatability and estimated standard error of drought response. Numbers in bold indicate significant repeatability values; Ves, general environmental variance; Vres, residual variance; Rep, repeatability; Vpro, variance arising due to the incorporation of ‘provenance’ as a covariate; N.E., no estimate.
| Provenance | Variance component | Res | Rec | Rsl | rRsl |
|---|---|---|---|---|---|
| I | Ves | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.081 ± 0.075 | 0.027 ± 0.067 |
| I | Vres | 0.503 ± 0.093 | 0.574 ± 0.106 | 0.429 ± 0.091 | 0.535 ± 0.113 |
| I | Rep | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.159 ± 0.134 | 0.049 ± 0.118 |
| II | Ves | 0.116 ± 0.116 | 0.025 ± 0.076 | 0.118 ± 0.136 | 0.061 ± 0.148 |
| II | Vres | 0.608 ± 0.139 | 0.558 ± 0.128 | 0.778 ± 0.178 | 1.071 ± 0.245 |
| II | Rep | 0.160 ± 0.146 | 0.043 ± 0.128 | 0.132 ± 0.143 | 0.054 ± 0.130 |
| III | Ves | 0.000 ± 0.000 | 0.058 ± 0.112 | 0.275 ± 0.178 | 0.320 ± 0.206 |
| III | Vres | 0.494 ± 0.100 | 0.749 ± 0.173 | 0.575 ± 0.134 | 0.686 ± 0.159 |
| III | Rep | 0.000 ± 0.000 | 0.072 ± 0.135 | 0.324 ± 0.159 | 0.318 ± 0.158 |
| 2 | Ves | 0.532 ± 0.301 | 0.000 ± 0.000 | 0.514 ± 0.284 | 0.000 ± 0.000 |
| 2 | Vres | 0.882 ± 0.195 | 0.390 ± 0.074 | 0.799 ± 0.176 | 0.654 ± 0.126 |
| 2 | Rep | 0.376 ± 0.150 | 0.000 ± 0.000 | 0.391 ± 0.149 | N.E. |
| 15 | Ves | 0.135 ± 0.169 | 0.000 ± 0.000 | 0.190 ± 0.127 | 0.026 ± 0.114 |
| 15 | Vres | 1.001 ± 0.226 | 1.736 ± 0.337 | 0.468 ± 0.106 | 0.898 ± 0.202 |
| 15 | Rep | 0.119 ± 0.141 | 0.000 ± 0.000 | 0.289 ± 0.155 | 0.028 ± 0.123 |
| 16 | Ves | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.186 ± 0.219 | 0.000 ± 0.000 |
| 16 | Vres | 1.390 ± 0.270 | 2.402 ± 0.471 | 1.232 ± 0.281 | 1.813 ± 0.356 |
| 16 | Rep | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.131 ± 0.146 | 0.000 ± 0.000 |
| 17 | Ves | 0.000 ± 0.000 | 0.014 ± 0.058 | 0.047 ± 0.069 | 0.000 ± 0.000 |
| 17 | Vres | 0.467 ± 0.092 | 0.454 ± 0.103 | 0.444 ± 0.101 | 0.458 ± 0.091 |
| 17 | Rep | 0.000 ± 0.000 | 0.030 ± 0.123 | 0.095 ± 0.136 | 0.000 ± 0.000 |
| 19 | Ves | 0.252 ± 0.178 | 0.049 ± 0.106 | 0.390 ± 0.257 | 0.240 ± 0.178 |
| 19 | Vres | 0.684 ± 0.155 | 0.751 ± 0.170 | 0.908 ± 0.206 | 0.738 ± 0.167 |
| 19 | Rep | 0.270 ± 0.154 | 0.061 ± 0.130 | 0.300 ± 0.155 | 0.245 ± 0.153 |
| 20 | Ves | 0.091 ± 0.128 | 0.080 ± 0.117 | 0.222 ± 0.180 | 0.000 ± 0.000 |
| 20 | Vres | 0.846 ± 0.187 | 0.767 ± 0.169 | 0.874 ± 0.193 | 1.359 ± 0.261 |
| 20 | Rep | 0.097 ± 0.132 | 0.095 ± 0.132 | 0.203 ± 0.145 | N.E. |
| 22 | Ves | 0.022 ± 0.145 | 0.000 ± 0.000 | 0.027 ± 0.122 | 0.013 ± 0.140 |
| 22 | Vres | 1.078 ± 0.266 | 1.412 ± 0.304 | 0.883 ± 0.218 | 1.083 ± 0.267 |
| 22 | Rep | 0.020 ± 0.133 | 0.000 ± 0.000 | 0.030 ± 0.135 | 0.012 ± 0.131 |
| Overall | Vpro | 0.096 ± 0.055 | 0.005 ± 0.014 | 0.059 ± 0.042 | 0.005 ± 0.012 |
| Ves | 0.074 ± 0.039 | 0.000 ± 0.000 | 0.209 ± 0.052 | 0.036 ± 0.039 | |
| Vres | 0.832 ± 0.059 | 0.989 ± 0.062 | 0.733 ± 0.052 | 0.954 ± 0.068 | |
| Rep | 0.074 ± 0.039 | 0.000 + 0.000 | 0.209 ± 0.047 | 0.036 ± 0.039 |
Overall evaluation of provenances
Final evaluation of provenances as illustrated in Figure 6 revealed that provenances with a high mean resistance and mean resilience also show high competitive force in terms of diameter and height growth (provenances I, II and 2). Even though recovery was not significantly different among provenances, there was a notable trend that provenances with a higher average recovery had weaker diameter and height growth (e.g., provenances 19, 20 and 22). When we incorporated the common variance of the ranks of drought reaction across the four drought events (Huehn 1990) in the evaluation scheme, three different archetypes emerged: (i) provenances with a high ability to withstand drought or to immediately reach pre-drought levels after drought (Res and Rsl, respectively) and a relatively low deviation of their mean response across time (I, II, 2), (ii) provenances with relatively low resistance and/or resilience, but with remarkable rank shifts through time (15, 16, 19, 22) and (iii) provenances that showed consistently bad performance for Res and/or Rsl through time (III, 17 and 20).
Figure 6.
Axes are given in standard deviations, since data were z-normalized. Dashed line is indicating the centered mean.
Discussion
Intra-specific variation in drought response
The genetic variation in drought response is crucial for understanding the species’ capacity to adapt to changing climate conditions and for developing tree breeding programs and assisted migration guidelines. For European larch, a montane and alpine species with limited natural distribution, recent studies revealed only a limited capacity to deal with soil water deficit and drought events (Eilmann and Rigling 2012, Levesque et al. 2013). In the present study, we analyzed drought response of ten provenances that originate from its southern to its northern distribution limit and quantified the genetic variation among provenances as well as the degree of genetic determination of drought reaction.
Drought response in Larix decidua significantly varied among provenances for two of the four analyzed drought response measures (Resistance and Resilience). Across all drought periods, provenances from the Polish lowlands (I, II) had higher mean values for resistance and resilience than provenances from the alpine area. These provenances also had a high to moderate stability of drought response and showed superior growth performance for DBH and height growth. All these differences between provenances from the Polish lowlands and from the alpine area indicate that phenotypic divergence might not only be driven by local adaptation, but also by different population histories. Recent phylogeographic analysis (Wagner et al. 2015a , 2015b ) and also earlier studies (Maier 1992) support this hypothesis as they revealed a clear phylogenetic split between Alpine and eastern central European populations of L. decidua due to the species’ survival in different refugia during the last glacial maximum. But also provenances within the alpine domain revealed some degree of variation as provenances #2 and #15 showed consistently better drought performance than the other alpine genotypes. Since the alpine occurrence of larch consists of up to four phylogeographic lineages (Wagner et al. 2015a ), we cannot exclude that inner-alpine differences in drought sensitivity are also shaped by long-term isolation in separate refugia. However, it seems more likely that local adaptation and natural selection has shaped the observed differentiation pattern. For example, provenance #17 (Pergine) from a low elevation (600–800 m) performed much worse in three out of four drought periods compared to its high-altitude equivalent #19 (1300–1400 m). Although the low-elevation site is warmer and probably more drought prone in summer, the better drought reaction of the high-elevation genotype may be explained by its high-altitude habitat, which likely consists of steeper and rocky surfaces with reduced soil layer horizon and water availability. Such conditions could have resulted in stronger environmental selection which is also evident from the significant differences in repeatability values among provenances: provenance #17 did not show any degree of determination for resistance or resilience, whereas #19 showed considerable genetic determination for resistance, resilience, and relative resilience, indicating recent or ongoing selection pressure. Relationships between drought response and source climate were weak and did not allow identifying a single obvious cause for better or worse drought performance or higher degree of genetic determination (e.g., see Figure S2 available as Supplementary Data at Tree Physiology Online). Also, our data do not support an advantage of populations from the species' southern border since all southern populations (Embrun, Cavedine, Pergine, and Cavalese) showed average- or below-average drought performance, whereas populations from the northern distribution limit in the Polish lowlands were most productive and revealed highest drought resistance and resilience. Besides that, human-mediated translocations of seed material within the last 300 years, often from Alpine populations to lower altitudes (Wagner et al. 2015b , Jansen and Geburek 2016), might have resulted in local maladaptation. For example, provenance #III (Wienerwald) originated from the dry northeastern foothills, but showed unexpectedly high drought vulnerability and thus raises doubts on its natural occurrence. In fact, larch is considered to occur naturally within the western part of the Viennese basin according to vegetation history (e.g., Tschermak 1935), but also due to recent admixture translocation studies, where populations from as far as 15°55′E were found to contain only small amounts of artificially transferred migrants from other regions (Wagner et al. 2015b ). Provenance #III, however, originated from 16°10′E and may be of non-autochthonous origin. Another factor that could have contributed to differences between local adaptations at the seed origin and the provenances’ realized drought performance at the trial site could relate to distinctions in type, structure or chemistry of soils between seed origin and planting site. For example, soil characteristics could have caused different development of the root system (e.g., Hopkins and Donahue 1939) and thus affected the ability to maintain a constant water supply under water-limited conditions.
Intra-specific pattern of drought sensitivity compared to other conifers
When we compare the general drought response pattern of L. decidua with data from other European conifers, we find major differences: first, intra-specific variation in resistance and resilience in European larch is more strongly pronounced than, for example, in silver fir (George et al. 2015) or in Scots pine (Taeger et al. 2013). On the other hand, European larch showed no intra-specific differences (neither overall nor pairwise) for recovery and rel. resilience, while there were high to moderate differences in silver fir and Scots pine. We presume that this contrasting pattern is caused by the different life strategies of these species: Larix decidua, as the only deciduous conifer in Europe, loses its needles at the end of the growing season, while Abies alba and Pinus sylvestris will maintain their needles during winter. As soon as the new growing season starts, shoots and needles from previous years in A. alba and P. sylvestris can quickly contribute to diameter growth, while it takes 3–4 weeks after needle appearance before Larix decidua accelerates stem growth and new xylem conduits (Moser et al. 2009). This explanation is also supported by observations that Larix sp. has significantly lower concentrations of non-structural carbohydrates in branch sapwood at the beginning of the growing season compared to the evergreen Abies sp. and Pinus sp. (Hoch et al. 2003), underpinning maybe its greater dependency on current-year photosynthates, especially when reserves were depleted shortly before by drought. Another unique feature of European larch seems to be its biogeographical pattern of drought tolerance. Eilmann et al. (2013), for example, reported a north-to-south downward trend in drought susceptibility for Douglas-fir with the most drought-sensitive provenances found in the northern distribution range. European larch, in contrast, showed highest susceptibility for southern provenances and lowest overall sensitivity for provenances originating from the most northern distribution limit. Finally, the overall mean value for relative resilience was much lower in European larch compared to silver fir (George et al. 2015) and to Douglas-fir (Montwe et al. 2015). Even though the relative resilience is to some degree inversely related to resistance at individual level, it was helpful in illustrating that drought effects at species level are possibly longer lasting in European larch than in silver fir and Douglas-fir and confirmed earlier findings of a species-specific inability of L. decidua to convalesce after drought events (Eilmann and Rigling 2012).
Stability of drought response and genotype-by-time interactions
Despite significant genetic differences in drought response for Res and Rsl after four consecutive drought events, it was obvious that rank shifts occurred between the various drought events resulting in significant interactions between the factors ‘provenance’ and ‘drought year’ for all four response measures. In particular, within the multiyear drought event in 1990–1994 provenances showed the most unexpected drought reaction in terms of resistance compared to the two previous droughts of 1977 and 1981 and the later occurring drought in 2003. Here, provenances I and II had nearly equal mean resistance as provenance 17 (orange, brown and blue lines in Figure 5a), although they significantly differed in their overall performance after all analyzed drought events. Even when the multiyear drought event was excluded from the analysis, interactions between ‘provenance’ and ‘drought year’ were still significant and rank shifts occurred between the droughts 1977 to 1981. For example, provenance #15 shifted from the lowest rank for resistance to the second highest between these two events. Since drought events analyzed in this study varied in their duration (single-year vs. multi-year), their intensity levels (SPI-values from −2.09 to −2.96) as well as in time of occurrence (April to August), they were helpful in illustrating how drought heterogeneity might affect trees’ stress response and its stability through time. Overall, the interaction between ‘provenance’ and ‘drought year’ indicate that the potential randomness of meteorological drought regimes in space, time, and intensity is an important factor that needs to be considered when selecting forest reproductive material for future climate (Iverson and Mckenzie 2013, Williams and Dumroese 2013). So far, studies analyzing trees’ response to repeated stress within a tree's life are scarce (e.g., George et al. 2015) and often restricted to seedling-adult comparisons (e.g., Cavender-Bares and Bazzaz 2000, review of Niinemets 2010). Hence, we argue that tree physiology and its related disciplines should focus more strongly on how to set up phenology-based long-term experiments to gain a better understanding of time-dependent stress response of forest trees in the future.
Repeatability of intra-specific drought response
Genetic repeatability values confirmed results from the repeated measure ANOVA and allowed to estimate the upper limit of the degree of genetic determination (i.e., heritability) of drought response for individual provenances as well as its variation within the species. Both resistance and resilience were shown to be genetically determined while relative resilience was significant only for two provenances and recovery was not repeatable at all. Remarkably, repeatability for Res and Rsl significantly varied among provenances. This indicates variable selection pressures and may confirm the adaptation of certain populations to their local environments. The maximum value of repeatability ranged up to 0.38 for Res, 0.39 for Rsl, and to 0.32 for rRsl, suggesting that selecting parent trees within the respective populations or outstanding individuals for crossings among provenances could maybe substantially increase the drought performance of the following generation. Comparable data on drought response for larch or other tree species are scarce and were mostly obtained from seedling tests or young plantations and/or from specific physiological measurements, but not from repeated drought occurrence in long-term experiments. When available, heritability estimates of physiological drought parameters were on a similar medium level as in our study: for example, Marguerit et al. (2014) analyzed the water-use-efficiency in Pinus pinaster by using carbon isotope composition δ13C at three test sites and obtained heritability values between 0.23 and 0.41. Similar analyses of δ13C in other tree species revealed heritabilities of 0.54 for Picea mariana (Johnsen et al. 1999), 0.09 for Pinus taeda (Baltunis et al. 2008), and 0.34–0.48 for Quercus robur (Brendel et al. 2002). Although these measurements are not fully comparable, they demonstrate that traits related to drought stress generally have medium genetic determination and could be valuable for tree breeding. Repeatability only gives an upper limit of heritability and thus does not necessarily indicate that the degree of genetic determination can be fully realized (Falconer and Mackay 1996). On the other hand, our estimates could also underestimate the true genetic component, since the repeated drought events varied in intensity, time of occurrence and duration and thus may not constitute ‘true repeated measures’ (Dohm 2002). The tested larch provenances may possess different genetic and physiological mechanisms to cope with these variable drought characteristics making comparisons between drought events difficult. Also, heritability and genetic correlations for other traits and wood properties were found to depend on the cambial age (e.g., Zamudio et al. 2002, Hong et al. 2015). If this would also hold true for traits involved in mitigation of drought stress, it might have influenced the obtained repeatability values in our study. Thus, we see our results only as a first step in understanding intra-specific variation in drought response of this ecologically and economically important conifer.
Conclusions
Understanding effects of climatic extreme events on tree productivity, vitality and survival at different stages of a tree's life requires long-term trial and observation plots. Although 50 year old experiments still cover less than half of the regular rotation period of the mountainous Larix decidua, it gives first indications on provenance-specific drought reactions within a meaningful planning horizon for forest managers under changing environmental conditions. Our analysis indicates that simple seed transfer guidelines for forest adaptation to climate change, for example transfers from South to North, might be not sufficient for larch and other tree species. Since the adaptive diversity of tree species is often affected by population history and local adaption patterns, as our data suggest for L. decidua, seed transfer guidelines and genetic conservation measures need to consider the underlying causes of adaptive diversity for developing useful adaptation guidelines.
Despite high vulnerability of L. decidua to drought events, we found high genetic variation among populations and promising high degrees of genetic determination, as a basis for future breeding activities. Moreover, our analysis indicates that special attention on the long-term conservation should be paid to productive and drought resistant provenances from the scattered distribution in the Polish lowlands.
Supplementary data
Supplementary data for this article are available at Tree Physiology Online.
Acknowledgments
This study is part of the project ‘Softwood for the future’ and was funded by the Austria Science Fund (FWF, Translational Research Program 122). For providing meteorological data, we would like to thank the Austrian Central Institute for Meteorology and Geodynamics (ZAMG). We kindly acknowledge Thomas Thalmayr (BFW) for technical support during sampling. Finally, we would like to thank two anonymous reviewers for their critical comments on how to improve the manuscript.
Conflict of interest
None declared.
References
- Aitken SN, Yeaman S, Holliday JA, Wang T, Curtis-McLane S (2008) Adaptation, migration or extirpation: climate change outcomes for tree populations. Evol Appl 1:95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberto FJ, Aitken SN, Alia R et al. (2013) Potential for evolutionary responses to climate change-evidence from tree populations. Glob Change Biol 19:1645–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alia R, Moro J, Denis JB (1997) Performance of Pinus pinaster provenances in Spain: interpretation of the genotype-by-environment interaction. Can J Forest Res 27:1548–1559.
- Allen CD, Macalady AK, Chenchouni H et al. (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecol Manag 259:660–684. [Google Scholar]
- Anekonda TS, Lomas MC, Adams WT, Kavanagh KL, Aitken SN (2002) Genetic variation in drought hardiness of coastal Douglas-fir seedlings from British Columbia. Can J Forest Res 32:1701–1716. [Google Scholar]
- Anderegg WRL, Hicke JA, Fisher RA et al. (2015) Tree mortality from drought, insects and their interactions in a changing climate. New Phytol 208:674–683. [DOI] [PubMed] [Google Scholar]
- Arend M, Kuster T, Günthardt-Goerg MS, Dobbertin M (2011) Provenance-specific growth responses to drought and air-warming in three European oak species (Quercus robur, Q. petraea and Q. pubescens). Tree Physiol 31:287–297. [DOI] [PubMed] [Google Scholar]
- Baltunis BS, Martin TA, Huber DA, Davis JM (2008) Inheritance of foliar stable carbon isotope discrimination and third-year height in Pinus taeda clones on contrasting sites in Florida and Georgia. Tree Genet Genomes 4:797–807. [Google Scholar]
- Barbeta A, Ogaya R, Penuelas J (2013) Dampening effects of long-term experimental drought on growth and mortality rates of a holm oak forest. Glob Change Biol 19:3133–3144. [DOI] [PubMed] [Google Scholar]
- Boake CRB. (1989) Repeatability: its role in evolutionary studies of mating behavior. Evol Ecol 3:173–182. [Google Scholar]
- Brendel O, Pot D, Plomion C, Rozenberg P, Guehl JM (2002) Genetic parameters and OTL analysis of δ13C and ring width in maritime pine. Plant Cell Environ 25:945–953. [Google Scholar]
- Breshears DD, Cobb NS, Rich PM et al. (2005) Regional vegetation die-off in response to global-change-type drought. Proc Natl Acad Sci USA 102:15144–15148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn AG. (2010) Statistical and visual crossdating in R using the dplr library. Dendrochronologia 28:251–258. [Google Scholar]
- Calanca P. (2007) Climate change and drought occurrence in the Alpine region: how severe are becoming the extremes. Glob Planet Change 57:151–160. [Google Scholar]
- Cavender-Bares J, Bazzaz FA (2000) Changes in drought response strategies with ontogeny in Quercus rubra: implications for scaling from seedlings to mature trees. Oecologia 124:8–18. [DOI] [PubMed] [Google Scholar]
- Cook BI, Ault TR, Smerdon JE (2015) Unprecedented 21st century drought risk in the American Southwest and Central Plains. Sci Adv 1:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corzo Perez GA, van Huijgevoort MHJ, Voß F, van Lanen HAJ (2011) On the spatio-temporal analysis of hydrological droughts from global hydrological models. Hydrol Earth Syst Sci 15:2963–2978. [Google Scholar]
- Desplanque C, Rolland C, Schweingruber FH (1999) Influence of species and abiotic factors on extreme tree ring modulation: Picea abies and Abies alba in Tarentaise and Maurienne (French Alps). Trees 13:218–227. [Google Scholar]
- Dohm MR. (2002) Repeatability estimates do not always set an upper limit to heritability. Funct Ecol 16:273–280. [Google Scholar]
- Eilmann B, de Vries SMG, den Ouden J, Mohren GMJ, Sauren P, Sass-Klaassen U (2013) Origin matters! Difference in drought tolerance and productivity of coastal Douglas-fir (Pseudotsuga menziesii (Mirb.)) provenances. For Ecol Manag 302:133–143. [Google Scholar]
- Eilmann B, Rigling A (2012) Tree-growth analyses to estimate tree species’ drought tolerance. Tree Physiol 32:178–187. [DOI] [PubMed] [Google Scholar]
- Estrela MJ, Penarrocha D, Millan M (2000) Multi-annual drought episodes in the Mediterranean (Valenica region) from 1950–1996. A spatio-temporal analysis. Int J Climatol 20:1599–1618. [Google Scholar]
- Falconer DS, Mackay TFC (eds) (1996) Introduction to quantitative genetics. Pearson, Harlow. [Google Scholar]
- Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009) Plant drought stress: effects, mechanisms and management. Agron Sustain Dev 29:185–212. [Google Scholar]
- George JP, Schueler S, Karanitsch-Ackerl S, Mayer K, Klumpp RT, Grabner M (2015) Inter- and intra-specific variation in drought sensitivity in Abies spec. and its relation to wood density and growth traits. Agric For Meteorol 214–215:430–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour AR, Gogel BJ, Cullis BR, Thompson R (2009) ASReml user guide release 3.0. VSN International Ltd., Hemel Hempstead, UK: www.bsni.co.uk. [Google Scholar]
- Gleason SM, Westoby M, Jansen F et al. (2016) Weak trade-off between xylem safety and xylem-specific hydraulic efficiency across the world's woody plant species. New Phytol 209:123–136. [DOI] [PubMed] [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PJ, Jarvis J (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978. [Google Scholar]
- Hoch G, Richter A, Körner C (2003) Non-structural carbon compounds in temperate forest trees. Plant Cell Environ 26:1067–1081. [Google Scholar]
- Hong Z, Fries A, Wu HX (2015) Age trend of heritability, genetic correlation, and efficiency of early selection for wood quality traits in Scots pine. Can J For Res 45:817–825. [Google Scholar]
- Hopkins HT, Donahue RL (1939) Forest tree root development as related to soil morphology. Soil Sci Soc Amer Proc 4:353. [Google Scholar]
- Huehn M. (1990) Nonparametric measures of phenotypic stability. Part 1: theory. Euphytica 47:189–194. [Google Scholar]
- Hsiao TC. (1973) Plant responses to water stress. Annu Rev Plant Physiol 24:519–570. [Google Scholar]
- Forests ICP. (2010) Manual on methods and criteria for harmonized sampling, assessment, monitoring and analysis of the effects of air pollution on forests. UNECE ICP Forests Programme Co-Ordinating Centre Hamburg. http://www.icp-forests.org/Manual.htm (11 October 2012, data last accessed)
- Iverson LR, McKenzie D (2013) Tree-species range shifts in a changing climate: detecting, modeling, assisting. Landscape Ecol 28:879–889. [Google Scholar]
- Jansen S, Geburek T (2016) Historic translocations of European larch (Larix decidua Mill.) genetic resources across Europe – A review from the 17th until the mid-20th century. Forest Ecol Manag 379:114–123. [Google Scholar]
- Johnsen KH, Flanagan LB, Huber DA, Major JE (1999) Genetic variation in growth, carbon isotope discrimination, and foliar N concentration in Picea mariana: analyses from a half-diallel mating design using field-grown trees. Can J For Res 29:1727–1735. [Google Scholar]
- Laird NM, Ware JH (1982) Random-effects models for longitudinal data. Biometrics 38:963–974. [PubMed] [Google Scholar]
- Levesque M, Saurer M, Siegwolf R, Eilmann B, Brang P, Bugmann H, Rigling A (2013) Drought response of five conifer species under contrasting water availability suggests high vulnerability of Norway spruce and European larch. Glob Change Biol 19:3184–3199. [DOI] [PubMed] [Google Scholar]
- Lloret F, Keeling EG, Sala A (2011) Components of tree resilience: effects of successive low-growth episodes in old ponderosa pine forests. Oikos 120:1909–1920. [Google Scholar]
- Lloyd-Hughes B, Saunders MA (2002) A drought climatology for Europe. Int J Climatol 22:1571–1592. [Google Scholar]
- Maier J. (1992) Genetic variation in European larch (Larix decidua Mill.). Ann Sci For 49:39–47. [Google Scholar]
- Marguerit E, Bouffier L, Chancerel E et al. (2014) The genetics of water-use efficiency and its relation to growth in maritime pine. J Exp Bot 65:4757–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Vilalta J, Pinol J (2002) Drought-induced mortality and hydraulic architecture in pine populations of the NE Iberian Peninsula. Forest Ecol Manag 161:247–356. [Google Scholar]
- Matras J, Pâques L (2008) EUFORGEN technical guidelines for genetic conservation and use for European larch (Larix decidua). Bioversity International, Rome, Italy, p 6. [Google Scholar]
- Mátyás C. (1996) Climatic adaptation of trees: rediscovering provenance tests. Euphytica 92:45–54. [Google Scholar]
- McCarty JP. (2001) Ecological consequences of recent climate change. Conserv Biol 15:320–331. [Google Scholar]
- McKee TBN, Doesken J, Kleist J (1993) The relationship of drought frequency and duration to time scales In: Eighth Conference on Applied Climatology. American Meteorological Society, Anaheim, CA, pp 179–184. [Google Scholar]
- Merilä J, Sheldon BC (2000) Lifetime reproductive success and heritability in nature. Am Nat 155:301–310. [DOI] [PubMed] [Google Scholar]
- Mishra AK, Singh VP (2010) A review of drought concepts. J Hydrol 391:202–216. [Google Scholar]
- Montwé D, Spiecker H, Hamann A (2015) Five decades of growth in a genetic field trial of Douglas-fir reveal trade-offs between productivity and drought tolerance. Tree Genet Genomes 11:1–11. [Google Scholar]
- Moser EB, Saxton AM, Pezeshki SR (1990) Repeated measure analysis of variance: application to tree research. Can J For Res 20:524–535. [Google Scholar]
- Moser L, Fonti P, Büntgen U et al. (2009) Timing and duration of European larch growing season along altitudinal gradients in the Swiss Alps. Tree Physiol 30:225–233. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Schielzeth S (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev 85:935–956. [DOI] [PubMed] [Google Scholar]
- NDMC (2014) A Program to Calculate Standardized Precipitation Index. National Drought Mitigation Centre, Lincoln NE. http://drought.unl.edu/MonitoringTools/DownloadableSPIProgram.aspx (19 February 2016, date last accessed).
- Neuwirth B, Esper J, Schweingruber FH, Winiger M (2004) Site ecological differences to the climatic forcing of spruce pointer years from the Lötschental, Switzerland. Dendrochronologia 21:69–78. [Google Scholar]
- Niinemets Ü. (2010) Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: past stress history, stress interactions, tolerance and acclimation. Forest Ecol Manag 260:1623–1639. [Google Scholar]
- Park Williams A, Allen CD, Macalady AK et al. (2012) Temperature as a potent driver of regional forest drought stress and tree mortality. Nat Clim Change 3:292–297. [Google Scholar]
- Pâques L, Foffová E, Heinze B (2013) Larches (Larix sp.) In: Pâques L. (ed) Forest tree breeding in Europe-current state-of-the-art and perspectives. Springer, Dordrecht, pp 13–122. [Google Scholar]
- Pasho E, Camarero JJ, de Luis M, Vicente-Serrano SM (2011) Impacts of drought on different time scales on forest growth across a wide climatic gradient in north-eastern Spain. Agric For Meteorol 151:1800–1811. [Google Scholar]
- Peng C, Ma Z, Lei X et al. (2011) A drought-induced pervasive increase in tree mortality across Canada's boreal forests. Nat Clim Change 1:467–471. [Google Scholar]
- R Development Core Team (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org. [Google Scholar]
- Schmidtling RC. (1994) Use of provenance tests to predict response to climate change: loblolly pine and Norway spruce. Tree Physiol 14:805–817. [DOI] [PubMed] [Google Scholar]
- Schober R. (ed) (1977) Vom zweiten internationalen Lärchenprovenienzversuch In: Ein Beitrag zur Lärchenherkunftsfrage. Sauerlaender Verlag, Frankfurt. [Google Scholar]
- Schweingruber FHD, Eckstein F, Serre-Bachet F, Bräker OU (1990) Identification, presentation and interpretation of event years and pointer years in dendrochronology. Dendrochronologia 8:9–38. [Google Scholar]
- Seki M, Narusaka M, Ishida J et al. (2002) Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J 31:279–292. [DOI] [PubMed] [Google Scholar]
- Sheffield J, Wood EF (2008) Projected changes in drought occurrence under future global warming from multi-model, multi-scenario, IPCC AR4 simulations. Clim Dyn 31:79–105. [Google Scholar]
- Smirnoff N. (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58. [DOI] [PubMed] [Google Scholar]
- Svenning JC, Skov F (2004) Limited filling of potential range in European tree species. Ecol Lett 7:565–573. [Google Scholar]
- Taeger S, Zang C, Liesebach M, Schneck V, Menzel A (2013) Impact of climate and drought events on the growth of Scots pine (Pinus sylvestris L.) provenances. Forest Ecol Manag 307:30–42. [Google Scholar]
- Tschermak L. (ed) (1935) Die natürliche Verbreitung der Lärche in den Ostalpen. Springer, Wien. [Google Scholar]
- Vicente-Serrano SM, Begueria S, Lopez-Moreno JI (2010) A multiscalar drought index sensitive to global warming: the standardized precipitation evapotranspiration index. J Climate 23:1696–1718. [Google Scholar]
- Vranckx G, Jacquemyn H, Mergeay J et al. (2014) The effect of drought stress on heterozygosity-fitness correlations in pedunculate oak (Quercus robur). Ann Bot 113:1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Litt T, Sanchez-Goni MF, Petit RJ (2015a) History of Larix decidua Mill. (European larch) since 130ka. Quatenary Sci Rev 124:224–247. [Google Scholar]
- Wagner S, Liepelt S, Gerber S, Petit RJ (2015b) Within-range translocations and their consequences in European larch. PLoS ONE 10:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Hamann A, Yanchuk A, O'Neill GA, Aitken SN (2006) Use of response functions in selecting lodgepole pine populations for future climates. Glob Change Biol 12:2402–2416. [Google Scholar]
- White TL. (1987) Drought tolerance of southwestern Oregon Douglas-fir. For Sci 33:283–293. [Google Scholar]
- Williams MI, Dumrose RK (2013) Preparing for climate change: forestry and assisted migration. J Forest 111:287–297. [Google Scholar]
- Zamudio F, Baettyg R, Vergara A, Guerra F, Rozenberg P (2002) Genetic trends in wood density and radial growth with cambial age in a radiata pine progeny test. Ann For Sci 59:541–549. [Google Scholar]