Abstract
Trichloroethylene (TCE) is a persistent environmental contaminant proposed to contribute to autoimmune disease. Experimental studies in lupus-prone MRL+/+ mice have suggested that TCE exposure can trigger autoimmune hepatitis. The vast majority of studies examining the connection between TCE and autoimmunity utilize this model, and the impact of TCE exposure in other established models of autoimmune liver disease is not known. We tested the hypothesis that TCE exposure exacerbates experimental hepatic autoimmunity in dominant negative transforming growth factor beta receptor type II (dnTGFBRII) mice, which develop serological and histological features resembling human primary biliary cholangitis. Female 8-week-old wild-type and dnTGFBRII mice were exposed to TCE (0.5 mg/ml) or vehicle (1% ethoxylated castor oil) in the drinking water for 12 or 22 weeks. Liver histopathology in 20- and 30-week-old wild-type mice was unremarkable irrespective of treatment. Mild portal inflammation was observed in vehicle-exposed 20-week-old dnTGFBRII mice and was not exacerbated by TCE exposure. Vehicle-exposed 30-week-old dnTGFBRII mice developed anti-mitochondrial antibodies, marked hepatic inflammation with necrosis, and hepatic accumulation of both B and T lymphocytes. To our surprise, TCE exposure dramatically reduced hepatic parenchymal inflammation and injury in 30-week-old dnTGFBRII mice, reflected by changes in hepatic proinflammatory gene expression, serum chemistry, and histopathology. Interestingly, TCE did not affect hepatic B cell accumulation or induction of the anti-inflammatory cytokine IL10. These data indicate that TCE exposure reduces autoimmune liver injury in female dnTGFBRII mice and suggests that the precise effect of environmental chemicals in autoimmunity depends on the experimental model.
Keywords: trichloroethylene, autoimmune, immunotoxicology, primary biliary, cholangitis, liver, systems toxicology.
Trichloroethylene (TCE) is an industrial degreaser and common environmental contaminant. Strong experimental and epidemiological evidence links TCE exposure to a variety of adverse human health effects, including cancer (Chiu et al., 2013; Yuksel et al., 2014). Indeed, TCE has been classified by the International Agency for Research on Cancer (IARC) as a Group 1 human carcinogen (IARC, 2014). However, exacerbation of autoimmune disease after TCE exposure remains a topic of increasing interest (Cooper et al., 2009; Germolec et al., 2012). In particular, there has been considerable attention focused on the possibility that exposure to TCE induces autoimmune hepatitis, an organ-specific autoimmune disease. Animal studies suggest that at relatively high doses, and in one specific mouse model of systemic autoimmunity (MRL+/+), exposure to TCE can induce an autoimmune hepatitis-like syndrome (Gilbert et al., 2009; Griffin et al., 2000b,c; Khan et al., 1995). Missing from the literature are studies examining the effect of TCE exposure in validated models of autoimmune liver injury. There is also a lack of definitive epidemiological evidence linking TCE exposure with hepatic autoimmunity. Thus, the true relevance of TCE as a trigger of hepatic autoimmunity in humans is not yet known, and additional studies are required.
The liver plays a central role in the metabolism of nutrients and chemicals. It also has an exceedingly important function in maintenance of immune tolerance (Beuers and Gershwin, 2015; Bogdanos et al., 2013; Doherty, 2016; Invernizzi, 2013; Shuai et al., 2016). To address the potential for TCE to induce hepatic autoimmunity, we utilized a widely used mouse model of autoimmune liver disease that has not yet been considered in studies linking TCE with autoimmunity (Ando et al., 2012; Huang et al., 2014; Kawata et al., 2013; Oertelt et al., 2006; Wang et al., 2015; Yang et al., 2016a,b). In particular, we attempted to determine whether exposure to TCE in drinking water would alter the development of an autoimmune liver disease called primary biliary cholangitis (PBC) in an experimental setting. To do this, we exposed mice whose lymphocytes express a dominant negative form of transforming growth factor beta receptor type II (dnTGFBRII) to TCE. dnTGFBRII mice develop key immunopathological features of PBC, including lymphocytic liver infiltration with periportal inflammation, production of anti-mitochondrial autoantibodies, and a proinflammatory cytokine profile (Oertelt et al., 2006). These mice have been used extensively to decipher the cellular mechanisms of PBC (Katsumi et al., 2015; Leung et al., 2012; Wang et al., 2014a). The exposure paradigm followed the route and dose of TCE previously shown to exacerbate liver inflammation in lupus-prone MRL+/+ mice (Gilbert et al., 2009). We report here that TCE failed to exacerbate PBC, and surprisingly, reduced the severity of disease in the dnTGFBRII mice. Our data suggest that the link between TCE exposure and hepatic autoimmunity may not be definitive. Dichotomous findings in different mouse models emphasize the conclusions of recent panels convened by the National Institute of Environmental Health Sciences (NIEHS) (Germolec et al., 2012; Miller et al., 2012; Selmi et al., 2012), which stress the need for more studies focused on autoimmune liver disease.
MATERIALS AND METHODS
Mice
Mice possessing a dominant negative form of human transforming growth factor beta receptor Type II (B6.Cg-Tg(Cd4-TGFBR2)16Flv/J) (Gorelik and Flavell, 2000), from now on referred to as dnTGFBRII mice, and age-matched wild-types on an identical genetic background (C57BL/6J), were purchased from The Jackson Laboratory (Bar Harbor, Maine) and maintained by breeding transgene positive males with wild-type females. Wild-type and transgene-positive female mice from the vendor or derived from the colony were randomly assigned to experimental exposure groups at 8 weeks of age. Mice were housed in a specific pathogen-free vivarium routinely tested negative for helicobacter at an ambient temperature of 22 ± 2 °C with alternating 12-h light/dark cycles. In line with previous studies (Oertelt et al., 2006), the mice were fed medicated (metronidazole, bismuth, amoxicillin), sterile rodent chow (Bio-Serv, Flemington, New Jersey) ad libitum, and were given access to reverse osmosis-purified drinking water ad libitum. Mice were housed in Association for Assessment and Accreditation of Laboratory Animal Care-approved facilities at Michigan State University (MSU). All animal procedures were approved by the MSU Institutional Animal Care and Use Committee.
TCE Exposure and Tissue Collection
Female 8-week-old mice were exposed to 0.5 mg/ml TCE (ACS, 99.5% min, ThermoFisher, Ward Hill Massachusetts) or vehicle (1% ethoxylated castor oil [Spectrum Chemical Manufacturing Corp., New Brunswick, New Jersey]) via the drinking water. TCE and vehicle water were made fresh and changed twice weekly to minimize TCE degradation (Griffin et al., 2000a). Blood and tissues were collected after 12 or 22 weeks of TCE exposure. Mice were anesthetized using isoflurane and blood was collected from the caudal vena cava into sodium citrate (final 0.38%) or an empty syringe for the collection of plasma and serum, respectively. The liver was excised, weighed, and washed in phosphate-buffered saline (PBS). The left lateral lobe was fixed in 10% neutral-buffered formalin for 96 h, and then stored in 70% ethanol before paraffin embedding. Three small pieces (approximately 20 mg total) were taken from the remaining lobes to be used for RNA isolation and cDNA synthesis. The remaining liver was snap frozen in liquid nitrogen.
RNA Isolation, cDNA Synthesis and Quantitative Real-Time PCR
Total RNA was isolated from snap-frozen liver using TRI Reagent according to the manufacturer’s protocol (Molecular Research Center, Cincinnati, Ohio). Total RNA (1 μg) was used to synthesize cDNA in the presence of RNase inhibitor (ThermoFisher) using a High Capacity cDNA Reverse Transcription Kit (Life Technologies, Foster City, California) and a C1000 Thermal Cycler (Bio-Rad Laboratories, Hercules, California). SYBR Green quantitative real-time PCR (qPCR) amplification was performed using a CFX Connect thermal cycler (Bio-Rad) with primers (Supplementary Table 1) purchased from IDT (Coralville, Iowa). HPRT mRNA was utilized as a housekeeper gene and relative fold change was determined using the ΔΔCt method.
Histopathology and Immunohistochemistry
Paraffin sections were prepared and stained by the MSU Investigative Histopathology Laboratory, as described previously in Joshi et al. (2015) and Moritoki et al. (2009b). CD45R (B220, B cells) immunohistochemistry was performed on sections after antigen retrieval with heated citrate buffer (pH 6.0) using a primary rat anti-mouse CD45R antibody (BD Biosciences). Detection was accomplished using a rat-on-mouse HRP polymer system and AEC chromogen. Scoring of hepatic inflammation and injury was performed on hematoxylin and eosin (H&E)-stained sections by a board-certified veterinary pathologist (K.J.W.), based on criteria established previously (Moritoki et al., 2009b). Paraffin-embedded livers were cut at 5 µm and labeled with anti-CD3 (T cells) anti-body as previously described in Joshi et al. (2015). For quantification of hepatic CD3+ and CD45R+ cell populations, images of stained liver sections were captured using a Virtual Slide System VS110 (Olympus, Hicksville, New York) with a 20× objective. Random images (>500 per sample) totaling the entire area of the left-lateral liver lobe were sampled from the digitized slides. The number of CD3 and CD45R expressing cells per image was determined in an unbiased fashion using a batch macro involving the color deconvolution and analyze particles tools to identify positive staining.
Determination of Serum ALT Activity and Plasma Anti-mitochondrial Autoantibody Levels
Serum alanine aminotransferase (ALT) was measured using a commercial reagent (Infinity ALT/GPT, Thermo Fisher, Waltham, Massachusetts). Plasma levels of anti-mitochondrial antibodies (AMAs) to the inner lipoyl domain of the E2 unit of pyruvate dehydrogenase (Moteki et al., 1996), the major AMA epitope of PBC, were quantified as previously described in Leung et al. (2016) and Wang et al. (2014b). Briefly, 96-well ELISA plates were coated with recombinant protein of the human PDC-E2 inner lipoyl domain (10 μg/ml) in carbonate coating buffer, pH 9.6 at 4 °C overnight, blocked with 3% nonfat dry milk in PBS and incubated with 1:250 dilution of the plasma samples for 1 h. The plates were then washed with PBS containing 0.05% Tween 20 (PBS-T) and incubated for 1 h with a predetermined optimized dilution of horseradish peroxidase conjugated anti-mouse IgG, IgM and IgA (Invitrogen, Carlsbad, California). Unbound HRP-conjugated antibody was removed by washing with PBS-T and HRP activity determined after 10 minute incubation with 3,3′,5,5′ tetramethylbenzidine (BD Biosciences) as substrate and the reaction was terminated using 2N sulfuric acid. The optical density was measured using an ELISA plate reader at 450 nm. Positive and negative controls were included throughout.
Statistics
Comparison of groups was performed using 2-way analysis of variance and posthoc comparisons were made with Student–Newman–Keuls test. Analysis of prevalence of plasma autoantibodies was made using a Fisher’s exact test. Differences were considered significant when P < .05.
RESULTS
Impact of TCE Exposure on Liver Histopathology in dnTGFBRII Mice
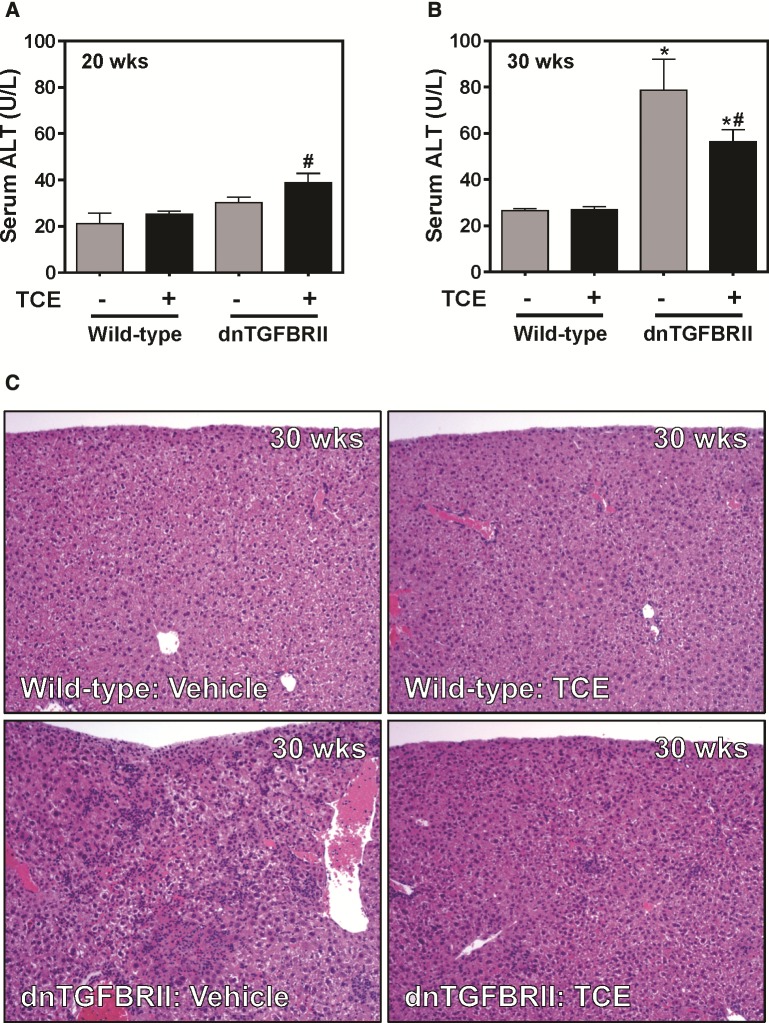
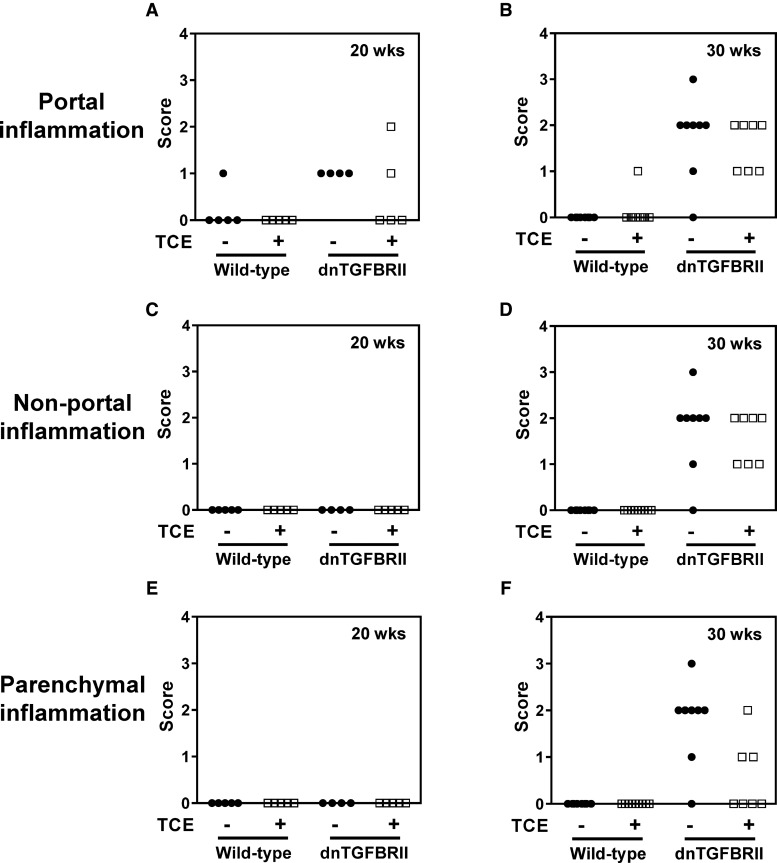
When compared with mice exposed to vehicle, TCE exposure (0.5 mg/ml, drinking water) had no significant effect on serum ALT activity in either 20- (12 weeks exposure) or 30-week-old (22 weeks exposure) wild-type mice (Figs. 1A and B) . Likewise, liver histopathology in wild-type mice was unremarkable regardless of age or exposure (Figure 1C and data not shown). Serum ALT activity was not significantly increased in vehicle-exposed 20-week-old dnTGFBRII mice. Serum ALT was slightly increased in TCE-exposed 20-week-old dnTGFBRII mice (Figure 1A). Serum ALT activity increased significantly in vehicle-exposed 30-week-old dnTGFBRII mice (Figure 1B) and interestingly, was attenuated by exposure to TCE (Figure 1B). Twenty-week-old dnTGFBRII mice exposed to vehicle developed mild hepatic inflammation localized to the portal tracts, and TCE had minimal effect on this lesion (Figure 2A). Thirty-week-old dnTGFBRII mice exposed to vehicle developed marked portal, non-portal and parenchymal inflammation and single cell hepatocellular necrosis (Figs. 1C, 2B, D, and F). Hepatic inflammation was reduced in TCE-exposed 30-week-old dnTGFBRII mice, with the most obvious reduction observed in parenchymal inflammation (Figs. 1C and 2F). Consistent with the generalized protection from liver disease, the liver weight to body weight ratio was significantly reduced in 30-week-old TCE-exposed mice compared with vehicle-exposed mice (data not shown). Thus, TCE exposure did not exacerbate early features of hepatic autoimmunity in dnTGFBRII mice. In contrast, TCE exposure reduced hepatic inflammation and injury in 30-week-old dnTGFBRII mice.
FIG. 1.
Impact of TCE exposure on liver injury and histopathology in dnTGFBRII mice. 8-week-old wild-type and dnTGFBRII mice were exposed to TCE (0.5 mg/ml) or vehicle (1% ethoxylated castor oil) via drinking water until 20 or 30 weeks of age. Serum ALT activity was determined at (A) 20 or (B) 30 weeks of age. (C) Representative photomicrographs show H&E-stained liver sections of 30-week old mice. N = 4–8 mice per group for 20 weeks of age and 7–15 mice per group for 30 weeks of age. *Significantly different from wild-type mice in the same exposure group. #Significantly different from vehicle-exposed mice of the same genotype.
FIG. 2.
Impact of TCE exposure on hepatic inflammation in dnTGFBRII mice. 8-week-old wild-type and dnTGFBRII mice were exposed to TCE (0.5 mg/ml) or vehicle (1% ethoxylated castor oil) via drinking water until 20 or 30 weeks of age. (A,B) Portal, (C,D) non-portal, and (E,F) parenchymal inflammation in H&E-stained liver sections was scored (0–4) in a masked fashion as described in Materials and Methods section.
Impact of TCE Exposure on the Development of Anti-PDCE2 Autoantibodies in dnTGFBRII Mice
Anti-PDCE2 antibodies were not detected in plasma of 20-week-old dnTGFBRII mice regardless of vehicle or TCE exposure (data not shown). In contrast, 6 of 9 vehicle-exposed 30-week-old dnTGFBRII mice and 7 of 7 TCE-exposed 30-week-old dnTGFBRII mice had detectable titers of AMAs (Table 1). This represented a statistically significant increase in prevalence of serum antiPDCE2 in dnTGFBRII mice compared with wild-type mice, but there was no significant effect of TCE on anti-PDCE2 in mice of either genotype. The results indicate that despite a reduction in hepatic inflammation and injury, TCE exposure did not have a significant effect on AMA titer in dnTGFBRII mice.
TABLE 1.
Detection of Plasma Anti-PDCE2 Antibodies in TCE-Exposed TGFBRII Mice
| Group | Age | anti-PDCE2 Ab (% positive) |
|---|---|---|
| Wild-type | 30 weeks | 2 of 11 (18%) |
| Wild-type + TCE | 30 weeks | 4 of 11 (36%) |
| dnTGFBRII | 30 weeks | 6 of 9 (66%) |
| dnTGFBRII + TCE | 30 weeks | 7 of 7 (100%) |
Eight week-old wild-type and dnTGFBRII mice were exposed to TCE (0.5 mg/ml) or vehicle (1% ethoxylated castor oil) via drinking water until 30 weeks of age. Plasma levels of anti-PDCE2 autoantibodies were determined as described in Materials and Methods section. P < .05 for genotype, but not exposure-related effects.
Effect of TCE Exposure on Hepatic Inflammatory Cytokine Gene Expression
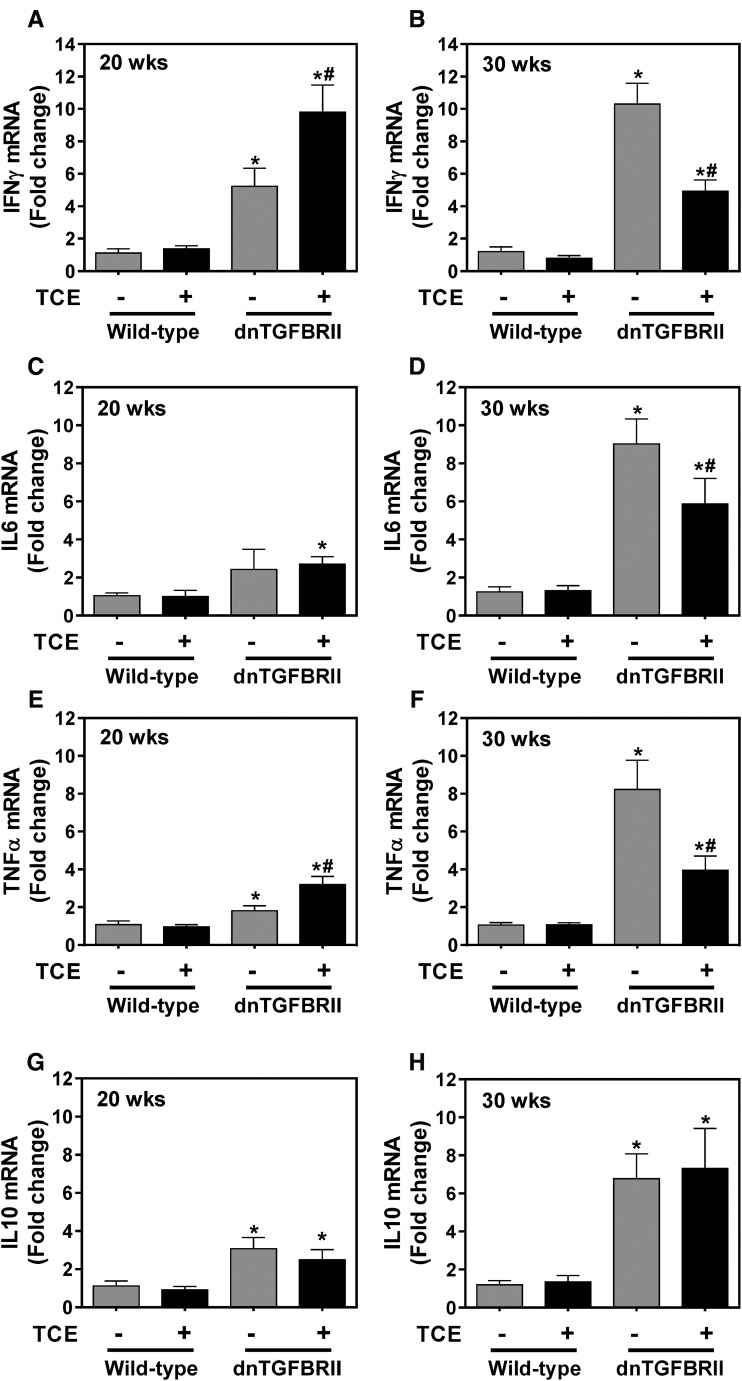
Although anti-PDCE2 autoantibodies and marked histopathological changes were not evident in 20-week-old dnTGFBRII mice, hepatic expression of mRNAs encoding several cytokines (IFNγ, TNFα, IL10) increased in vehicle-exposed 20-week-old dnTGFBRII mice compared with wild-type mice (Figure 3A, E, and G), while there was no significant change in IL6 mRNA expression (Figure 3C). TCE exposure significantly increased hepatic expression of IFNγ and TNFα mRNAs in 20-week-old dnTGFBRII mice (Figs. 3A and E). However, reflecting the reduction in histological inflammation, TCE exposure significantly attenuated the robust induction of each of IFNγ, TNFα, and IL-6 in 30-week-old dnTGFBRII mice (Figs. 3B, D, and F). Interestingly, TCE exposure had no effect on hepatic expression of mRNA encoding the anti-inflammatory cytokine IL10 in either 20- or 30-week-old dnTGFBRII mice (Figs. 3G and H). The results suggest that the reduction in hepatic inflammation in TCE-exposed dnTGFBRII mice is accompanied by a reduction in proinflammatory gene induction without an effect on anti-inflammatory IL10 mRNA expression.
FIG. 3.
Impact of TCE exposure on hepatic gene induction in dnTGFBRII mice. 8-week-old wild-type and dnTGFBRII mice were exposed to TCE (0.5 mg/ml) or vehicle (1% ethoxylated castor oil) via drinking water until 20 or 30 weeks of age. Hepatic levels of mRNAs encoding (A,B) IFNγ, (C,D) IL6, (E,F) TNFα, and (G,H) IL10 were determined by qPCR as described in Materials and Methods section. N = 4–8 mice per group for 20 weeks of age and 7–15 mice per group for 30 weeks of age. *Significantly different from wild-type mice in the same exposure group. #Significantly different from vehicle-exposed mice of the same genotype.
Effect of TCE Exposure on Hepatic T and B Lymphocyte Accumulation in dnTGFBRII Mice
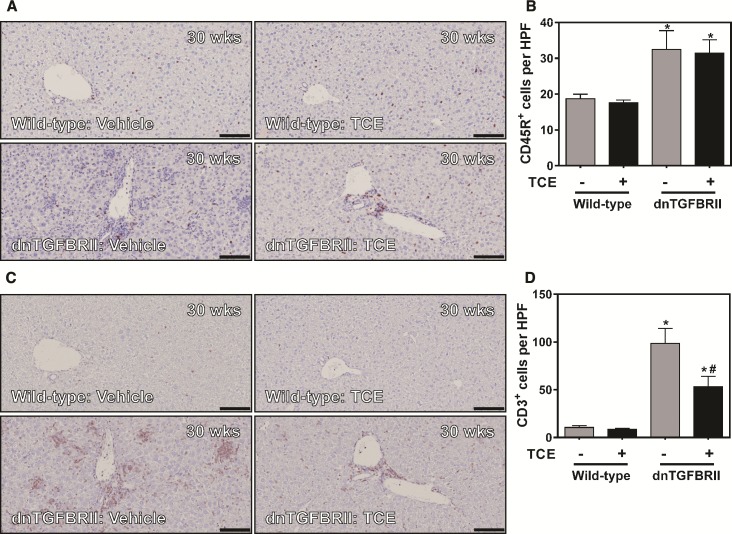
The role of B and T-lymphocytes in autoimmune hepatitis in dnTGFBRII mice has been described previously in Moritoki et al. (2009a,b) and Yang et al. (2008). We determined whether TCE exposure affected infiltration of CD3+ and CD45R+ lymphocytes in livers of dnTGFBRII mice. Increased numbers of CD45R+ (B cells) was observed in livers of 30-week-old vehicle-exposed dnTGFBRII mice (Figure 4A). TCE exposure had no effect on the number of CD45R+ cells in livers of either 30-week-old wild-type mice or dnTGFBRII mice (Figure 4B). TCE exposure did not affect CD3+ cell numbers in livers of 30-week-old wild-type mice (Figure 4D). When compared with vehicle-exposed wild-type mice, livers from 30-week-old vehicle-exposed dnTGFBRII mice showed marked panlobular CD3+ lymphocyte infiltration (Figure 4C). In agreement with our analysis of liver histopathology, TCE exposure significantly reduced hepatic CD3+ lymphocytes in 30-week-old dnTGFBRII mice (Figure 4D). Interestingly, the impact of TCE appeared greatest on the foci of CD3+ lymphocytes scattered throughout the parenchyma, whereas portal CD3+ labeling seemed less affected in TCE-exposed dnTGFBRII mice.
FIG. 4.
Impact of TCE exposure on hepatic B and T cell accumulation in dnTGFBRII mice. 8-week-old wild-type and dnTGFBRII mice were exposed to TCE (0.5 mg/ml) or vehicle (1% ethoxylated castor oil) via drinking water until 30 weeks of age. (A and B), Representative photomicrographs (scale bar = 100 microns) and quantification of CD45R+ B cell accumulation. (C and D), Representative photomicrographs (scale bar = 100 microns) and quantification of CD3+ T cell accumulation. Quantification of B and T cell accumulation per high power field was determined as described in Materials and Methods section. N = 7–15 mice per group. *Significantly different from wild-type mice in the same exposure group. #Significantly different from vehicle-exposed mice of the same genotype.
DISCUSSION
The potential for TCE exposure to precipitate autoimmune disease is an area of intense interest. This is highlighted by inclusion of this topic in the recent IARC monograph classifying TCE as a Group 1 carcinogen (IARC, 2014) and by several recent reviews (Cooper et al., 2009; Germolec et al., 2012; Parks and De Roos, 2014). However, there is very limited epidemiological evidence supporting a connection between TCE exposure and autoimmune hepatitis. The majority of evidence comes from studies in mice, which found that exposure to TCE evokes some features of autoimmune hepatitis in lupus-prone MRL+/+ mice (Cooper et al., 2009; Gilbert, 2010; Gilbert et al., 2009; Griffin et al., 2000b,c; Khan et al., 1995). The impact of TCE in MRL+/+ mice is robust and repeatable, and the mechanisms underlying this response are emerging (Gilbert et al., 2016). To date, exposure of these autoimmune-prone mice to TCE has formed the basis for the connection between TCE exposure and autoimmune hepatitis. However, the nature of hepatic autoimmunity elicited by TCE in MRL+/+ mice may not precisely resemble humans with autoimmune hepatitis. Mutations driving autoimmune hepatitis in humans have yet to be linked with genomic changes leading to disease in MRL+/+ mice (Cordell et al., 2015; Gulamhusein et al., 2015; Marzorati et al., 2016). Indeed, the MRL+/+ mouse is not widely utilized to model autoimmune hepatitis, nor is it used as an experimental setting to validate treatments for this organ-specific autoimmune disease (Yuksel et al., 2014). Overall, while a very strong case can be made for TCE triggering hepatic autoimmunity in the MRL+/+ mouse model, significant questions remain as to the impact of TCE on autoimmune liver disease in humans.
dnTGFBRII mice develop autoimmune liver disease bearing serological and histological resemblance to PBC in humans (Oertelt et al., 2006; Wang et al., 2014a), providing compelling reason to investigate the impact of TCE exposure on disease development and progression in this model. Contrasting observations in TCE-exposed MRL+/+ mice, TCE exposure did not exacerbate histologic evidence of inflammation or injury in livers of 20-week-old dnTGFBRII mice. Rather, as disease progressed, we observed a robust reduction in liver pathology in TCE-exposed dnTGFBRII mice. This was characterized by reduced hepatic inflammatory gene induction, attenuation of parenchymal lymphocytic inflammation, and reduced hepatocellular damage noted biochemically and by histopathological analysis. Despite conferring protection from these aspects of liver pathology, TCE exposure did not reduce anti-mitochondrial autoantibody prevalence. Of importance, several previous studies have shown that marked liver pathology in dnTGFBRII mice can occur without a change in AMA levels (Chuang et al., 2008; Yoshida et al., 2009; Zhang et al., 2009). Collectively, these results suggest that the effect of TCE on liver pathology was not related to a global reduction in autoimmunity in the dnTGFBRII mice.
Our results suggest some selectivity in the mechanism whereby TCE exposure reduces T cell accumulation and liver injury in dnTGFBRII mice. For example, TCE had no effect on hepatic B cell accumulation in 30-week-old dnTGFBRII mice. The precise role of B cells in liver pathology of dnTGFBRII mice has been addressed previously by 2 different approaches. Whereas B cell depletion in young mice reduces PBC-like pathology, potentially by reducing AMA levels, B cell depletion in older mice had no effect (Moritoki et al., 2009a,b). However, B cell deficient (Igmu−/−) dnTGFBRII mice developed exacerbated liver pathology, suggesting that B cells might suppress inflammation in this experimental setting (Moritoki et al., 2009b). In addition, despite a strong reduction in multiple genes encoding proinflammatory cytokines, TCE exposure had no effect on induction of IL10 mRNA. This result is somewhat surprising, insofar as IL10 expression typically counterbalances the expression of proinflammatory cytokines like TNFα. These observations suggest that the reduction of liver disease in dnTGFBRII mice is somewhat selective; however, additional studies are required to address the precise mechanism whereby TCE limits hepatic pathology in this model.
There are limitations in our study that require long-term follow-up and further work. For example, there are potential changes in the metabolism of TCE driven by the presence of concurrent liver disease in dnTGFBRII mice, which can potentially lead to less toxic metabolites being produced in this setting. Moreover, defining the precise changes in lymphocyte subsets in liver and other immune tissues alongside global gene expression profiling of liver and other compartments (eg, blood) may offer opportunity to phenotypically anchor critical gene expression changes to relevant shifts in specific lymphocyte subsets. Comparison of transcriptional and immunological changes driven by TCE in the dnTGFBRII and the MRL+/+ mice has strong potential to (1) identify the mechanism whereby TCE reduces liver injury in dnTGFBRII mice and (2) determine critical differences between these 2 mice underlying the divergent effects of TCE.
The observation that TCE exposure reduces multiple indices of hepatic autoimmunity in dnTGFBRII mice does not challenge the observation that TCE exposure precipitates hepatic autoimmunity in MRL+/+ mice. These are entirely distinct mouse models, each driven by different triggers (ie, Faslpr mutation vs blocking TGFβ signaling in CD4+ and CD8+ T cells). However, the observed dichotomy provides reason to pause, and examine the collective evidence supporting a role for TCE in human autoimmune liver diseases, such as PBC. Because epidemiologic evidence supporting a connection between TCE and autoimmune hepatitis in humans is limited (Cooper et al., 2009), experimental studies using animals form the primary evidence supporting a link between TCE exposure and autoimmune hepatitis. This connection is largely based on experimental studies in MRL+/+ mice, which may not approximate the human condition. The observation that TCE exposure reduces multiple indices of hepatic autoimmunity in dnTGFBRII mice does not challenge the observation that TCE exposure precipitates hepatic autoimmunity in MRL+/+ mice. Rather, this highlights a model-specific response and the importance of defining the impact of TCE on autoimmunity across multiple models. Interestingly, in nonobese diabetic (NOD) mice, which also develop autoimmunity, TCE exposure did not accelerate autoimmunity and the mice did not develop autoimmune hepatitis (Ravel et al., 2005). In NOD mice, TCE exposure reduced lymphocyte proliferation and cytokine production, suggesting a protective effect (Ravel et al., 2005). Notably, the protection conferred by TCE in NOD mice aligns with our observation that TCE reduces hepatic autoimmune-mediated pathology in dnTGFBRII mice. Additional studies are required to explore the basis for differential effects of TCE in these different models (ie, dnTGFBRII, NOD, MRL), and to define the precise relevance of these results to humans. Indeed, such comparisons are complex given involvement of other risk factors such as genetic variants among populations and races (Dong et al., 2015; Hirschfield and Siminovitch, 2015; Podda et al., 2013; Xie et al., 2016).
Establishing features of the mechanism and precise nature of autoimmunity may determine the observed effect of TCE in different experimental settings. MRL+/+ mice, while autoimmune-prone, do not typically develop hepatic autoimmunity in the age-range explored for TCE challenge. In contrast, dnTGFBRII mice develop marked PBC-like liver pathology by approximately 6 months of age. This difference highlights the inherent potential for TCE to trigger liver pathology in one model (ie, MRL+/+ mice) and inhibit pathology in another (ie, dnTGFBRII mice). Autoimmune pathologies in MRL+/+ mice are primarily driven by CD4+ T cells (Giese and Davidson, 1995; Koh et al., 1995; Santoro et al., 1988), and TCE exposure selectively expands CD4+, but not CD8+ T cells, in lymph nodes and spleens of MRL+/+ mice (Griffin et al., 2000b). In dnTGFBRII mice, much like patients with PBC, CD8 + T cells play a key role in liver damage (Bernuzzi et al., 2010; Yang et al., 2008). Because the role and effect of TCE on T cell subtypes could be model-dependent, future studies should include side-by-side exposure of both dnTGFBRII and MRL+/+ mice to TCE to examine the precise changes in lymphocyte subsets not only in liver, but also in spleen and lymph node. It is conceivable that this analysis will reveal the basis for the lack of similarity in TCE-driven responses in these 2 mouse models.
The observation that TCE reduces liver pathology in dnTGFBRII mice represents an important addition to the field by demonstrating the impact of TCE on the course of experimental autoimmune liver disease. Moreover, the present study addresses the need for evaluation of TCE exposure in additional mouse models of autoimmunity, called for in recent years by an NIEHS expert panel workshop (Germolec et al., 2012). The dichotomy of results from studies of TCE exposure in 3 different mouse models of autoimmunity illustrates the complexity of gene-environment interaction imposed by the selected experimental setting. Additional research using dnTGFBRII mice, or other established mouse models resembling human autoimmune liver disease (Bae et al., 2016; Hsueh et al., 2016; Leung et al., 2012; Liberal et al., 2016; Pollheimer and Fickert, 2015; Wakabayashi et al., 2008; Yao et al., 2014), has a high potential to inform on the specific risks associated with TCE in populations prone to autoimmunity.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
FUNDING
This work was supported by grants from the National Institutes of Health, National Institute of Environmental Health Sciences (R21 ES024470; R01 ES017537). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
Supplementary Material
REFERENCES
- Ando Y., Yang G. X., Tsuda M., Kawata K., Zhang W., Nakajima T., Tsuneyama K., Leung P., Lian Z. X., Okazaki K., et al. (2012). The immunobiology of colitis and cholangitis in interleukin-23p19 and interleukin-17A deleted dominant negative form of transforming growth factor beta receptor type II mice. Hepatology 56, 1418–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae H. R., Leung P. S., Tsuneyama K., Valencia J. C., Hodge D. L., Kim S., Back T., Karwan M., Merchant A. S., Baba N., et al. (2016). Chronic expression of interferon gamma leads to murine autoimmune cholangitis with a female predominance. Hepatology. 644, 1189–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernuzzi F., Fenoglio D., Battaglia F., Fravega M., Gershwin M. E., Indiveri F., Ansari A. A., Podda M., Invernizzi P., Filaci G. (2010). Phenotypical and functional alterations of CD8 regulatory T cells in primary biliary cirrhosis. J. Autoimmun. 35, 176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuers U., Gershwin M. E. (2015). Unmet challenges in immune-mediated hepatobiliary diseases. Clin. Rev. Allergy Immunol. 48, 127–131. [DOI] [PubMed] [Google Scholar]
- Bogdanos D. P., Gao B., Gershwin M. E. (2013). Liver immunology. Compr. Physiol. 3, 567–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu W. A., Jinot J., Scott C. S., Makris S. L., Cooper G. S., Dzubow R. C., Bale A. S., Evans M. V., Guyton K. Z., Keshava N., et al. (2013). Human health effects of trichloroethylene: Key findings and scientific issues. Environ. Health Perspect. 121, 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang Y. H., Lian Z. X., Yang G. X., Shu S. A., Moritoki Y., Ridgway W. M., Ansari A. A., Kronenberg M., Flavell R. A., Gao B., et al. (2008). Natural killer T cells exacerbate liver injury in a transforming growth factor beta receptor II dominant-negative mouse model of primary biliary cirrhosis. Hepatology 47, 571–580. [DOI] [PubMed] [Google Scholar]
- Cooper G. S., Makris S. L., Nietert P. J., Jinot J. (2009). Evidence of autoimmune-related effects of trichloroethylene exposure from studies in mice and humans. Environ. Health Perspect. 117, 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell H. J., Han Y., Mells G. F., Li Y., Hirschfield G. M., Greene C. S., Xie G., Juran B. D., Zhu D., Qian D. C., et al. (2015). International genome-wide meta-analysis identifies new primary biliary cirrhosis risk loci and targetable pathogenic pathways. Nat. Commun. 6, 8019–8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty D. G. (2016). Immunity, tolerance and autoimmunity in the liver: A comprehensive review. J. Autoimmun. 66, 60–75. [DOI] [PubMed] [Google Scholar]
- Dong M., Li J., Tang R., Zhu P., Qiu F., Wang C., Qiu J., Wang L., Dai Y., Xu P., et al. (2015). Multiple genetic variants associated with primary biliary cirrhosis in a Han Chinese population. Clin. Rev. Allergy Immunol. 48, 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germolec D., Kono D. H., Pfau J. C., Pollard K. M. (2012). Animal models used to examine the role of the environment in the development of autoimmune disease: Findings from an NIEHS Expert Panel Workshop. J. Autoimmun. 39, 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese T., Davidson W. F. (1995). In CD8+ T cell-deficient lpr/lpr mice, CD4+B220+ and CD4+B220- T cells replace B220+ double-negative T cells as the predominant populations in enlarged lymph nodes. J. Immunol. 154, 4986–4995. [PubMed] [Google Scholar]
- Gilbert K. M. (2010). Xenobiotic exposure and autoimmune hepatitis. Hepat. Res. Treat. 2010, 248157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert K. M., Blossom S. J., Erickson S. W., Reisfeld B., Zurlinden T. J., Broadfoot B., West K., Bai S., Cooney C. A. (2016). Chronic exposure to water pollutant trichloroethylene increased epigenetic drift in CD4(+) T cells. Epigenomics 8, 633–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert K. M., Przybyla B., Pumford N. R., Han T., Fuscoe J., Schnackenberg L. K., Holland R. D., Doss J. C., Macmillan-Crow L. A., Blossom S. J. (2009). Delineating liver events in trichloroethylene-induced autoimmune hepatitis. Chem. Res. Toxicol. 22, 626–632. [DOI] [PubMed] [Google Scholar]
- Gorelik L., Flavell R. A. (2000). Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 12, 171–181. [DOI] [PubMed] [Google Scholar]
- Griffin J. M., Blossom S. J., Jackson S. K., Gilbert K. M., Pumford N. R. (2000a). Trichloroethylene accelerates an autoimmune response by Th1 T cell activation in MRL +/+ mice. Immunopharmacology 46, 123–137. [DOI] [PubMed] [Google Scholar]
- Griffin J. M., Gilbert K. M., Lamps L. W., Pumford N. R. (2000b). CD4(+) T-cell activation and induction of autoimmune hepatitis following trichloroethylene treatment in MRL+/+ mice. Toxicol. Sci. 57, 345–352. [DOI] [PubMed] [Google Scholar]
- Griffin J. M., Gilbert K. M., Pumford N. R. (2000c). Inhibition of CYP2E1 reverses CD4+ T-cell alterations in trichloroethylene-treated MRL+/+ mice. Toxicol. Sci. 54, 384–389. [DOI] [PubMed] [Google Scholar]
- Gulamhusein A. F., Juran B. D., Lazaridis K. N. (2015). Genome-wide association studies in primary biliary cirrhosis. Semin. Liver Dis. 35, 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfield G. M., Siminovitch K. A. (2015). Genetics in PBC: What do the “risk genes” teach us?. Clin. Rev. Allergy Immunol. 48, 176–181. [DOI] [PubMed] [Google Scholar]
- Hsueh Y. H., Chang Y. N., Loh C. E., Gershwin M. E., Chuang Y. H. (2016). AAV-IL-22 modifies liver chemokine activity and ameliorates portal inflammation in murine autoimmune cholangitis. J. Autoimmun. 66, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Kachapati K., Adams D., Wu Y., Leung P. S., Yang G. X., Zhang W., Ansari A. A., Flavell R. A., Gershwin M. E., et al. (2014). Murine autoimmune cholangitis requires two hits: Cytotoxic KLRG1(+) CD8 effector cells and defective T regulatory cells. J. Autoimmun. 50, 123–134. 10.1016/j.jaut.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC. (2014). IARC monographs on the evaluation of carcinogenic risks to humans: Trichloroethylene, tetrachloroethylene, and some other chlorinated agents. 106, 1–525. [PMC free article] [PubMed] [Google Scholar]
- Invernizzi P. (2013). Liver auto-immunology: The paradox of autoimmunity in a tolerogenic organ. J. Autoimmun. 46, 1–6. [DOI] [PubMed] [Google Scholar]
- Joshi N., Kopec A. K., O'Brien K. M., Towery K. L., Cline-Fedewa H., Williams K. J., Copple B. L., Flick M. J., Luyendyk J. P. (2015). Coagulation-driven platelet activation reduces cholestatic liver injury and fibrosis in mice. J. Thromb. Haemost. 13, 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumi T., Tomita K., Leung P. S., Yang G. X., Gershwin M. E., Ueno Y. (2015). Animal models of primary biliary cirrhosis. Clin. Rev. Allergy Immunol. 48, 142–153. [DOI] [PubMed] [Google Scholar]
- Kawata K., Yang G. X., Ando Y., Tanaka H., Zhang W., Kobayashi Y., Tsuneyama K., Leung P. S., Lian Z. X., Ridgway W. M., et al. (2013). Clonality, activated antigen-specific CD8(+) T cells, and development of autoimmune cholangitis in dnTGFbetaRII mice. Hepatology 58, 1094–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. F., Kaphalia B. S., Prabhakar B. S., Kanz M. F., Ansari G. A. (1995). Trichloroethene-induced autoimmune response in female MRL +/+ mice. Toxicol. Appl. Pharmacol. 134, 155–160. [DOI] [PubMed] [Google Scholar]
- Koh D. R., Ho A., Rahemtulla A., Fung-Leung W. P., Griesser H., Mak T. W. (1995). Murine lupus in MRL/lpr mice lacking CD4 or CD8 T cells. Eur. J. Immunol. 25, 2558–2562. [DOI] [PubMed] [Google Scholar]
- Leung P. S., Choi J., Yang G., Woo E., Kenny T. P., Gershwin M. E. (2016). A contemporary perspective on the molecular characteristics of mitochondrial autoantigens and diagnosis in primary biliary cholangitis. Expert Rev. Mol. Diagn. 16, 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung P. S., Yang G. X., Dhirapong A., Tsuneyama K., Ridgway W. M., Gershwin M. E. (2012). Animal models of primary biliary cirrhosis: Materials and methods. Methods Mol. Biol. 900, 291–316. [DOI] [PubMed] [Google Scholar]
- Liberal R., Krawitt E. L., Vierling J. M., Manns M. P., Mieli-Vergani G., Vergani D. (2016). Cutting edge issues in autoimmune hepatitis. J. Autoimmun. 75, 6–19. [DOI] [PubMed] [Google Scholar]
- Marzorati S., Lleo A., Carbone M., Gershwin M. E., Invernizzi P. (2016). The epigenetics of PBC: The link between genetic susceptibility and environment. Clin. Res. Hepatol. Gastroenterol. 40, 650–659. [DOI] [PubMed] [Google Scholar]
- Miller F. W., Pollard K. M., Parks C. G., Germolec D. R., Leung P. S., Selmi C., Humble M. C., Rose N. R. (2012). Criteria for environmentally associated autoimmune diseases. J. Autoimmun. 39, 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritoki Y., Lian Z. X., Lindor K., Tuscano J., Tsuneyama K., Zhang W., Ueno Y., Dunn R., Kehry M., Coppel R. L., et al. (2009a). B-cell depletion with anti-CD20 ameliorates autoimmune cholangitis but exacerbates colitis in transforming growth factor-beta receptor II dominant negative mice. Hepatology 50, 1893–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritoki Y., Zhang W., Tsuneyama K., Yoshida K., Wakabayashi K., Yang G. X., Bowlus C., Ridgway W. M., Ueno Y., Ansari A. A., et al. (2009b). B cells suppress the inflammatory response in a mouse model of primary biliary cirrhosis. Gastroenterology 136, 1037–1047. [DOI] [PubMed] [Google Scholar]
- Moteki S., Leung P. S., Coppel R. L., Dickson E. R., Kaplan M. M., Munoz S., Gershwin M. E. (1996). Use of a designer triple expression hybrid clone for three different lipoyl domain for the detection of antimitochondrial autoantibodies. Hepatology 24, 97–103. [DOI] [PubMed] [Google Scholar]
- Oertelt S., Lian Z. X., Cheng C. M., Chuang Y. H., Padgett K. A., He X. S., Ridgway W. M., Ansari A. A., Coppel R. L., Li M. O., et al. (2006). Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. J. Immunol. 177, 1655–1660. [DOI] [PubMed] [Google Scholar]
- Parks C. G., De Roos A. J. (2014). Pesticides, chemical and industrial exposures in relation to systemic lupus erythematosus. Lupus 23, 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podda M., Selmi C., Lleo A., Moroni L., Invernizzi P. (2013). The limitations and hidden gems of the epidemiology of primary biliary cirrhosis. J. Autoimmun. 46, 81–87. [DOI] [PubMed] [Google Scholar]
- Pollheimer M. J., Fickert P. (2015). Animal models in primary biliary cirrhosis and primary sclerosing cholangitis. Clin. Rev. Allergy Immunol. 48, 207–217. [DOI] [PubMed] [Google Scholar]
- Ravel G., Christ M., Perron-Lepage M. F., Condevaux F., Descotes J. (2005). Trichloroethylene does not accelerate autoimmune diabetes in NOD mice. J. Immunotoxicol. 1, 141–148. [DOI] [PubMed] [Google Scholar]
- Santoro T. J., Portanova J. P., Kotzin B. L. (1988). The contribution of L3T4+ T cells to lymphoproliferation and autoantibody production in MRL-lpr/lpr mice. J. Exp. Med. 167, 1713–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmi C., Leung P. S., Sherr D. H., Diaz M., Nyland J. F., Monestier M., Rose N. R., Gershwin M. E. (2012). Mechanisms of environmental influence on human autoimmunity: A National Institute of Environmental Health Sciences expert panel workshop. J. Autoimmun. 39, 272–284. [DOI] [PubMed] [Google Scholar]
- Shuai Z., Leung M. W., He X., Zhang W., Yang G., Leung P. S., Eric Gershwin M. (2016). Adaptive immunity in the liver. Cell Mol. Immunol. 13, 354–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K., Lian Z. X., Leung P. S., Moritoki Y., Tsuneyama K., Kurth M. J., Lam K. S., Yoshida K., Yang G. X., Hibi T., et al. (2008). Loss of tolerance in C57BL/6 mice to the autoantigen E2 subunit of pyruvate dehydrogenase by a xenobiotic with ensuing biliary ductular disease. Hepatology 48, 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Yang G. X., Tsuneyama K., Gershwin M. E., Ridgway W. M., Leung P. S. (2014a). Animal models of primary biliary cirrhosis. Semin. Liver Dis. 34, 285–296. [DOI] [PubMed] [Google Scholar]
- Wang J. J., Yang G. X., Zhang W. C., Lu L., Tsuneyama K., Kronenberg M., Vela J. L., Lopez-Hoyos M., He X. S., Ridgway W. M., et al. (2014b). Escherichia coli infection induces autoimmune cholangitis and anti-mitochondrial antibodies in non-obese diabetic (NOD).B6 (Idd10/Idd18) mice. Clin. Exp. Immunol. 175, 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. H., Yang W., Yang J. B., Jia Y. J., Tang W., Gershwin M. E., Ridgway W. M., Lian Z. X. (2015). Systems biologic analysis of T regulatory cells genetic pathways in murine primary biliary cirrhosis. J. Autoimmun. 59, 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y. Q., Ma H. D., Lian Z. X. (2016). Epigenetics and primary biliary cirrhosis: A comprehensive review and implications for autoimmunity. Clin. Rev. Allergy Immunol. 50, 390–403. [DOI] [PubMed] [Google Scholar]
- Yang G. X., Lian Z. X., Chuang Y. H., Moritoki Y., Lan R. Y., Wakabayashi K., Ansari A. A., Flavell R. A., Ridgway W. M., Coppel R. L., et al. (2008). Adoptive transfer of CD8(+) T cells from transforming growth factor beta receptor type II (dominant negative form) induces autoimmune cholangitis in mice. Hepatology 47, 1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G. X., Sun Y., Tsuneyama K., Zhang W., Leung P. S., He X. S., Ansari A. A., Bowlus C., Ridgway W. M., Gershwin M. E. (2016a). Endogenous interleukin-22 protects against inflammatory bowel disease but not autoimmune cholangitis in dominant negative form of transforming growth factor beta receptor type II mice. Clin. Exp. Immunol. 185, 154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. B., Wang Y. H., Yang W., Lu F. T., Ma H. D., Zhao Z. B., Jia Y. J., Tang W., Tsuneyama K., Ridgway W. M., et al. (2016b). Successful treatment of murine autoimmune cholangitis by parabiosis: Implications for hematopoietic therapy. J. Autoimmun. 66, 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Yang W., Yang Y. Q., Ma H. D., Lu F. T., Li L., Tao Y. Y., Tsuneyama K., Zhang W., Friedman S., et al. (2014). Distinct from its canonical effects, deletion of IL-12p40 induces cholangitis and fibrosis in interleukin-2Ralpha(-/-) mice. J. Autoimmun. 51, 99–108, 10. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Yang G. X., Zhang W., Tsuda M., Tsuneyama K., Moritoki Y., Ansari A. A., Okazaki K., Lian Z. X., Coppel R. L., et al. (2009). Deletion of interleukin-12p40 suppresses autoimmune cholangitis in dominant negative transforming growth factor beta receptor type II mice. Hepatology 50, 1494–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuksel M., Laukens D., Heindryckx F., Van Vlierberghe H., Geerts A., Wong F. S., Wen L., Colle I. (2014). Hepatitis mouse models: From acute-to-chronic autoimmune hepatitis. Int. J. Exp. Pathol. 95, 309–320. 10.1111/iep.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Moritoki Y., Tsuneyama K., Yang G. X., Ilan Y., Lian Z. X., Gershwin M. E. (2009). Beta-glucosylceramide ameliorates liver inflammation in murine autoimmune cholangitis. Clin. Exp. Immunol. 157, 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.