Abstract
The clinical application of nanoparticular Gd(III) based contrast agents for tumor molecular MRI has been hindered by safety concerns associated with prolonged tissue retention, although they can produce strong tumor enhancement. In this study, a targeted well-defined cyclodextrin-based nanoglobular contrast agent was developed through self-assembly driven by host-guest interactions for safe and effective cancer molecular MRI. Multiple β-cyclodextrins attached POSS (polyhedral oligomeric silsesquioxane) nanoglobule was used as host molecule. Adamantane–modified macrocyclic Gd(III) contrast agent, cRGD (cyclic RGDfK peptide) targeting ligand and fluorescent probe was used as guest molecules. The targeted host-guest nanoglobular contrast agent cRGD-POSS-βCD-(DOTA-Gd) specifically bond to αvβ3 integrin in malignant 4T1 breast tumor and provided greater contrast enhancement than the corresponding non-targeted agent. The agent also provided significant fluorescence signal in tumor tissue. The histological analysis of the tumor tissue confirmed its specific and effective targeting to αvβ3 integrin. The targeted imaging agent has a potential for specific cancer molecular MR and fluorescent imaging.
Keywords: cancer molecular imaging, magnetic resonance imaging, gadolinium contrast agent, αvβ3 integrin, cyclodextrin, host-guest interaction
1. Introduction
Molecular imaging of cancer biomarkers allows detection and differential diagnosis of cancer and monitoring disease progression and facilitates more effective personalized patient care.(1, 2) MRI is a non-invasive clinical imaging modality, produces high-resolution anatomic images of soft tissues, and has the potential for high-resolution molecular imaging of cancer biomarkers for early cancer detection and characterization. Gadolinium(III) based contrast agents have been used to shorten the relaxation times of the surrounding water protons and therefore to enhance image contrast for accurate diagnostic imaging.(3, 4) Small molecular Gd(III) chelates with high chelation stability are routinely used in clinical practice for cancer imaging. However, these chelates are not specific to cancer biomarkers and not suitable for cancer molecular imaging with MRI. Thus, there is a need of safe and effective targeted MRI contrast agents for high-resolution cancer molecular imaging.
Recently, we have shown that small molecular targeted MRI contrast agents specific to biomarkers abundant in tumor extracellular matrix is effective to produce strong signal enhancement for detection of primary tumors and micrometastases in animal models.(5, 6) However, small molecular MRI contrast agents are not effective to detect the biomarkers expressed on cancer cells because of the low concentration of the biomarkers and low sensitivity of MRI. In order to produce MRI detectable signal enhancement for the biomarkers on cancer cell surface, nanocarriers including polymers, dendrimers, liposomes, and nanoparticles have been used to load a large amount of Gd(III) chelates with targeting groups to increase the agent concentration around the cancer biomarkers.(7–9) Although these nanocarrier-based targeted contrast agents have many advantages over small molecular contrast agents, including high relaxivity,(10, 11) high tumor delivery efficiency,(12) strong signal enhancement,(13, 14) and prolonged imaging window, safety concerns associated with their slow excretion and prolonged body retention have hampered clinical development.(15)
Cyclodextrins (CDs) is a class of cyclic oligosaccharides with molecular hydrophobic cavities that have been used to form inclusion complexes by selective host-guest interactions.(16–19) A number of drug-CD complexes have been marketed using the features of CDs, including good bioavailability, reversible binding and release of guest drugs governed by the thermodynamics of the complexes.(16) We hypothesize that loading small molecular MRI contrast agents to a cyclodextrin-containing nanocarrier by the reversible host-guest interaction would benefit from these advantageous features to develop targeted nanoparticular contrast agents for effective cancer molecular MRI and to facilitate the excretion of the contrast agents by their release from the host to minimize the potential contrast related toxic side effects. (20, 21) We intend to design and develop a targeted contrast agent for cancer MR molecular imaging using CD based host-guest chemistry and a well-defined and biocompatible polyhedral silsesquioxane (POSS). This novel targeted host-guest nanosized contrast agent has the potential to provide effective signal enhancement for MR molecular imaging of the biomarkers expressed on cancer cells, while addressing the safety concerns associated with slow excretion of Gd(III)-based nanosized MRI contrast agents.
Here, we synthesized a targeted cyclodextrin-based nanosized MRI contrast agent by host-guest interaction of β-cyclodextrin (βCD) (host molecule) with an adamantane (Ad) (hydrophobic guest molecule) modified targeting agent and imaging agents. Multiple βCDs were conjugated to the surface of a POSS to obtain a nanocarrier with well-defined nanosize.(22) A cyclic peptide containing Arg-Gly-Asp-Phe(D)-Lys (cRGD) was used as a targeting group to selectively bind to αvβ3 intergrins, which are overexpressed on tumor vascular endothelial cells and as well as various tumor cells, such as mammary carcinoma cells.(23–26) A macrocyclic Gd(III) chelate (Gd-DOTA monoamide) and a fluorescent dye Cy5 were used as bimodal imaging probes. The targeted bimodal contrast agent was readily synthesized by host-guest interaction. The effectiveness of the agent for MR molecular imaging was demonstrated in a mouse 4T1 breast cancer model.
2. Materials and methods
2.1. Materials
All reagents were used without further purification unless otherwise stated. Octa(3-aminopropyl) silsesquioxane hydrochloride (OctaAmmonium POSS·HCl) was purchased from Hybrid Plastics (Hattiesburg, MS). β-cyclodextrin (βCD) and 5-hexynoic acid were purchased from Fisher Scientific (Pittsburgh, PA). 1,4,7,10-Tetraazacyclododecane-1,4,7-tris-tert-butyl acetate-10-acetic acid [DOTA-tris(t-Bu)] was purchased from Macrocyclics (Dallas, TX). 1-Adamantylamine, dicyclohexylcarbodiimide (DCC), tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (TBTA) and tetrakis(acetonitrile) copper(I) hexafluorophosphate were purchased from Sigma Aldrich (St. Louis, MO). 2,3,4,5,6-Pentafluorophenol and trifluoroacetic acid were purchased from Oakwood Products (West Columbia, SC). Fluorophore sulfo-cyanine5 (Cy5) mono-reactive NHS ester was purchased from GE Healthcare (Buckinghamshire, UK). NH2-PEG-COOH (MW = 5000 Da) was purchased from Nanocs (NewYork, NY). Benzotriazol-1-yl-oxy-tris(pyrrolidino) phosphonium hexafluorophosphate (PyBOP), 2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU), and 1-hydroxybenzotriazole hydrate (HOBt) were purchased from Chem-Impex International (Wood Dale, IL). N,N-Diisopropylethyl amine (DIPEA), tetrahydrofuran anhydrous (THF) and N,N-dimethylformamide anhydrous (DMF) were purchased from Alfa Aesar (Ward Hill, MA). Cyclic peptide RGDfK (cRGD) and non-specific control cyclic peptide RADfK (cRAD) were synthesized using standard solid phase peptide chemistry from Fmoc-protected amino acids on a 2-chlorotrityl chloride resin followed by cleavage, cyclization and deprotection.(27, 28)
2.2. Methods
1H NMR and 13C NMR spectra were acquired on a 400 MHz Varian Inova NMR spectrometer with deuterated solvent as noted. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectra were acquired on a Bruker Autoflex III MALDI-TOF MS in a linear mode with 2,5-dihydroxybenzoic acid (2,5-DHB) as a matrix. Analytical ion-pairing reverse-phase HPLC (RP-HPLC) was performed on an Agilent 1100 series HPLC system fitted with a Beckman Coulter Ultrasphere ODS 4.6 mm × 25 cm, 5 μm pore size column. Preparative runs were performed on a ZORBAX PrepHT C-18 column. Gradient elution was used to characterization and purification. Eluent A was water, B was acetonitrile and flow rate was 1.0 or 5.0 ml min−1. HPLC method: 0–10 min 100% A, 10–30 min 60% A, 30–35 min 0% A, 35–40 min 0% A, 40–45 min 100% A. Relaxation times of the aqueous solution of the contrast agents with different concentrations were measured at 60 MHz (1.5 T) using a Bruker minispec relaxometer at 37 °C. T1 was measured with an inversion-recovery pulse sequence. T2 was measured using a Carr-Purcell-Meiboom-Gill sequence with 500 echoes collected. The T1 and T2 relaxivities of the agents were calculated from the slopes of the plots of 1/T1 and 1/T2 versus the Gd concentrations.
2.3. Synthesis and characterization
2.3.1. Synthesis of pentafluorophenyl 5-hexynoate
5-Hexynoic acid (2.0 g, 17.8 mmol) and pentafluorophenol (3.7 g, 20 mmol) were dissolved in anhydrous THF (200 ml) at room temperature (RT) with stirring. DCC (4.9 g, 24 mmol) dissolved in anhydrous THF (40 ml) was added to the solution and the mixture was stirred at RT overnight. The solution was filtered through celite and the filtrate was concentrated and purified by column chromatography (SiO2, hexane/EtOAc=10:1) to give pentafluorophenyl 5-hexynoate as colorless oil (4.4 g, yield 89%). 1H NMR (400 MHz, CDCl3, δ):2.84 (t, 2H, CH2CO), 2.37 (dt, 2H, CH≡C-CH2), 2.04–1.96 (m, 3H, CH≡C + CH2CH2CH2). 13C NMR (400 MHz, CDCl3, δ): 169.16 (s, 1C, CH2CO), 142.60–136.85 (m, 6C, pentafluorophenyl), 82.53 (s, 1C, CH≡C), 69.80 (s, 1C, CH≡C), 31.99 (s, 1C, CH2CO), 23.49 (s, 1C, CH2CH2CH2), 17.72 (s, 1C, CH≡CCH2). (Figure S1 and S2).
2.3.2. Synthesis of octa(3-(5-hexynamido)propyl) POSS (octapropargyl POSS)
OctaAmmonium POSS·HCl (0.2 g, 0.17 mmol) and DIPEA (0.35 ml, 2.04 mmol) was dissolved in DMF (10 ml). Pentafluorophenyl 5-hexynoate (0.57 g, 2.04 mmol) was added to the solution and the mixture was stirred at RT under N2 overnight. The solution changed from cloudy to clear and it was poured into cold ethyl ether (200 ml) to precipitate the product. The product was collected by centrifugation, washed with ethyl ether and dried in vacuum to give Octapropargyl POSS as white solid (0.26 g, yield 94%). MALDI-TOF (m/z, [M]+): 1631.9 (obsd), 1632.6 (calcd); (m/z, [M + Na]+): 1653.8 (obsd), 1655.6 (calcd). 1H NMR (400 MHz, CDCl3, δ): 3.22 (br, 16H, CH2NHCO), 2.35 (br, 16H, CH2CONH), 2.26 (br, 16H, CH≡CCH2), 2.00 (br, 8H, CH≡C), 1.87 (br, 16H, CH2CH2CH2CO), 1.78 (br, 16H, SiCH2CH2), 0.64 (br, 16H, SiCH2CH2).
2.3.3. Synthesis of mono-6-deoxy-6-azido-β-cyclodextrin (azido-βCD)
The synthesis of mono-6-deoxy-6-(p-tolylsulfonyl)-β-cyclodextrin (Tosyl-βCD) was according to the literature.(29) MALDI-TOF (m/z, [M + Na]+): 1310.6 (obsd), 1311.0 (calcd); (m/z, [M + K]+): 1349.8 (obsd), 1350.0 (calcd). 1H NMR (400 MHz, DMSO-d6, δ) of tosyl-βCD: 7.71 (d, 2H), 7.41 (d, 2H), 5.85–5.64 (m, 14H), 4.81–4.73 (m, 7H), 4.49–4.14 (m, 6H), 3.72–3.43 (m, 28H), 3.40–3.18 (m, overlap with HDO, 14H), 2.43 (s, 3H).
Tosyl-βCD (5 g, 3.89 mmol) and sodium azide (5 g, 7.70 mmol) were mixed in deionized water (500 ml) and the mixture was refluxed with stirring for 72 h. Water was removed by rotary evaporation and the crud product was purified by recrystallization in hot water. The product was collected by filtration and dried in vacuum to give azido-βCD as white solid (3.1 g, yield 69%). MALDI-TOF (m/z, [M]+): 1159.1 (obsd), 1159.0 (calcd); (m/z, [M + Na]+): 1181.6 (obsd), 1182.0 (calcd). 1H NMR (400 MHz, DMSO-d6, δ) of Azido-βCD: 5.80–5.60 (m, 14 H), 4.84 (br, 1H), 4.79 (br, 6H), 4.52–4.48 (m, 2H), 4.46–4.40 (br, 5H), 3.76–3.46 (m, 28H), 3.40- 3.22 (br, overlap with H2O, 14H).
2.3.4. Synthesis of POSS-β-cyclodextrin (POSS-βCD)
Octapropargyl POSS (0.1 g, 0.06 mmol) and Azido-βCD (0.67 g, 0.58 mmol) was dissolved in a solution of t-butanol (15 ml) and water (10 ml). The solution was bubbled with nitrogen to remove oxygen and then heated to 40 °C. Tetrakis(acetonitrile)copper(I) hexafluorophosphate (6.0 mg, 0.016 mmol) and tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (TBTA) (8.5 mg, 0.016 mmol) was added to the solution. The mixture was stirred under a nitrogen atmosphere and kept at 40 °C for 2 days. The solution was filtered and the solvent was removed by rotary evaporator. The product was dissolved in water and dialyzed (Spectra/Pro MWCO 5,000) against water (1 L × 4). The solution was lyophilized to give POSS-βCD as white solid (0.37 g, yield 56%). MALDI-TOF (m/z): 7543.1 (POSS-(βCD)5), 8678.0 (POSS-(βCD)6), 9844.0 (POSS-(βCD)7), 10975.9 (POSS-(βCD)8). 1H NMR (400 MHz, DMSO-d6, δ) of POSS-βCDn: 5.80–5.60 (m, 14H×n, OCH(CH)O), 4.80 (br, 6H×n), 4.50–4.45 (m, 2H×n), 4.46–4.40 (br, 5H×n), 3.80–3.45 (m, 28H×n), 3.40–3.20 (br, overlap with H2O, 14H×n), 3.20 (br, 16H, CH2NHCO), 2.42–1.85 (br, 32H, CH2CONH, CH2CH2CH2CO), 1.80 (br, 16H, SiCH2CH2), 0.64 (br, 16H, SiCH2CH2).
2.3.5. Synthesis of tri-tert-butyl 2,2′,2″-(10-(2-(1-adamantylamino)-2-oxoethyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl) triacetate (Ad-DOTA-tris(t-Bu))
DOTA-tris(t-Bu) (2.0 g, 3.36 mmol) was dissolved in 30 ml DMF. 1-Adamantylamine (0.7 g, 4.63 mmol), PyBOP (2.08 g, 4.0 mmol) and DIPEA (1 ml, 5.8 mmol) was added to the solution and the mixture was stirred at room temperature for 5 h. Saturated NaHCO3 solution (120 ml) was added to the previous solution and the mixture was extracted by ethyl acetate (200 ml × 3). The organic solution was dried over anhydrous Na2SO4 and the solvent was removed by rotary evaporator. The crude product was purified by column chromatography (SiO2, CH2Cl2/ethyl acetate 5:1) to get Ad-DOTA-tris(t-Bu) as white solid (2.4 g, yield 100%). MALDI-TOF (m/z, [M]+): 705.9 (obsd), 705.5 (calcd); (m/z, [M + Na]+): 727.9 (obsd), 728.5 (calcd). (Figure S3).
2.3.6. Synthesis of 2,2′,2″-(10-(2-(1-adamantylamino)-2-oxoethyl)-1,4,7,10-tetraazacyclo dodecane-1,4,7-triyl) triacetic acid (Ad-DOTA)
Ad-DOTA-tris(t-Bu) (2.0 g, 2.8 mmol) was dissolved in formic acid (150 ml). The solution was heated to 50 °C and stirred overnight. The solvent was removed by rotary evaporator. Further dryness was achieved by adding water (10 ml) and followed by rotary evaporation. The procedure was repeated 3 times. The product was dissolved in water (15 ml) and dried by lyophilization to get Ad-DOTA as white solid (2.2 g, yield 100%). MALDI-TOF (m/z, [M]+): 537.9 (obsd), 537.3 (calcd).
2.3.7. Synthesis of 2,2′,2″-(10-(2-(1-Adamantylamino)-2-Oxoethyl)-1,4,7,10-Tetraazacyclo-Dodecane-1,4,7-triyl)Triacetate Gadolinium(III) (Ad-(Gd-DOTA))
Ad-DOTA (2.0 g, 3.7 mmol) was dissolved in water (120 ml) and pH of the solution was adjusted to 6.5 by NaOH (3 M). Gd(OAc)3·4H2O (2.3 g, 7.6 mmol) in water (40 ml) was added in the solution. The mixture was heated to 50 °C and pH of the solution was adjusted to 6.5 every 3–10 h until pH didn’t decrease (typically 1–2 day). pH of the solution was then adjusted to 11 to precipitate out the excessive Gd(III). The precipitate (Gd(OH)3) was removed by centrifuge and the solution was dried by lyophilization. The crude product containing NaOAc was purified by preparative HPLC to give Ad-(Gd-DOTA) as white solid (1.5 g, yield 62%). MALDI-TOF (m/z, [M]+): 692.61 (obsd), 692.22 (calcd). ICP-OES (Gd3+ content): 22.56% (obsd), 22.73% (calcd). (Figure S4).
2.3.8. Synthesis of 4-((adamantan-1-yl)amino)-4-oxobutanoic acid (Ad-COOH)(30)
1-Adamantylamine (2.0 g, 13.2 mmol) and DIPEA (2.7 ml, 15.8 mmol) was dissolved in CH2Cl2 (30 ml) and stirred for 30 min. The solution was dropped into a solution of succinic anhydride (1.58 g, 15.8 mmol) in 40 ml CH2Cl2 and the mixture was stirred at RT overnight. The solvent was removed by rotary evaporator. The residue was dissolved in an aqueous solution of NaOH (30 ml, 1.0 M) and the solution was filtered. The pH of the filtrate was adjusted to 7.0 using 1.0 M HCl. The precipitate was collected, washed with deionized water and dried in vacuum overnight to get Ad-COOH as white solid (2.5 g, yield 75%). MALDI-TOF (m/z, [M]+): 251.50 (obsd), 251.15 (calcd); (m/z, [M + Na]+): 273.48 (obsd), 274.15 (calcd). (Figure S5)
2.3.9. Synthesis of pentafluorophenyl 4-((adamantan-1-yl)amino)-4-oxobutanonate (Ad-PFP)
Ad-COOH (2.0 g, 8.0 mmol) and pentafluorophenol (1.47 g, 8.0 mmol) was dissolved in dioxane (50 ml). DCC (2.0 g, 9.6 mmol) in dioxane (10 ml) was added to the previous solution and the mixture was stirred at RT overnight. The solution was filtered and the solvent was removed by rotary evaporator. The product was purified by recrystallization in hexanes to get Ad-PFP as white solid (3.0 g, 90%).1H NMR (400 MHz, CDCl3, δ): 5.20 (br, 1H, NHCO), 3.0 (t, 2H, CH2CH2CO), 2.53 (t, 3H, CH2CH2CO), 2.08 (m, 3H, CH(CH2)3), 1.99 (d, 6H, CHCH2C), 1.68 (t, 6H, CHCH2CH). 13C NMR (400 MHz, CDCl3, δ): 169.28 (s, 1C, NHCO), 169.21 (s, 1C, COO), 142.47–136.51 (weak m, 6C, pentafluorophenyl), 52.36 (s, 1C, (CH2)3CNH), 41.77 (s, 3C, CHCH2C), 36.46 (s, 3C, CHCH2CH), 31.70 (s, 1C,CH2CH2CO), 29.60 (s, 3C, CH(CH2)3), 28.97 (s, 1C, CH2CH2CO). MALDI-TOF (m/z, [M]+): 417.629 (obsd), 417.14 (calcd); (m/z, [M + Na]+): 439.617 (obsd), 440.14 (calcd). (Figure S6–S8).
2.3.10. Synthesis of Ad-PEG-cRGD or Ad-PEG-cRAD
NH2-PEG-COOH (Mw = 5000, 0.5 g, 0.1 mmol) and Ad-PFP (62 mg, 0.15 mmol) was dissolved in CH2Cl2 (10 ml). DIPEA (35 μL, 0.2 mmol) was added to the solution and the mixture was stirred at RT overnight. The solution was poured into cold ethyl ether (150 ml) and the precipitate was collected and washed with cold ethyl ether. The product was dried in vacuum overnight to get Ad-PEG-COOH (0.48 g, yield 92%) as white solid.
Ad-PEG-COOH (0.4 g, 0.076 mmol) and pentafluorophenol (28 mg, 0.152 mmol) was dissolved in dioxane (10 ml). DCC (32 mg, 0.152 mmol) was added to the solution and the mixture was stirred at RT overnight. The solution was poured into cold ethyl ether (150 ml) and the precipitate was collected and washed with cold ethyl ether. The product was dried in vacuum overnight to get Ad-PEG-PFP (0.35 g, yield 85%) as white solid.
cRGDfK (50 mg, 0.083 mmol) and DIPEA (29 μL, 0.17 mmol) was dissolved in DMF (5 ml). To this solution, Ad-PEG-PFP (0.33 g, 0.062 mmol) was added and the solution was stirred at RT overnight. The solution was poured into cold ethyl ether (100 ml) and the precipitate was collected and washed with cold ethyl ether. The crude product was dried in vacuum overnight and then dissolved in water and purified by Centricon® filter with a 3000-molecular-weight cutoff (Amicon, Bedford, MA, no. YM-3). The solution was dried by lyophilization to get Ad-PEG-cRGD (0.21 g, yield 58%) as white solid. Ad-PEG-cRAD was synthesized similarly. (Figure S9)
2.3.11. Synthesis of adamantyl sulfo-cyanine5 (Ad-Cy5)
1-Adamantylamine (4 mg, 26 μmol) and sulfo-Cy5-NHS (2 mg, 2.6 μmol) were dissolved in anhydrous DMF (2 ml). The mixture was kept in dark and stirred at RT overnight. The solution was poured into cold ethyl ether (30 ml). The precipitate was collected by centrifuge and washed with ethyl ether. The product was dried in vacuum to get Ad-Cy5 as dark blue solid. MALDI-TOF (m/z, [M+K]+): 836.97 (obsd), 836.28 (calcd). (Figure S10).
2.3.12. Self-assembly of POSS-βCD and Ad-(Gd-DOTA)
POSS-βCD (12 mg) and Ad-(Gd-DOTA) (5.5 mg, 8 μmol) were dissolved in 2 ml deionized water and the mixture was stirred at RT for 30 min. The solution (4 mM Gd3+) and its serial dilutions (2, 1, 0.5 and 0.25 mM Gd3+) were directly used for relaxivity measurement. The Gd3+ content of each sample was confirmed by ICP-OES after the MR measurement. The self-assembled βCD and Ad-(Gd-DOTA) was prepared similarly.
2.3.13. Self-assembly of POSS-βCD with Ad-(Gd-DOTA), Ad-PEG-cRGD and Ad-Cy5
POSS-βCDn (12 mg, average Mn=9976 Da, n=7.2, 1.2 μmol) and Ad-PEG-cRGD (10 mg, Mn=5800 Da, 1.72 μmol) were mixed in 2 ml deionized water and stirred at RT for 30 min. To this solution, Ad-Cy5 (60 μg in 0.1 ml water, 0.072 μmol)) and Ad-(Gd-DOTA) (5.5 mg, 8 μmol) were added and the mixture was stirred for another 30 min. The solution was filtered through a Centricon filter with 3000-molecular-weight cutoff at 2600 g for 1 h. The Gd3+ content of the residue was confirmed by ICP-OES and the Cy5 content was measured by UV. The control agent with cRAD or PEG-only was prepared similarly.
2.4. Cells and animals
The 4T1-GFP-Luc2 (metastatic murine mammary carcinoma, catalog number: 128090) breast cancer cell line were purchased from Caliper Life Sciences (Hopkinton, MA) and have been authenticated. Cells were maintained as recommended by the provider. All the mice were obtained from Charles River and housed in the Animal Core Facility at Case Western Reserve University. All animal experiments were performed in accordance with the animal protocol approved by the CWRU Institutional Animal Care and Use Committee.
2.5. Western Blot
Cells were seeded onto 6-well plates (5000 cells/well) and allowed to adhere overnight. The cells were then incubated with or without TGF-β (10 ng ml−1) for 5 days at 37 °C and 5% CO2. The detergent-solubilized whole-cell extracts (WCE) were prepared by lysing the cells in cell extraction buffer (FNN0011, Invitrogen). The supernatants were collected, and the protein concentration was determined by protein assay (Bio-Rad). SDS-PAGE and western blotting were performed using 30 μg proteins. The blots were incubated with a rabbit monoclonal anti-integrin αv antibody antibody (ab179475, Abcam) or anti-integrin β3 antibody (ab75872, Abcam); the anti-β-actin antibody was used for the loading control. The blots were washed and incubated with Rhodamine-Red-X conjugated goat anti-rabbit IgG (H + L). Typoon scanner was used for processing the membrane blotted with Rhodamine-Red conjugated secondary antibody.
2.6. Cellular uptake studied by confocal fluorescence microscope
Cellular uptakes of sulfo-Cy5 labeled nanoglobules were observed using confocal fluorescence microscopy. 4T1 cells were pretreated with TGF-β (15 ng ml−1, 5 days) to induce up-regulation of αvβ3 integrin. Cells were incubated with nanoglobules (30 μg ml−1) at pH 7.4 for 4 h. Hoechst 33342 (Molecular Probes, Eugene, OR) (1 μg ml−1) was then added and cells were incubated for 5 min. Cells were then thoroughly washed with PBS at 4 °C for three times. Images were obtained using Olympus FV1000 confocal laser scanning microscope. Hoechst 33342 was observed using 405 nm laser and the emission wavelength was read from 430 to 470 nm and expressed as blue. Cy5 was observed using 635 nm laser and the emission wavelength was read from 655 to 755 nm and expressed as red. Images were produced using the lasers sequentially with a 40× objective lens.
2.7. Cellular uptake measured by flow cytometry
4T1 cells were pretreated with TGF-β (10 ng ml−1) for 5 days. Cells were seeded onto twelve-well plates (2.0 ml of cell suspension per well) at 2 × 105 cells ml−1 and allowed to grow for 24 h. Sulfo-Cy5 labeled nanoglobules were added to each well at pH 7.4 and incubated with cells for 4 h. Cells were then washed with cold PBS twice, harvested by 0.005% trypsin-EDTA, pelleted in Eppendorf tubes and centrifuged at 1000 g for 4 min at 4 °C, and then resuspended in PBS with 2% FBS and filtered through cell strainers. Each sample was analyzed on BD LSRII flow cytometer (BD Biosciences, San Jose, CA) using the 633 nm laser for excitation and the emitted 660/20 fluorescence for detection. Files were collected of 10,000 gated events and analyzed with FlowJo Vx software.
2.8. Animal model
Six-week-old femal BALB/c mice (Charles River) were anaesthetized and 4T1-GFP-Luc2 breast cancer cells (5 × 105 in 100 μl mixture of PBS and Matrigel) were injected into the flank of mice. The MRI study and fluorescent imaging were performed when the tumor size reached 0.5–1.0 cm in diameter in about 2 weeks.
2.9. Contrast enhanced MR tumor imaging
The MRI study was performed using a BrukerBiospec7T MRI scanner (Bruker Corp., Billerica, MA, USA) with a volume radio frequency (RF) coil. Mice were anesthetized with a 2% isoflurane-oxygen mixture in an isoflurane induction chamber. The tail vein of mouse was catheterized with a 30 gauge needle connected with 1.6m tubing filled with heparinized saline. The animal was then moved into the magnet and kept under inhalation anesthesia with 1.5% isoflurane-oxygen via a nose cone. A respiratory sensor connected to a monitoring system (SA Instruments, Stony Brook, NY) was placed on the back to monitor rate and depth of respiration. The body temperature was maintained at 37 °C by blowing hot air into the magnet through a feedback control system. A group of 3 mice was used for each agent. Sagittal section images were acquired with a localizing sequence to identify the tumor location, followed by a fat suppression three-dimensional (3D) FLASH sequence and a 2D T1-weighted gradient fat suppression sequence before injection. After pre-injection baseline MR imaging acquisition, the targeted agent, the non-targeted scrambled control or ProHance® was injected at a dose of 0.1 mmol of Gd kg−1 by flushing with 80 μl of saline. T1-weighted 3D FLASH images and 2D axial images were then acquired at different time points after the injection for up to 30 min. Parameters of 3D FLASH sequence were repetition time (TR) = 8.5 ms, echo time (TE) = 2.6 ms, in-plane field of view (FOV) = 10 cm, slice thickness = 25 mm, average = 3, flip angle = 30°. Parameters of the 2D T1-weighted gradient echo sequence were TR/TE = 151.2/1.9 ms, FOV = 3.0 cm, slice thickness = 1.2 mm, slice number = 12, average = 1, flip angle = 80°, matrix = 128 ×128. MR image analysis was performed using Paravision 4.0 software. Signal enhancement ratio (ER) in tumor, heart and kidneys at each time point were calculated using the equation ER = S post/Spre, Where Spre and Spost are the signal intensities calculated from tissues pre-injection and post-injection images, and averaged from three different mice (N=3).
2.10. Tumor fluorescence imaging
After MR imaging, the mice were sacrificed at 4 h post-injection. Tumors and major organs and tissues, including spleen, liver, lung, brain, muscle and heart were dissected, washed with PBS and imaged on the Maestro FLEX In Vivo imaging system. GFP fluorescence images were obtained using green light filters (excitation: 444–490 nm; emission: 515 nm long-pass filter; acquisition settings: 500–720 in 10 nm steps) and yellow light filters for Cy5 (excitation, 576–612 nm; emission: 635 nm long-pass filter, acquisition settings: 630–800 in 10 nm steps). The Cy5 signal was spectrally extracted from the multispectral fluorescence images with Maestro software (Cambridge Research & Instrumentation, Inc, Woburn, MA) after subtracting the background auto-fluorescence. Regions of interest (ROIs) were selected over the tumors.
2.11. Tumor immunofluorescence
Tumors from mice injected with Cy5 labeled targeting agent or non-targeting agent were embedded in optimal cutting temperature compound (O.C.T) and cryo-sectioned into 5-μm slices. For αv or β3 integrin immunostaining, tissues were stained with a rabbit monoclonal anti-integrin αv antibody antibody (ab179475, Abcam) or anti-integrin β3 antibody (ab75872, Abcam), followed by Rhodamine-Red-X conjugated goat anti-rabbit IgG (H + L) (Jackson Immuno Research Lab, West Grove, PA). Cell nucleus of the tissues were stained with 4′,6-diamidino-2-phenylindole (DAPI). Tissue slides were imaged on an Olympus FV1000 confocal laser scanning microscope. GFP was observed using 405 nm laser, the emission wavelength was read from 480–495nm and is expressed as green. DAPI was observed using 405 nm laser and the emission wavelength was read from 450–470 nm and is expressed as blue. RhodamineRed was observed using 543 nm laser, the emission wavelength was read from 560–620 nm and is expressed as yellow. Cy5 was observed using 635 nm laser, the emission wavelength was read from 655–755 nm and is expressed as red.
3. Results and discussions
3.1. Synthesis and characterization
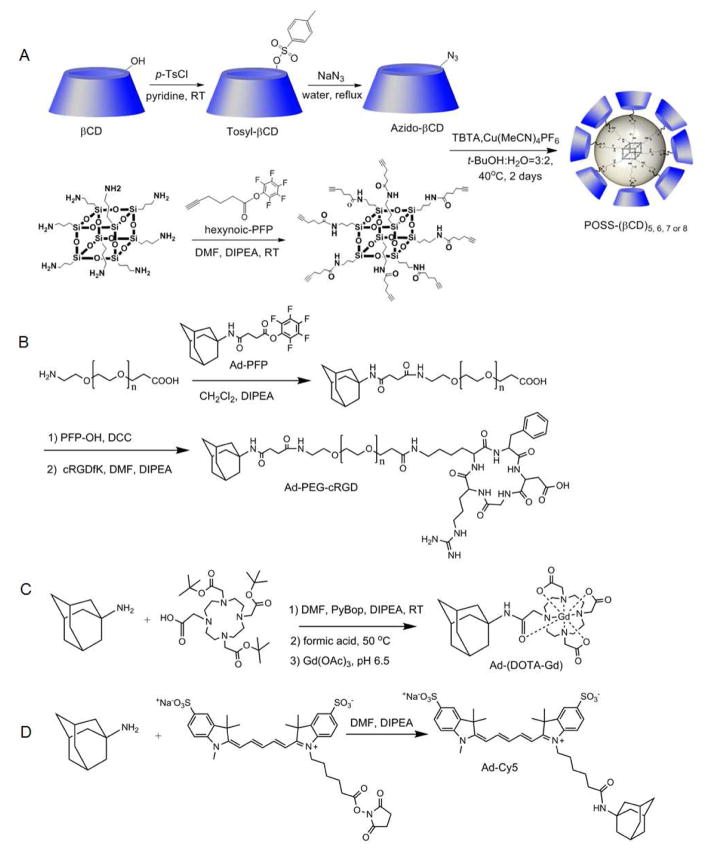
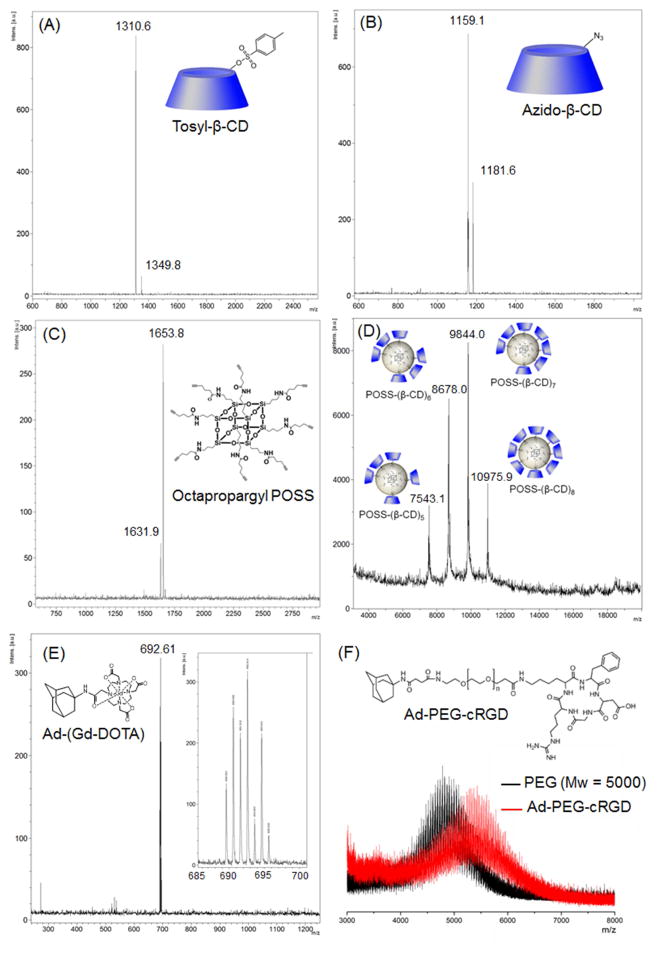
The targeted host-guest contrast agent cRGD-POSS-βCD-(DOTA-Gd)-Cy5 was designed to form by self-assembly of β-cyclodextrin (βCD) conjugated polyhedral silsesquioxane (POSS-βCD) and adamantane-functionalized imaging agents and a targeting agent via the host-guest interaction (Fig. 1). Among many hydrophobic molecules used as guests of βCD cavity, the spherical adamantyl group with a diameter of 7 Å fits inside the βCD cavity and form physical complexes with acceptable association constant (103–105 M−1).(31, 32) Thus, βCD-adamantane complexes have been used in various supramolecular systems for biomedical applications.(33–35) The synthesis of the intermediates for the targeted host-guest imaging agent is shown in Fig. 2. The chemicals were characterized by 1H NMR, 13C NMR, MALDI-TOF mass and HPLC spectra (Fig. 3, Figure S1–S10). The oct(3-aminopropyl)silsesquioxane core (POSS) has a size around 1 nm in diameter with eight functional groups at each corner.(22) POSS-βCD was synthesized by click chemistry between the alkyne-functionalized POSS (Fig. 2A and 3C, Figure S1 and S2) and azide-attached βCD (Fig. 2A and 3). POSS was coupled with 5 to 8 βCD as determined by MALDI-TOF mass spectrum (Fig. 3D). Due to the steric effect, not all the eight branches of POSS were conjugated with βCD. The average molecular weight of POSS-βCD is determined as 9976 Da with around 7.2 βCD per POSS molecule, calculated from its 1H NMR spectrum from the integration ratio of the peaks at 5.80–5.60 ppm (-OCHO-, βCD) and 0.64 ppm (-SiCH2-, POSS). Cyclic RGD as a tumor targeting agent was attached to adamantine group through a PEG spacer with molecular weight of 5000 (Fig. 2B). Ad-PEG-cRGD was designed to incorporate the targeting probe on the POSS surface. Mass spectrometry revealed that the average molecular weight increase from PEG to Ad-PEG-cRGD was about 700 Da, indicating the successful attachment of cRGD and Ad to PEG (Fig. 3F and Figure S9). The PEG spacer was introduced between cRGD and POSS core to prepare the targeting system without significantly diminished binding affinity of the cRGD to αvβ3 integrin on cancer cells.(27, 36) Macrocyclic chelates, 4,7,10-tris(carboxymethyl)-1,4,7,10- tetraazacyclododecyl gadolinium (DOTA-Gd), with high thermodynamic and kinetic stability was chosen as MRI contrast agent (Fig. 2C and 3E, Figure S3 and S4). DOTA and adamantane were coupled through an acetamide bond. Water soluble sulfo-Cy5 was used as a fluorescence probe and attached to an adamantyl group. An excess of adamantylamine was used to react with N-hydroxysuccinimide active ester of sulfo-Cy5 to give Ad-Cy5 (Fig. 2D and Figure S10). The targeted dual imaging agent, cRGD-POSS-βCD-(DOTA-Gd)-Cy5, was obtained by the self-assembly of Ad-PEG-cRGD, Ad-(DOTA-Gd), Ad-Cy5, and POSS-βCD through host-guest interaction at a designated ratio. The agent contains around 5.7 molecules of Gd3+, 1.4 molecules of cRGD, and 0.05 molecule of Cy5 per molecule of cRGD-POSS-βCD-(DOTA-Gd)-Cy5 on average, as determined by ICP-OES and UV spectrum. An untargeted dual imaging agent, cRAD-POSS-βCD-(DOTA-Gd)-Cy5, was synthesized with similar content of Gd-DOTA, cRAD and Cy5.
Figure 1.
A targeted host-guest MRI contrast agent, cRGD-POSS-βCD-(DOTA-Gd)-Cy5, self-assembled from POSS-βCD, Ad-PEG-cRGD, Ad-(DOTA-Gd) and Ad-Cy5 by host-guest interaction. This agent can preferentially bind to αvβ3 receptors on tumor vascular endothelial cells and as well as the malignant breast cancer cells, and produce strong MR signal enhancement in tumor tissue.
Figure 2.
The synthesis of POSS-βCD (A), Ad-PEG-cRGD (B), Ad-(DOTA-Gd) (C) and Ad-Cy5 (D).
Figure 3.
MALDI-TOF mass spectra of Tosyl-βCD (A), Azido-βCD (B), Octapropargyl POSS (C), POSS-βCD, Ad-(Gd-DOTA) (E), PEG5000 and Ad-PEG5000-cRGD (F).
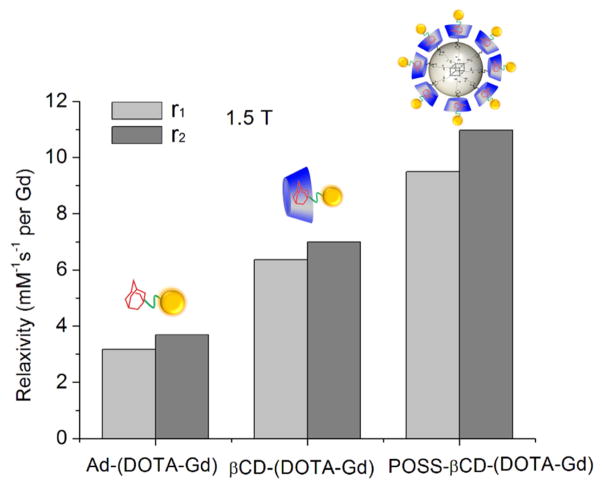
A host-guest POSS MRI contrast agent without the targeting agent, POSS-βCD-(DOTA-Gd), was synthesized by mixing the aqueous solutions of POSS-βCD and Ad-(DOTA-Gd). Ad-(DOTA-Gd) has higher solubility in water than POSS-βCD. The solution of POSS-βCD (6 mg ml−1) changed from cloudy to clear after the addition of Ad-(DOTA-Gd), which indicated the self-assembly of the host and guest molecules. The r1 and r2 relaxivity values of Ad-(DOTA-Gd), βCD-(DOTA-Gd) and POSS-βCD-(DOTA-Gd) at 1.5 T are shown in Fig. 4. The r1 relaxivity of Ad-(DOTA-Gd) was 3.17 mM−1s−1, measured at 1.5 T and 37 °C, comparable with reported values for clinical small molecular contrast agents.(37) The r1 relaxivity increased to 6.36 mM−1s−1 after its complexation with βCD to form βCD-(DOTA-Gd). The r1 value further increased to 9.50 mM−1s−1 when Ad-(DOTA-Gd) was complexed with POSS-βCD to form POSS-βCD-(DOTA-Gd). Comparing with POSS nanoglobular dendrimers POSS-G2-(DOTA-Gd) with covalently attached DOTA-Gd, (13) which has r1 relaxivity of 7.92 mM−1s−1 measured at 3T, POSS-βCD-(DOTA-Gd) with DOTA-Gd loaded by host-guest interaction showed comparable r1 relaxivity. Covalent binding of small molecular Gd(III) chelates to macromolecules slows down the rotational motion of the complexes, thus increases relaxivities.(3) It can be concluded from this study that the non-covalent binding of small molecular Gd(III) chelates to macromolecules or nanoparticules via host-guest interaction will also increase relaxivity. This result is consistent with the previous report that the non-covalent interaction of Ad-(DOTA-Gd) with the cyclodextrin hosts increases relaxivity and their high binding affinity has been approved.(10) The r1 relaxivity of βCD-(DOTA-Gd) and POSS-βCD-(DOTA-Gd) was two and three times of Ad-(DOTA-Gd), respectively, which indicate the relaxivity increasing rate was size dependent. The addition of the targeting probe Ad-PEG-cRGD and fluorescent probe Ad-Cy5 to POSS-βCD-(DOTA-Gd) has little effect on their relaxivity. cRGD-POSS-βCD-(DOTA-Gd)-Cy5 had similar relaxivity as POSS-βCD-(DOTA-Gd). The results demonstrate that admantane-functionalized small molecular contrast agent, Ad-(DOTA-Gd), forms host-guest complexes with βCD and POSS-βCD. The nanosized host-guest contrast agents with high relaxivity would provide high contrast enhancement for effective molecular imaging at same dose in target tissue.
Figure 4.
Longitudinal (r1) and transverse (r2) relaxivities of Ad-(DOTA-Gd), βCD-(DOTA-Gd) and POSS-βCD-(DOTA-Gd) in PBS, measured at 1.5 T and 37°C.
3.2. Binding of the targeted agent to αvβ3 integrin in 4T1 cancer cells
Integrin αvβ3 is overexpressed in tumor vascular endothelial cells as well as malignant breast cancer and particularly pronounced in mesenchymal cancer cells, which plays an important role in tumor angiogenesis, progression, and metastasis.(38–40) TGF-β promotes epithelial mesenchymal transition (EMT) of cancer cells and cancer metastasis through its effects on tumor microenvironment.(40–43) The treatment of 4T1 breast cancer cells with TGF-β resulted in a fibroblast-like mesenchymal phenotype (Fig. 5A), which is a phenomenon in the initiation of invasion and metastases of high-risk breast cancer.(5) The treatment of 4T1 cells with TGF-β was accompanied by increased expression of αvβ3 integrin as determined by western blot (Fig. 5B) as compared with untreated 4T1 cells and U87 and MCF-7 control cells.
Figure 5.
Binding of Cy5 labeled cRGD-POSS-βCD-(Gd-DOTA) to TGF-β pre-treated 4T1 cancer cells overexpressing αvβ3 integrin. (A) The morphology of 4T1 cells with and without TGF-β treatment (15 ng ml−1, 5 days). Images were taken by phase-contrast microscopy. (B) Western blots showing αvβ3 expressing in TGF-β treated 4T1 cells, untreated 4T1, and control MCF-7 and U87 cells. (C) Cellular uptake measured by flow cytometry after 4T1 cells were treated 30 μg ml−1 of cRGD-POSS-βCD-(Gd-DOTA)-Cy5 (a), cRGD-POSS-βCD-(Gd-DOTA)-Cy5 co-cultured with free cRGD (b), cRAD-POSS-βCD-(Gd-DOTA)-Cy5 (c), PEG-modified POSS-βCD-(Gd-DOTA)-Cy5 (d), and PBS (e) for 4 h. (D) Confocal fluorescence images of 4T1 cells treated with cRGD-POSS-βCD-(Gd-DOTA)-Cy5 (a), cRGD-POSS-βCD-(Gd-DOTA)-Cy5 co-cultured with free cRGD (b) and cRAD-POSS-βCD-(Gd-DOTA)-Cy5 (c) at 30 μg ml−1 for 4 h, cell nucleus were stained by Hoechst 33342 and expressed as blue. (All scale bar = 100 μm).
The cellular uptake of cRGD-POSS-βCD-(Gd-DOTA)-Cy5 in TGF-β pre-treated 4T1 cancer cells was studied by flow cytometry. As shown in Fig. 5C, cells cultured with cRGD-POSS-βCD-(Gd-DOTA)-Cy5 showed much higher fluorescent intensity than those cultured with cRAD-POSS-βCD-(Gd-DOTA)-Cy5 and PEG-modified POSS-βCD-(Gd-DOTA)-Cy5. The cells cultured with cRGD-POSS-βCD-(Gd-DOTA)-Cy5 had a fluorescent intensity of 2.5 times higher than that of the cells cultured with the non-targeted controls. The addition of excessive free cRGD blocked the αvβ3 integrin binding site and greatly reduced the uptake of cRGD-POSS-βCD-(Gd-DOTA)-Cy5. There is no difference in fluorescence intensity for the cells cultured with cRAD-POSS-βCD-(Gd-DOTA)-Cy5 and PEG-modified POSS-βCD-(Gd-DOTA)-Cy5. The cellular uptake of the different agents to the 4T1 cells was further investigated by confocal laser scanning microscopy (Fig. 5D). cRGD-POSS-βCD-(Gd-DOTA)-Cy5 showed significantly higher binding to 4T1 cells than cRAD-POSS-βCD-(Gd-DOTA)-Cy5. The fluorescence images also revealed that cRGD-POSS-βCD-(Gd-DOTA)-Cy5 was localized in the cell cytoplasm rather than the nuclei, which was consistent with the reported intracellular distribution of nanoparticle by integrin-mediated endocytosis.(44, 45) The uptake of cRGD-POSS-βCD-(Gd-DOTA)-Cy5 greatly decreased in the presence of excessive free cRGD. These studies demonstrated the enhanced cellular uptake of cRGD-POSS-βCD-(Gd-DOTA)-Cy5 by receptor mediated endocytosis.
3.3. In vivo MR tumor molecular imaging
In vivo MRI experiments were carried out in mice bearing 4T1-GFP-Luc2 flank tumors (Fig. 6A). Fat-suppressed T1-weighted 2D spin echo MR images of the tumor enhanced with cRGD-POSS-βCD-(DOTA-Gd)-Cy5, a non-targetd control agent cRAD-POSS-βCD-(DOTA-Gd)-Cy5, and a clinical control Gd(HP-DO3A) (ProHance®) are shown in Fig. 6B and C. The The targeted contrast agent, cRGD-POSS-βCD-(DOTA-Gd)-Cy5, resulted in stronger contrast enhancement than both controls, especially in the tumor periphery. It was noticed that the contrast enhancement in the tumor periphery with the targeted agent was much higher than that in the tumor interior, because the tumor periphery was rich of angiogenic vasculatures where αvβ3 integrin were highly expressed on endothelial cells. Quantitative analysis of the signal enhancement ratio (ER) in the tumor before and after the injection of contrast agents were measured and plotted in Fig. 6D. The targeted nanoglobular contrast agent produced strong and prolonged contrast enhancement in the tumor in comparison with the control agents due to high relaxivity and specific target binding in tumor. The ER of targeted agent gradually increased to approximately 1.5-fold during the first 10 min and slowly dropped to about 1.3-fold in 30 min, whereas the nontargeted agent generated about 1.3-fold ER at first 5 min and dropped to around 1.2-fold in 30 min. ProHance™ produced lower ER in tumor than that of the nanoglobular contrast agents because of its nonspecific binding and low relaxivity. The targeted agent cRGD-POSS-βCD-(DOTA-Gd)-Cy5 produced maximum signal enhancement in the tumor at the first 10 min post-injection and then the signal gradually decreased over time, suggesting that Ad-(DOTA-Gd) could gradually dissociate from the cyclodextrin hosts and clear from the target sites. Quantitative analysis of MR signal in the blood of the heart revealed approximately 2.5 and 2.4-fold of increased enhancement ratio (ER) at 2 min post-injection of targeted and non-targeted nanoglobular contrast agent, respectively, whereas the ER of the ProHance™ control was about 1.9-fold after 2 min and dropped to background levels within 30 min (Fig. 6E). The difference of the enhancement ratios in the blood among the nanoglobular agents was not significant. The nanoglobular contrast agents produced stronger and more prolonged signal enhancement in heart than that of small molecular contrast agent ProHance™ because of their greater relaxivity and larger size. Furthermore, ER observed in the kidney for the targeted contrast agents gradually decreased from 2.2–2.3 to 1.7–1.8, while ProHance™ dropped rapidly from 2.0 to 1.3 (Fig. 6F). It can be concluded that the host-guest targeted contrast agents can be excluded from the body by the renal filtration, which may partly attribute to the releasing of small molecular Ad-(DOTA-Gd) loaded by the reversible host-guest interaction. Rapid excretion of Gd(III) based contrast agents after the diagnostic imaging is essential to minimize potential toxic side effects and thus critical for clinical applications.(3)
Figure 6.
MR molecular imaging with cRGD-POSS-βCD-(DOTA-Gd)-Cy5 in mice bearing 4T1-Luc2-GFP tumor xenografts. (A) Illustration of experimental procedure of MRI, ex vivo fluorescent imaging, and histological analysis in mice bearing 4T1-Luc2-GFP breast tumor. (B) Representative 2D axial T1-weighted spin-echo MR images before (pre) and at 5, 15, 30 min post-injection of ProHance® (a), cRAD-POSS-βCD-(DOTA-Gd)-Cy5 (b), and cRGD-POSS-βCD-(DOTA-Gd)-Cy5 (c), at 0.1 mmol-Gd/kg. The arrows point to the tumor. (C) Representative color coded maps of MR images at 5 min administration of each agent. Signal enhancement ratio (ER) of tumor (D), heart (E) and kidney (F) before and at different time points after administration of each agent. (N=3).
It is generally challenging to image biomarkers expressed on cancer cell surface with contrast enhanced MRI due to their low concentration of the biomarkers and low sensitivity of MRI. Although conjugation of Gd(III) chelates to nanosized carriers can deliver a large amount of Gd(III) contrast agents to the biomarkers to generate sufficient signal enhancement for detection, safety concerns associated with prolonged tissue retention hinder the translational development of such targeted contrast agents. Comparing with traditional nanosized contrast agents, the host-guest nanostructure in cRGD-POSS-βCD-(DOTA-Gd)-Cy5 allows facile loading of multiple Gd(III) chelates by self-assembly to increase the local concentration of the contrast agent, producing detectable signal enhancement for molecular imaging of αvβ3 integrin on breast cancer cells. The reversible interactions of the host molecules and the nanosized guest carrier can facilitate the clearance of Gd(III) based contrast agent after the diagnostic imaging and dissociation of the contrast agents, and alleviate potential toxic side effects.(21, 33, 46) The host-guest nanostructure also has the versatility to load different imaging probes for multimodal molecular imaging.
The effectiveness of molecular MRI with targeted nanosized host-guest MRI contrast agents can be improved by increasing the loading of the contrast agent in the design of next generation of the targeted host-guest contrast agents. We have recently synthesized nanoglobules with well-defined structures and high surface functionalities based on the POSS core.(22, 47) The nanoglobules have been used in the synthesis of nanosized targeted contrast agents for molecular imaging.(13, 14, 22) High surface functionality of the nanoglobules will also increase the load of contrast agents in targeted nanosized host-guest MRI contrast agents to further improve the sensitivity for MR molecular imaging of the biomarkers expressed on cancer cell surface. High functionalities of the nanoglobular host-guest structures will further expand versatility for both diagnostic and therapeutic applications. Molecular probes for other imaging modality and therapeutics can also loaded in the highly functionalized nanoglobular host-guest structures for multi-modal imaging and targeted drug delivery. Further studies will focus on the optimization of the structures of the targeted nanosized host-guest MRI contrast agents and comprehensive assessment of their tumor targeting efficiency, pharmacokinetics, biodistribution, and safety.
3.4. Ex vivo fluorescent imaging
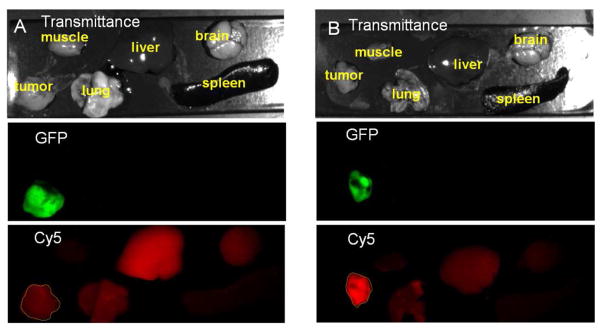
Binding of the cRGD targeted host-guest imaging agent was further confirmed by fluorescence imaging. Fig. 7 shows the GFP and Cy5 fluorescence images of the tumor and major organs acquired 4 h after the injection of cRGD-POSS-βCD-(DOTA-Gd)-Cy5. 4T1-Luc2-GFP tumor tissue was labeled with GFP and expressed as green. Substantial Cy5 red fluorescence was detected in the breast tumor of mice injected with cRGD-POSS-βCD-(DOTA-Gd)-Cy5, whereas little red fluorescence was found in the tumor from the mice injected with cRAD-POSS-βCD-(DOTA-Gd)-Cy5. No significant fluorescence was detected in healthy organs and tissues except the kidney for the targeted agent.
Figure 7.
Ex vivo fluorescence imaging showing specific binding of cRGD-POSS-βCD-(DOTA-Gd)-Cy5 to the tumor tissue. After MRI, the mice bearing 4T1-GFP-Luc2 tumor xenografts were sacrificed at 4 h post-injection and the tumor, muscle, lung, liver, spleen, and brain were collected and imaged with the Maestro FLEX In Vivo Imaging System. Transmittance, GFP, and Cy5 images of the tumor and other tissues with cRAD-POSS-βCD-(DOTA-Gd)-Cy5 (A) and cRGD-POSS-βCD-(DOTA-Gd)-Cy5 (B) at dose of 0.1 mmol-Gd/kg and 0.88 μmol-Cy5/kg are shown.
3.5. Histological analysis
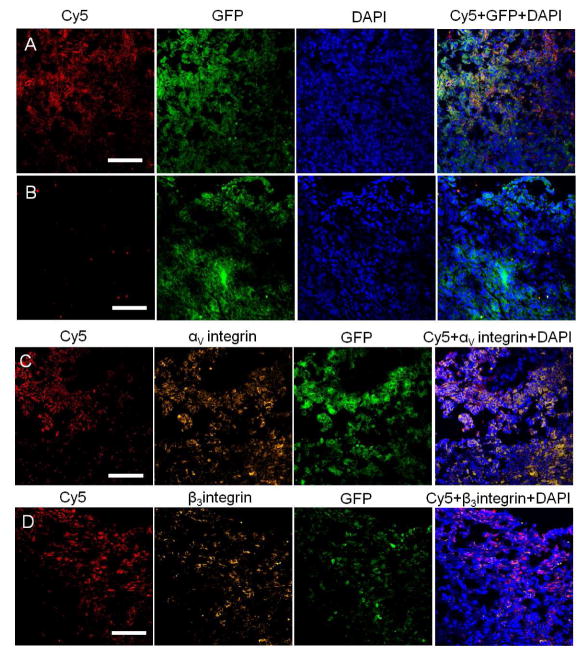
Tumor specific targeting ability of cRGD-POSS-βCD-(DOTA-Gd)-Cy5 was further demonstrated by high-resolution fluorescence imaging of tumor sections (Fig. 8 A and B). Significant Cy5 fluorescence was observed in the tumor tissues from the mice injected with targeted agent, while little Cy5 fluorescence was shown in the tumor tissues with the non-targeted control agent. The Cy5 fluorescence intensity in tumors from the mice injected with the cRGD targeted agent was 2.62 times of that in the tumor injected with the cRAD-loaded agent. Immunostaining of tumor sections with the antibodies against αv and β3 integrins showed abundant αvβ3 integrin in the tumor tissue. Cy5 fluorescence from cRGD-POSS-βCD-(DOTA-Gd)-Cy5 colocalized with αv or β3 integrin immunostaining (Fig. 8 C and D). These results confirmed that the cRGD targeted host-guest contrast agent cRGD-POSS-βCD-(DOTA-Gd)-Cy5 specifically bound to the αvβ3 integrin in malignant breast cancer. Target specific binding of cRGD-POSS-βCD-(DOTA-Gd)-Cy5 in the tumor produced significant MR signal enhancement for high-resolution MR molecular imaging.
Figure 8.
Fluorescent imaging of 4T1-luc2-GFP tumor histological sections. Frozen section of 4T1-luc2-GFP breast tumor from mice injected with cRGD-POSS-βCD-(DOTA-Gd)-Cy5 (A) or cRAD-POSS-βCD-(DOTA-Gd)-Cy5 (B). Frozen sections of 4T1-luc2-GFP breast tumor from mice injected cRGD-POSS-βCD-(DOTA-Gd)-Cy5 was stained for αv (C) or β3 (D) integrin. The slides were imaged by confocal microscopy. (All scale bar = 100 μm).
4. Conclusions
We developed a nanosized targeted host-guest contrast agent cRGD-POSS-βCD-(DOTA-Gd)-Cy5 for cancer molecular imaging of αvβ3 integrin in tumor vascular endothelial cells and as well as on various tumor cells. The contrast agent was formed by host-guest interactions between the adamantine modified imaging agents and targeting agent and β-cyclodextrin. cRGD-POSS-βCD-(DOTA-Gd)-Cy5 resulted in strong and prolonged tumor contrast enhancement. The combination of MRI, fluorescent imaging and histological analysis demonstrated that cRGD-POSS-βCD-(DOTA-Gd)-Cy5 could effectively and specifically target to αvβ3 integrin overexpressed in tumors. This agent has a potential for accurate detection and localization of breast cancer.
Supplementary Material
Acknowledgments
This research was supported in part by the National Institute of Health grant R01 EB00489.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weissleder R. Molecular imaging in cancer. Science. 2006;312:1168–1171. doi: 10.1126/science.1125949. [DOI] [PubMed] [Google Scholar]
- 2.Mishra A, Verma M. Cancer biomarkers: Are we ready for the prime time? Cancers. 2010;2:190–208. doi: 10.3390/cancers2010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Z, Lu ZR. Gadolinium-based contrast agents for magnetic resonance cancer imaging. Wires Nanomed Nanobi. 2013;5:1–18. doi: 10.1002/wnan.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chem Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Z, Qutaish M, Han Z, Schur RM, Liu Y, Wilson DL, Lu ZR. MRI detection of breast cancer micrometastases with a fibronectin-targeting contrast agent. Nat Commun. 2015;6:7984. doi: 10.1038/ncomms8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Z, Wu X, Kresak A, Griswold M, Lu ZR. Peptide targeted tripod macrocyclic Gd(III) chelates for cancer molecular MRI. Biomaterials. 2013;34:7683–7693. doi: 10.1016/j.biomaterials.2013.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim KS, Park W, Hu J, Bae YH, Na K. A cancer-recognizable MRI contrast agents using pH-responsive polymeric micelle. Biomaterials. 2014;35:337–43. doi: 10.1016/j.biomaterials.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Chan MH, Lin HM. Preparation and identification of multifunctional mesoporous silica nanoparticles for in vitro and in vivo dual-mode imaging, theranostics, and targeted tracking. Biomaterials. 2015;46:149–58. doi: 10.1016/j.biomaterials.2014.12.034. [DOI] [PubMed] [Google Scholar]
- 9.Nicholls FJ, Rotz MW, Ghuman H, MacRenaris KW, Meade TJ, Modo M. DNA-gadolinium-gold nanoparticles for in vivo T1 MR imaging of transplanted human neural stem cells. Biomaterials. 2016;77:291–306. doi: 10.1016/j.biomaterials.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Battistini E, Gianolio E, Gref R, Couvreur P, Fuzerova S, Othman M, Aime S, Badet B, Durand P. High-relaxivity magnetic resonance imaging (MRI) contrast agent based on supramolecular assembly between a gadolinium chelate, a modified dextran, and poly-β-cyclodextrin. Chem Eur J. 2008;14:4551–4561. doi: 10.1002/chem.200701587. [DOI] [PubMed] [Google Scholar]
- 11.Bruckman MA, Hern S, Jiang K, Flask CA, Yu X, Steinmetz NF. Tobacco mosaic virus rods and spheres as supramolecular high-relaxivity MRI contrast agents. J Mater Chem B. 2013;1:1482–1490. doi: 10.1039/C3TB00461A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim KS, Park W, Na K. Gadolinium-chelate nanoparticle entrapped human mesenchymal stem cell via photochemical internalization for cancer diagnosis. Biomaterials. 2015;36:90–97. doi: 10.1016/j.biomaterials.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Tan M, Wu X, Jeong EK, Chen Q, Lu ZR. Peptide-targeted nanoglobular Gd-DOTA monoamide conjugates for magnetic resonance cancer molecular imaging. Biomacromolecules. 2010;11:754–761. doi: 10.1021/bm901352v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan M, Ye Z, Lindner D, Brady-Kalnay SM, Lu ZR. Synthesis and evaluation of a targeted nanoglobular dual-modal imaging agent for MR imaging and image-guided surgery of prostate cancer. Pharm Res. 2014;31:1469–1476. doi: 10.1007/s11095-013-1008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Zhang N. Gadolinium loaded nanoparticles in theranostic magnetic resonance imaging. Biomaterials. 2012;33:5363–75. doi: 10.1016/j.biomaterials.2012.03.084. [DOI] [PubMed] [Google Scholar]
- 16.Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: Past, present and future. Nat Rev Drug Discov. 2004;3:1023–1035. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Z, Mondjinou Y, Hyun SH, Kulkarni A, Lu ZR, Thompson DH. Gd3+-1,4,7,10-tetraazacyclododecane-1,4,7-triacetic-2-hydroxypropyl-β-cyclodextrin/pluronic polyrotaxane as a long circulating high relaxivity MRI contrast agent. ACS Appl Mater Inter. 2015;7:22272–22276. doi: 10.1021/acsami.5b05393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Q, Zhang L, Liu M, Zhang X, Zhang X, Xu X, Chen S, Li X, Zhang J. Reversion of multidrug resistance by a pH-responsive cyclodextrin-derived nanomedicine in drug resistant cancer cells. Biomaterials. 2015;67:169–82. doi: 10.1016/j.biomaterials.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 19.Chen WH, Luo GF, Lei Q, Cao FY, Fan JX, Qiu WX, Jia HZ, Hong S, Fang F, Zeng X, Zhuo RX, Zhang XZ. Rational design of multifunctional magnetic mesoporous silica nanoparticle for tumor-targeted magnetic resonance imaging and precise therapy. Biomaterials. 2016;76:87–101. doi: 10.1016/j.biomaterials.2015.10.053. [DOI] [PubMed] [Google Scholar]
- 20.Hu J, Liu S. Engineering responsive polymer building blocks with host-guest molecular recognition for functional applications. Acc Chem Res. 2014;47:2084–2095. doi: 10.1021/ar5001007. [DOI] [PubMed] [Google Scholar]
- 21.Brewster ME, Loftsson T. Cyclodextrins as pharmaceutical solubilizers. Adv Drug Deliv Rev. 2007;59:645–666. doi: 10.1016/j.addr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Z, Lu ZR. Dendritic nanoglobules with polyhedral oligomeric silsesquioxane core and their biomedical applications. Nanomedicine-Uk. 2014;9:2387–2401. doi: 10.2217/nnm.14.133. [DOI] [PubMed] [Google Scholar]
- 23.Meyer T, Marshall JF, Hart IR. Expression of αv integrins and vitronectin receptor identity in breast cancer cells. Br J Cancer. 1998;77:530–536. doi: 10.1038/bjc.1998.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu X, Guan X, Li J, Pei Q, Liu M, Xie Z, Jing X. Hybrid polymer micelles capable of cRGD targeting and pH-triggered surface charge conversion for tumor selective accumulation and promoted uptake. Chem Commun. 2014;50:9188–9191. doi: 10.1039/c4cc04056b. [DOI] [PubMed] [Google Scholar]
- 25.Ke TY, Jeong EK, Wang XL, Feng Y, Parker DL, Lu ZR. RGD targeted poly(L-glutamic acid)-cystamine-(Gd-DO3A) conjugate for detecting angiogenesis biomarker αvβ3 integrin with MR T1 mapping. Int J Nanomed. 2007;2:191–199. [PMC free article] [PubMed] [Google Scholar]
- 26.Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KCP. Detection of tumor angiogenesis in vivo by αvβ3-targeted magnetic resonance imaging. Nat Med. 1998;4:623–626. doi: 10.1038/nm0598-623. [DOI] [PubMed] [Google Scholar]
- 27.Chen XY, Park R, Shahinian AH, Bading JR, Conti PS. Pharmacokinetics and tumor retention of I-125-labeled RGD peptide are improved by pegylation. Nucl Med Biol. 2004;31:11–19. doi: 10.1016/j.nucmedbio.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Mandal D, Shirazi AN, Parang K. Cell-penetrating homochiral cyclic peptides as nuclear-targeting molecular transporters. Angew Chem Int Ed. 2011;50:9633–9637. doi: 10.1002/anie.201102572. [DOI] [PubMed] [Google Scholar]
- 29.Petter RC, Salek JS, Sikorski CT, Kumaravel G, Lin FT. Cooperative binding by aggregated mono-6-(alkylamino)-β-cyclodextrins. J Am Chem Soc. 1990;112:3860–3868. [Google Scholar]
- 30.Wang K, Liu Y, Li C, Cheng SX, Zhuo RX, Zhang XZ. Cyclodextrin-responsive micelles based on poly(ethylene glycol)-polypeptide hybrid copolymers as drug carriers. ACS Macro Lett. 2013;2:201–205. doi: 10.1021/mz300568b. [DOI] [PubMed] [Google Scholar]
- 31.Granadero D, Bordello J, Perez-Alvite MJ, Novo M, Al-Soufi W. Host-guest complexation studied by fluorescence correlation spectroscopy: Adamantane-cyclodextrin inclusion. Int J Mol Sci. 2010;11:173–188. doi: 10.3390/ijms11010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paolino M, Ennen F, Lamponi S, Cernescu M, Voit B, Cappelli A, Appelhans D, Komber H. Cyclodextrin-adamantane host-guest interactions on the surface of biocompatible adamantyl-modified glycodendrimers. Macromolecules. 2013;46:3215–3227. [Google Scholar]
- 33.Hu QD, Tang GP, Chu PK. Cyclodextrin-based host-guest supramolecular nanoparticles for delivery: From design to applications. Acc Chem Res. 2014;47:2017–2025. doi: 10.1021/ar500055s. [DOI] [PubMed] [Google Scholar]
- 34.Lee JH, Chen KJ, Noh SH, Garcia MA, Wang H, Lin WY, Jeong H, Kong BJ, Stout DB, Cheon J, Tseng HR. On-demand drug release system for in vivo cancer treatment through self-assembled magnetic nanoparticles. Angew Chem Int Ed. 2013;52:4384–4388. doi: 10.1002/anie.201207721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perl A, Gomez-Casado A, Thompson D, Dam HH, Jonkheijm P, Reinhoudt DN, Huskens J. Gradient-driven motion of multivalent ligand molecules along a surface functionalized with multiple receptors. Nat Chem. 2011;3:317–322. doi: 10.1038/nchem.1005. [DOI] [PubMed] [Google Scholar]
- 36.Liu S. Radiolabeled cyclic RGD peptides as integrin αvβ3-targeted radiotracers: Maximizing binding affinity via bivalency. Bioconjugate Chem. 2009;20:2199–2213. doi: 10.1021/bc900167c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005;40:715–724. doi: 10.1097/01.rli.0000184756.66360.d3. [DOI] [PubMed] [Google Scholar]
- 38.Knowles LM, Gurski LA, Engel C, Gnarra JR, Maranchie JK, Pilch J. Integrin αvβ3 and fibronectin upregulate slug in cancer cells to promote clot invasion and metastasis. Cancer Res. 2013;73:6175–6184. doi: 10.1158/0008-5472.CAN-13-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brooks PC, Clark RAF, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 40.Mamuya FA, Duncan MK. αv Integrins and TGF-ss-induced EMT: A circle of regulation. J Cell Mol Med. 2012;16:445–455. doi: 10.1111/j.1582-4934.2011.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scaffidi AK, Petrovic N, Moodley YP, Fogel-Petrovic M, Kroeger KM, Seeber RM, Eidne KA, Thompson PJ, Knight DA. αvβ3 Integrin interacts with the transforming growth factor β (TGF-β) type II receptor to potentiate the proliferative effects of TGF-β1 in living human lung fibroblasts. J Biol Chem. 2004;279:37726–37733. doi: 10.1074/jbc.M403010200. [DOI] [PubMed] [Google Scholar]
- 42.Galliher AJ, Schiemann WP. β3 Integrin and Src facilitate transforming growth factor-β mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res. 2006;8:R42. doi: 10.1186/bcr1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salvo E, Garasa S, Dotor J, Morales X, Pelaez R, Altevogt P, Rouzaut A. Combined targeting of TGF-β1 and integrin β3 impairs lymph node metastasis in a mouse model of non-small-cell lung cancer. Mol Cancer. 2014;13:112. doi: 10.1186/1476-4598-13-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nasongkla N, Shuai X, Ai H, Weinberg BD, Pink J, Boothman DA, Gao JM. cRGD-functionalized polymer micelles for targeted doxorubicin delivery. Angew Chem Int Ed. 2004;43:6323–6327. doi: 10.1002/anie.200460800. [DOI] [PubMed] [Google Scholar]
- 45.Desgrosellier JS, Cheresh DA. Integrins in cancer: Biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang YR, Tu Q, Wang DE, Chen Y, Lu BZ, Yuan MS, Wang JY. Adamantyl-terminated dendronized molecules: Synthesis and interaction with β-cyclodextrin-functionalized poly(dimethylsiloxane) interface. New J Chem. 2013;37:2358–2368. [Google Scholar]
- 47.Kaneshiro TL, Jeong EK, Morrell G, Parker DL, Lu ZR. Synthesis and evaluation of globular Gd-DOTA-monoamide conjugates with precisely controlled nanosizes for magnetic resonance angiography. Biomacromolecules. 2008;9:2742–2748. doi: 10.1021/bm800486c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.