Abstract
Sepsis continues to result in high morbidity and mortality. General anesthesia is often administered to septic patients, but the impacts of general anesthesia on host defense are not well understood. General anesthesia can be given by volatile and intravenous anesthetics. Our previous in vitro study showed that volatile anesthetic isoflurane directly inhibits leukocyte function-associated antigen-1 (LFA-1) and macrophage-1 antigen (Mac-1), critical adhesion molecules on leukocytes. Thus, the role of isoflurane exposure on in vivo LFA-1 and Mac-1 function was examined using polymicrobial abdominal sepsis model in mice. As a comparison, intravenous anesthetic propofol was given to a group of mice. Wild type, LFA-1, Mac-1, and adhesion molecule-1 knockout mice were used. Following the induction of polymicrobial abdominal sepsis by cecal ligation and puncture, groups of mice were exposed to isoflurane for either 2 or 6 h, or to propofol for 6 h, and their outcomes were examined. Bacterial loads in tissues and blood, neutrophil recruitment to the peritoneal cavity and phagocytosis were studied. Six hours of isoflurane exposure worsened the outcome of abdominal sepsis (P < .0001) with higher bacterial loads in tissues, but 2 h of isoflurane or 6 h of propofol exposure did not. Isoflurane impaired neutrophil recruitment to the abdominal cavity by inhibiting LFA-1 function. Isoflurane also impaired bacterial phagocytosis via complement receptors including Mac-1. In conclusion, prolonged isoflurane exposure worsened the outcome of experimental polymicrobial abdominal sepsis and was associated with impaired neutrophil recruitment and bacterial phagocytosis via reduced LFA-1 and Mac-1 function.
Keywords: anesthesia, sepsis, neutrophil, leukocyte function-associated antigen-1, macrophage-1 antigen.
Sepsis continues to be associated with high morbidity and mortality with annual cost of $20.3 billion, accounting for nearly one-fifth of the total aggregate costs in all the hospitalizations in the United States (Torio and Andrews, 2013). A large number of clinical trials to modify the systemic inflammatory response in sepsis were without apparent success (Marshall, 2014). The Surviving Sepsis Campaign’s guidelines for the management of severe sepsis and septic shock have been widely implemented (Dellinger et al., 2004, 2008, 2013), but the mortality from sepsis is still high, ranging from 20 to 30% (Levy et al., 2012; Peake et al., 2014; Yealy et al., 2014) with questionable benefit of goal-directed protocol-based care (Peake et al., 2014; Yealy et al., 2014). These findings imply that our understanding of sepsis pathophysiology remains quite limited. The number of patients suffering from sepsis has been on the rise by 1.5% annually due to the increasing number of patients with treatment-resistant organisms, compromised immune systems, or undergoing prolonged high-risk surgery (Angus et al., 2001; Hecker et al., 2014; Martin et al., 2003). Severe sepsis is the leading cause of death in the non-cardiac intensive care unit (Angus et al., 2001; Eissa et al., 2010). Strategies to improve outcomes are an urgent priority.
Identification and control of the source of infection is the mainstay of sepsis management (Schorr and Dellinger, 2014). Source control procedures such as drainage of infected fluids, debridement of infected tissues, and/or removal of infected devices are usually undertaken under general anesthesia on an emergent or urgent basis. In pediatric patients, diagnostic imaging studies for the source of infection are often undertaken under general anesthesia. General anesthesia can be provided by volatile and intravenous anesthetics. Volatile anesthetics such as isoflurane and sevoflurane are the most commonly used drugs despite their undetermined mechanism of action (Campagna et al., 2003; Hemmings et al., 2005; Perouasnsly et al., 2009). The literature suggests that they have targets outside of the central nervous system, including targets on immune cells (Kurosawa and Kato, 2008). Propofol is a very popular intravenous anesthetic drug and provides anesthetic effect mainly via its interaction with gamma-aminobutyric acid A receptor (Jurd et al., 2003). Our previous in vitro investigations have demonstrated that isoflurane bound to and impaired adhesion molecules leukocyte function-associated antigen-1 (LFA-1, αLβ2, or CD11a/CD18) and macrophage-1 antigen (Mac-1, αMβ2, or CD11b/CD18, complement receptor 3) (Yuki et al., 2008; 2012). In contrast, propofol blocked LFA-1 only at the supraclinical concentrations in vitro (Yuki et al., 2013). LFA-1 is expressed ubiquitously on all leukocytes and plays a major role in leukocyte arrest. Mac-1 is expressed largely on neutrophils, monocytes, and macrophages and primarily mediates intraluminal crawling and complement-mediated phagocytosis (Wagner and Frenette, 2008; Phillipson and Kubes, 2011). Both LFA-1 and Mac-1 bind to intercellular adhesion molecule-1 (ICAM-1) on the endothelium for leukocyte recruitment. The clinical significance of these molecules is illustrated in the rare genetic disorder leukocyte adhesion deficiency, which is characterized by their functional deficiency and often associated with severe infection (Fischer et al., 1988; Hogg et al., 1999).
Any iatrogenic modulation of adhesion molecule functions could have significant consequences on the outcomes of septic patients, but there is a paucity of research investigating the impact of volatile and intravenous anesthetics on sepsis pathophysiology. Because isoflurane and propofol are commonly administered to patients, typically without specific consideration about their underlying diseases, it is important to understand their impact on immune function, particularly on neutrophils, the first-defense innate immune cells (Alves-Filho et al., 2008; Williams et al., 2011). Patients with intra-abdominal sepsis have a particularly high mortality, ranging from 22 to 90% (Hecker et al., 2014). Here, using a using a murine cecal ligation and puncture (CLP) polymicrobial abdominal sepsis model, we tested the hypothesis that isoflurane, not propofol worsens the outcome of experimental sepsis, and found that 6-h isoflurane exposure after CLP led to increased mortality, with higher bacterial levels in tissues, decreased neutrophil recruitment and phagocytosis, and impaired LFA-1 and Mac-1 function. Understanding in vivo effect of anesthetics and their mechanism in preclinical sepsis models will allow us to provide future direction in patient-based research and to hopefully optimize our patient care.
MATERIALS AND METHODS
Mice
Mice were from Jackson Laboratory (Bar Harbor, Maine, USA) and inbred in our animal facilities. CD11a knockout mice (=LFA-1 KO mice) (Ding et al., 1999), CD11b (=Mac-1) KO mice (Coxon et al., 1996), and ICAM-1 KO mice (Bullard et al., 2007) were previously described. All mice were on the C57BL/6 background and housed under specific pathogen-free conditions, with 12-h light and dark cycles. Male mice at 8–10 weeks of age were used for the experiments.
CLP Model
All the experimental procedures complied with institutional and federal guidelines regarding the use of animals in research. Polymicrobial abdominal sepsis was induced by CLP, as previously described in Rittirsch et al. (2009) and Feng et al. (2011). Mice were anesthetized with ketamine 60 mg/kg and xylazine 5 mg/kg given intraperitoneally. Following exteriorization, the cecum was ligated at 1.0 cm from its tip and subjected to a single, through and through puncture using an 18-gauge needle. A small amount of fecal material was expelled with gentle pressure to maintain the patency of puncture sites. The cecum was reinserted into the abdominal cavity. 0.1 ml/g of warmed saline was administered subcutaneously. Buprenorphine was given subcutaneously to alleviate postoperative surgical pain. Some groups of mice were placed on a nose cone to be continuously exposed to 1% isoflurane using isoflurane vaporizer (VetQuip; New South Wales, Australia) or were given propofol (10 mg/kg/h over 6 h) intravenously for the indicated duration. Isoflurane is often used at the concentration of 1–2% in clinical practice. 10 mg/kg/h of propofol is above the dose used in a typical clinical scenario. Mice were continously observed during anesthetic exposure and until they were fully recovered from anesthesia.
Complete Blood Count and Chemistry Measurement
Complete blood count was measured using VetScan HM2 (Abaxis; Union City, California, USA). Blood chemistry was measured using VetScan VS2 (Abaxis).
Serum and Peritoneal Cytokine Measurement
Levels of various serum cytokines were determined by using mouse Th1/Th2 kit (Meso Scale Discovery; Gaithersburg, Maryland, USA) per the company protocol.
Quantitative Organ Culture
To determine the bacterial loads in the organs and blood, tissue homogenates or blood were loaded on 5% blood agar plates (Teknova; Hollister, California, USA) after serial dilutions and incubated for 18 hours as previously described (Liu et al., 2014). Colonies of all morphologies on plates were counted.
Peritoneal Leukocyte Count
At 6, 12, and 24 h after CLP, mice were euthanized and peritoneal lavage was performed. The number of peritoneal leukocytes was counted using TURK blood diluting fluid (Ricca Chemical; Arlington, Texas, USA), and leukocyte differential was determined by Giemsa stain (Thermo Fischer; Waltham, Massachusetts, USA).
Whole Blood Phagocytosis Assay
Mouse whole blood phagocytosis assay using fluorescein isothiocyanate labeled opsonized E. coli was performed using phagotest kit (Glycotope Biotechnology; Hidelberg, Germany) per the company protocol.
Neutrophil Binding Assay to ICAM-1
Mouse neutrophils from bone marrow were obtained using negative sorting with MACS separation column using anti-CD45R, anti-CD5, anti-CD8, and anti-CD4 antibodies (all from BD Biosciences; Franklin Lakes, New Jersey, USA) as described previously in Carbo et al. (2013). A total of 96 wells were coated with 1 µg/ml of ICAM-1 (R&D systems; Minneapolis, Minnesota, USA) and blocked with HEPES buffered saline (HBS) containing 2% BSA. After washing, 50 µl each of HBS with either 10 mM EDTA, 2 mM Mg2+/Ca2+, or 2 mM Mn2+ was given to each well. Some plates were pre-exposed to isoflurane for 30 min. 50 µl of neutrophil suspension in HBS (1.0 × 105 cells/50 µl) was added to each well and incubated with or without 1% isoflurane for 30 min. Wells were washed and attached cells were visualized and counted.
Mouse Whole Blood Flow Cytometry for Neutrophils
Following incubation with Fc blocking antibody, surface expressions of LFA-1, Mac-1, and α4β1 were probed using M17/4 (anti-CD11a) antibody, M1/70 (anti-CD11b) antibody, and 9C10 (anti-CD49d) antibodies (BD Biosciences), respectively. Erythrocytes were lyzed using lysis buffer. Neutrophil population was gated as anti-Gr-1 antibody positive cells.
Cells
Human embryonic kidney (HEK) cells were cultured in HEPES modified Dulbecco’s modified Eagle medium/10% fetal bovine serum (FBS), and U937 cells were cultured in RPMI1640/10% FBS at 37°C, 5% CO2. Both cells were obtained from ATCC (Manassa, Virginia, USA).
V-Bottom Well vascular cell adhesion molecule-1 Binding Assay
V-bottom well binding assay was performed as previously described (Weitz-Schmidt and Chreng 2012). Briefly, HEK cells transiently transfected with α4β1 or U937 cells stained with 2′,7′- bis- (2-carboxyethyl)-5-(and 6-)-carboxyfluorescein, acetoxymethyl ester (Life Technologies) were incubated in 96 wells coated with 1 µg/ml of vascular cell adhesion molecule-1 (VCAM-1)-Fc (R&D systems; Minneapolis, Minnesota, USA) with 5 mM EDTA, 1 mM Mg2+/Ca2+, or 1 mM Mn2+ with or without 4% isoflurane for 30 min. Then plates were centrifuged at 200 × g for 5 min. Cells that did not bind to VCAM-1 were accumulated at the center of the well. As a control, 10 µM of α4β1 inhibitor TCS2314 (Tocris Bioscience; Bristol, UK) was used. Fluorescence was read with excitation 485 nm and emission 538 nm. The binding % was defined as [(fluorescence intensity of samples)-(fluorescence intensity of sample in EDTA)]/[fluorescence intensity of sample in EDTA] × 100 (%).
Statistics
Data were analyzed as indicated in the corresponding figure legends. Statistical analysis was defined as P < .05. All the statistical calculations were performed using PRISM 5 software (GraphPad Software; La Jolla, California, USA).
RESULTS
Long Isoflurane Exposure Worsens the Outcome of Polymicrobial Abdominal Sepsis
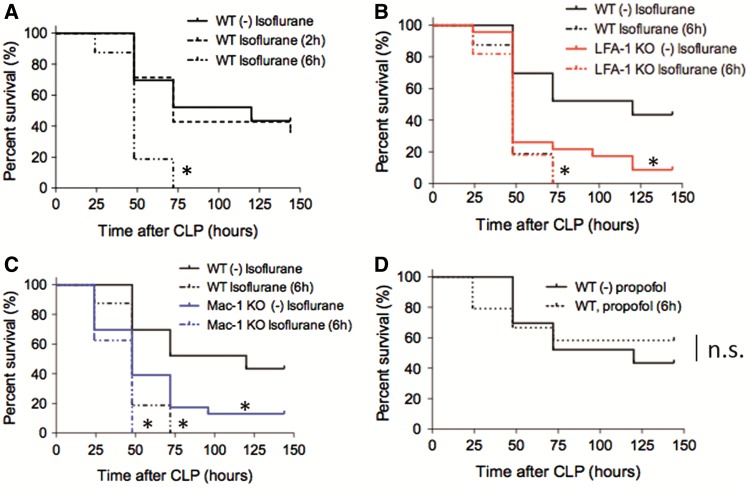
Wild-type (WT) mice exposed to 1% isoflurane for 6 h after CLP (long exposure group) had significantly higher mortality than WT mice without isoflurane (no exposure group) or those exposed to 1% isoflurane for 2 h (short exposure group)(Figure 1A). Two-hour exposure and 6-h exposure were chosen to reflect “short” and “long” anesthesia cases in our clinical practice. Previously we demonstrated in vitro that isoflurane directly inhibited adhesion molecule LFA-1 and Mac-1 (Yuki et al., 2008). As a reference, and in order to parse out the mechanism of isoflurane effects, we performed CLP experiments in LFA-1 and Mac-1 KO mice. LFA-1 KO mice had poorer survival than WT mice without isoflurane exposure (Figure 1B). However, the mortality of LFA-1 KO mice exposed to 1% isoflurane for 6 h after CLP was not different from that of WT mice exposed to 1% isoflurane for the same duration (Figure 1B). Similarly, Mac-1 KO mice had higher mortality than WT mice without isoflurane exposure (Figure 1C). Exposure to 1% isoflurane for 6 h increased mortality of both WT and Mac-1 KO mice to the same level (Figure 1C). In contrast, high-dose propofol exposure after CLP did not affect the outcome of WT mice (Figure 1D). In the following, we studied mice exposed to 1% isoflurane for 6 h to understand the underlying mechanism of their poorer outcomes.
FIG. 1.
The impact of isoflurane exposure on mortality in experimental polymicrobial abdominal sepsis. A–C, The outcomes of polymicrobial abdominal sepsis induced by CLP in WT mice without isoflurane exposure (n = 25), with isoflurane exposure for 2 h (short exposure, n = 14) and 6 h (long exposure, n = 20), LFA-1 KO mice without isoflurane exposure (n = 24), LFA-1 KO mice with isoflurane exposure for 6 h (n = 16), Mac-1 KO mice without isoflurane exposure (n = 24) and Mac-1 KO mice with isoflurane exposure for 6 h (n = 15) are shown. D, The outcomes of mice after CLP in WT mice with propofol exposure (n = 20) or without propofol exposure (n = 20) for 6 h. Statistical significance was evaluated using Log-rank test. * and ** represent P < .05 and .01, respectively versus WT without isoflurane (or propfol). CLP, cecal ligation and puncture.
Long Isoflurane Worsens Kidney Function
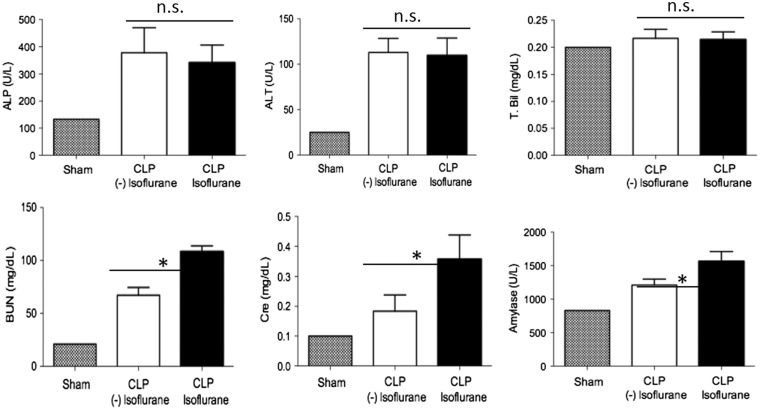
Because long isoflurane exposure after CLP significantly worsened the outcome of mice, we examined liver and kidney functions. Although liver function was not different at 6 and 24 h after CLP, kidney function was significantly worse in isoflurane-exposed mice (Figure 2). This result was in line with the previous mortality result.
FIG. 2.
The effect of isoflurane on liver and kidney functions after CLP. Liver and kidney functions were assessed after CLP in no and long isoflurane exposure groups of mice. Data represent mean ± SD of 8 mice. Statistical analysis was performed using 1-way analysis of variance with Bonferroni post hoc analysis.
Long Isoflurane Exposure Increases Bacterial Loads after CLP
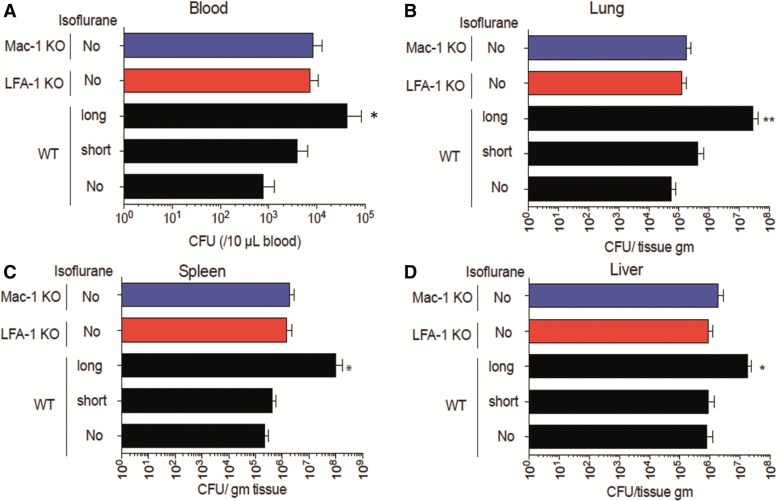
We next examined bacterial loads in the lung, spleen, liver, and blood. The long exposure group in WT mice had higher bacterial loads in all the tested tissues than the no and short exposure groups in WT, LFA-1, or Mac-1 KO mice (Figs. 3A–D).
FIG. 3.
The effect of isoflurane exposure on tissue and blood bacterial levels after CLP. Bacterial loads in blood (A), lung (B), spleen (C), and liver (D) at 6 h after CLP was compared among WT mice in no, short and long isoflurane exposure groups, LFA-1 KO and Mac-1 KO mice. Blood and tissue bacterial loads were shown as mean ± SD of 8 mice. Statistical significance was evaluated using Kruskal Wallis test with Dunn’s multiple comparisons. In figures, * and ** represent P < .05 and .01, respectively versus WT mice without isoflurane exposure. CFU, colony forming unit.
Isoflurane Exposure Reduces Neutrophil Recruitment to the Abdominal Cavity
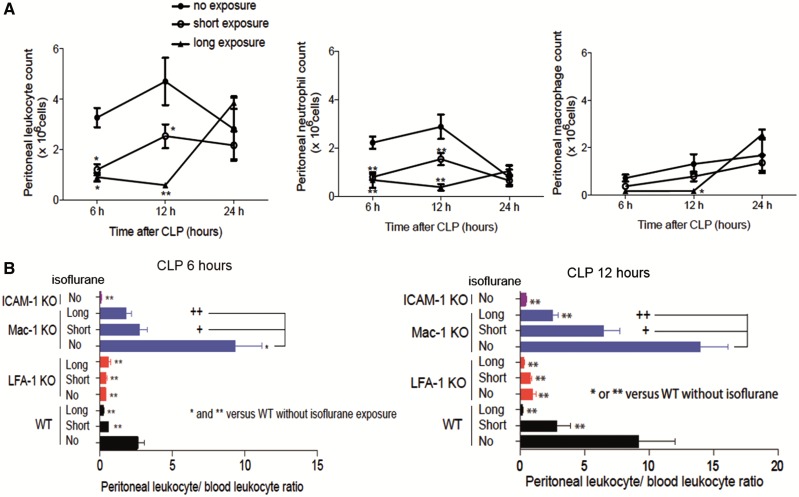
To understand the cause of the increased mortality and higher bacterial loads following long isoflurane exposure, we first examined the impact of isoflurane on leukocyte counts in peripheral blood and the abdominal cavity. Isoflurane exposure did not result in differences in peripheral blood leukocyte counts (Supplementary Figure S1). In the abdominal cavity, neutrophils were the major population at 6 and 12 h after CLP, while macrophages were the primary constituents at 24 h (Figure 4A). Both short and long isoflurane exposures reduced the number of recruited neutrophils at 6 and 12 h after CLP while only long exposure reduced the number of macrophages at 12 h after CLP (Figure 4A). By 24 h, isoflurane exposure did not have any effect on neutrophil or macrophage recruitment (Figure 4A). Our findings thus far demonstrated that isoflurane impairment of leukocyte recruitment, while only transient, has profound effects on sepsis outcomes.
FIG. 4.
The effect of isoflurane exposure on leukocyte migration to the abdominal cavity. A, The number of total leukocytes, neutrophils and macrophages in the peritoneal cavity at 6, 12, and 24 h after CLP. The data represent mean ± SD of 4 mice. Statistical analysis was performed using 2-way analysis of variance with Bonferroni post hoc analysis. * and ** represent P < .05 and .01, respectively versus WT mice without isoflurane exposure at the same time point. B, The peritoneal leukocyte/blood leukocyte ratio 12 h after CLP. Data represent mean ± SD of 4 mice. Statistical analysis was evaluated using 1-way analysis of variance with Bonferroni post hoc analysis versus WT mice without isoflurane exposure. In figures, * and ** represent P < .05 and .01 versus WT without isoflurane exposure. Separate, statistical analysis was also performed among Mac-1 KO mice. + and ++ denote P < .05 and .01, respectively versus Mac-1 KO mice without isoflurane exposure.
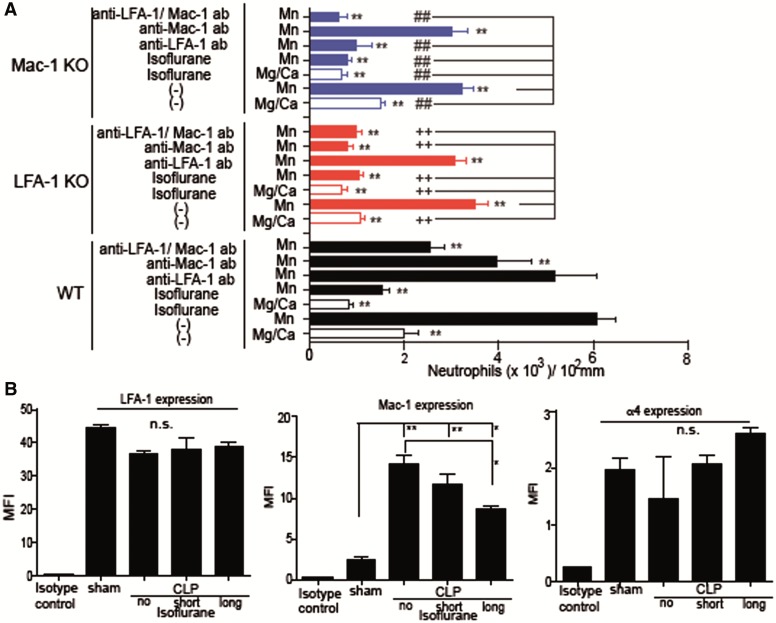
Previously Prince et al. (2001) defined the peritoneal emigration (recruitment) ratio as peritoneal leukocyte number/peripheral leukocyte number to compare the degree of leukocyte migration to the abdominal cavity in mice with different total numbers of leukocytes (Supplementary Table S1). We evaluated the emigration ratio in studies with KO mice. In WT mice, both short and long exposures led to lower emigration ratios at 6 and 12 h after CLP, consistent with the previous experiment (Figure 4B). LFA-1 KO mice showed very limited recruitment, while Mac-1 KO mice did not demonstrate any limitation in leukocyte recruitment but had reduction after isoflurane exposure, as in WT mice, suggesting that LFA-1 would be the major determinant for leukocyte recruitment within the peritoneum. Both LFA-1 and Mac-1 bind to ICAM-1 on the endothelium for leukocyte recruitment. As expected, ICAM-1 KO mice had very limited leukocyte recruitment (Figure 4B).
Isoflurane Exposure Impairs Bacterial Phagocytosis in Neutrophils
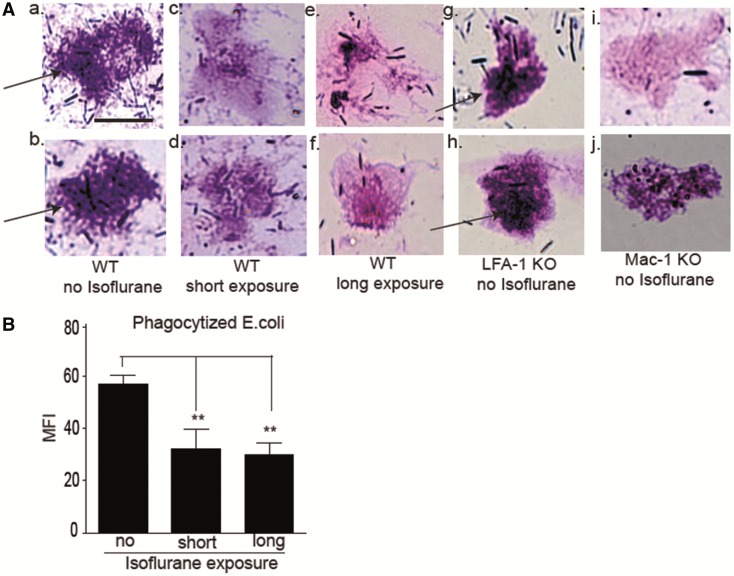
Mac-1 binds to several ligands including ICAM-1 and iC3b. Its binding to iC3b facilitates complement-mediated phagocytosis. Because our data suggest the impairment of Mac-1 function by isoflurane, we examined bacterial phagocytosis. Peritoneal neutrophils from mice exposed to isoflurane revealed less bacterial phagocytosis as fewer neutrophils were seen containing engulfed bacteria (Figure 5A). Although LFA-1 KO mice phagocytized normally, Mac-1 KO mice had impairment. Neutrophils exposed to isoflurane ex vivo phagocytized fluorescently labeled E. coli less (Figure 5B).
FIG. 5.
The effect of isoflurane exposure on bacterial phagocytosis. A, Representative images of peritoneal leukocytes from mice 12 h after CLP. (a) and (b), WT mice with no isoflurane exposure; (c) and (d), WT mice with isoflurane short exposure; (e) and (f), WT mice with long isoflurane exposure; (g) and (h), LFA-1 KO mice with no isoflurane exposure; (i) and (j), Mac-1 KO mice with no isoflurane exposure. Arrows indicate bacteria. Scale bar 10 µm. B, Whole blood phagocytosis assay using fluorescent E. coli. Mice in the short isoflurane exposure group received 1% isoflurane for 2 h and then were kept in cages for 4 h before blood collection. The long exposure group received 1% isoflurane for 6 h and then blood was collected immediately. Gated on neutrophil population. Data represent mean ± SD of 4 mice. Statistical significance was analyzed using 1-way analysis of variance with Bonferroni post hoc analysis. * and ** denote P < .05 and .01, respectively versus mice without isoflurane exposure. MFI, mean fluorescence intensity.
Isoflurane Inhibits LFA-1 and Mac-1 Binding to ICAM-1
We previously reported that isoflurane blocked the binding of LFA-1 and Mac-1 to ICAM-1 in a cell-free system (Yuki et al., 2008). The direct binding of isoflurane onto LFA-1 was structurally confirmed (Yuki et al., 2008, 2012; Zhang et al., 2009). LFA-1 and Mac-1 presumably takes different conformations depending on its activation status and binds to ICAM-1 only when activated (Nishida et al. 2006). Here we examined the effect of isoflurane on LFA-1 or Mac-1 binding to ICAM-1 using neutrophils. Mn2+ was used as an activator because it activates these adhesion molecules directly without the involvement of inside-out signals and allows us to exclude the possible impact of isoflurane on inside-out signals (Dransfield et al., 1992; Sanchez-Martin et al., 2004). Isoflurane blocked the binding of neutrophils from WT, LFA-1 KO and Mac-1 KO mice to ICAM-1, suggesting its binding inhibition of LFA-1 and Mac-1 to ICAM-1 (Figure 6A). Importantly, isoflurane did not change LFA-1 expression on neutrophils in CLP model (Figure 6B), which further supports the direct interaction between isoflurane and LFA-1. In contrast, isoflurane attenuated Mac-1 expression in CLP model, suggesting isoflurane can perturb Mac-1 activity using multiple mechanisms (Figure 6B). In addition to LFA-1, integrin α4β1 reportedly plays some role in neutrophil migration by binding to its ligand VCAM-1 (Burns et al., 2001). The expression of α4 integrin on neutrophils was relativity low compared with other surface markers (Figure 6B), but LFA-1 KO mice were still able to recruit a very small number of leukocytes, implying α4β1 involvement. Isoflurane did not change the binding of α4β1 to VCAM-1 (Supplementary Figure S2). Taken together, these results suggest the underlying mechanism of reduced recruitment by isoflurane was primarily due to the change of LFA-1 function.
FIG. 6.
The effect of isoflurane on LFA-1 and Mac-1 on primary neutrophils. A, The binding of ICAM-1 to LFA-1 and Mac-1 was evaluated using neutrophils from WT, LFA-1 KO and Mac-1 KO mice with or without isoflurane exposure. As controls, LFA-1 blocking antibody M17/4 (15 µg/ml) and Mac-1 blocking antibody M1/70 (15 µg/ml) were used. Data are shown as mean ± SD of quadruplicates. Statistical analysis was performed using 1-way analysis of variance with Bonferroni post hoc analysis. * and ** denote P < .05 and .01, respectively versus WT neutrophils with Mn2+ activation group. Among LFA-1 KO neutrophils, + and ++ denote P < .05 and .01, respectively versus LFA-1 KO neutrophils with Mn2+ activation. Among Mac-1 KO neutrophils, # and ## denote P < .05 and .01, respectively versus Mac-1 KO neutrophils with Mn2+ activation. B, The expression of LFA-1, Mac-1, and α4β1 on neutrophils were examined at 12 h after CLP using flow cytometry. Data show mean ± SD of 4 mice. Statistical analysis was performed using 1-way analysis of variance with Bonferroni post hoc analysis. MFI, mean fluorescence intensity. * and ** denote P < .05 and .01, respectively.
Long Isoflurane Exposure Group Did Not Attenuate Systemic Inflammation
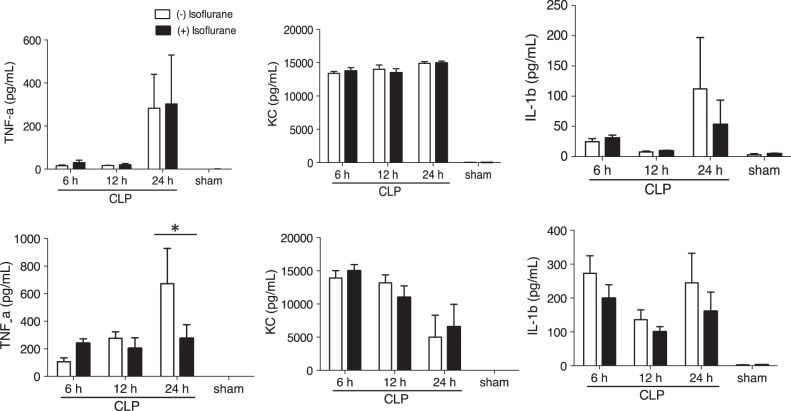
The previous report by Herrmann et al. (2013) demonstrated that volatile anesthetics reduced systemic proinflammatory cytokine levels after CLP with survival benefit in mice. Six hours of isoflurane exposure did not affect systemic proinflammatory cytokine levels (Figure 7A). However, it attenuated TNF-α production in the peritoneal fluid (Figure 7B). TNF-α could active peritoneal neutrophils and macrophages. Therefore, the reduction in TNF-α in the peritoneal fluid might attenuate their functions at the site of infection.
FIG. 7.
The effect of prolonged isoflurane exposure on proinflammatory cytokines. The proinflammatory cytokine profiles in blood (A) and peritoneal fluid (B) were compared between non-isoflurane exposure group and prolonged isoflurane exposure group. The data represent mean ± SD of 8 mice. Statistical analysis was performed using 2-way analysis of variance with Bonferroni post hoc analysis.
DISCUSSIONS
Here we demonstrated that prolonged isoflurane exposure worsened the outcome of experimental polymicrobial abdominal sepsis. Short isoflurane exposure and prolonged propofol exposure did not affect the outcome of septic mice. Prolonged isoflurane exposure was associated with higher bacterial loads in tissues along with the impairment of neutrophil recruitment to the site of infection and bacterial phagocytosis. Isoflurane induced the attenuation of LFA-1 and Mac-1 functions, and prolonged isoflurane exposure might have led into the condition analog to leukocyte adhesion deficiency I temporarily that was significant enough to affect the outcome of septic mice.
In infection, neutrophils are first recruited to the site of infection and migrate across the endothelium. In our study, deficiency of LFA-1, not Mac-1 significantly impaired neutrophil recruitment to the peritoneal cavity. Two major transmigration pathways have been known. In “paracellular pathway”, neutrophils arrest on the endothelium via LFA-1: ICAM-1 interaction, crawl for the cell-cell junctions via Mac-1: ICAM-1 interaction and transmigrate there (Phillipson et al., 2006). In “transcellular pathway”, neutrophils pass through the endothelial cells, which do not necessitate crawling but still requires the LFA-1: ICAM-1 interaction. Blockade of LFA-1 would hinder both pathways, but blocking only Mac-1 spares transcellular pathway. This can explain our finding of the near abolishment of migration to the abdominal cavity in LFA-1 KO while Mac-1 KO mice had normal migration. Integrin α4β1 is also suggested to play a role in neutrophil migration (Burns et al., 2001), but isoflurane did not affect the expression of α4β1 or its binding to VCAM-1. Taken together, we can conclude that the impairment of neutrophil migration by isoflurane was due to the functional impairment of LFA-1. We did not show direct interaction of isoflurane with LFA-1 in vivo. However, we believe there is a direct interaction in vivo because isoflurane blocked the binding of extracellularly activated LFA-1 (via Mn2+) to ICAM-1 on primary neutrophils, and isoflurane did not affect LFA-1 expression in vivo.
Neutrophils engulf IgG-opsonized targets via Fcγ receptor and complement-opsonized targets via complement receptors, including Mac-1 (Nordenfelt and Tapper, 2011). A prior study demonstrated that neutrophil bacterial phagocytosis in a CLP model was complement receptor-dependent (Prodeus et al., 1997). As expected, phagocytosis in Mac-1 KO mice was impaired in our model. And isoflurane attenuated bacterial phagocytosis in vivo and ex vivo. However, isoflurane attenuated Mac-1 surface expression in this study, and we cannot conclude a direct interaction of isoflurane with Mac-1 in vivo.
Significantly higher bacterial loads in tissues were observed in 6 h of isoflurane exposure group. The suppression in leukocyte migration seen after isoflurane exposure was reversible and recovered to nonexposure levels after 24 h. The fact that a fairly short suppression in immune function by 6 h of isoflurane can lead to increased bacterial levels and increased mortality presumably relates to the short doubling time of bacteria. Two hours of suppression would not be long enough to demonstrate significant difference in bacteria loads. We did not monitor the blood pressure of the mice during anesthetic exposure in this study. It is possible that isoflurane administration may have caused more hemodynamic compromise than propofol administration, leading to tissue hypoperfusion. We cannot exclude this as an alternative mechanism to explain our findings. Our result was compatible with the previous study by Herrmann et al. (2013). They tested 2 h of isoflurane exposure in CLP model, and noted no difference in bacterial loads and outcomes. Six hours of isoflurane exposure is not uncommon, and our result may indicate the possible disadvantage of using isoflurane for a longer duration.
Sevoflurane is another volatile anesthetic used commonly and also inhibits LFA-1 in vitro (Yuki et al., 2010), and it is necessary to evaluate in vivo. The study by Herrmann et al. showed that 2 h of sevoflurane exposure attenuated systemic proinflammatory cytokines and improved survival. However, it is unclear if longer exposure poses a similar effect. Further investigation is required. We also tested commonly used intravenous anesthetic propofol at high dose for 6 h, and did not find any difference in outcomes. Notably, one small study that compared the intravenous anesthetic propofol versus isoflurane anesthesia in patients undergoing abdominal surgeries demonstrated that the isoflurane arm had increased incidence of postoperative infection (Von Dossow et al., 2007). The sample size in this study was small and additional studies are needed to validate this finding.
In conclusion, we demonstrated that prolonged isoflurane exposure worsened the outcome of experimental polymicrobial abdominal sepsis in mice and was associated with impaired neutrophil recruitment and bacterial phagocytosis via reduced LFA-1 and Mac-1 function. In contrast, propofol did not affect the outcome of septic mice.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Xiaohui Han, RN, Matthew Chamberlain, BS, Kazumasa Tazawa, MD and Wei Wang, PhD (all from the Department of Anesthesiology, Perioperative and Pain Medicine, Boston Children’s Hospital, Boston, Massachusetts, USA) for technical assistance.
FUNDING
This work was supported by CHMC Anesthesia Foundation (K.Y.), William F. Milton Fund (K.Y.), National Institute of General Medical Sciences (GM101345 and GM118277 to K.Y.) and National Heart, Lung, and Blood Institute (HL092515 to G.P.P.), a Grant-in-Aid (S1311011) from the Foundation of Strategic Research Projects in Private Universities from the MEXT (T.Y.), MEXT/JSPS KAKENHI (15H05901, 15H05904, and 15H04708) (T.Y.), (16K08596, 15KK0320) (T.O.) and Takeda Science Foundation (T.Y. and T.O.).
REFERENCES
- Alves-Filho J. C., de Freitas A., Spiller F., Souto F. O., Cunha F. Q. (2008). The role of neutrophils in severe sepsis. Shock 30(Suppl 1), 3–9. [DOI] [PubMed] [Google Scholar]
- Angus D. C., Linde-Zwirble W. T., Lidicker J., Clermont G., Carcillo J., Pinsky M. R. (2001). Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29, 1303–1310. [DOI] [PubMed] [Google Scholar]
- Bullard D. C., Hu X., Schoeb T. R., Collins R. G., Beaudet A. L., Barnum S. R. (2007). Intercellular adhesion molecule-1 expression is required on multiple cell types for the development of experimental autoimmune encephalomyelitis. J. Immunol. 178, 851–857. [DOI] [PubMed] [Google Scholar]
- Burns J. A., Issekutz T. B., Yagita H., Issekutz A. C. (2001). The alpha 4 beta 1 (very late antigen (VLA)-4, CD49d/CD29) and alpha 5 beta 1 (VLA-5, CD49e/CD29) integrins mediate beta 2 (CD11/CD18) integrin-independent neutrophil recruitment to endotoxin-induced lung inflammation. J. Immunol. 166, 4644–4649. [DOI] [PubMed] [Google Scholar]
- Campagna J. A., Miller K. W., Forman S. A. (2003). Mechanisms of actions of inhaled anesthetics. N. Engl. J. Med. 348, 2110–2124. [DOI] [PubMed] [Google Scholar]
- Carbo C., Yuki K., Demers M., Wagner D. D., Shimaoka M. (2013). Isoflurane inhibits neutrophil recruitment in the cutaneous Arthus reaction model. J. Anesth 27, 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coxon A., Rieu P., Barkalow F. J., Askari S., Sharpe A. H., von Andrian U. H., Arnaout M. A., Mayadas T. N. (1996). A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: A homeostatic mechanism in inflammation. Immunity 5, 653–666. [DOI] [PubMed] [Google Scholar]
- Dellinger R. P., Carlet J., Masur M., Gerlach H., Calandra H., Cohen T., Gea-Banacloche J., Keh J. D., Marshall J. C., Parker M., Ramsay M. G., Zimmerman J. L., Vincent J. L., Levy M. M. (2004). Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit. Care Med. 32, 858–873. [DOI] [PubMed] [Google Scholar]
- Dellinger R. P., Levy M. M., Carlet J. M., Bion J., Parker M. M., Jaeschke R., Reinhart K., Angus D. C., Brun-Buisson C., Beale R., et al. (2008). Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit. Care Med. 36, 296–327. [DOI] [PubMed] [Google Scholar]
- Dellinger R. P., Levy M. M., Rhodes A., Annane D., Gerlach H., Opal S. M., Sevransky J. E., Sprung C. L., Douglas I. S., Jaeschke R., et al. (2013). Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 41, 580–637. [DOI] [PubMed] [Google Scholar]
- Ding Z. M., Babensee J. E., Simon S. I., Lu H., Perrard J. L., Bullard D. C., Dai X. Y., Bromley S. K., Dustin M. L., Entman M. L.. et al. (1999). Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J. Immunol. 163, 5029–5038. [PubMed] [Google Scholar]
- Dransfield I., Cabanas C., Craig A., Hogg N. (1992). Divalent cation regulation of the function of the leukocyte integrin LFA-1. J. Cell Biol. 116, 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissa D., Carton E. G., Buggy D. J. (2010). Anaesthetic management of patients with severe sepsis. Br. J. Anaesth. 105, 734–743. [DOI] [PubMed] [Google Scholar]
- Feng Y., Zou L., Zhang M., Li Y., Chen C., Chao W. (2011). MyD88 and Trif signaling play distinct roles in cardiac dysfunction and mortality during endotoxin shock and polymicrobial sepsis. Anesthesiology 115, 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A., Lisowska-Grospierre B., Anderson D. C., Springer T. A. (1988). Leukocyte adhesion deficiency: Molecular basis and functional consequences. Immunodefic. Rev. 1, 39–54. [PubMed] [Google Scholar]
- Hecker A., Uhle F., Schwandner T., Padberg W., Weigand M. A. (2014). Diagnostics, therapy and outcome prediction in abdominal sepsis: Current standards and future perspectives. Langenbecks Arch. Surg. 399, 11–22. [DOI] [PubMed] [Google Scholar]
- Hemmings H. C. Jr, Akabas M. H., Goldstein P. A., Trudell J. R., Orser B. A., Harrison N. L. (2005). Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol. Sci. 26, 503–510. [DOI] [PubMed] [Google Scholar]
- Herrmann I. K., Castellon M., Schwartz D. E., Hasler M., Urner M., Hu G., Minshall R. D., Beck-Schimmer B. (2013). Volatile anesthetics improve survival after cecal ligation and puncture. Anesthesiology 119, 901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg N., Stewart M. P., Scarth S. L., Newton R., Shaw J. M., Law S. K., Klein N. (1999). A novel leukocyte adhesion deficiency caused by expressed but nonfunctional beta2 integrins Mac-1 and LFA-1. J. Clin. Invest. 103, 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurd R., Arras M., Lambert S., Drexler B., Siegwart R., Crestani F., Zaugg M., Vogt K. E., Ledermann B., Antkowiak B., Rudolph U. (2003). General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. faseb J. 17, 250–252. [DOI] [PubMed] [Google Scholar]
- Kurosawa S., Kato M. (2008). Anesthetics, immune cells, and immune responses. J. Anesth. 22, 263–277. [DOI] [PubMed] [Google Scholar]
- Levy M. M., Artigas A., Phillips G. S., Rhodes A., Beale R., Osborn T., Vincent J. L., Townsend S., Lemeshow S., Dellinger R. P. (2012). Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: A prospective cohort study. Lancet Infect. Dis. 12, 919–924. [DOI] [PubMed] [Google Scholar]
- Liu J., Han X., Soriano S. G., Yuki K. (2014). The role of macrophage-1 antigen in polymicrobial sepsis. Shock 42, 532–539. [DOI] [PubMed] [Google Scholar]
- Marshall J. C. (2014). Why have clinical trials in sepsis failed? Trends Mol. Med. 20, 195–203. [DOI] [PubMed] [Google Scholar]
- Martin G. S., Mannino D. M., Eaton S., Moss M. (2003). The epidemiology of sepsis in the United States from 1979 through 2000. N Engl. J. Med. 348, 1546–1554. [DOI] [PubMed] [Google Scholar]
- Nishida N., Xie C., Shimaoka M., Cheng Y., Walz T., Springer T. A. (2006). Activation of leukocyte beta2 integrins by conversion from bent to extended conformations. Immunity 25, 583–594. [DOI] [PubMed] [Google Scholar]
- Nordenfelt P., Tapper H. (2011). Phagosome dynamics during phagocytosis by neutrophils. J. Leukoc. Biol. 90, 271–284. [DOI] [PubMed] [Google Scholar]
- Peake S. L., Delaney A., Bailey M., Bellomo R., Cameron P. A., Cooper D. J., Higgins A. M., Holdgate A., Howe B. D., Webb S. A., Williams P. (2014). Goal-directed resuscitation for patients with early septic shock. N. Engl. J. Med. 371, 1496–1506. [DOI] [PubMed] [Google Scholar]
- Perouasnsly M., Peaece R. A., Hemmings H. C. Jr. (2009). Inhaled anesthetics: Mechanisms of action In Miller’s Anesthesia (R. Miller, Ed.), 7th ed. Elsevier, Atlanta, GA. [Google Scholar]
- Phillipson M., Heit B., Colarusso P., Liu L., Ballantyne C. M., Kubes P. (2006). Intraluminal crawling of neutrophils to emigration sites: A molecularly distinct process from adhesion in the recruitment cascade. J. Exp. Med. 203, 2569–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson M., Kubes P. (2011). The neutrophil in vascular inflammation. Nat. Med. 17, 1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince J. E., Brayton C. F., Fossett M. C., Durand J. A., Kaplan S. L., Smith C. W., Ballantyne C. M. (2001). The differential roles of LFA-1 and Mac-1 in host defense against systemic infection with Streptococcus pneumoniae. J. Immunol. 166, 7362–7369. [DOI] [PubMed] [Google Scholar]
- Prodeus A. P., Zhou X., Maurer M., Galli S. J., Carroll M. C. (1997). Impaired mast cell-dependent natural immunity in complement C3-deficient mice. Nature 390, 172–175. [DOI] [PubMed] [Google Scholar]
- Rittirsch D., Huber-Lang M. S., Flierl M. A., Ward P. A. (2009). Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 4, 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Martin L., Sanchez-Sanchez N., Gutierrez-Lopez M. D., Rojo A. I., Vicente-Manzanares M., Perez-Alvarez M. J., Sanchez-Mateos P., Bustelo X. R., Cuadrado A., Sanchez-Madrid F., Rodriguez-Fernandez J. L., Cabanas C. (2004). Signaling through the leukocyte integrin LFA-1 in T cells induces a transient activation of Rac-1 that is regulated by Vav and PI3K/Akt-1. J. Biol. Chem. 279, 16194–16205. [DOI] [PubMed] [Google Scholar]
- Schorr C. A., Dellinger R. P. (2014). The Surviving Sepsis Campaign: Past, present and future. Trends Mol. Med. 20, 192–194. [DOI] [PubMed] [Google Scholar]
- Torio C., Andrews R. M. (2013) National inpatient hospital costs: The most expensive conditions by payer 2011 HUCP statistical brief #160. Agenecy for healthcare research and quality, Rockville, MD: Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb160.pdf. [PubMed] [Google Scholar]
- Von Dossow V., Baur S., Sander M., Tonnesen H., Marks C., Paschen C., Berger G., Spies C. D. (2007). Propofol increased the interleukin-6 to interleukin-10 ratio more than isoflurane after surgery in long-term alcoholic patients. J. Int. Med. Res. 35, 395–405. [DOI] [PubMed] [Google Scholar]
- Wagner D. D., Frenette P. S. (2008). The vessel wall and its interactions. Blood 111, 5271–5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz-Schmidt G., Chreng S. (2012). Cell adhesion assays. Methods Mol. Biol. 757, 15–30. [DOI] [PubMed] [Google Scholar]
- Williams M. R., Azcutia V., Newton G., Alcaide P., Luscinskas F. W. (2011). Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 32, 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yealy D. M., Kellum J. A., Huang D. T., Barnato A. E., Weissfeld L. A., Pike F., Terndrup T., Wang H. E., Hou P. C., LoVecchio F., Filbin M. R., Shapiro N. I., Angus D. C. (2014). A randomized trial of protocol-based care for early septic shock. N. Engl. J. Med. 370, 1683–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki K., Astrof N. S., Bracken C., Soriano S. G., Shimaoka M. (2010). Sevoflurane binds and allosterically blocks integrin lymphocyte function-associated antigen-1. Anesthesiology 113, 600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki K., Astrof N. S., Bracken C., Yoo R., Silkworth W., Soriano S. G., Shimaoka M. (2008). The volatile anesthetic isoflurane perturbs conformational activation of integrin LFA-1 by binding to the allosteric regulatory cavity. faseb J. 22, 4109–4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki K., Bu W., Xi J., Sen M., Shimaoka M., Eckenhoff R. G. (2012). Isoflurane binds and stabilizes a closed conformation of the leukocyte function-associated antigen-1. faseb J. 26, 4408–4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki K., Bu W., Xi J., Shimaoka M., Eckenhoff R. (2013). Propofol shares the binding site with isoflurane and sevoflurane on leukocyte function-associated antigen-1. Anesth. Analg. 117, 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Astrof N. S., Liu J. H., Wang J. H., Shimaoka M. (2009). Crystal structure of isoflurane bound to integrin LFA-1 supports a unified mechanism of volatile anesthetic action in the immune and central nervous systems. faseb J. 23, 2735–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.