Abstract
Propionimicrobium lymphophilum is an anaerobic Gram-positive bacillus that exists in human skin and urinary tract. The pathogenicity is, however, not well known. Only two cases of urinary tract infection have been described recently. In the case presented here, the bacterium was isolated, concomitant with Actinotignum schaalii, from blood culture of a patient with fever and difficulty of urination. The bacteria were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and 16S rRNA sequencing. The case was successfully treated with ampicillin/sulbactam.
Keywords: Actinotignum schaalii, bacteraemia, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, Propionimicrobium lymphophilum, 16S rRNA sequencing
Introduction
Propionimicrobium lymphophilum was originally described as a member of the Corynebacterium. However, because it growths under anaerobic condition and produces propionic acid, this bacteria was classified as Propionibacterium and then was reclassified in 2002 as a single species in the genus Propionimicrobium [1]. This organism colonizes in the human skin and genital tract [2], [3], [4].
Actinotignum schaalii is a facultative anaerobic Gram-positive bacillus and originally was described as a member of Actinobaculum in 1997 [5]. As of 2015, the genus Actinobaculum was divided into Actinotignum and Actinobaculum [6]. A. schaalii is currently considered to be an emerging uropathogen because it has been associated with urinary tract infections (UTIs) in many cases [7], [8], [9], [10]. In contrast to that of A. schaalii, the pathogenicity of P. lymphophilum remains poorly defined. A single case report was published of nonbacteraemic UTI of the anaerobe [11].
Here we report the first case of P. lymphophilum bacteraemia with A. schaalii. The organisms were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) and 16S rRNA sequencing, and the case was successfully treated with ampicillin/sulbactam.
Case Report
An 80-year-old man visited our emergency department complaining of fever, chills and general fatigue after several days with difficulty urinating. The patient regularly visited the outpatient clinic for postsurgical follow-up of colon cancer with pulmonary metastasis. He also had bladder dysfunction due to diabetes mellitus neuropathy.
At admission, he had no significant physical signs, but his vital signs were as follows: body temperature, 38.9°C; blood pressure, 164/74 mm Hg; pulse rate, 95/min; and respiratory rate, 24/min. Laboratory data were as follows: white blood cell count, 9300/μL (neutrophil 71.9%); serum creatinine, 1.79 mg/dL; and C-reactive protein, 7.01 mg/dL. Urinary analysis revealed 3+ leukocyte esterase and positivity for nitrites. Abdominal computed tomography revealed bilateral hydronephrosis and bilateral dilatation of the ureters. The presence of fluid around the seminal gland and thickening of the urinary bladder were also observed.
Insertion of a urinary catheter resulted in the discharge of more than 1000 mL of turbid urine. The patient was clinically diagnosed with postrenal pyelonephritis by urinary stasis due to neurogenic bladder. After the collection of two sets of blood culture bottles with aseptic procedure and one urine specimen for culturing, ceftriaxone therapy was initiated (day 0). On day 2, the urine culture detected only α-streptococci on sheep’s blood/chocolate agar (Eiken Chemicals, Tokyo, Japan), incubated at 37°C in 5% CO2. Although it was inoculated for subculture on sheep’s blood/chocolate agar again, no colony growth was observed. The α-streptococci could not be isolated and identified. No anaerobic culture was performed in routine analysis.
Two anaerobic bottles of two sets of blood cultures (BacT/ALERT; SYSMEX bioMérieux, Tokyo, Japan) collected on day 0 yielded one each of two Gram-positive bacilli on day 4 and day 5. After a further 72 hours under anaerobic conditions, streaks of both of the isolates grew as small grey-colored, smooth colonies on Brucella agar (Fig. 1, Fig. 2). Both anaerobes were identified as Actinomyces meyeri by VITEK2 ANC identification card (SYSMEX bioMérieux). However, MALDI-TOF MS (using the MALDI Biotyper; Bruker Daltonics, Bremen, Germany) identified one of the anaerobes as Actinotignum schaalii and the other as Propionimicrobium lymphophilum.
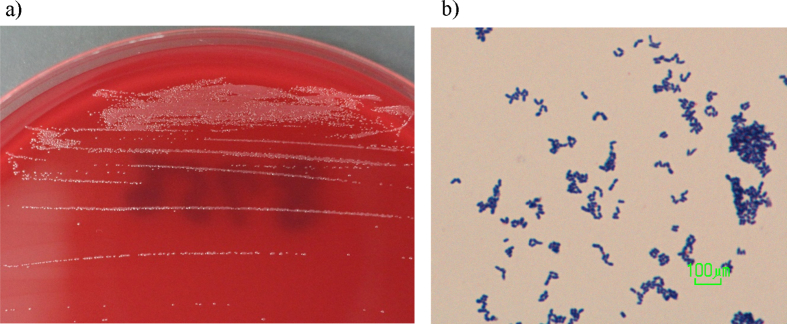
Fig. 1.
Morphology of Propionimicrobium lymphophilum. (a) Appearance of colonies was small, grey and tiny after 72 hours' growth on Brucella agar medium under anaerobic conditions. (b) Gram staining of (a) revealed the presence of short Gram-positive bacilli with coccoid form.
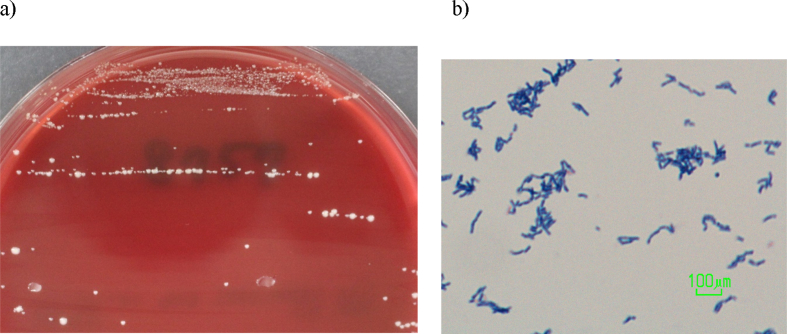
Fig. 2.
Morphology of Actinotignum schaalii. (a) Appearance of colonies was small, light yellow to grey-colored and smooth after 72 hours' growth on Brucella agar medium under anaerobic conditions. (b) Gram staining of (a) revealed the presence of Gram-positive slightly curved rods that seemed to be longer than P. lymphophilum.
16S rRNA sequencing was also performed [12] and identified these bacteria as A. schaalii and P. lymphophilum, respectively. Specifically, comparison to sequences in the BLAST database (http://www.ncbi.nlm.nih.gov/BLAST) revealed that the case isolates showed 99% homology with GenBank accession numbers HQ992948 and HM135521 respectively. Antimicrobial susceptibility testing was performed anaerobically by Etest (SYSMEX bioMérieux) on Brucella HK agar plates (Kyokuto Pharm Ind, Tokyo, Japan). The minimum inhibitory concentration (MIC) results are shown in Table 1.
Table 1.
Antimicrobial susceptibility of Propionimicrobium lymphophilum and Actinotignum schaalii
| Antimicrobial | Minimum inhibitory concentrations (mg/L) |
|
|---|---|---|
| A. schaalii | P. lymphophilum | |
| Ampicillin | <0.016 | <0.016 |
| Cefotaxime | 0.012 | 0.064 |
| Cefepime | 0.016 | 0.25 |
| Cefoxitin | 0.016 | 0.19 |
| Meropenem | 0.008 | <0.002 |
| Vancomycin | 0.094 | 0.064 |
| Clindamycin | <0.016 | 0.032 |
| Clarithromycin | <0.016 | <0.016 |
| Minocycline | 0.023 | <0.016 |
| Levofloxacin | 1 | 0.5 |
| Metronidazole | >256 | >256 |
Minimum inhibitory concentrations were determined by Etest on Brucella HK agar plates.
The antibiotic therapy was changed on day 9 to ampicillin/sulbactam. Two sets of blood culture bottles were collected at the time of changeover in antimicrobial agent; both of these blood cultures were sterile. On day 12, abdominal computed tomography revealed improvement of bilateral pyelonephritis, and magnetic resonance imaging indicated the presence of prostate cancer and invasion of the seminal vesicles. A biopsy of the prostate was performed, and the presence of prostate cancer was pathologically confirmed. Therefore, we suspected that P. lymphophilum and A. schaalii might cause bacteraemia through UTI with invasive prostate cancer and neurogenic bladder. Recurrence of bacteraemia by these anaerobes was not observed after completion of 32 days of antimicrobial therapy.
Discussion
We describe here a case of bacteraemia due to P. lymphophilum and A. schaalii. A. schaalii has already been recognized as an emerging human uropathogen [7], [8], [9], [10]. Increased risk of A. schaalii infection has been reported for the elderly, especially those with diseases of the genitourinary region, and for newborns using diapers [10], [13]. The present case was considered as high risk for A. schaalii infection because the patient was elder with prostate cancer.
P. lymphophilum has been reported as a possible urogenital pathogen based on metagenomic analysis using 16S rRNA sequencing [4]. Two cases have been reported of nonbacteraemic UTI associated with P. lymphophilum [11]. In this case, P. lymphophilum caused bacteraemia from the patient with UTI. Further cases are needed to clear the clinical characteristics and risk factors for P. lymphophilum infection.
MALDI-TOF MS has been reported to provide more accurate identification of bacteria, even anaerobes, compared to phenotypic methods based on biochemical reactions (e.g. VITEK2) [14]. Indeed, in the present work, A. schaalii and P. lymphophilum were misidentified as A. meyeri by the VITEK2 system. Although the reference standard for bacterial identification is 16S rRNA sequencing, such sequencing is not routinely used in clinical microbial laboratories. MALDI-TOF MS was useful for the rapid correct identification of these anaerobic bacilli in this case.
A. schaalii is susceptible to penicillin, cephalosporin and carbapenem [10], [13] but has high MICs of sulfamethoxazole/trimethoprim and fluoroquinolones, which are antibiotics often used to treat UTIs. In the present case, P. lymphophilum showed low MICs of β-lactam antibiotics and a high MIC of levofloxacin. Clinical breakpoints of antimicrobial susceptibility against P. lymphophilum or A. schaalii are not provided. In the present case, anaerobic polymicrobial bacteraemia was diagnosed; given that β-lactam antibiotics are the preferred therapy for A. schaalii, treatment with ampicillin/sulbactam was selected.
We report here the first case and successful treatment of bacteraemia due to P. lymphophilum and A. schaalii. MALDI-TOF MS was useful for the rapid correct identification of these anaerobic bacilli.
MALDI-TOF MS spectrum accession number
The MALDI-TOF MS spectrum of the Propionimicrobium lymphophilum isolate (Propionimicrobium lymphophilum 8756) and the Actinotignum schaalii isolate (Actinotignum schaalii 8757) are available online (http://mediterranee-infection.com/article.php?laref=256&titre=urms-database).
Nucleotide sequence accession number
The 16S rRNA gene sequences were deposited in DDBJ/EMBL/GenBank databases under the accession numbers LC222741 for Propionimicrobium lymphophilum and LC222742 for Actinotignum schaalii.
Ethics statement
Written informed consent for publication of the clinical details was obtained from the patient.
Conflict of Interest
None declared.
References
- 1.Stackebrandt E., Schumann P., Schaal K.P., Weiss N. Propionimicrobium gen. nov., a new genus to accommodate Propionibacterium lymphophilum (Torrey 1916) Johnson and Cummins 1972, 1057AL as Propionimicrobium lymphophilum comb. nov. Int J Syst Evol Microbiol. 2002;52:1925–1927. doi: 10.1099/00207713-52-6-1925. [DOI] [PubMed] [Google Scholar]
- 2.Pitcher D.G., Collins M.D. Phylogenetic analysis of some ll-diaminopimelic acid–containing coryneform bacteria from human skin: description of Propionibacterium innocuum sp. nov. FEMS Microbiol Lett. 1991;68:295–300. doi: 10.1016/0378-1097(91)90372-h. [DOI] [PubMed] [Google Scholar]
- 3.Torrey J.C. Bacteria associated with certain types of abnormal lymph glands. J Med Res. 1916;34 65–80.1. [PMC free article] [PubMed] [Google Scholar]
- 4.Imirzalioglu C., Hain T., Chakraborty T., Domann E. Hidden pathogens uncovered: metagenomic analysis of urinary tract infections. Andrologia. 2008;40:66–71. doi: 10.1111/j.1439-0272.2007.00830.x. [DOI] [PubMed] [Google Scholar]
- 5.Lawson P.A., Falsen E., Akervall E., Vandamme P., Collins M.D. Characterization of some Actinomyces-like isolates from human clinical specimens: reclassification of Actinomyces suis (Soltys and Spratling) as Actinobaculum suis comb. nov. and description of Actinobaculum schaalii sp. nov. Int J Syst Bacteriol. 1997;47:899–903. doi: 10.1099/00207713-47-3-899. [DOI] [PubMed] [Google Scholar]
- 6.Yassin A.F., Spröer C., Pukall R., Sylvester M., Siering C., Schumann P. Dissection of the genus Actinobaculum: reclassification of Actinobaculum schaalii Lawson et al. 1997 and Actinobaculum urinale Hall et al. 2003 as Actinotignum schaalii gen. nov., comb. nov. and Actinotignum urinale comb. nov., description of Actinotignum sanguinis sp. nov. and emended descriptions of the genus Actinobaculum and Actinobaculum suis; and re-examination of the culture deposited as Actinobaculum massiliense CCUG 47753T ( =DSM 19118T), revealing that it does not represent a strain of this species. Int J Syst Evol Microbiol. 2015;65:615–624. doi: 10.1099/ijs.0.069294-0. [DOI] [PubMed] [Google Scholar]
- 7.Lotte L., Lotte R., Durand M., Degand N., Ambrosetti D., Michiels J.F. Infections related to Actinotignum schaalii (formerly Actinobaculum schaalii): a 3-year prospective observational study on 50 cases. Clin Microbiol Infect. 2016;22:388–390. doi: 10.1016/j.cmi.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 8.Bank S., Søby K.M., Kristensen L.H., Voldstedlund M., Prag J. A validation of the Danish microbiology database (MiBa) and incidence rate of Actinotignum schaalii (Actinobaculum schaalii) bacteraemia in Denmark. Clin Microbiol Infect. 2015;21 doi: 10.1016/j.cmi.2015.08.006. 1097.e1–1097.e4. [DOI] [PubMed] [Google Scholar]
- 9.Barberis C., Cittadini R., del Castillo M., Acevedo P., García Roig C., Ramírez M.S. Actinobaculum schaalii causing urinary tract infections: report of four cases from Argentina. J Infect Dev Ctries. 2014;8:240–244. doi: 10.3855/jidc.3748. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen H., Senneby E., Rasmussen M. Clinical and microbiological features of Actinotignum bacteremia: a retrospective observational study of 57 cases. Eur J Clin Microbiol Infect Dis. 2017;36(5):791–796. doi: 10.1007/s10096-016-2862-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams G.D. Two cases of urinary tract infection caused by Propionimicrobium lymphophilum. J Clin Microbiol. 2015;53:3077–3080. doi: 10.1128/JCM.00438-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lane D.J. 16S/23S rRNA sequencing. In: Stackebrandt E., Goodfellow M., editors. Nucleic acid techniques in bacterial systematics. Wiley; Chichester: 1991. pp. 115–175. [Google Scholar]
- 13.Lotte R., Lotte L., Ruimy R. Actinotignum schaalii (formerly Actinobaculum schaalii): a newly recognized pathogen—review of the literature. Clin Microbiol Infect. 2016;22:28–36. doi: 10.1016/j.cmi.2015.10.038. [DOI] [PubMed] [Google Scholar]
- 14.Yunoki T., Matsumura Y., Nakano S., Kato K., Hotta G., Noguchi T. Genetic, phenotypic and matrix-assisted laser desorption ionization time-of-flight mass spectrometry–based identification of anaerobic bacteria and determination of their antimicrobial susceptibility at a University Hospital in Japan. J Infect Chemother. 2016;22:303–307. doi: 10.1016/j.jiac.2016.01.014. [DOI] [PubMed] [Google Scholar]