Abstract
Background: More than 1 million women per year in the United States with benign breast biopsies are known to be at elevated risk for breast cancer (BC), with risk stratified on histologic categories of epithelial proliferation. Here we assessed women who had serial benign biopsies over time and how changes in the histologic classification affected BC risk.
Methods: In the Mayo Clinic Benign Breast Disease Cohort of 13 466 women, 1414 women had multiple metachronous benign biopsies (10.5%). Both initial and subsequent biopsies were assessed histologically. BC risk for clinical and prognostic factors was assessed using subdistribution models to account for competing risks, and logistic regression/Wilcoxon/chi-square tests to assess covariates. All statistical tests were two-sided.
Results: Breast cancer risk for women with serial biopsies, stratified by histologic category in the later biopsies, was similar to women with a single biopsy. We found that changes in histological category between initial and subsequent biopsy statistically significantly impacted BC risk. Women with nonproliferative initial findings and subsequent proliferative findings had an increased risk (hazard ratio [HR] = 1.77, 95% confidence interval [CI] = 1.06 to 2.94, P = .03) compared with no change. Among women with proliferative disease without atypia at initial biopsy, risk decreased if later biopsy regressed to nonproliferative (HR = 0.49, 95% CI = 0.25 to 0.98) and increased if later biopsy showed progression to atypical hyperplasia (HR = 1.49, 95% CI = 0.73 to 3.05) compared with no change (P = .04).
Conclusions: We found that breast cancer risk increases in women with progressive epithelial proliferation over time and decreases in women whose biopsies show less proliferation. This finding has important implications for effective clinical management of the 100 000 women per year who have multiple benign breast biopsies.
One to 2 million women per year in the United States have a breast biopsy with benign findings (1,2) and are thus classified as having benign breast disease (BBD). As a group, women with BBD are known to have a higher risk for subsequent development of breast cancer (BC), and this risk can be stratified by degree of histological abnormality in the benign biopsy (3–5). Histological findings in BBD comprise a highly diverse spectrum of changes involving both terminal duct lobular unit (TDLU) epithelium and accompanying stromal/connective tissue constituents. Over half of benign biopsies in clinical practice are characterized by nonproliferative (NP) changes, consisting mostly of simple cysts, fibrosis, or fibroadenoma; these women are at only slightly increased risk for subsequent BC development (4). Most of the remaining biopsies demonstrate one or more forms of conventional (ie, nonatypical) epithelial hyperplasia, collectively referred to as proliferative disease without atypia (PDWA). PDWA conveys an approximately twofold increased likelihood for subsequent BC compared with age-matched population controls (4,6–8). The remaining 10% of benign breast biopsies show atypical hyperplasia (AH); for women with AH, BC risk is elevated approximately fourfold (9–11).
Because histological subtype of BBD is strongly associated with risk, this information is central to the assessment and management of BC risk. Other factors that affect BC risk for women with BBD include BRCA1/2 mutations, family history of BC, extent of age-related lobular involution, mammographic breast density, and parity (4,12–14), and models developed for prediction of BC risk have identified complex interactions of these factors for risk assessment (15–18). Analysis of cohorts of women with BBD indicate that a substantial fraction have undergone a prior benign breast biopsy (4,19), and two commonly used models for BC risk assessment, the Gail/Breast Cancer Risk Assessment Tool (BCRAT) and International Breast cancer Intervention Study (IBIS) models, incorporate number of benign biopsies as a risk prediction feature (15,16). However, there has not previously been information regarding how specific characteristics of the prior BBD impact BC risk at subsequent biopsy for women with multiple serial benign biopsies. The fact that breast cancers in women with any form of BBD typically develop on average eight to 12 years after their benign biopsy (4) implies that breast carcinogenesis is lengthy and complex. Risk assessments based on pathologic findings observed at a single time point may lack potentially relevant data.
Because most women will undergo only one benign biopsy in clinical practice, the natural history of the microscopic lesions that comprise BBD is obscure. It is unknown, for example, whether and how often proliferative or atypical lesions persist, “regress” (to nonproliferative), or “progress” (ie, from proliferative to atypical). Knowledge of histological changes in women with BBD over time might be useful not only for improved BC risk assessment, but also for substantially improved understanding of the natural history of BC development overall.
This study reports the pathologic findings and breast cancer occurrence in a single institutional cohort of women with BBD who underwent metachronous benign biopsies. We hypothesized that histological category would change in at least some follow-up biopsies (ie, compared with the initial biopsy) and that risk status might change in a clinically significant manner for groups defined by different pathologic findings in second biopsies.
Methods
Study Population
The Mayo Benign Breast Disease Cohort is an ongoing internal review board–approved initiative that presently consists of 13 466 women age 18 to 85 years who underwent a benign breast biopsy for clinical indication at Mayo Clinic Rochester during the years 1967 to 2001, as detailed previously (4). Within this cohort, women were identified who had undergone more than one benign biopsy prior to any diagnosis of breast cancer (n = 1649). Biopsies occurring within 60 days of the index biopsy were excluded (n = 168), as well as 67 subjects without complete histology information, resulting in a final sample size of 1414. Outcomes and demographic data were obtained via medical records, tumor registries, and serial questionnaires. Family history was categorized as none, weak, or strong. Women were categorized as strong family history if they had at least one first-degree relative with breast cancer before the age of 50 years or two or more relatives with breast cancer, with at least one being a first-degree relative. Any lesser degree of family history of breast cancer was categorized as weak. Appropriate documentation of research authorization as per Mayo Clinic Institutional Review Board policies was obtained.
Histological Examination
Original archival hematoxylin and eosin–stained tissue sections from all biopsies were reviewed by a single study pathologist (DWV) who was blinded to initial pathologic diagnoses and all clinical data, including outcome. Overall histological classifications were assigned to all biopsies as described previously (Supplementary Table 1, available online) (4,14).
Statistical Analyses
Among all women, we compared those in the multiple biopsy cohort (MBC) with those with just one biopsy using chi-square tests for categorical variables and Wilcoxon rank sum tests for continuous variables. We calculated age-adjusted P values using logistic regression. Similar comparisons were made across development of a subsequent breast cancer (yes vs no) for women in the MBC. The duration of follow-up was calculated as days from benign biopsy to breast cancer diagnosis, death, prophylactic mastectomy, reduction mammoplasty, LCIS, or last contact. We examined comparisons of time from initial and follow-up biopsy to breast cancer using Fine and Gray’s proportional subdistribution hazards model, accounting for death as a competing risk (20). Bowker’s test of symmetry was used to compare impression at index and second biopsy.
We examined associations of clinical and pathological characteristics at index biopsy with change in overall impression using logistic regression, stratified by histologic impression. Each variable was initially examined univariately; subsequent analyses adjusted for all variables found to be statistically significant in univariate models.
We fit Fine and Gray models stratified by impression at index biopsy as NP women are not able to regress and AH women are not able to progress. Analyses adjusted for age at index biopsy, year of index biopsy, extent of lobular involution, family history of breast cancer, and time between index and second biopsy. One final model was fit including women of all index impressions. Cumulative incidence curves accounting for death as a competing risk were created by impression at second biopsy for illustrative purposes. Breast cancer risk at follow-up biopsy in the MBC was compared with risk at index biopsy for women not in the MBC using 1 degree of freedom Wald tests on hazard ratios (HRs) stratified by presence in the MBC. All analyses were performed using SAS version 9.4. A P value of less than .05 was considered statistically significant, and all statistical tests were two-sided.
Results
Demographic, Histological, and Outcome Characteristics of the Multiple Biopsy Cohort
Overall, women in the multiple biopsy cohort (MBC) comprised 10.5% of the Mayo BBD cohort (1414/13 466) (Supplementary Table 2, available online). Age at time of (initial) biopsy was statistically significantly younger for patients in the MBC compared with the overall BBD cohort (48% vs 31% who were younger than age 45 years, P < .001). Women in the MBC were also statistically significantly more likely to have a family history of breast cancer (49.3% vs 37.9%, P = .001). At initial biopsy, 61.0% of MBC women were classified histologically as NP, 34.4% as PDWAs, and 4.6% as AH. This distribution was not statistically significantly different from the Mayo BBD cohort overall.
The median time between biopsies was 5.6 years (Table 1). About half of the second biopsies occurred within five years of the initial biopsy (21.4% were < 2 years, 25.3% were 2–5 years) but a substantial proportion (30%) occurred more than 10 years later (indications for performance of subsequent biopsy in Supplementary Table 3, available online). Second biopsies were statistically significantly more likely than initial biopsies to harbor proliferative and atypical histological findings (Table 2): 41.4% of second biopsies were PDWA or AH compared with 34.4% of initial biopsies, and indication for subsequent biopsy differed by histologic impression of subsequent biopsy (Supplementary Table 4, available online). Similarly, AH classification was more common among follow-up samples (10.3% vs 4.6%, P ≤ .001). Although most patients (56.1%) retained the same overall histological classification in both samples, 29.9% progressed from NP or PDWA at second biopsy, and 14.0% regressed from AH or PDWA.
Table 1.
Clinical and pathological characteristics by later breast cancer status
| Covariate | Unaffected (n = 1274) | Breast cancer (n = 140) | Total (n = 1414) | Age adjusted* |
|
|---|---|---|---|---|---|
| HR (95% CI) | P† | ||||
| Duration of follow-up‡ | |||||
| From index biopsy | – | – | |||
| Median | 20.6 | 16.1 | 20.3 | ||
| Q1, Q3 | 15.0, 29.0 | 9.3, 25.3 | 14.6, 28.7 | ||
| Range | 0.7–47.5 | 2.2–40.4 | 0.7–47.5 | ||
| From follow-up biopsy | – | – | |||
| Median | 13.7 | 9.5 | 13.4 | ||
| Q1, Q3 | 8.2, 19.4 | 4.2, 16.4 | 7.8, 19.2 | ||
| Range | 0.0–40.6 | 0.5–35.8 | 0.0–40.6 | ||
| Index biopsy characteristics | |||||
| Age of BBD, No. (%), y | .56 | ||||
| <45 | 622 (48.8) | 59 (42.1) | 681 (48.2) | 1.00 (ref) | |
| 45–55 | 402 (31.6) | 49 (35.0) | 451 (31.9) | 0.97 (0.64 to 1.47) | |
| >55 | 250 (19.6) | 32 (22.9) | 282 (19.9) | 1.30 (0.73 to 2.30) | |
| Year of biopsy, No. (%) | .15 | ||||
| 1967–1981 | 397 (31.2) | 53 (37.9) | 450 (31.8) | 1.00 (ref) | |
| 1982–1991 | 402 (31.6) | 45 (32.1) | 447 (31.6) | 1.06 (0.67 to 1.67) | |
| 1992–2001 | 475 (37.3) | 42 (30.0) | 517 (36.6) | 1.59 (0.95 to 2.65) | |
| Overall impression, No. (%) | <.001 | ||||
| NP | 798 (62.6) | 65 (46.4) | 863 (61.0) | 1.00 (ref) | |
| PDWA | 426 (33.4) | 60 (42.9) | 486 (34.4) | 1.79 (1.20 to 2.66) | |
| AH | 50 (3.9) | 15 (10.7) | 65 (4.6) | 4.60 (2.41 to 8.79) | |
| Involution, No. (%) | |||||
| Missing | 135 | 17 | 152 | .46 | |
| None | 285 (25.0) | 29 (23.6) | 314 (24.9) | 1.00 (ref) | |
| Partial | 653 (57.3) | 79 (64.2) | 732 (58.0) | 1.03 (0.66 to 1.62) | |
| Complete | 201 (17.6) | 15 (12.2) | 216 (17.1) | 0.69 (0.33 to 1.45) | |
| Second biopsy characteristics | |||||
| Time between biopsies | .86 | ||||
| Median | 5.7 | 4.6 | 5.6 | 1.00 (0.97 to 1.02) | |
| Q1, Q3 | 2.3, 11.1 | 2.1, 10.1 | 2.3, 11.1 | ||
| Range | 0.2, 43.0 | 0.2, 25.3 | 0.2, 43.0 | ||
| Age, No. (%), y | .39 | ||||
| <45 | 333 (26.1) | 31 (22.1) | 364 (25.7) | 1.00 (ref) | |
| 45–55 | 427 (33.5) | 51 (36.4) | 478 (33.8) | 1.25 (0.80 to 1.94) | |
| >55 | 514 (40.4) | 58 (41.4) | 572 (40.5) | 1.35 (0.88 to 2.07) | |
| Overall impression, No. (%) | <.001 | ||||
| NP | 636 (49.9) | 46 (32.9) | 682 (48.2) | 1.00 (ref) | |
| PDWA | 517 (40.6) | 69 (49.3) | 586 (41.4) | 1.77 (1.22 to 2.57) | |
| AH | 121 (9.5) | 25 (17.9) | 146 (10.3) | 3.40 (2.08 to 5.55) | |
| Involution, No. (%) | |||||
| Missing§ | 93 | 9 | 102 | .16 | |
| None | 82 (6.9) | 16 (12.2) | 98 (7.5) | 1.00 (ref) | |
| Partial | 611 (51.7) | 76 (58.0) | 687 (52.4) | 0.81 (0.47 to 1.38) | |
| Complete | 488 (41.3) | 39 (29.8) | 527 (40.2) | 0.60 (0.34 to 1.07) | |
Follow-up variables were used as the time covariate in the regression modeling. AH = atypical hyperplasia; CI = confidence interval; HR = hazard ratio; NP = nonproliferative disease; PDWA = proliferative disease without atypia.
The P value was calculated using a two-sided type 3 Wald test.
Proportional subdistribution hazards model with death as a competing risk. Time was modeled as time from index biopsy to cancer for index biopsy characteristics and time from second biopsy to cancer for secondary biopsy characteristics. Age at index biopsy was used as an adjustment term for the characteristics at index biopsy, and age at second biopsy was used for characteristics at second biopsy.
Involution status was not assessed in samples with fewer than four background lobules.
Table 2.
Overall impression at index and second biopsy
| Impression at second biopsy | No. (%) | Impression at index biopsy |
P* | ||
|---|---|---|---|---|---|
| NP (n = 863, 61.0%) No. (%) | PDWA (n = 486, 34.3%) No. (%) | AH (n = 65, 4.6%) No. (%) | |||
| NP | 682 (48.2) | 509 (59.0) | 156 (32.1) | 17 (26.2) | <.001 |
| PDWA | 586 (41.4) | 300 (34.8) | 261 (53.7) | 25 (38.5) | |
| AH | 146 (10.3) | 54 (6.3) | 69 (14.2) | 23 (35.4) | |
Two-sided Bowker's test of symmetry. AH = atypical hyperplasia; NP = nonproliferative disease; PDWA = proliferative disease without atypia.
Breast cancers developed (ie, after second biopsy) in 140 of the 1414 women (9.9%). Women who developed breast cancer were more likely to have AH at either the first or second biopsy compared with women who did not develop cancer; furthermore, the highest frequencies of PDWA and AH (49.3% and 17.9%, respectively) were present among the follow-up biopsies of patients who subsequently developed breast cancer (Table 1). Breast cancer risk at initial and subsequent biopsies was not affected by age of biopsy, year of biopsy, or lobular involution. Histologic impression in adjacent benign tissue was available for 124 of the 140 breast cancer cases (Supplementary Table 5, available online). In these, the presence of atypical findings was far more common than what was found in the previous benign biopsies: 80 (64.5%) had either atypical hyperplasia or lobular carcinoma in situ adjacent to the cancerous tissue, another 35 (28.2%) had proliferative disease without atypia, and nine (7.3%) had nonproliferative findings.
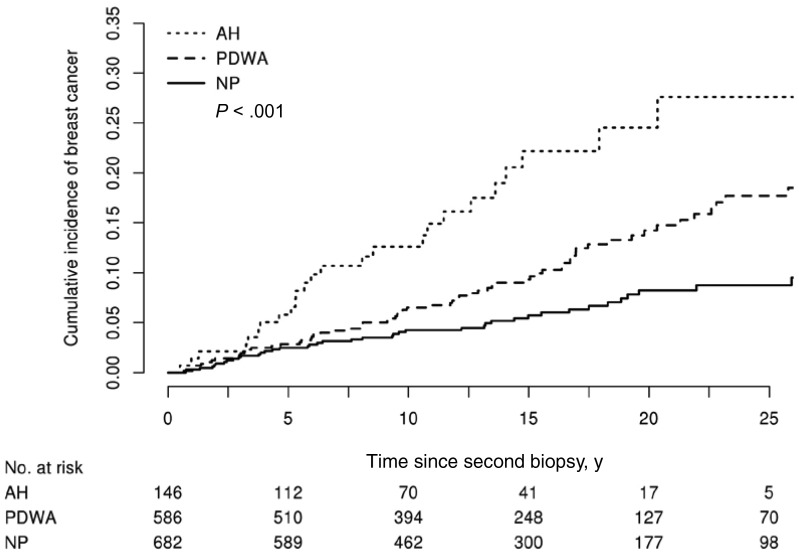
Women with AH had increased risk of breast cancer, whether present at the initial (HR = 4.60, 95% confidence interval [CI] = 2.41 to 8.79, P < .001) or follow-up biopsy (HR = 3.40, 95% CI = 2.08 to 5.55, P < .001) (Table 1). Long-term risk for women based on histologic classification in the second biopsy was stratified by histologic category (Figure 1), similar to prior findings in the overall BBD cohort (4).
Figure 1.
Incidence of breast cancer by histological impression in the multiple biopsy cohort. Cumulative incidence of breast cancer post–second biopsy by histologic impression category at second biopsy using the competing risk approach with death modeled as a competing event. The P value was calculated using a two-sided Gray’s test for equality. AH = atypical hyperplasia; NP = nonproliferative disease; PDWA = proliferative disease without atypia.
Joint Effects of Histologic Impression at Initial and Second Biopsy
Multivariable survival analyses examining the independent effects of histologic classification at initial biopsy and at second biopsy for those in the MBC are provided in Supplementary Table 6 (available online). After adjustment for relevant demographic and clinical characteristics and for histologic impression at second biopsy, impression at first biopsy remained a statistically significant predictor of breast cancer risk (PDWA HR = 1.56, 95% CI = 1.04 to 2.35; AH HR = 3.24, 95% CI = 1.61 to 6.52; P = .003). Histologic impression at second biopsy was similarly independently associated with risk after adjustment for demographic and clinical variables and impression at initial biopsy (PDWA HR = 1.60, 95% CI = 1.06 to 2.42; AH HR = 2.42, 95% CI = 1.37 to 4.29; P = .007).
Analysis of Factors Associated With Changed Histological Impression in Second Biopsy
For women with NP at index biopsy, age at biopsy (<45 years less likely and 45–55 years more likely), time between biopsies (5+ years more likely, <5 years less likely), presence of columnar alteration, and contralaterality of the nonsynchronous biopsies were statistically significantly associated with changes in histological impression (Supplementary Table 7, available online). In women with PDWA in their first sample, age (55+ years more likely) and usual ductal hyperplasia (none more likely) were statistically significantly associated with changes in impression, but not interval to second biopsy or laterality. In AHs, only age (55+ years less likely) and number of atypical foci (3+ less likely) were marginally associated with change in impression.
Changes in Overall Histological Classification and Breast Cancer Risk in the MBC Group
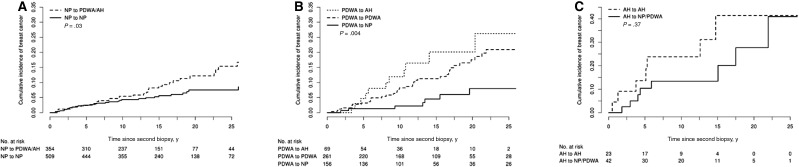
Overall risk estimates by histological category were similar for women with multiple biopsies compared with women with only a single biopsy (Supplementary Table 8, available online). Breast cancer risk was uniformly modified in the expected direction as a result of changes in overall histological classification between first and second biopsy (Table 3). When subset to NP on initial biopsy, women with PDWA/AH at follow-up biopsy were more likely to develop breast cancer than women with NP in both initial and later biopsies (HR = 1.77, 95% CI = 1.06 to 2.94, P = .03) (Table 3 and Figure 2A).
Table 3.
Associations between changes in histologic impression and breast cancer risk*
| Subgroup | No. | HR (95% CI) | P† |
|---|---|---|---|
| NP at initial biopsy | .03 | ||
| NP at later biopsy | 509 | 1.00 (ref) | |
| PDWA/AH at later biopsy | 354 | 1.77 (1.06 to 2.94) | |
| PDWA at initial biopsy | .04 | ||
| NP at later biopsy | 156 | 0.49 (0.25 to 0.98) | |
| PDWA at later biopsy | 261 | 1.00 (ref) | |
| AH at later biopsy | 69 | 1.49 (0.73 to 3.05) | |
| AH at initial biopsy | .53 | ||
| NP/PDWA at later biopsy | 42 | 0.57 (0.10 to 3.32) | |
| AH at later biopsy | 23 | 1.00 (ref) | |
| All women | <.001 | ||
| NP to NP | 509 | 1.00 (ref) | |
| NP to PDWA/AH | 354 | 1.69 (1.01 to 2.82) | |
| PDWA to NP | 156 | 1.12 (0.54 to 2.34) | |
| PDWA to PDWA | 261 | 2.32 (1.38 to 3.88) | |
| PDWA to AH | 69 | 3.23 (1.53 to 6.85) | |
| AH to NP/PDWA | 41 | 3.36 (1.34 to 8.45) | |
| AH to AH | 23 | 7.30 (2.68 to 19.86) |
All analyses adjusted for age at index biopsy, year of index biopsy, extent of lobular involution, family history of breast cancer, and time between index and second biopsy. AH = atypical hyperplasia; CI = confidence interval; HR = hazard ratio; NP = nonproliferative disease; PDWA = proliferative disease without atypia.
Proportional subdistribution hazards model with death as a competing risk. Time was modeled as time from index biopsy to cancer for index biopsy characteristics and time from second biopsy to cancer for secondary biopsy characteristics. The P value was calculated using a two-sided type 3 Wald test.
Figure 2.
Incidence of breast cancer by histological impression by change in histological impression. A) Cumulative incidence of breast cancer post–second biopsy by histologic progression at subsequent biopsy among women with nonproliferative disease at index biopsy. B) Cumulative incidence of breast cancer post–second biopsy by histologic progression at subsequent biopsy among women with proliferative disease without atypia at index biopsy. C) Cumulative incidence of breast cancer post–second biopsy by histologic progression at subsequent biopsy among women with atypical hyperplasia at index biopsy. All curves used the competing risk approach with death modeled as a competing event. Each P value was calculated using a two-sided Gray’s test for equality. AH = atypical hyperplasia; NP = nonproliferative disease; PDWA = proliferative disease without atypia.
Similarly, when subset to the category of PDWA on initial biopsy (Table 3 and Figure 2B), breast cancer risk decreased in women whose second biopsy showed NP (HR = 0.49, 95% CI = 0.25 to 0.98) and increased in women whose second biopsy showed AH (HR = 1.49, 95% CI = 0.73 to 3.05), compared with the referent group of women whose second biopsy remained PDWA (overall P = .04).
Among the 65 women diagnosed with AH at initial biopsy in the MBC, breast cancers developed in seven out of 23 (30.4%) who retained atypia vs eight out of 42 (19.0%) of those without atypia at subsequent biopsy. Although not statistically significant (possibly because of small numbers), the hazard ratio was decreased in women whose later biopsies did not show AH (HR = 0.57, 95% CI = 0.10 to 3.32, vs 1.0 ref for persistent AH) (Table 3 and Figure 2C). However, when comparing with a reference group with NP in both samples, women with AH at first biopsy only had hazard ratio of 3.36, which increased to 7.30 among women with AH in both initial and later biopsy. There was no evidence supporting risk differences between the MBC at follow-up biopsy and women without a follow-up biopsy for any of the modeled characteristics (P = .77 for PDWA histologic impression vs NP, P = .90 for AH vs NP, P = .34 for age at biopsy 45–55 vs <45 years, P = .85 for age 56+ vs <45 years, P = .53 for year of biopsy 1982–1991 vs 1967–1981, P = .49 for 1992–2014 vs 1967–1981, P = .46 for partial lobular involution vs none, P = .47 for complete lobular involution vs none, P = .20 for weak family history vs none, P = .82 for strong family history vs none) (Supplementary Table 9 and Supplementary Figure 1, available online).
To determine if our results were robust to the changes in the minimum time interval required between biopsies, we ran sensitivity analyses that re-examined associations of change in histologic impression with breast cancer risk after requiring a minimum of one year (resulting in a sample size of n = 1288), three years (n = 983), and five years (n = 754) between biopsies. Results are presented in Supplementary Table 8 (available online). We see some attenuation in effect for women progressing from PDWA at index biopsy to AH at second biopsy, but all other associations were similar to those reported in Table 3.
Discussion
It has long been known that a substantial proportion of women with BBD will have chronic, recurring, or worsening clinical and/or radiographic abnormalities. Hence, many will undergo more than one biopsy over their lifetime in order to rule out malignancy. Although some studies have observed clinical breast cancer risk association to this subset of BBD (15), the frequency and pathological aspects of these cases have not been adequately addressed. This study is the first to systematically describe the histological features and outcome in a well-characterized cohort of such women with long-term follow-up.
For women in the Mayo BBD cohort with multiple nonsynchronous biopsies, the overall histological classification changed from initial to second breast biopsy in nearly half (43.9%). Changes in histological classification overall were about twice as likely to involve progression to a higher-risk category as regression to a lower-risk category. Importantly, altered histological classification statistically significantly modified risk for developing breast cancer in the expected or intuitive direction, according to findings in the second biopsy in each of the major histological categories of BBD.
A sizable proportion of women with NP in their initial biopsy progressed to PDWA or AH in their second sample (41.0%). This finding is clinically significant owing to increased cancer risk in the group of women who “progressed” to PDWA and adds to our understanding of NP. Most women with BBD—about 65% across multiple studies—are diagnosed with NP. Our results imply that breast cancer risk status can evolve in many women with NP and that BC risk can increase for some of these women.
Despite a lack of systematic studies that have tracked longitudinal histological changes over time among women with BBD, it is presumed that some changes will occur. For example, there are well-described alterations in mammographic appearances of benign breast tissue, possibly related to physiological involution/atrophy (21). Our group has also observed progression of lobular involution in patients who have undergone more than one benign biopsy (22). Such findings are consistent with the concept that breast cancer risk for individual women changes over time and is reflected in tissue characteristics.
One potential limitation to our study is that a breast biopsy provides a small sample of any patient’s total breast tissue, and small proliferative or atypical lesions may not be present in the biopsy. However, we have observed statistically significant changes in breast cancer risk based upon such samples, suggesting that diagnostic breast biopsy samples may reasonably reflect the larger field of at-risk tissue. Moreover, the finding of additional AH in 35% of second biopsies of patients who had AH in their initial biopsy further supports the notion that evolution of pathologic findings is not merely driven by random sampling effects but favors a “field effect” hypothesis.
Another potential limitation of our study is that the findings were necessarily made in a clinically distinct subset of BBD women who underwent more than one benign biopsy for clinical reasons. Although the MBC subset does not appear greatly different from other subjects in the Mayo BBD cohort on the basis of initial overall histology or cancer incidence, the two differed statistically significantly by age and family history of breast cancer and the fact that they had clinically actionable recurrent ongoing clinical abnormalities. We cannot exclude the possibility that women with successive benign biopsies are more likely to develop histologically progressive breast lesions.
An additional limitation that should be noted is that these breast biopsies were reviewed and characterized by a single pathologist with particular expertise in breast pathology. Assessment of breast cancer risk at the individual level should consider the possibility of variability by individual pathologists in characterization of breast lesions into nonproliferative, PDWA, and atypia categories; this issue also emphasizes the importance of clear parameters for histological classification.
In summary, we found that overall risk estimates by histological category were similar for women with multiple biopsies at their subsequent biopsy as compared with women with only a single biopsy. However, for women with multiple breast biopsies, the histologic classification in prior biopsies stratified their long-term BC risk estimates. Women with progressive epithelial proliferation have increased risk, and those with less proliferation have decreased risk. These findings have important implications for optimization of clinical management for the approximately 100 000 women per year in the United States who have multiple, metachronous benign biopsies and provide insight into the natural history of benign breast disease and its role in breast carcinogenesis.
Funding
This work was supported by the National Cancer Institute (CA187112 to ACD); Bankhead-Coley Foundation (5BC02 to DCR and AN); Mayo Clinic Breast Cancer–Specialized Program of Research Excellence (SPORE CA116201 to DWV, DCR, AN, and LCH).
Notes
The study sponsors had no role in design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The authors disclose no potential conflicts of interest related to this study.
Supplementary Material
References
- 1.Gutwein LG, Ang DN, Liu H, et al. Utilization of minimally invasive breast biopsy for the evaluation of suspicious breast lesions. Am J Surg. 2011;202:127–132. [DOI] [PubMed] [Google Scholar]
- 2.Silverstein M. Where's the outrage? J Am Coll Surg. 2009;208:78–79. [DOI] [PubMed] [Google Scholar]
- 3.Dupont WD, Parl FF, Hartmann WH, et al. Breast cancer risk associated with proliferative breast disease and atypical hyperplasia. Cancer. 1993;71:1258–1265. [DOI] [PubMed] [Google Scholar]
- 4.Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229–237. [DOI] [PubMed] [Google Scholar]
- 5.McDivitt RW, Stevens JA, Lee NC, Wingo PA, Rubin GL, Gersell D.. Histologic types of benign breast disease and the risk for breast cancer. The Cancer and Steroid Hormone Study Group. Cancer. 1992;69:1408–1414. [DOI] [PubMed] [Google Scholar]
- 6.Dupont WD, Page DL.. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med 1985;312:146–151. [DOI] [PubMed] [Google Scholar]
- 7.Dupont WD, Page DL, Parl FF, et al. Long-term risk of breast cancer in women with fibroadenoma. N Engl J Med. 1994;331:10–15. [DOI] [PubMed] [Google Scholar]
- 8.Dyrstad SW, Yan Y, Fowler AM, Colditz GA.. Breast cancer risk associated with benign breast disease: Systematic review and meta-analysis. Breast Cancer Res Treat. 2015;149:569–575. [DOI] [PubMed] [Google Scholar]
- 9.Page DL, Dupont WD, Rogers LW, Rados MS.. Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer. 1985;55:2698–2708. [DOI] [PubMed] [Google Scholar]
- 10.Hartmann LC, Degnim AC, Santen RJ, Dupont WD, Ghosh K.. Atypical hyperplasia of the breast—risk assessment and management options. N Engl J Med. 2015;372:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartmann LC, Radisky DC, Frost MH, et al. Understanding the premalignant potential of atypical hyperplasia through its natural history: A longitudinal cohort study. Cancer Prev Res (Phila). 2014;7:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colditz GA, Rosner B.. Cumulative risk of breast cancer to age 70 years according to risk factor status: Data from the Nurses' Health Study. Am J Epidemiol. 2000;152:950–964. [DOI] [PubMed] [Google Scholar]
- 13.Dupont WD, Page DL.. Breast cancer risk associated with proliferative disease, age at first birth, and a family history of breast cancer. Am J Epidemiol. 1987;125:769–779. [DOI] [PubMed] [Google Scholar]
- 14.Milanese TR, Hartmann LC, Sellers TA, et al. Age-related lobular involution and risk of breast cancer. J Natl Cancer Inst. 2006;98:1600–1607. [DOI] [PubMed] [Google Scholar]
- 15.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–1886. [DOI] [PubMed] [Google Scholar]
- 16.Tyrer J, Duffy SW, Cuzick J.. 2004. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med 23:1111–1130. [DOI] [PubMed] [Google Scholar]
- 17.Pankratz VS, Degnim AC, Frank RD, et al. Model for individualized prediction of breast cancer risk after a benign breast biopsy. J Clin Oncol. 2015;33:923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tice JA, Miglioretti DL, Li CS, Vachon CM, Gard CC, Kerlikowske K.. Breast density and benign breast disease: Risk assessment to identify women at high risk of breast cancer. J Clin Oncol. 2015;33:3137–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quante AS, Whittemore AS, Shriver T, Strauch K, Terry MB.. Breast cancer risk assessment across the risk continuum: Genetic and nongenetic risk factors contributing to differential model performance. Breast Cancer Res. 2012;14:R144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fine JP, Gray RJ.. A proportional hazards model for the subdistribution of a competing risk. JAMA. 1999;94:496–509. [Google Scholar]
- 21.Work ME, Reimers LL, Quante AS, Crew KD, Whiffen A, Terry MB.. Changes in mammographic density over time in breast cancer cases and women at high risk for breast cancer. Int J Cancer. 2014;135:1740–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radisky DC, Visscher DW, Frank RD, et al. Natural history of age-related lobular involution and impact on breast cancer risk. Breast Cancer Res Treat. 2016;155:423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.