Abstract
The brain is increasingly appreciated to be a constantly rewired organ that yields age-specific behaviors and responses to the environment. Adolescence in particular is a unique period characterized by continued brain maturation, superimposed with transient needs of the organism to traverse a leap from parental dependence to independence. Here we describe how these needs require immune maturation, as well as brain maturation. Our immune system, which protects us from pathogens and regulates inflammation, is in constant communication with our nervous system. Together, neuro-immune signaling regulates our behavioral responses to the environment, making this interaction a likely substrate for adolescent development. We review here the identified as well as understudied components of neuro-immune interactions during adolescence. Synaptic pruning, neurite outgrowth, and neurotransmitter release during adolescence all regulate—and are regulated by—immune signals, which occur via blood-brain barrier dynamics and glial activity. We discuss these processes, as well as how immune signaling during this transitional period of development confers differential effects on behavior and vulnerability to mental illness.
1. Introduction
One of the most influential ideas in behavioral neuroscience has been the idea that the brain continues to develop throughout adolescence and into adulthood. Decades of research have unveiled adolescence as a period of transient differences that yield increased risk-taking, reward seeking, and vulnerability to affective disorders (see below, and in this issue). Important for this review, behaviors and mental illness do not originate singularly from neuronal activity, or even just from the brain itself. In contrast, the brain is in constant communication with peripheral factors, including the immune system. Together, neuronal and immune mechanisms regulate cognitive and behavioral function as well as dysfunction throughout the lifespan. Here we propose that a full understanding of the adolescent brain can only be achieved through a rigorous developmental investigation of both peripheral and central immune mechanisms. The study of immune development has lagged behind that of the brain, with most research focusing on embryonic, perinatal, or senescent stages (Barrientos et al., 2010, Bilbo et al., 2011, Harry and Kraft, 2012). Only recently has it been suggested that the immune system also undergoes important and distinct changes throughout adolescence. As a result, the adolescent immune system has begun to gain attention as a potential mediator of developmental programming and adolescent-specific behavior (Crews et al., 2007, Crews and Vetreno, 2011). We will first review emerging evidence that both the peripheral and central immune systems undergo important development through the adolescent stage. Then, we will present mechanisms by which immune modulators influence adolescent neuronal circuitry and behavior. Finally, these mechanisms will be explored in the context of adolescence as a window of vulnerability to, and an opportunity to prevent, psychiatric illnesses.
2. A brief overview of the immune system
Our immune system is designed to recognize and defend our bodies against invasion from viruses, bacteria, and other antigens. The immune system can eliminate the presence of a pathogen via a specialized, robust molecular and cellular response. As part of this immune response, immune cells secrete elevated levels of immune molecules, resulting in inflammation that coordinates a cellular attack against the pathogen. Many of the symptoms we experience during an infection are the direct result of our immune system’s response to the pathogen, and not the pathogen itself. For example, peripheral immune molecules including cytokines and chemokines activate the immune cells in the brain, which in turn affect neuronal function to initiate generalized “sickness behaviors” (fever, malaise, decreased appetite, and cognitive dysfunction). This behavioral response to immune activation is conserved across many species and is the mechanism by which our bodies coordinate our brain and our behavior during sickness, allowing us to rest and recover from infection. Thus, neurons exhibit a marked sensitivity to the inflammatory signals produced in the periphery and the brain; and if left unchecked, these molecules can induce serious neuronal dysfunction, cognitive dysfunction, and even neuronal cell death.
Peripheral immune responses can be divided into two types: either adaptive immune responses or innate immune responses, both of which can have the ability to impact neural function. Adaptive immune responses are acquired, specific responses that are the result from exposure to specific components of bacteria or virus and require days to develop, but confer an immunological memory for a lifetime. As a result of an adaptive immune response, a second exposure to the same bacteria or virus (or antigen) results in a very specific and immediate immune response (Berczi, 1998). Adaptive immune responses are largely orchestrated by white blood cells called lymphocytes, which mature in the thymus or bone marrow. After they mature, T (thymus-derived) and B (bone-derived) cells circulate through the blood and lymph where they respond to the foreign antigen via antibody production (B-cells) and other T-cell specific functions including assisting other lymphocytes, eliminating infected host cells, and expressing unique subsets of cytokines and chemokines. After infection, these cells survey for re-exposure to the previously detected pathogens. As their name and function implies, and important for this particular review, adaptive immune responses develop throughout the lifespan, dependent upon exposure to specific signals. Thus, even into adolescence and beyond, our adaptive immune system continues to develop specific immunity to environmental factors that can subsequently impact the function of the brain and associated behaviors.
In contrast, innate immune responses refer to nonspecific resistance to pathogens that we all possess even prior to birth. This type of immunity occurs via the recognition of pathogens (and other environmental factors) via highly conserved receptors and adapter proteins expressed on the surface of immune cells (including most famously the Toll-like receptors, TLRs). These innate immune receptors respond to a specific molecular pattern expressed by a pathogen and allow an individual to mount a rapid, robust immune response to the invasion without dependence on prior exposure during the individual’s lifetime (Berczi, 1998, Brodsky and Medzhitov, 2009). Though we are all born with the capacity to mount an innate immune response, there is evidence that the innate immune response matures and changes throughout the lifespan, potentially even into adolescence, thereby differentially impacting the brain and behavior (Ellis et al., 2005, Levy, 2007, Ortega et al., 2010). Innate immune responses are largely carried out by a number of cells including macrophages, monocytes, neutrophils, and other phagocytic proteins, as well as microglia in the brain. Exposure to any insult that activates innate immune cells will result in the induction of innate immune molecules such as cytokines and chemokines, a process known as the “inflammatory response”.
There are many different cytokines, chemokines and related immune molecules that have been identified. These molecules are typically classified as either pro-inflammatory, meaning that they help to stimulate an immune response (e.g. Interleukin (IL)-1β, IL-6 or TNFα), or anti-inflammatory, meaning that they can control or attenuate an immune response (e.g. IL-10). Both the pro- and anti-inflammatory responses are necessary for proper immune function, because if left unchecked, inflammation can cause significant tissue damage and cell death, particularly within the brain. Cytokines are often redundant in their function and yet rarely work alone, as they orchestrate a set of physiological changes throughout the entire body, including the brain and central nervous system (CNS) (Dantzer et al., 1998a, Dantzer et al., 1998b), which is the focus of this review.
The central nervous system also has its own immune cells called microglia that are capable of responding not only to insult or injury via the expression of innate immune receptors, but also to peripheral immune activation via their communication with circulating immune molecules. Astrocytes in the brain also produce cytokines and chemokines (McKimmie and Graham, 2010) that—in conjunction with the immune molecules produced by microglia—are important for immune function, but also can influence neuronal function and behavior [see (Rivest, 2009) for review]. This neural immune response is often referred to as “neuroinflammation”, and while this term is used in the interest of simplicity to refer to immune signaling in the CNS (Liu et al., 2014), inflammation classically refers to injurious infiltration of immune cells to an affected area. Thus the use of the term “neuroinflammation” is better targeted towards immune responses associated with neurodegeneration, traumatic brain injury, or other types of progressive CNS damage. Therefore, in this review, we will simply refer to immune signaling and cytokine production in the brain as “neuro-immune signaling” or “neuro-immune activation” as an effort to encourage further precision and less confusion in the neuroscience literature.
3. Defining adolescence
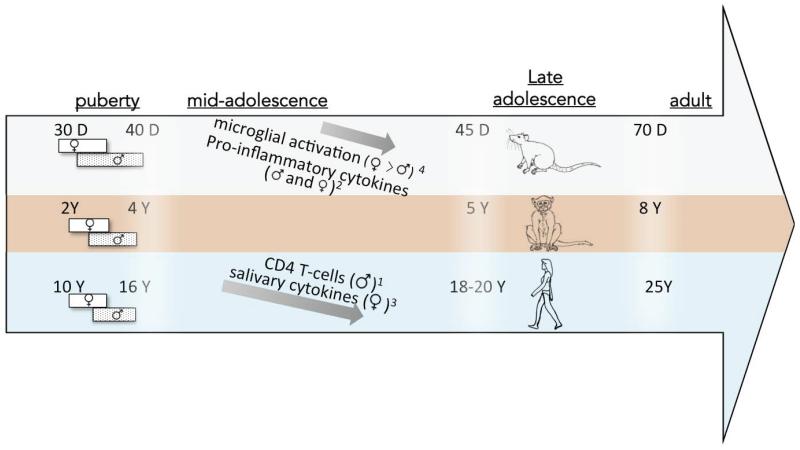
Adolescence is generally defined as the period between 10-19 years in humans, between three-five years in rhesus monkeys, and between 35–60 days of age in rodents (Brenhouse and Andersen, 2011a; McCutcheon and Marinelli, 2009; Schwandt et al., 2010). Mammals spanning from rodents to humans all experience a tumultuous transitional period where navigation through puberty and decreased parental influence is coupled with increased peer influence, sexual competition, and new decision-making challenges (reviewed by (Spear, 2000a) and in this issue). Importantly, these needs differentially manifest at different stages of adolescence, making an important distinction between puberty (early adolescence), late adolescence, and emerging adulthood (Figure 1; (McCutcheon and Marinelli, 2009)). These developmental milestones and transitions can also differ between sexes; for example, females typically display full puberty earlier than males across several species (Figure 1). Age distinctions are thus also important in regards to immune development; however the ages reported in studies of adolescent immune development often span from pre-adolescent ages through emerging adulthood ages. Thus it will be important in future studies to follow the trajectory of immune development throughout these distinct phases of adolescence rather than analyzing it as one time-point.
Figure 1. Ages of adolescent maturation across species, and reported age-related immune changes.
Ages of adolescence in rats (top), monkeys (middle), and humans (bottom) are shown. Bars represent male and female puberty. Arrows depict reported changes in immune mediators over adolescence, in the species they were measured. Superscripts indicate citations: 1(Tollerud et al., 1990)*; 2(Grassi-Oliveira et al., in press); 3(Riis et al., 2014); 4(Schwarz and Bilbo, 2011). *Note: The Tollerud et al. study found female CD4 T-cells to increase during adolescence, however a study by Bartlett et al. (Bartlett et al., 1998) measured inducers of CD4 T-cells (CD29), and did not find the same sex differences.
4. Missing “links”: The blood-brain interface
4.1. Peripheral immune function during adolescence and the effect of puberty
There are a number of “missing links” in our understanding that, if uncovered, would have the potential to shed important light on the role of immune function in the ontogeny of adolescent neural circuits and associated behaviors. Most notably, we understand very little about how peripheral immune function may be different in adolescence relative to earlier stages of development or adulthood; thus we understand perhaps even less about how peripheral immune function may impact central immune and neural immune function at these ages. There is evidence that peripheral immunity changes during adolescence in animals and humans, particularly with the onset of puberty, but there also evidence that early-life events can precipitate changes in immune function specifically during adolescence. Both ideas are discussed below.
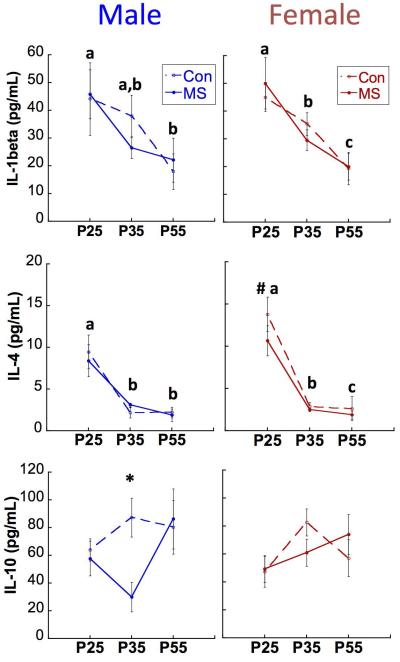
Preclinical studies investigating immune influences on neuronal function have largely focused on cytokines and glial function within the brain; however, clinical studies often rely on circulating immune molecules as biomarkers of immune activation in the brain (e.g., (Hodes et al., 2014; Miller and Cole, 2012). Closer investigation of these circulating biomarkers has begun to reveal that not all, but rather only a subset of patients with psychiatric illness display a pro-inflammatory phenotype or altered cytokine expression in the periphery (Dennison et al., 2012; Fillman et al., 2015). Therefore, peripheral immune dysregulation likely plays a role in the pathogenesis of certain psychiatric illnesses that are also dependent on other lifetime environmental experiences, biological variables or risk factors. For example, we recently observed that postnatal stress in rats caused by maternal separation from postnatal day (P) 2-20 yields a decrease in circulating levels of the anti-inflammatory cytokine IL-10 at P35, but not P25 nor P55 (Figure 2). This transient decrease occurred in adolescent males, but not females (Grassi-Oliveira et al., in press). In support of the idea that adolescent changes in circulating cytokines can predict altered behavioral outcomes, we found that lower levels of circulating peripheral IL-10 at P35 predicted poor performance in the win-shift cognitive behavioral task two weeks later, only in maternally separated male rats (Grassi-Oliveira et al., in press). Adolescence may therefore be a period during which early-life stressors or events that “derail” development can manifest in immune dysfunction, with consequences for the brain and long-term behavioral outcomes. Interestingly, female rats did not exhibit the same profile of circulating IL-10 in adolescence following previous early-life stress, which may suggest that either females are not as sensitive to this particular early-life stressor or that, only in females, adolescent peripheral biomarkers may not predict the risk of negative cognitive outcomes that have yet to be identified. Importantly, these findings also highlight how little we understand regarding whether and how circulating peripheral cytokines may directly impact brain development in adolescence.
Figure 2. Age, Group, and Sex effects on cytokine expression in rats.
Means ± SEM expression levels of IL-1ß (top), IL-4 (middle) and IL-10 (bottom) across development. P: postnatal day. *p<0.05 difference between MS and CON. Different letters (a,b,c) designate p<0.05 difference between ages within the same sex (no effect of group). #p<0.05 difference between males and females, collapsed across groups. n=15-20/group/sex/time-point. (Grassi-Oliveira et al., in press)
Adolescence is also characterized by pubertal increases in circulating gonadal hormones (Sisk and Foster, 2004). As a result, puberty is a sensitive period for both organizational and activational actions of sex steroid hormones (Schulz et al., 2009), including estrogens and androgens, both of which have well-known modulatory effects on the peripheral immune system and brain during this time. Sex hormones affect the function of lymphocytes and macrophages either directly by binding to surface hormone receptors or indirectly via their effects on other hormone-responsive target tissues such as the hypothalamic pituitary axis that in turn influence immune responses [reviewed by (Shames, 2002)]. As a result, puberty is a time during which changes in immune function confer a risk (often sex-specific risk) to a number of immune related disorders including autoimmune diseases such as lupus or multiple sclerosis, and allergies and asthma (Ahn et al., 2015, Canguven and Albayrak, 2011, Shah, 2012), as well as a sex-specific risk to many infections (Guerra-Silveira and Abad-Franch, 2013). Oftentimes, these immune-related disorders are more prevalent or robust in females following the onset of puberty. In fact, testosterone can increase the production of IL-10 by T-lymphocytes—an effect that may be driven directly by androgen receptors that are expressed on the lymphocytes themselves (Liva and Voskuhl, 2001) or indirectly by receptor expression on innate immune cells such as macrophages. In doing so, testosterone can have an anti-inflammatory effect on peripheral immune function, and this may be one way by which males are less susceptible to immune-related disorders following the onset of puberty. In our model of early-life maternal separation stress, decreases in peripheral IL-10 observed in adolescent male rats were also associated with significantly lower testosterone levels compared to controls (Grassi-Oliveira et al., in press). Therefore, the early-life events linked to achieving important pubertal milestones may also involve and modulate the orchestrated maturation of the immune system during adolescence (in this example, the induction of IL-10 in the periphery that occurs specifically at this time). Conversely, it has also been postulated that early life adversity can produce long-term changes in responses to circulating sex hormones via their impact on the immune system (Blaustein and Ismail, 2013, Blaustein et al., 2015). While it is clear that sex steroid hormones have a significant effect on peripheral immune function, it remains to be determined exactly how these pubertal hormones influence the full maturation of the peripheral and central immune systems, and how this may impact both physiological and behavioral outcomes during the period of adolescence.
4.2. The blood-brain barrier
The peripheral immune system is largely devoted to defending the host from invasion by pathogens and responding to injury. Given the exquisite sensitivity of neurons to immune molecules, the passive leakage of immune cells or immune molecules (such as cytokines, chemokines, and growth factors) into the brain is typically thought to occur only in severe pathogenic states (Muller and Ackenheil, 1998, Yarlagadda et al., 2009). However, our immune system is also designed to regulate allostasis and respond to threats in our environment with orchestrated changes in behaviors, including feeding, sleep, cognition and social interaction (Farrar et al., 1987, Kubota et al., 2000, Vitkovic et al., 2000a, Vitkovic et al., 2000b). To that end, there are several pathways of active communication that allow controlled immune signaling between the periphery and the brain. Peripheral immune mediators can thereby influence behavioral and neuroendocrine responses to environmental stimuli during both normal and abnormal development. Brain responses to peripheral immune activity occur either via vagal afferents, directly at the blood brain barrier (BBB), or at circumventricular organs (Blatteis, 1992, Vitkovic et al., 2000b). This review will focus on the BBB, since vagal afferents also play a large role in the gut-brain axis, of which development has been discussed elsewhere (Borre et al., 2014) and in this issue.
The BBB is a dynamic system that controls the access of a number of factors, including blood-borne immune mediators, into the brain parenchyma. Most of our understanding of BBB changes and peripheral cytokine influences on the brain is in the context of systemic inflammatory responses leading to a febrile response, rather than in a healthy, non-inflammatory state [e.g., (Galic et al., 2012)]. However, regulated transport of cytokines and chemokines across the BBB is necessary for healthy brain function (Banks, 2015; Banks and Kastin, 1991; Sui et al., 2014). Under typical physiologic conditions, the BBB adapts to the changing needs of the brain that occur throughout development, during aging, and following other life events (Banks, 2015). While this idea is generally accepted, the mechanisms that underpin healthy-state BBB dynamics throughout development are not clear. Recent work supports the idea that circulating immune molecules play critical roles in adolescent brain function (do Prado et al., 2015; Wieck et al., 2013), therefore one might hypothesize that the BBB has evolved to allow differential access to specific immune actors throughout adolescence to influence proper neural development at this time.
One example of the importance of BBB transport for adolescent brain function is seen in the developmental expression of Insulin Growth Factor (IGF)-1, which is a growth hormone mediator in the periphery that has well-known neuroprotective activity in the brain. Circulating IGF1 is relatively low during childhood, but increases to high levels in adolescence, and then progressively decreases with age (Smith et al., 1989). Importantly, declining circulating levels of IGF1 during adulthood are implicated in cognitive impairments in mice (Svensson et al., 2006), which suggests that proper blood-to-brain transport of IGF1 is necessary for proper brain function. Yan and colleagues (Yan et al., 2011) determined in rats that the rise of circulating IGF1 during adolescence is necessary for the increase in IGF1 levels measured in the brain at this time. Furthermore, increases in circulating IGF1 were necessary for the induction of genes involved in brain development and regulation of synaptic plasticity (Yan et al., 2011). To date, we have no knowledge of whether BBB transport mechanisms behave differently during adolescent development. However, active transport of IGF1, cytokines, and other regulatory proteins is largely driven by glutamate-dependent neuronal activity (Nishijima et al., 2010); and therefore adolescent-specific neuronal activity could have a bi-directional control of BBB permeability to circulating immune mediators during this time.
BBB function is maintained and regulated by the neurovascular unit, which is comprised of neuronal synapses, astrocytes, and the vascular endothelial cells (Abbott et al., 2006). The release of neurotransmitters, in particular glutamate, leads to fluctuations in intracellular calcium within astrocytes, which in turn results in the subsequent release of diffusible mediators such as nitric oxide (NO) and arachidonic acid derivatives like cyclooxygenase (COX)-2 that modulate BBB permeability (Nishijima et al., 2010). The synaptic refinement of glutamatergic synapses that occurs during adolescence is a major part of the neural network pruning necessary to navigate through the sensitive period of puberty that also coincides with decreased parental influence [reviewed by (Spear, 2000b) and (Brenhouse and Andersen, 2011a)]. Thus, the active pruning of glutamatergic synapses that is so prevalent in the adolescent brain may be one potential mechanism by which BBB permeability could be changed during this time; thereby altering the passage of important immune and other signaling molecules into the adolescent brain.
Therefore while there is virtually no knowledge of differential BBB dynamics during adolescence, there is evidence to support it, including peripheral changes in circulating immune modulators, changes in the neuronal activity that control BBB permeability, as well as differential necessity for the entry of these circulating modulators into the brain. It is also surprising just how little is known about BBB function during adolescence given that the BBB also controls access of drugs to the brain and given the increasing prevalence of pharmacological treatments for mental and medical illness in pediatric and adolescent populations (Vitiello, 2008).
5. Adolescent development and glia
While relatively ignored for years by the neuroscience community, the growing amount of literature on glia now indicates they can no longer be ignored and that they are important mediators of neuronal function and behavior. Microglia are the primary immune cells of the brain, existing under non-pathological conditions in the adult brain in a resting, ramified state, expressing low or undetectable levels of activation markers (e.g. CD11b and MHCII) on the cell surface and producing low or undetectable levels of immune molecules. In response to a pathogen, insult or injury, microglia become active, at which point they can up-regulate a number of cell surface receptors and increase the production of cytokines and chemokines, resulting in neuro-immune activation.
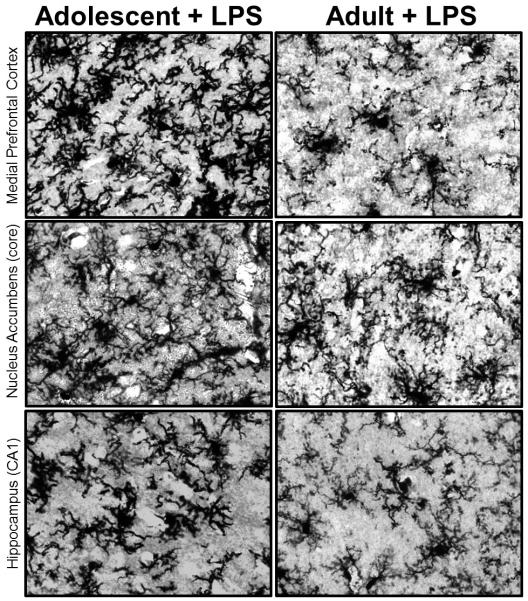
Microglial cells are of mesodermal lineage (similar to other immune cells) and they first colonize the developing rodent brain around embryonic day (E) 9-10 via the infiltration of primitive macrophage precursors from the yolk sac of the embryo (Chan et al., 2007, Ginhoux et al., 2010). Microglia within the developing brain have a larger, round morphology, consistent with their role as macrophages (Male and Rezaie, 2001; Rezaie et al., 1999); however, they change their morphology and their function quite rapidly as they mature within the context of the brain, to a more mature shape characterized by a smaller cell body with thinner, longer processes (Schwarz et al., 2011). Despite that, microglia within certain regions of the adolescent brain continue to show what appears to be a more “activated” or immature morphology, with thick variegated branches, suggesting that certain brain regions, and the neurons and glia within them are continuing to undergo maturational changes long after the postnatal period (Schwarz et al., 2011), which could significantly impact the function of these cells following immune activation (Figure 3).
Figure 3. Microglia in the adolescent brain have a unique morphology compared to microglia in the adult brain.
Microglia stained with an antibody for the Ionized calcium binding adaptor molecule (Iba) 1 exhibit a very full and seemingly “activated” morphology within the adolescent (P30) medial prefrontal cortex, nucleus accumbens, and hippocampus, 4 hours post injection with a low dose of lipopolysaccharide (LPS, 25 μg/kg intraperitoneal). In contrast, microglia in the adult brain appear fewer and exhibit a very different, more ramified appearance even 4 hours post injection with the same dose of immunogenic compound, LPS. Data are representative of a work in progress.
Because microglia colonize the brain early in development and represent a relatively permanent population with very little turnover (Male and Rezaie, 2001), events that impact the function of microglia during development (including adolescence) can have long-term consequences for their function (Bilbo and Schwarz, 2009). Microglial cells have the capacity to “remember” events or become “primed” (Town et al., 2005), similar to peripheral immune cells. While microglial priming has not been fully defined or characterized, it is understood that the pro-inflammatory response produced by primed microglia to a subsequent challenge is significantly exaggerated when compared to other, unprimed glia (Perry et al., 2003). Via priming, microglia are forever bound to and influenced by the events that occur during perinatal and adolescent development, with significant consequences for later-life neural function and behavior.
Though they are often lumped together as “glia”, microglia and astrocytes are actually quite distinct. Astrocytes are derived from specific populations of neural progenitor cells within the rodent brain later in embryonic development (Zhang and Barres, 2010). Astrocytes continue to proliferate in select niches of the adolescent and adult rodent brain, though the proliferation potential of astrocytes is dependent upon their function and location within the brain (Kriegstein and Alvarez-Buylla, 2009). Astrocytes develop long primary processes relatively rapidly during the course of early brain development. During juvenile development, astrocytes display multiple primary processes, with all processes being rather thin and ramified (Bushong et al., 2004). Astrocytes have established distinct boundaries from neighboring astrocytes by this age, though each astrocyte can interact with and impact the function of nearly 2 million synapses (Freeman, 2010). To date, it is unclear whether and in what manner astrocytes may continue to change throughout adolescent development or, perhaps more importantly, how these changes in morphology can impact astrocyte and neuronal function during this time.
6. Immune regulation of adolescent brain development
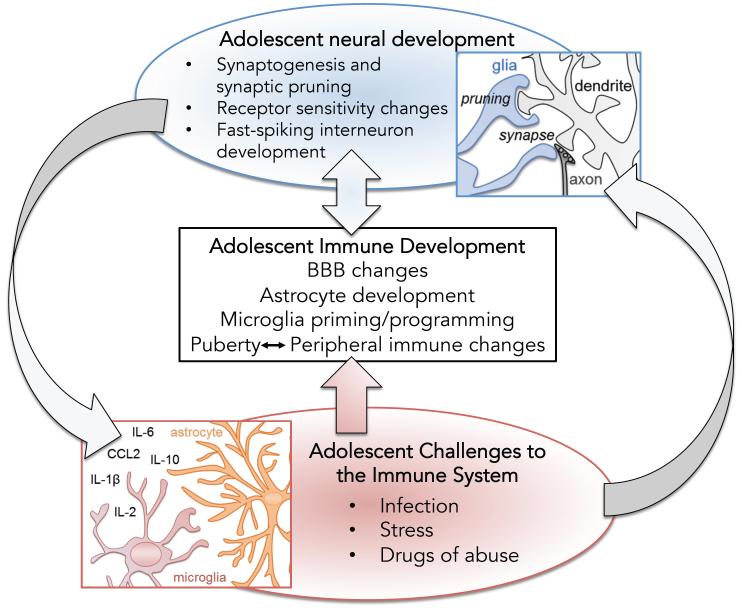
As microglia and astrocytes mature, they function in distinct ways to influence and encourage the on-going development of the brain (Bilbo and Schwarz, 2009, Clarke and Barres, 2013, Stipursky et al., 2011). The adolescent brain in particular is a complex milieu of synaptic pruning in some brain regions (Selemon, 2013), emerging connections in others (e.g., (Cunningham et al., 2002), and receptor repurposing in still others (Tseng and O'Donnell, 2007) [reviewed by (Brenhouse and Andersen, 2011a), and in this issue]. These processes of substantial neural remodeling occur predominantly in brain areas involved in emotion and learning including the prefrontal cortex, hippocampus, amygdala, and nucleus accumbens. As a result, provoking pro-inflammatory activity during adolescence can have robust effects on brain development and behavior that do not occur if the same immune challenge were to be experienced earlier or later in development (Schwarz and Bilbo, 2013). These observations have begun to inform us about mechanisms that are in-place for immune regulation of typical brain development (Figure 4).
Figure 4. A schematic representation of potential interactions between adolescent neural development, immune development, and immune activation at this time.
Adolescence is a complex period of development that includes important neurodevelopmental processes such as synapse formation (synaptogenesis) and the pruning of other exuberant synapses. Similarly, neurotransmitter receptor sensitivity and expression change throughout adolescent brain development, while important inhibitory interneurons finalize their rhythm and functionality. Simultaneously and likely in response to changes mentioned above, the immune system is undergoing its own maturational changes including alterations in blood brain barrier (BBB) permeability, astrocyte and microglial development, and peripheral immune changes that are likely dependent upon their interaction with pubertal sex hormones at this time. Activation of the immune system and immune cells in the brain (microglia and astrocytes) via either infection, stress, or drugs of abuse can result in the release of immune molecules (cytokines and chemokines) that in turn impact both neural and immune function during this unique period of adolescent immune function and neuro-behavioral vulnerability.
Many researchers believe that the developmental trajectory and function of glial cells is dependent upon the on-going processes of neural development described above. For example, the migration and proliferation of microglia has been closely tied to the naturally occurring apoptosis of cells in the developing brain (Ashwell, 1990; Ashwell, 1991; Perry et al., 1985). Developing glia also produce elevated levels of diffusible immune factors, such as cytokines, chemokines, and growth factors, all of which have a demonstrated role in many neurodevelopmental processes (Arnoux and Audinat, 2015, Clarke and Barres, 2013, Michell-Robinson et al., 2015, Squarzoni et al., 2015, Wu et al., 2015). For example, live imaging of healthy rodent cortex has revealed intricate bi-directional signaling between microglia and neurons that can modify dendrite morphology during development and into adolescence (Tremblay et al., 2010) [reviewed by (Eyo and Wu, 2013)]. Glia can influence these processes via the identification of other immune molecules produced by neurons (Boulanger, 2009; Deverman and Patterson, 2009).
Importantly, age-specific effects of immune activation have also revealed how immune mechanisms may confer adolescent vulnerabilities to environmental insults such as stress, drugs of abuse, and traumatic injury (Crews et al., 2007, Crews and Vetreno, 2011). Real and perceived threats (i.e., stress), drugs of abuse, physical injury, and pathogenic infection all result in secretion of molecular signals that activate innate immune receptors such as TLRs. These signals include pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs). DAMPs—also called alarmins—are released endogenously from damaged, dying, or dead cells and can trigger innate immune signaling by activating the TLR pathway (Gadani et al., 2015). TLRs have been found to play important roles in adolescent brain development, which is a likely mechanism through which neuronal remodeling is tailored to the environmental demands of each individual. While much of what is known about TLR effects in adolescence is from models of insult or injury as described in the next section, it is of upmost importance to acknowledge the role that TLRs and DAMPs have in typical brain development, neurogenesis, and neuroplasticity (Barak et al., 2014). Taken together, additional research is necessary to further our understanding of how glial cells and immune molecules mediate these on-going and necessary processes associated with adolescent neural development. Only then can we understand the full extent of cell-to-cell communication in the context of immune function and behavior during this time.
7. Psychiatric disorders with an adolescent onset: Adding the neuroimmune filter
Affective and psychotic disorders such as major depressive disorder, schizophrenia, and drug addiction have a strikingly disproportionate rise in presentation and diagnosis during adolescence and early adulthood compared to other life stages. Each of these disorders has also been associated with atypical immune activation, and some have been associated with an earlier immune challenge as a risk factor. Here we will discuss each disorder with regard to the possibility of adolescence as a window of differential neuro-immune regulation.
7.1 Drug Addiction
Studies in humans and experimental animals have demonstrated the particular vulnerability of the adolescent brain to the actions of drugs of abuse, the long-term consequences of drug taking, and the risk for addiction [e.g., (McClory and Spear, 2014); reviewed by (Andersen and Teicher, 2009; Stanis and Andersen, 2014)]. Vulnerability during adolescence can be partially attributed to transient developmental processes such as the maturation of connectivity, receptor overproduction, and pruning in corticolimbic regions including the nucleus accumbens and prefrontal cortex (e.g., (Brenhouse et al., 2008; Smith et al., 2015)). Additionally, drugs of abuse such as ethanol (Fernandez-Lizarbe et al., 2013), opioids (Hutchinson et al., 2007), and psychostimulants (Northcutt et al., 2015) activate pro-inflammatory gene transcription as well as receptors for DAMPs and PAMPs in these same brain regions. It is likely that neuro-immune activation by drugs of abuse during adolescence interferes with typical brain development, because pro-inflammatory signaling mediates neural activity. For example, TLR activation by cocaine has been shown to directly contribute to cocaine-induced dopamine release in the nucleus accumbens; blocking TLR during cocaine exposure prevented dopamine release as well as cocaine conditioned place preference in adult rodents (Northcutt et al., 2015). Taken together, the emerging concept that rewarding, and possibly addictive, effects of abused drugs requires stimulation of both neuronal and glial cell functioning (Northcutt et al., 2015) incites two paths of investigation: First, it is possible that typical neuro-immune events during adolescent development instigate risk-taking, novelty-seeking, and other behaviors that lead to drug-taking. Second, exogenous activation of neuro-immune processes during adolescence may make the adolescent brain more vulnerable to the immediate and enduring effects of abused drugs, thus increasing the risk of addiction long-term. We discuss here data that directly or indirectly speak to these lines of research.
Woefully little is known about neuro-immune changes that are distinct to the adolescent period. However, recent findings suggest that developmental events during adolescence share a bi-directional relationship with immune mechanisms. For example, the pro-inflammatory cytokine IL-2 has been shown to modulate dopamine levels and dopamine-mediated behaviors in developing and adult rodents (Karrenbauer et al., 2011; Lapchak, 1992). This impact of IL-2 on dopamine signaling is of particular clinical importance because IL-2 is a current cancer treatment that is complicated by marked neurobehavioral side-effects involving anhedonia and depression (Capuron et al., 2000). Interestingly, IL-2 administration during adolescence potentiates later displays of addiction-like behaviors, such as novelty-induced stereotypy and motor responsiveness to dopamine agonists like GBR12909 (Rankin et al., 2013). However, a single adolescent exposure to IL-2 was found to prevent stereotypy in response to IL-2 in adulthood. One could hypothesize that IL-2 in adolescence interferes with typical developmental changes in dopamine receptor circuitry, illustrating one way in which the adolescent brain is poised for neuro-immune interactions that affect later behavior and could have important medical consequences.
Compared to our understanding of typical adolescent neuro-immune development, much more work has been dedicated to the impact of inflammatory damage to the adolescent brain. The relative immaturity of microglia during adolescence together with their ability to be “primed” for extended lengths of time (Town, Nikolic, & Tan, 2005) make microglia an important substrate for enduring effects on addictive-like behaviors. In one example, exposing rats to morphine during adolescence (but not adulthood) was shown to cause an increased risk of relapse to drug-seeking behavior in adulthood, along with increased expression of the toll-like receptor TLR4 specifically on microglia within the nucleus accumbens (Schwarz and Bilbo, 2013). While the activation of TLR4 by bacteria typically results in the synthesis of many cytokines and chemokines, TLR4 binding by morphine induces a robust increase in the synthesis of a select group of cytokines and chemokines within the nucleus accumbens that is necessary for the relapse to drug-seeking behaviors (Schwarz et al., 2011). Therefore, it appears that microglia within the adolescent brain are more plastic relative to microglia within the adult brain, such that their activation in adolescence can influence cell function acutely and subsequently have a long-term impact on TLR4 signaling and microglia function.
Further evidence that a “plastic” phenotype of adolescent microglia may contribute to addiction vulnerability comes from work with rodent models of alcohol binge drinking. Using a model of intermittent ethanol administration, Crews and colleagues reported that binge-like exposure induced expression of the DAMP high-mobility group box 1 (HMGB1) in prefrontal cortex neurons that endured into adulthood and correlated with deficits in reversal learning (Vetreno and Crews, 2012). The toll-like-receptors TLR4 and TLR3, which bind HMGB1, were also increased as a result of adolescent ethanol exposure. HMGB1 plays different roles depending on its localization in the nucleus—where it plays an architectural role and regulates neurite outgrowth and migration during development, or in the extracellular matrix—where it plays a pro-inflammatory role via activation of TLR (Fang et al., 2012). The authors hypothesized that ethanol activates adolescent microglia to secrete cytokines that promote HMGB1 release from neuronal nuclei; extracellular HMGB1 takes on a pro-inflammatory role to further activate microglia, leading to an enduring neuro-immune loop that endures through adulthood. A recent report reproduced and expanded these findings, demonstrating that TLR4 knock-out mice were protected from effects of adolescent intermittent ethanol on enduring pro-inflammatory activity and cognitive deficits (Montesinos et al., 2015). Notably, these studies did not compare the effects of adolescent and adult binge drinking and therefore does not directly illustrate a distinct vulnerability to induction of this immunological cascade during adolescence. However, this likelihood is illustrated in the reports that neuro-immune signaling can drive developmental trajectories and that other TLR-activating drugs have effects specifically in adolescence. Furthermore, recent clinical data from Silveri and colleagues (personal communication, submitted ms) illustrate that male early adolescent, but not adult, binge-drinkers have elevated levels of prefrontal glutathione—an indirect measure of oxidative stress and pro-inflammatory activity (reviewed in (Leza et al., 2015); providing even more support for adolescence as a specific period of enhanced neuro-immune function and vulnerability that significantly impacts behavior.
7.2 Schizophrenia
Schizophrenia is a disorder with adolescent or young-adult onset that has been directly associated with altered immune responsivity at the onset of psychosis (Müller et al., 2015). For example, increased microglial activation and increased inflammatory cytokine mRNA expression are detected in the dorsolateral prefrontal cortex of 40% patients with schizophrenia, an effect that was greatest in patients who had been most recently diagnosed (Fillman et al., 2013). Furthermore, anti-inflammatory treatments for schizophrenia reportedly have their highest effectiveness during the earliest stages of the disease (Müller et al., 2010) and typical anti-psychotic treatments in turn lower peripheral cytokine levels in first-episode psychosis patients (Noto et al., 2015). Therefore, it appears that immune mechanisms may play a particular role in the onset of schizophrenia during adolescence, provoking the question: What is special about the adolescent neuro-immune environment? While strong evidence for immune signaling in the pathophysiology of schizophrenia is presented elsewhere (e.g., (Leza et al., 2015; Müller et al., 2015)), here we will examine the growing data supporting adolescence as a vulnerable window for these neuro-immune mechanisms.
Two leading and related theories on the pathogenesis of schizophrenia are the neurodevelopmental and 2-hit models. The neurodevelopmental model of schizophrenia is based on reports of an excess of adverse events such as immune activation (Knuesel et al., 2014) or stress (Bale, 2014; Selemon and Zecevic, 2015) occurring during the pre- and perinatal periods which lead to the presence of cognitive and behavioral prodromes in childhood, followed by symptoms of psychosis in adolescence and early adulthood (Tarbox et al., 2013). The 2-hit model expands this theory to acknowledge a strong interactive influence of early insults with subsequent insults occurring during adolescence (Keshavan, 1999). Taken together, it appears that early insults such as stress or immune activation have delayed effects that manifest in adolescent behavioral dysfunction or at least increased vulnerability to a second insult. To this end, animal studies have shed some light on whether delayed inflammatory effects lead to adolescent dysfunction.
Rodent models of early postnatal stress (Brenhouse and Andersen, 2011b) or neonatal ventral hippocampal lesions (Cabungcal et al., 2014) reportedly lead to a decrease in prefrontal cortex fast-spiking parvalbumin-positive interneurons, which resembles a phenomenon observed in schizophrenia and also correlates with schizophrenia-like behavioral impairments. This parvalbumin loss is seen in adolescence, and can be prevented with anti-inflammatory treatments that are administered during early adolescence (Brenhouse and Andersen, 2011b; Cabungcal et al., 2014). Further evidence for adolescent-specific immune activity indicates that parvalbumin interneurons in rats with neonatal ventral hippocampal lesions exhibit oxidative stress prior to symptom onset in adolescence (Cabungcal et al., 2014). Once again, we highlight the lack of knowledge as to the existence of an adolescent-specific immunological environment that may contribute to increased vulnerability. At present, we can refer to neurocircuitry development that is better understood; prefrontal fast-spiking interneurons do not mature until late adolescence (Tseng and O'Donnell, 2007), therefore the deleterious effects of immune insults to cortical function may not become evident until the transition through adolescence. Additionally, recent data have illustrated that prenatal immune activation alters the expression of genes for neurotrophic and neurodevelopmental regulators within the prefrontal cortex, but these expression changes were not seen prior to adolescence (Hemmerle et al., 2015). These findings further suggest that the effects of neuro-immune events leading to schizophrenia might manifest during a period of rapid and critical development of the prefrontal cortex.
7.3 Depression
Similar to schizophrenia, major depressive disorder typically first manifests during adolescence or early adulthood (reviewed in (Hagan et al., 2015; Sonuga-Barke et al., 2015)). Depression has long been associated with immune system dysregulation, a concept that originated partially due to the similarities between depressive symptoms and sickness behavior (Herbert and Cohen, 1993; Maes, 1995). Likely due to the long-studied immunological pathogenesis of depression as well as its well-characterized adolescent presentation, a valuable review of the albeit limited literature on inflammation in adolescent depression is already available (Mills et al., 2013). Therefore, here we will limit our discussion to potential mechanistic underpinnings of adolescence as a discrete period of vulnerability. Specifically, we recently showed that early life stress exposure in rats led to adolescent increases in circulating levels of the pro-inflammatory cytokine IL-6 (do Prado et al., 2015), which has been reported in depressed patients (Miller and Cole, 2012). Importantly, this increase in IL-6 is positively correlated with prefrontal cortex parvalbumin-positive interneuron loss as well as NMDA receptor subunit changes (do Prado et al., 2015). Central administration of the anti-inflammatory cytokine IL-10 during early adolescence prevented the NMDA changes and prevented parvalbumin loss (Wieck et al., 2013), suggesting that central inflammatory activity was a consequence of peripheral pro-inflammatory activity. However, our lack of understanding BBB mechanisms during adolescence mires our ability to speculate on how peripheral cytokines may produce neural effects. Taken together, it appears that early insults may sensitize inflammatory activity, as proposed previously (Bilbo and Schwarz, 2009). During adolescence, heightened immune activation may alter neural signaling during a critical developmental period and result in behavioral dysfunction such as that seen in depressive illness.
8. Suggestions for future research
Moving forward, we will need to further unveil the critical role that the adolescent immune system has in maturing neural circuits during this time, and expand our preliminary understanding of how typical and atypical immune development interacts with adolescent vulnerability to increase the risk of many neuropsychiatric disorders. We suggest setting the following goals in order to develop these concepts in a cohesive way.
8.1 Investigate the adolescent blood-brain interface
This review highlighted a paucity of research on how, or whether, peripheral immune function is unique during adolescence. Future research should seek to examine how the immaturity of certain brain structures may influence the function of the immune system or vice versa. We propose here that as the adolescent brain continues to develop, differences in neurotransmitter function may affect the function of the blood brain barrier dynamics (thus affecting the passage of immune molecules into the brain) or the function of glial cells in the brain. While an intriguing hypothesis, this has yet to be confirmed or examined. Thus future experiments may seek to use in vitro methods for examining this question and thereby determine the bi-directional influence of neural development on immune function and development.
8.2 Use age as a biological variable
An important priority for future research in this area should be to examine changes in peripheral immune function that occur specifically during adolescence. In the human literature, data from adolescents are often lumped together with data from children when examining disease risk or peripheral immune responses. In the rodent literature, peripheral immunity during adolescence has rarely been examined at all. Thus, preclinical and clinical researchers should pay close attention to age in order to interpret potential differences within the time of adolescence. This is particularly important given that there are a number of different developmental events described here (e.g. social maturation, pubertal maturation, etc.) that occur throughout this time in adolescent development, and many of these do not always occur at the same time in every species, or in every individual; nor do these events occur at the same age in both males and females (see Figure 1). In order to fully understand how adolescence is distinct from other ages of development, future basic research should take care to manipulate and test immune activation or behavioral endpoints in adolescence and also at other ages. In doing so, we can better understand whether adolescent immune and neural function is distinct or similar from other ages; we can also determine whether adolescence is a period of vulnerability or sensitivity relative to other ages.
8.3 Design studies with consideration of adolescence as a heterogeneous phase
As illustrated in Figure 1, adolescence includes several stages within itself; these stages are characterized (albeit incompletely) by behavioral, hormonal, neural, and immune changes. Therefore, investigation of adolescent neuroimmune development will benefit from more studies comparing measurements and manipulations between these phases. As suggested in the fields of child neuropsychology (Karmiloff-Smith and Thomas, 2008) and brain development (Andersen, 2003), understanding the trajectory of changes throughout adolescence provides rich information about neuroimmune development; it becomes possible to provide more in-depth descriptors in terms of delayed onsets, atypical rates, or normal changes with consistent differences between groups. At the minimum, findings should be reported in the context of a particular phase of adolescence (i.e., puberty, post-puberty, late adolescence) whenever possible, to facilitate comparisons between studies.
9. Conclusions
Evidence is growing in support of the idea that the immune system can significantly influence the brain, both in times of health and in times of disease. As a result, the immune system has an integral role in shaping neural development and subsequently altering neural function and behavior. Given the research presented here, we submit that adolescence is its own unique period of immune system function, with significant implications for the brain.
Highlights.
Maturation of the immune system continues through adolescence
Neuro-immune signaling controls neural development
We review evidence that adolescence is a unique stage of immune development
Neuro-immune development contributes to adolescent vulnerability to mental illness
We propose a need for understanding adolescence a period of neuro-immune signaling
Acknowledgements
This work was funded by 5R21MH097182 to HCB. We would like to thank Dr. Jennifer Honeycutt for her artistic contributions to Figure 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nature reviews. Neuroscience. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neuroscience and biobehavioral reviews. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Desperately driven and no brakes: developmental stress exposure and subsequent risk for substance abuse. Neuroscience and biobehavioral reviews. 2009;33:516–524. doi: 10.1016/j.neubiorev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL. Lifetime stress experience: transgenerational epigenetics and germ cell programming. Dialogues in clinical neuroscience. 2014;16:297–305. doi: 10.31887/DCNS.2014.16.3/tbale. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA. The blood-brain barrier in neuroimmunology: Tales of separation and assimilation. Brain, behavior, and immunity. 2015;44:1–8. doi: 10.1016/j.bbi.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Blood to brain transport of interleukin links the immune and central nervous systems. Life sciences. 1991;48:PL117–121. doi: 10.1016/0024-3205(91)90385-o. [DOI] [PubMed] [Google Scholar]
- Barak B, Feldman N, Okun E. Toll-like receptors as developmental tools that regulate neurogenesis during development: an update. Frontiers in neuroscience. 2014;8:272. doi: 10.3389/fnins.2014.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JA, Schleifer SJ, Demetrikopoulos MK, Delaney BR, Shiflett SC, Keller SE. Immune function in healthy adolescents. Clinical and diagnostic laboratory immunology. 1998;5:105–113. doi: 10.1128/cdli.5.1.105-113.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Frontiers in behavioral neuroscience. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borre YE, O'Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends in molecular medicine. 2014;20:509–518. doi: 10.1016/j.molmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neuroscience and biobehavioral reviews. 2011a;35:1687–1703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Nonsteroidal anti-inflammatory treatment prevents delayed effects of early life stress in rats. Biol Psychiatry. 2011b;70:434–440. doi: 10.1016/j.biopsych.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabungcal JH, Counotte DS, Lewis EM, Tejeda HA, Piantadosi P, Pollock C, Calhoon GG, Sullivan EM, Presgraves E, Kil J, Hong LE, Cuenod M, Do KQ, O'Donnell P. Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron. 2014;83:1073–1084. doi: 10.1016/j.neuron.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Dantzer R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18:2143–2151. doi: 10.1200/JCO.2000.18.10.2143. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. The Journal of comparative neurology. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Dennison U, McKernan D, Cryan J, Dinan T. Schizophrenia patients with a history of childhood trauma have a pro-inflammatory phenotype. Psychological medicine. 2012;42:1865–1871. doi: 10.1017/S0033291712000074. [DOI] [PubMed] [Google Scholar]
- do Prado CH, Narahari T, Holland FH, Lee HN, Murthy SK, Brenhouse HC. Effects of early adolescent environmental enrichment on cognitive dysfunction, prefrontal cortex development, and inflammatory cytokines after early life stress. Developmental psychobiology. 2015 doi: 10.1002/dev.21390. [DOI] [PubMed] [Google Scholar]
- Eyo UB, Wu LJ. Bidirectional microglia-neuron communication in the healthy brain. Neural plasticity. 2013;2013:456857. doi: 10.1155/2013/456857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang P, Schachner M, Shen YQ. HMGB1 in development and diseases of the central nervous system. Molecular neurobiology. 2012;45:499–506. doi: 10.1007/s12035-012-8264-y. [DOI] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Montesinos J, Guerri C. Ethanol induces TLR4/TLR2 association, triggering an inflammatory response in microglial cells. Journal of neurochemistry. 2013;126:261–273. doi: 10.1111/jnc.12276. [DOI] [PubMed] [Google Scholar]
- Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, Cairns M, Weickert CS. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18:206–214. doi: 10.1038/mp.2012.110. [DOI] [PubMed] [Google Scholar]
- Fillman SG, Weickert TW, Lenroot RK, Catts SV, Bruggemann JM, Catts VS, Weickert CS. Elevated peripheral cytokines characterize a subgroup of people with schizophrenia displaying poor verbal fluency and reduced Broca's area volume. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani SP, Walsh JT, Lukens JR, Kipnis J. Dealing with Danger in the CNS: The Response of the Immune System to Injury. Neuron. 2015;87:47–62. doi: 10.1016/j.neuron.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galic MA, Riazi K, Pittman QJ. Cytokines and brain excitability. Frontiers in neuroendocrinology. 2012;33:116–125. doi: 10.1016/j.yfrne.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi-Oliveira R, Holland FH, Honeycutt JA, Ganguly P, Brenhouse HC. Cognitive impairment effects of early life stress in adolescents can be predicted with early biomarkers: Impacts of sex, experience, and cytokines. Psychoneuroendocrinology. doi: 10.1016/j.psyneuen.2016.04.016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Silveira F, Abad-Franch F. Sex bias in infectious disease epidemiology: patterns and processes. PloS one. 2013;8:e62390. doi: 10.1371/journal.pone.0062390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan CC, Graham JM, Wilkinson PO, Midgley N, Suckling J, Sahakian BJ, Goodyer IM. Neurodevelopment and ages of onset in depressive disorders. The lancet. Psychiatry. 2015;2:1112–1116. doi: 10.1016/S2215-0366(15)00362-4. [DOI] [PubMed] [Google Scholar]
- Hemmerle AM, Ahlbrand R, Bronson SL, Lundgren KH, Richtand NM, Seroogy KB. Modulation of schizophrenia-related genes in the forebrain of adolescent and adult rats exposed to maternal immune activation. Schizophrenia research. 2015;168:411–420. doi: 10.1016/j.schres.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert TB, Cohen S. Depression and immunity: a meta-analytic review. Psychological bulletin. 1993;113:472–486. doi: 10.1037/0033-2909.113.3.472. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, Rebusi N, Heshmati M, Aleyasin H, Warren BL, Lebonte B, Horn S, Lapidus KA, Stelzhammer V, Wong EH, Bahn S, Krishnan V, Bolanos-Guzman CA, Murrough JW, Merad M, Russo SJ. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. TheScientificWorldJournal. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmiloff-Smith A, Thomas CS. The Importance of Tracing Developmental Trajectories for Clinical Child Neuropsychology. In: Reed J, Warner-Rogers J, editors. Child Neuropsychology: Concepts, Theory, and Practice. Wiley-Blackwell; 2008. [Google Scholar]
- Karrenbauer BD, Muller CP, Ho YJ, Spanagel R, Huston JP, Schwarting RK, Pawlak CR. Time-dependent in-vivo effects of interleukin-2 on neurotransmitters in various cortices: relationships with depressive-related and anxiety-like behaviour. Journal of neuroimmunology. 2011;237:23–32. doi: 10.1016/j.jneuroim.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Keshavan MS. Development, disease and degeneration in schizophrenia: a unitary pathophysiological model. Journal of psychiatric research. 1999;33:513–521. doi: 10.1016/s0022-3956(99)00033-3. [DOI] [PubMed] [Google Scholar]
- Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, Toovey S, Prinssen EP. Maternal immune activation and abnormal brain development across CNS disorders. Nature reviews. Neurology. 2014;10:643–660. doi: 10.1038/nrneurol.2014.187. [DOI] [PubMed] [Google Scholar]
- Lapchak PA. A role for interleukin-2 in the regulation of striatal dopaminergic function. Neuroreport. 1992;3:165–168. doi: 10.1097/00001756-199202000-00011. [DOI] [PubMed] [Google Scholar]
- Leza JC, Garcia-Bueno B, Bioque M, Arango C, Parellada M, Do K, O'Donnell P, Bernardo M. Inflammation in schizophrenia: A question of balance. Neuroscience and biobehavioral reviews. 2015;55:612–626. doi: 10.1016/j.neubiorev.2015.05.014. [DOI] [PubMed] [Google Scholar]
- Liu J, Buisman-Pijlman F, Hutchinson MR. Toll-like receptor 4: innate immune regulator of neuroimmune and neuroendocrine interactions in stress and major depressive disorder. Frontiers in neuroscience. 2014;8:309. doi: 10.3389/fnins.2014.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liva SM, Voskuhl RR. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. Journal of immunology (Baltimore, Md. : 1950) 2001;167:2060–2067. doi: 10.4049/jimmunol.167.4.2060. [DOI] [PubMed] [Google Scholar]
- Maes M. Evidence for an immune response in major depression: a review and hypothesis. Progress in neuro-psychopharmacology & biological psychiatry. 1995;19:11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- McClory AJ, Spear LP. Effects of ethanol exposure during adolescence or in adulthood on Pavlovian conditioned approach in Sprague-Dawley rats. Alcohol (Fayetteville, N.Y.) 2014;48:755–763. doi: 10.1016/j.alcohol.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Marinelli M. Age matters. The European journal of neuroscience. 2009;29:997–1014. doi: 10.1111/j.1460-9568.2009.06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cole SW. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biol Psychiatry. 2012;72:34–40. doi: 10.1016/j.biopsych.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills NT, Scott JG, Wray NR, Cohen-Woods S, Baune BT. Research review: the role of cytokines in depression in adolescents: a systematic review. Journal of child psychology and psychiatry, and allied disciplines. 2013;54:816–835. doi: 10.1111/jcpp.12080. [DOI] [PubMed] [Google Scholar]
- Montesinos J, Pascual M, Pla A, Maldonado C, Rodriguez-Arias M, Minarro J, Guerri C. TLR4 elimination prevents synaptic and myelin alterations and long-term cognitive dysfunctions in adolescent mice with intermittent ethanol treatment. Brain, behavior, and immunity. 2015;45:233–244. doi: 10.1016/j.bbi.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Müller N, Krause D, Dehning S, Musil R, Schennach-Wolff R, Obermeier M, Moller HJ, Klauss V, Schwarz MJ, Riedel M. Celecoxib treatment in an early stage of schizophrenia: results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophrenia research. 2010;121:118–124. doi: 10.1016/j.schres.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Müller N, Weidinger E, Leitner B, Schwarz MJ. The role of inflammation in schizophrenia. Frontiers in neuroscience. 2015;9:372. doi: 10.3389/fnins.2015.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima T, Piriz J, Duflot S, Fernandez AM, Gaitan G, Gomez-Pinedo U, Verdugo JM, Leroy F, Soya H, Nunez A, Torres-Aleman I. Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron. 2010;67:834–846. doi: 10.1016/j.neuron.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Northcutt AL, Hutchinson MR, Wang X, Baratta MV, Hiranita T, Cochran TA, Pomrenze MB, Galer EL, Kopajtic TA, Li CM, Amat J, Larson G, Cooper DC, Huang Y, O'Neill CE, Yin H, Zahniser NR, Katz JL, Rice KC, Maier SF, Bachtell RK, Watkins LR. DAT isn't all that: cocaine reward and reinforcement require Toll-like receptor 4 signaling. Mol Psychiatry. 2015;20:1525–1537. doi: 10.1038/mp.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noto C, Ota VK, Gouvea ES, Rizzo LB, Spindola LM, Honda PH, Cordeiro Q, Belangero SI, Bressan RA, Gadelha A, Maes M, Brietzke E. Effects of risperidone on cytokine profile in drug-naive first-episode psychosis. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2015;18 doi: 10.1093/ijnp/pyu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin JS, Zalcman SS, Zhu Y, Siegel A. Short- and long-term effects of interleukin-2 treatment on the sensitivity of periadolescent female mice to interleukin-2 and dopamine uptake inhibitor. PloS one. 2013;8:e64473. doi: 10.1371/journal.pone.0064473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riis JL, Out D, Dorn LD, Beal SJ, Denson LA, Pabst S, Jaedicke K, Granger DA. Salivary cytokines in healthy adolescent girls: Intercorrelations, stability, and associations with serum cytokines, age, and pubertal stage. Developmental psychobiology. 2014;56:797–811. doi: 10.1002/dev.21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Hormones and behavior. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwandt ML, Lindell SG, Chen S, Higley JD, Suomi SJ, Heilig M, Barr CS. Alcohol response and consumption in adolescent rhesus macaques: life history and genetic influences. Alcohol (Fayetteville, N.Y.) 2010;44:67–80. doi: 10.1016/j.alcohol.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Bilbo SD. The Immune System and the Developing Brain Morgan & Claypool Life Sciences. 2011.
- Schwarz JM, Bilbo SD. Adolescent morphine exposure affects long-term microglial function and later-life relapse liability in a model of addiction. J Neurosci. 2013;33:961–971. doi: 10.1523/JNEUROSCI.2516-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Hutchinson MR, Bilbo SD. Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. J Neurosci. 2011;31:17835–17847. doi: 10.1523/JNEUROSCI.3297-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Zecevic N. Schizophrenia: a tale of two critical periods for prefrontal cortical development. Translational psychiatry. 2015;5:e623. doi: 10.1038/tp.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shames RS. Gender differences in the development and function of the immune system. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2002;30:59–70. doi: 10.1016/s1054-139x(01)00382-2. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nature neuroscience. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Smith AR, Steinberg L, Strang N, Chein J. Age differences in the impact of peers on adolescents' and adults' neural response to reward. Developmental cognitive neuroscience. 2015;11:75–82. doi: 10.1016/j.dcn.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CP, Dunger DB, Williams AJ, Taylor AM, Perry LA, Gale EA, Preece MA, Savage MO. Relationship between insulin, insulin-like growth factor I, and dehydroepiandrosterone sulfate concentrations during childhood, puberty, and adult life. The Journal of clinical endocrinology and metabolism. 1989;68:932–937. doi: 10.1210/jcem-68-5-932. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Cortese S, Fairchild G, Stringaris A. Annual Research Review: Transdiagnostic neuroscience of child and adolescent mental disorders - differentiating decision making in attention-deficit/hyperactivity disorder, conduct disorder, depression, and anxiety. Journal of child psychology and psychiatry, and allied disciplines. 2015 doi: 10.1111/jcpp.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L. The adolescent brain and age-related behavioral manifestations. Neuroscience and biobehavioral reviews. 2000a;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear L. Modeling adolescent development and alcohol use in animals. Alcohol Res Health. 2000b;24:115–123. [PMC free article] [PubMed] [Google Scholar]
- Stanis JJ, Andersen SL. Reducing substance use during adolescence: a translational framework for prevention. Psychopharmacology. 2014;231:1437–1453. doi: 10.1007/s00213-013-3393-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui YT, Bullock KM, Erickson MA, Zhang J, Banks WA. Alpha synuclein is transported into and out of the brain by the blood-brain barrier. Peptides. 2014;62:197–202. doi: 10.1016/j.peptides.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson J, Diez M, Engel J, Wass C, Tivesten A, Jansson JO, Isaksson O, Archer T, Hokfelt T, Ohlsson C. Endocrine, liver-derived IGF-I is of importance for spatial learning and memory in old mice. The Journal of endocrinology. 2006;189:617–627. doi: 10.1677/joe.1.06631. [DOI] [PubMed] [Google Scholar]
- Tarbox SI, Addington J, Cadenhead KS, Cannon TD, Cornblatt BA, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Heinssen R, McGlashan TH, Woods SW. Premorbid functional development and conversion to psychosis in clinical high-risk youths. Development and psychopathology. 2013;25:1171–1186. doi: 10.1017/S0954579413000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollerud DJ, Ildstad ST, Brown LM, Clark JW, Blattner WA, Mann DL, Neuland CY, Pankiw-Trost L, Hoover RN. T-cell subsets in healthy teenagers: transition to the adult phenotype. Clinical immunology and immunopathology. 1990;56:88–96. doi: 10.1016/0090-1229(90)90172-m. [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS biology. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Crews FT. Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and Toll-like receptors in the adult prefrontal cortex. Neuroscience. 2012;226:475–488. doi: 10.1016/j.neuroscience.2012.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello B. Recent developments and strategies in pediatric pharmacology research in the USA. Child and adolescent psychiatry and mental health. 2008;2:36. doi: 10.1186/1753-2000-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieck A, Andersen SL, Brenhouse HC. Evidence for a neuroinflammatory mechanism in delayed effects of early life adversity in rats: relationship to cortical NMDA receptor expression. Brain, behavior, and immunity. 2013;28:218–226. doi: 10.1016/j.bbi.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Yan H, Mitschelen M, Bixler GV, Brucklacher RM, Farley JA, Han S, Freeman WM, Sonntag WE. Circulating IGF1 regulates hippocampal IGF1 levels and brain gene expression during adolescence. The Journal of endocrinology. 2011;211:27–37. doi: 10.1530/JOE-11-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]