Abstract
Childhood adversity increases vulnerability to psychiatric disorders that emerge in adolescence, in a sex-dependent manner. Early adversity modeled in rodents with maternal separation (MS) affects cognition and medial prefrontal cortex (mPFC) circuitry. Humans and animals exposed to early life adversity also display heightened circulating inflammatory cytokines, however the predictive relationship of these early measures with later behavioral deficits is unknown. Here, male and female rats were exposed to MS or control rearing during the postnatal period (P2–21). Blood samples were taken at distinct developmental time points for analysis of the pro-inflammatory cytokine IL-1β and the anti-inflammatory cytokines IL-4, and IL-10, followed by win-shift cognitive testing and analysis of mPFC parvalbumin (PVB) immunofluorescent interneurons in adolescence. Regression analyses were conducted to explore the relationship between early cytokines and adolescent behavioral measures. We observed sex- and age-dependent effects of MS on circulating cytokines. MS also yielded adolescent decreases in mPFC PVB and cognitive deficits, which were predicted by early cytokine expression in a sex- and experience-dependent manner. Taken together, the present data reveals that circulating cytokines and PVB levels are predictive of adolescent cognitive deficits, and therefore provide compelling evidence for a putative role of early biomarkers in mediating MS-induced behavioral dysfunction. Importantly, predictive relationships often depended on sex and on MS history, suggesting that early life experiences may yield individualistic mechanisms of vulnerability compared to the general population.
Keywords: maternal separation, development, cytokines, win-shift, prefrontal cortex, memory, interneurons, early life stress, parvalbumin
1. Introduction
Exposure to early life adversity increases vulnerability to psychiatric disorders later in life, including depression, drug abuse, and schizophrenia (Agid et al., 1999; Brake et al., 2004; Kessler et al., 1997; Kohut et al., 2009; Teicher et al., 2006). Clinical data show that the time between onset of child abuse and the onset of mental illness is approximately 11.5 years (usually during adolescence) (Teicher et al., 2009), suggesting that there may be a window of opportunity for intervention between childhood adversity and pathology emergence in adolescence. Childhood maltreatment is also related to executive dysfunction in both adolescence (Mothes et al., 2015) and middle adulthood (Nikulina and Widom, 2013), findings which can also be seen in rodent models of early life stress. However, identification of at-risk children who may not present any behavioral symptoms poses a significant challenge, and there is thus a great need for reliable early-life biomarkers of disease susceptibility.
Maternal separation (MS) in rodents is a well-established animal model of early life stress that induces long-lasting alterations at the behavioral, immunological, neuroanatomical, biochemical, and molecular levels [for review: (Brunton, 2015; Ganguly and Brenhouse, 2015)]. MS has been shown to induce cognitive dysfunction in rodents during adolescence including working memory (do Prado et al., 2015), spatial memory (Aisa et al., 2009), cognitive flexibility deficits (Thomas et al., 2015), reduced prepulse inhibition (Li et al., 2013), and dysfunctional reward-related behaviors (Hays et al., 2012). These effects are likely due to PFC and limbic dysfunction related to reduced synaptic plasticity (Aisa et al., 2009) and dopaminergic (Li et al., 2013), serotoninergic (Xue et al., 2013) and glutamatergic changes (O'Connor et al., 2015; Wieck et al., 2013). Moreover, cognitive dysfunction was recently associated with aberrant development of critical perisomatic inhibitory interneuron local microcircuitry (do Prado et al., 2015) in extension of previous findings showing a reduction of GABAergic parvalbumin-containing interneurons (PVB) in the prefrontal cortex of MS-exposed adolescent rats (Wieck et al., 2013). These lowered parvalbumin levels in MS adolescents were directly correlated with increased circulating levels of the pro-inflammatory cytokines IL-1β and IL-6, while administration of the anti-inflammatory cytokine interleukin IL-10 protected against such loss (Wieck et al., 2013).
Converging evidence suggests that MS induces enduring effects on the immune system (Avitsur et al., 2013; do Prado et al., 2016; Hennessy et al., 2011; Pinheiro et al., 2015; Roque et al., 2015; Wieck et al., 2013). For example, when the expression of 84 key genes involved in the inflammatory response was evaluated in MS and controls infant rats, gene expression of only two interleukins was affected by MS: IL-1β and IL-10 (Dimatelis et al., 2012). The negative effect on cognitive behavior of IL-1β is already known (Terrando et al., 2010), but recently it was discovered that Th2-related cytokine IL-4, in contrast, has a profound beneficial effect on cognition (Gadani et al., 2012). Therefore, despite the unknown mechanism, growing evidence suggests that behavioral deficits in early adversity-exposed adolescent or adult animals (Veenema et al., 2008; Wieck et al., 2013) and humans (Danese et al., 2007; Dennison et al., 2012; Slopen et al., 2013) may be related to peripherally circulating pro-inflammatory markers. Changes in the circulating levels of these proteins have been associated with many disease states, making them potentially valuable as functional biomarkers. Excessive or diminished cytokine levels are associated with schizophrenia and depression and are associated with a pro-inflammatory phenotype in patients with a history of childhood trauma, but not in patients without such history (Dennison et al., 2012; Miller and Cole, 2012). There are several pathways of active communication that allow controlled immune signaling between the periphery and the brain. Peripheral immune mediators can thereby influence behavioral and neuroendocrine responses to environmental stimuli during both normal and abnormal development (Miller et al., 2009). Brain responses to peripheral immune activity occur either via vagal afferents, directly at the blood brain barrier (BBB), active transport via saturable transport molecules, or activation of endothelial cells lining cerebral vasculature (Quan and Banks, 2007).Therefore, identification of individuals that have a peripheral pro-inflammatory phenotype during the relatively asymptomatic window of pre-adolescence could allow for proper intervention at a critical time.
Evidence suggests that immunodevelopment occurs on a different timeline in males and females (Rana et al., 2012), and that MS can impact persistence of HPA reactivity in a sex-specific manner (Meagher et al., 2010). Therefore, developmental differences in the immune environment, such as circulating cytokine expression may serve to better identify individuals at an elevated risk to develop later cognitive deficits. We hypothesized that there would be a sex-specific effect on immune regulation as a function of early life stress. In this regard, there are only few studies addressing whether early life stress affects male and female immune responsivity differently (Avitsur et al., 2013; Avitsur et al., 2009; Meagher et al., 2010), with little known about baseline differences in immune regulators. In the present study, we sought to identify sex- and age-specific relationships between juvenile cytokine levels, adolescent cognitive performance, and adolescent mPFC PVB expression.
2. Methods
All experiments were performed in accordance with the 1996 Guide for the Care and Use of Laboratory Animals (NIH) with approval from the Institutional Animal Care and Use Committee at Northeastern University.
2.1.Subjects and Maternal Separation
Forty gestational day 15 pregnant Sprague-Dawley rats were received from Charles River Laboratories (Wilmington MA), and were housed under constant temperature- and humidity-controlled conditions within a 12 h light/dark cycle (light period 0700h–1900h), with water and food available ad libitum. Litters were culled to 10 pups on postnatal day (P)1, maintaining as equal ratios as possible of males and females. Twenty litters were designated as maternal separation (MS), and were separated for 4 hours/day from P2–P20 in a thermo-regulated environment at 37°C. Apart from weighing and normal husbandry procedures, twenty control (CON) litters were undisturbed until weaning (P21) when all animals were rehoused with a cage-mate matched for age, sex, and group. Only one male and one female per litter were used, to avoid any litter effects. Therefore, each of twenty MS litters and twenty CON litters supplied one male and one female for each of two studies, as described below. Additionally, a separate cohort of 16 pregnant dams and their litters (eight MS and eight CON) were used for a post-hoc assessment of puberty timing, with no more than two pups per litter used for analyses.
2.2.Experimental Design
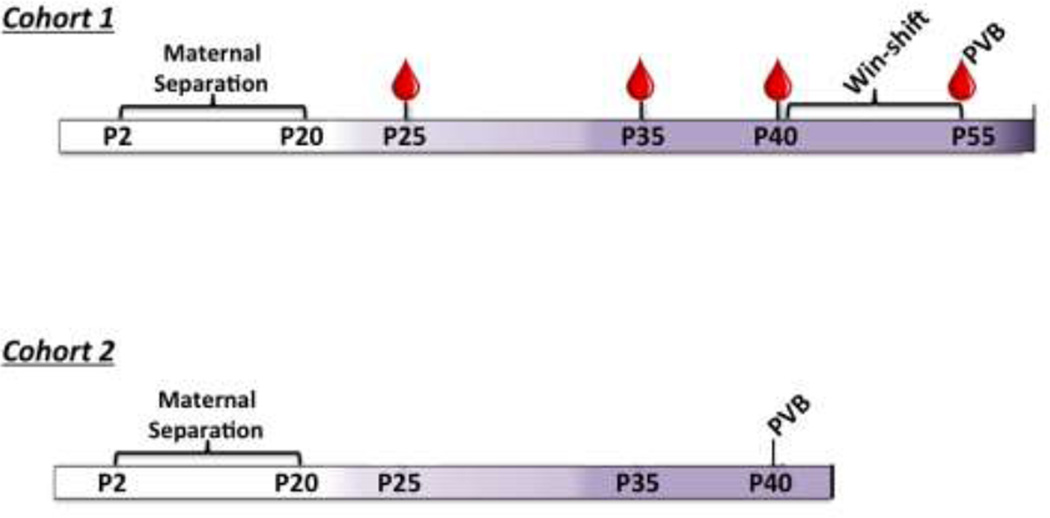
The experimental timeline is shown in Figure 1. In one cohort of offspring (n=15–20), blood was taken at P25, P35, and at sacrifice (P55). These subjects underwent behavioral training and testing on the win-shift paradigm before being sacrificed at P55 for analysis of PFC PVB immunofluorescence. A second cohort of offspring (n=12–15) was sacrificed at P40 for immunofluorescent analysis of PFC PVB expression.
Figure 1.
Experimental timelines. Blood draw time-points designated with droplet. Colors of timeline from left to right designates developmental stage: white: pre-weanling; light lavender: juvenile; dark lavender: adolescent; purple: adult. For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article
2.3.Blood Draws for Peripheral Cytokine and Hormone Measurements
Blood samples were collected from the saphenous vein (P25, P35), or heart (P55 sacrifice) between 10:00h and 13:00h in EDTA coated microcentrifuge tubes and spun at 1000 RCF for 10 minutes at 4°C. Plasma samples were collected and stored at −80°C until analysis. Saphenous blood draws were used due to their minimal invasiveness (total handling time= <2 min).
2.4.Cytometric Bead Array
Samples from 15–20 subjects/sex/group were analyzed at each age. Plasma levels of IL-1β, IL-4, and IL-10 were measured using Cytometric Bead Array (CBA) Rat Flex Sets according to the manufacturer’s instructions (BD Biosciences, San Jose CA). Acquisition was performed with a FACSCanto II flow cytometer (BD Biosciences). Quantitative results were generated using the FCAP Array v.3.0 software (BD Biosciences) with detection limits of 4.0 pg/ml for IL-1β, 3.4 pg/ml for IL-4 and 19.4 pg/ml for IL-10.
2.5.Testosterone ELISA
Since MS has not been shown to affect baseline estradiol levels in female rats but can alter peripubertal testosterone secretion (Bodensteiner et al., 2014), we also assessed plasma testosterone concentrations from blood samples at P35 using ELISA (R&D, Minneapolis, US). The mean minimum detectable dose was 0.030 ng/mL.
2.6.P40–P55 Win-shift
An 8-arm radial arm maze was used in a win-shift task to measure reference and working memory in MS and CON rats, as described previously (Brenhouse and Andersen, 2011). At P36 rats were food restricted to 15g of their daily Prolab® diet (RMA-3000, 5P00, TestDiet®, St. Louis, MO) in order to motivate the rats to consume the sucrose reward pellets (181135, TestDiet®, St. Louis, MO) presented within the maze. The food-restricted subjects’ body weights were maintained at 80% of ad libitum-fed. On P38–P39 rats (n=15–20/group/sex) were habituated to an 8-arm maze for 5 min each day. From P40–P55 rats were trained in the win-shift task. Briefly, in Phase 1, rats were introduced into the center of the maze and given 5 min to consume food rewards from four open arms, with the other four arms closed. After a 5-min delay period in the home cage, rats were reintroduced in Phase 2 to the maze with all 8 arms open but with rewards only available in the arms that had previously been blocked. An entry into an arm that did not contain a reward was marked as an error. After reaching criterion of two consecutive days with ≤ 1 error in Phase 2, subjects were tested for three consecutive days with varying delay times between Phase 1 and Phase 2 (5 minutes, 30 minutes, 3 hours). Delay sequence was counterbalanced across subjects. Subjects that had not reached criterion by P55 (9% of all subjects across groups) were designated as taking 16 days to reach criterion for data analysis purposes and were sacrificed without further testing. The maze was cleaned with 30% ethanol between each test and the configuration of open and closed doors changed daily.
2.7.PVB Immunohistochemstry
We analyzed PVB in two separate cohorts (n=12–15/group/sex): One cohort was sacrificed at P40 in order to replicate and extend previous findings from our lab that PVB is decreased at P40 in MS-exposed males (Brenhouse and Andersen, 2011; Holland et al., 2014; Wieck et al., 2013), and to obviate the possibility that food deprivation and/or learning the win-shift task could interact with MS experience to differentially affect PVB expression. For the second cohort, we analyzed PVB in the same animals that underwent win-shift testing in order to directly correlate PVB immunoreactivity with win-shift performance. After sacrifice and transcardial perfusion with 4% paraformaldehyde, 40µm coronal sections of the PFC were collected with a freezing microtome (Leica, Buffalo Grove, IL) and stored in cryoprotectant at -20 °C until staining. Every sixth section of the PFC was blocked in 5% normal donkey serum for one hour. Sections were then incubated with a monoclonal mouse anti-PVB antibody (1:10,000 Sigma-Aldrich, St.Louis MO) overnight at 4°C and incubated for one hour with an anti-mouse IgG antibody tagged with Alexa Flour® 568 (1:2,000, A11031, Life Technologies, Carlsbad, CA). Sections were washed between steps in phosphate buffered saline containing Triton X-100. Sections were then mounted on positively charged slides.
2.8.Stereological PVB Analysis
PVB interneurons were counted using an optical fractionator probe (StereoInvestigator; MBF Biosciences, Williston, Vermont). PVB expressing neurons were counted at 63× oil immersion magnification with a 100µm × 100µm counting frame and a 300µm × 300µm counting grid. Grid sizes were adapted during pilot studies to achieve an acceptable coefficient of error. Seven serial sections of the prelimbic (PL) and infralimbic (IL) PFC (inter-slice interval, 280µm) were counted for each subject. PVB immunoreactive cells were counted when the top of the cell was in focus within the counting frame. Estimated populations of cells were calculated for each subject using the mean section thickness.
2.9.Assessment of pubertal status
After finding several sex-dependent effects of MS on our physiological measurements, we ran a post-hoc study in a separate cohort of rats, assessing whether MS affects pubertal development differentially in males and females. Pubertal status was assessed daily in males and females (n=12–15/group/sex) from P25 until full puberty was achieved. Beginning on P25, the appearance of partial and complete balano-preputial separation were recorded in males on the days they were observed. The first day of partial balano-preputial separation was noted as puberty initiation, and the day of complete preputial separation was the endpoint used for full puberty in males. Beginning on PND 22, females were examined daily for vaginal opening. The appearance of a small “pinhole,” a vaginal thread, and complete vaginal opening were all recorded on the days they are observed. The first observance of a pinhole opening was designated as puberty initiation. The day of complete vaginal opening was the endpoint used for full puberty in females.
2.10. Statistical Analyses
All ANOVAs were performed using GraphPad Prism 6 software or IBM SPSS Statistics V.22, and all regression analyses were performed using SPSS. Cytokine levels across development where measures were taken longitudinally (P25, P25, and P55), as well as pup weights during MS were compared with mixed ANOVA (Sex × Group × [Age]) with age as a repeated measure. Group analyses within each developmental time point were performed using 2-way (Sex × Group) comparisons, with Bonferroni correction for multiple comparisons where appropriate. Multiplicity corrected p values are reported for Bonferroni tests. The number of days to reach win-shift criterion was analyzed using Kruskal Wallace survival analysis due to censorship at P55. Win-shift errors were compared between groups and sexes using a mixed ANOVA (Sex × Group × [delay]) with delay time (5 min, 30 min, 3hr) as a repeated measure. Group analyses within each delay were performed using 2-way (Sex × Group) comparisons. PVB immunoreactivity, adolescent testosterone levels, and age of puberty were analyzed using 2-way (Sex × Group) ANOVA. Pearson’s correlations and multiple regression analyses (using Sex and Group as modulating variables) were performed to correlate cytokine levels with win-shift performance and PVB immunoreactivity with win-shift performance. ANOVAs were conducted to test for significance of the regression lines. All correlations performed and their regression p values are shown in Supplemental Table 1.
3. Results
3.1.Developmental effects on circulating cytokine expression
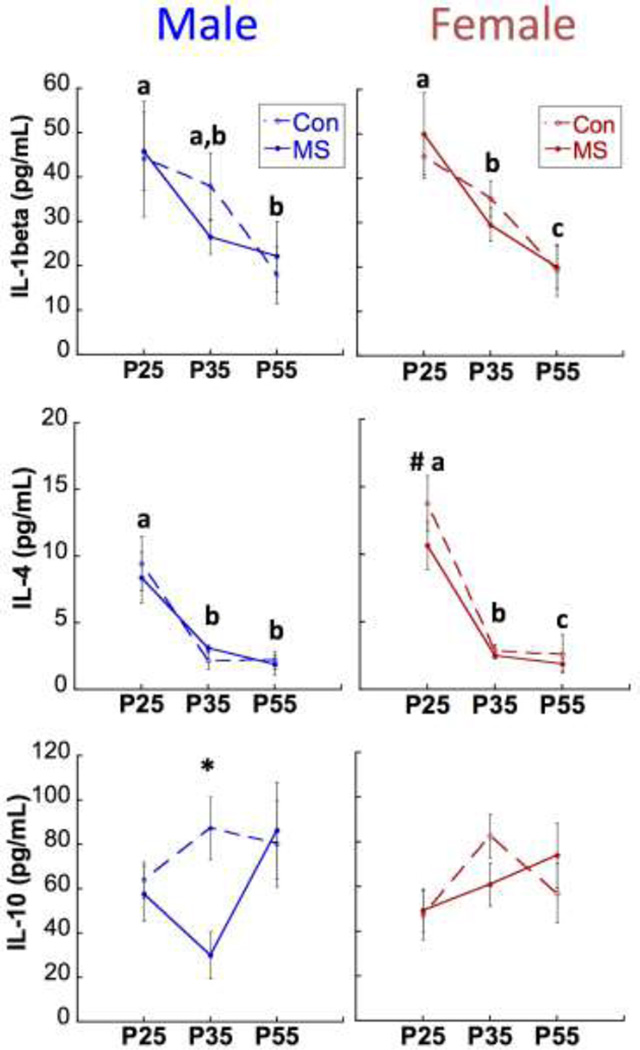
Subjects that were sacrificed on P55 were longitudinally analyzed for age, sex, and group differences in cytokine expression. As illustrated in Figure 2, circulating levels of IL-1β decreased significantly between P25 and P35 (Main Effect of Age F[1,104]=13.8; p<0.0001). Regardless of MS experience, a Sex × Age interaction (F1,104=4.84; p=0.03) revealed that females expressed higher levels of IL-4 than males at P25 (p=0.009). A Sex × Group Interaction for circulating IL-10 levels (F1,111=6.69; p=0.011) was driven by a lower expression in MS males compared to CON males at P35 (p= 0.001).
Figure 2.
Age, Group, and Sex effects on cytokine expression. Means ± SEM expression levels of IL-1β (top), IL-4 (middle) and IL-10 (bottom) across development. P: postnatal day. *p<0.05 difference between MS and CON. Different letters (a,b,c) designate p<0.05 difference between ages within the same sex (no effect of group). #p<0.05 difference between males and females, collapsed across groups. n=15–20/group/sex/time-point.
3.2.Effects on parvalbumin expression and win-shift performance at P55
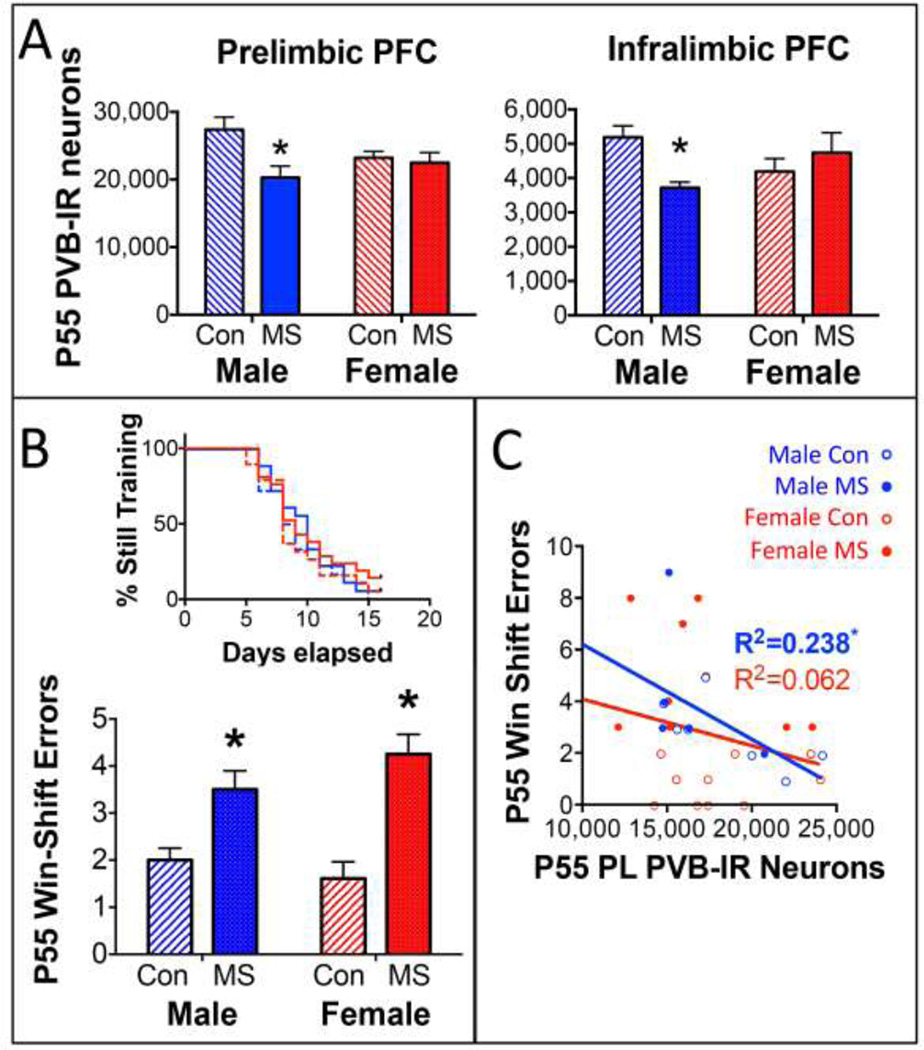
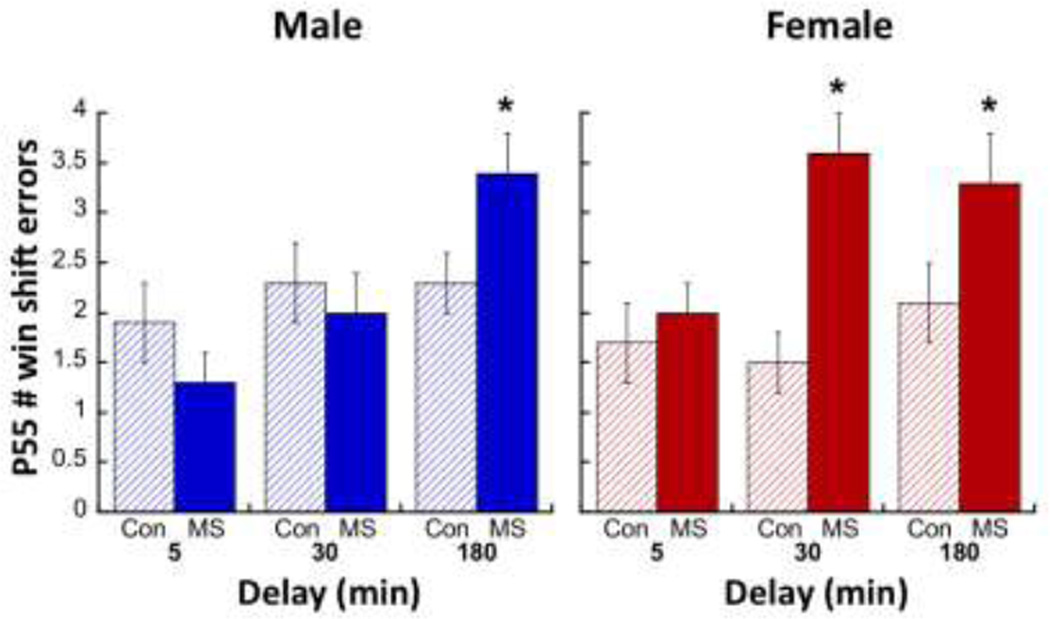
As illustrated in Figure 3A, P55 animals that had undergone win-shift training and testing displayed a sex-specific effect of MS on PVB. A Group × Sex interaction at P55 in both the PL (F1,25=6.18; p=0.019) and the IL (F1,25=4.48; p=0.044) PFC revealed that MS males displayed less PVB than CON (PL: p=0.038; IL: p=0.006), while this was not seen in females (PL: p>0.99; IL: p=0.67). Neither Sex nor Group affected the number of days subjects required to reach win-shift criterion (Figure 3B, top). However, both males and females exposed to MS committed more errors on the win-shift memory task compared to CON after a 3-h delay (Figure 3B, bottom). While no Group × Sex × Delay interaction was observed (p=0.463), a Group × Delay interaction (F2,120=3.84; p=0.024) was driven by increased errors committed after a 3-h delay between Phase 1 and Phase 2 (Main Effect of Group: F1,70=20.27; p<0.0001) in both males and females. Additionally, a Sex × Group interaction for errors committed after a 30-min delay (F1,64=6.72; p=0.012) revealed that MS-exposed females (p=0.001), but not males, committed more errors in the 30-min delay condition (Figure 4). Interestingly, PL PVB levels were significantly correlated with win-shift errors in the 3-h delay condition in males (Figure 3C; R=0.488; R2=0.238; F3,32=3.026; p=0.045), but not females (Interaction of Sex: p=0.039).
Figure 3.
Age, Group, and Sex effects on PVB and win-shift performance. A) Means ± SEM PVB expression levels in the PL (left) or IL (right) at P55. *p<0.05 difference between MS and CON. n=7–8/group/sex. B) Top: Kruskal Wallace chart demonstrating the number of training days required before subjects reached win-shift criterion. Bottom: Means ± SEM of errors made during Phase 2 of testing, following a 3-hr delay after Phase 1. *p<0.05 difference between MS and CON. n=15–20/group/sex. C) Regression analysis relating P55 PVB expression with win-shift performance. Male data is in blue and female data is in red. *p<0.05 significance of regression line. Due to an interaction of Sex but not Group, R2 values are provided for all male and all female subjects collapsed across MS and CON for clarity. For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article
Figure 4.
Effects of MS on number of errors committed in the win-shift task after varying delays. A Repeated Measures 2-way ANOVA failed to reveal a Delay × Group interaction; however, post-hoc comparisons show that MS males committed more errors than CON males after a 180-min delay, and MS females committed more errors than CON females after 30-min and 180-min delays. *p<0.05 difference from CON.
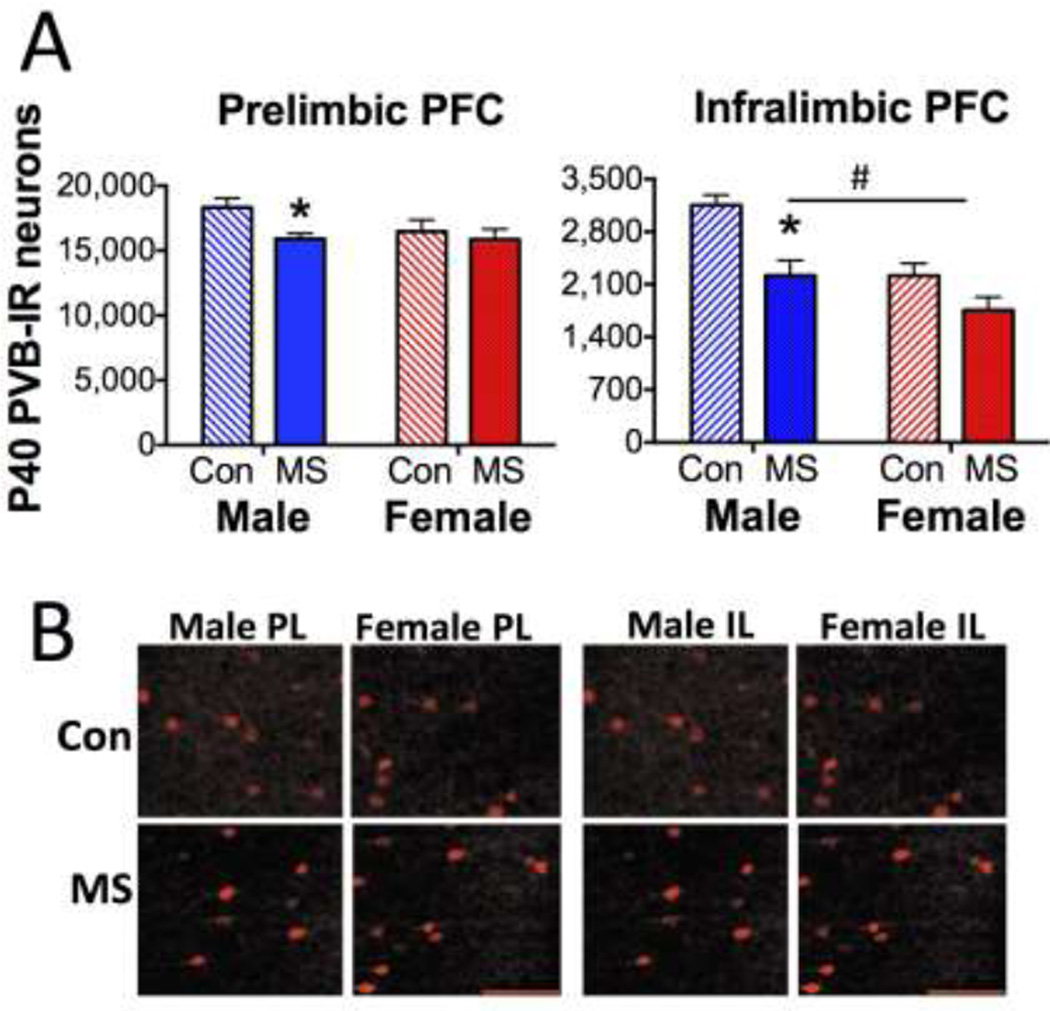
3.3.Effects on parvalbumin expression at P40
A similar pattern of MS effects was observed at P40 in a cohort of animals that did not undergo win-shift (Figures 5A and 5B). While no Sex × Group interaction was observed, a Main Effect of Group on PL PVB (F1,46=5.24; p=0.027) was driven by a PVB reduction in P40 MS males (p=0.002), but not females. Similarly, PVB expression in the IL was decreased in MS subjects (Main Effect of Group: F1,25=15.10; p=0.001) compared to CON. A Main Effect of Sex on IL PVB (F1,25=15.17; p=0.001) also revealed that females displayed less overall PVB in the IL. While no Sex × Group interaction was noted, only MS males (p=0.003), but not females (p=0.072) displayed a significant reduction of PVB in the IL.
Figure 5.
P40 PVB expression. A) Means ± SEM PVB expression levels in the PL (left) or IL (right) at P40. *p<0.05 difference between MS and Con. n=12–15/group/sex. #p<0.05 difference between males and females. B) Representative photomicrographs of PL and IL from CON or MS male and female subjects, labeled for PVB. Scale bar: 100µm.
Notably, we observed a higher population of PVB interneurons in the PL and IL of P55 subjects compared to P40 subjects (Main Effect of Age F1,79=43.54; p<0.001 in PL; F1,53=13.55; p=0.001 in IL), regardless of Group or Sex (2-way ANOVA Group × Age interaction p=0.870 in PL; p=0.171 in IL; Sex × Age interaction p=0.711 in PL; p= 0.116 in IL).
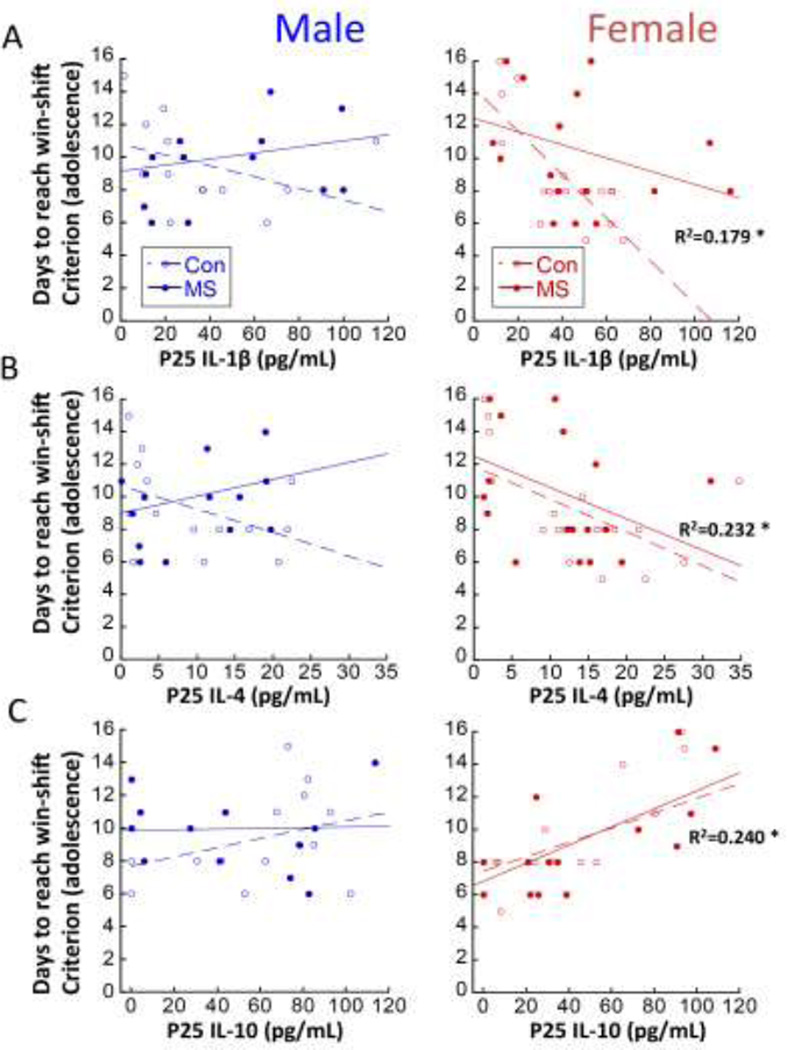
3.4.P25 cytokines predict adolescent cognitive performance
Juvenile circulating cytokines predicted later win-shift performance, but only in females. For example, P25 IL1β levels significantly predicted female, but not male, win-shift performance (Interaction of Sex: p<0.0001). Regardless of MS experience, females with lower levels of IL1β as juveniles took more days to reach win-shift criterion as adolescents (Figure 6A; R=0.5423; R2=0.179; F1,36=7.864; p=0.008). Similarly, P25 IL-4 levels predicted female, but not male win-shift performance (Figure 6B; Interaction of Sex: p=0.046). Lower IL-4 in juvenility predicted more difficulty learning the win-shift task in females (R=0.482; R2=0.232; F1,36=10.88; p=0.002). The predictive relationship between P25 IL-10 and cognitive performance was also only noted in females (Figure 6C; Interaction of Sex: p=0.031), in the opposite direction as IL-1β and IL-4. Higher levels of IL-10 at P25 predicted more difficulty learning the win-shift task in adolescent females (R=0.490; R2=0.240; F1,34=10.75; p=0.002).
Figure 6.
Regression analyses relating P25 cytokine levels with adolescent win-shift performance. Males are represented in left panels and females are represented in right panels A) P25 circulating IL-1β [n=14–16/group for males; n=17–19/group for females]; B) P25 circulating IL-4 [n=14–16/group for males; n=19/group for females]; and C) P25 circulating IL-10 [n=14–16 /group for males; n=14–19/group for females] with number of days to reach win-shift criterion in adolescence are presented. R2 values are indicated for significant regressions, and separately for MS and CON when there was a significant Group interaction; *p<0.05 significance of regression line.
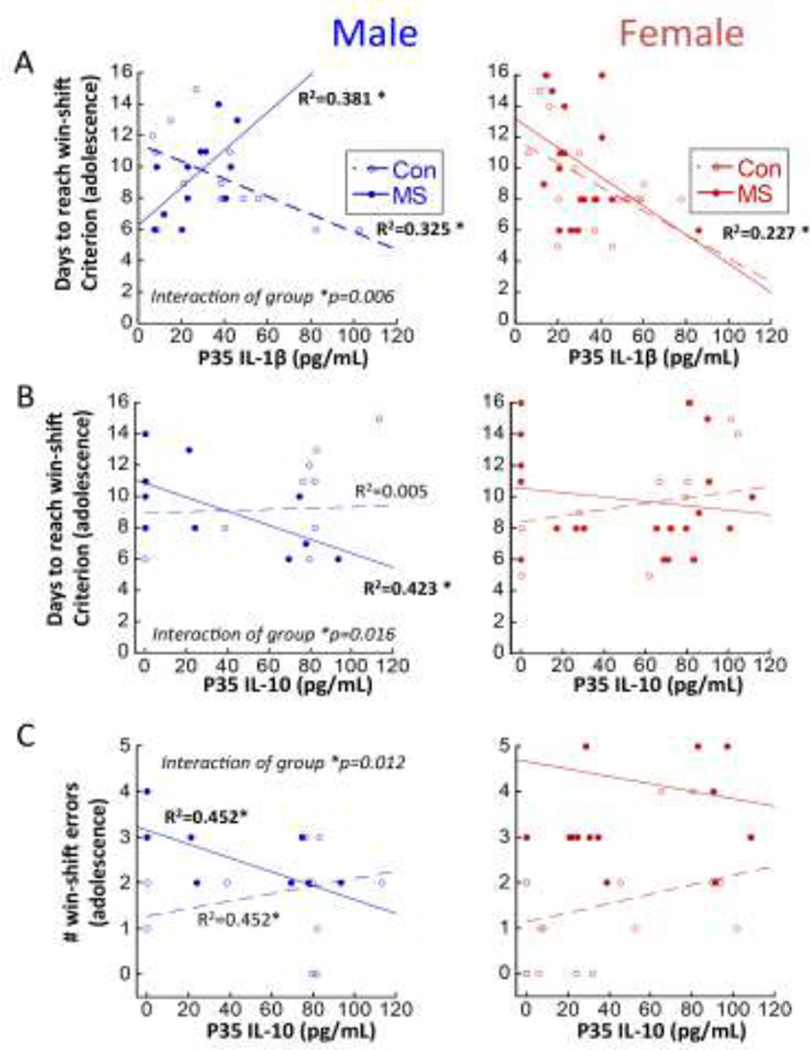
3.5.P35 cytokine levels predict adolescent cognitive performance
Figure 7A shows a negative relationship between IL-1β and days to reach criterion in CON males (R=0.570; R2=0.325; F[1,12]=5.77; p=0.033) and in all females regardless of experience (R=0.447; R2= 0.227; F[1,36]=8.98; p=0.005), with lower levels of IL-1β at P35 predicting more difficulty learning the win-shift task. However, a Sex × Group interaction (p=0.023) revealed that the predictive relationship between P35 IL-1β and win-shift performance was inverted in MS-exposed males, compared to all other subjects. Specifically, MS males with higher levels of IL-1β at P35 took longer to reach win-shift criterion (R=0.617; R2=0.381; F1,11=6.15; p=0.033). Figures 7B and 7C also both reveal Sex × Group interactions in predictive relationships of P35 IL-10 and win-shift performance. A Sex × Group interaction (p=0.042) revealed that higher IL-10 expression at P35 predicted fewer days to reach win-shift criterion in MS (R=0.650; R2= 0.423, F1,10=7.32; p=0.022) but not CON males (Figure 7B). No relationship was observed for females between P35 IL-10 and days to reach win-shift criterion. P35 IL-10 also significantly predicted the number of win-shift errors in MS males (R=0.672; R2=0.452; F1,9=6.593; p=0.033; Figure 7C), while neither CON males nor females displayed a relationship; however, a Sex × Group interaction was not significant in the P35 IL-10: Win shift error relationship.
Figure 7.
Regression analyses relating P35 cytokine levels with adolescent win-shift performance. Males are represented in left panels and females are represented in right panels. A) P35 circulating IL-1β [n=12–14/group for males; n=17–19/group for females]; and B) P35 circulating IL-10 [n=12–14/group for males; n=14–20/group for females] with number of days to reach win-shift criterion; and C) P35 circulating IL-10 with # errors on Phase 2 of the win-shift task after a 3-hr delay [n=10–12/group for males; n=14–18/group for females]. R2 values are indicated for significant regressions, and separately for MS and CON when there was a significant Group interaction; *p<0.05 significance of regression line. *p<0.05 significance of each regression line.
3.6.Relationship between cytokine levels at sacrifice and cognition
Levels of cytokines at sacrifice (P55) were not correlated with win-shift performance (see Supplemental Table 1).
3.7.Effects of MS on pubertal development
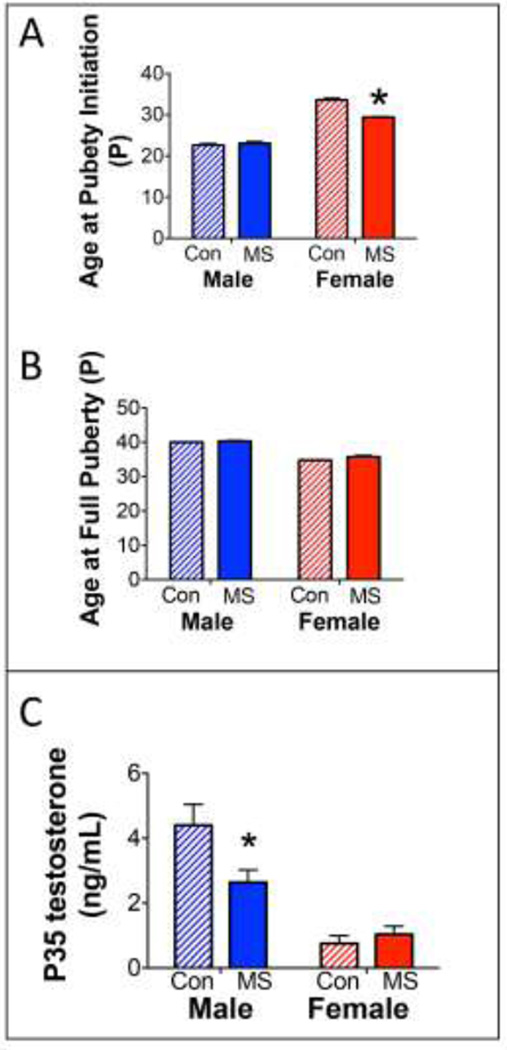
To our knowledge, this is the first assessment of pubertal development in MS males and females. Females exposed to MS displayed an early initiation of puberty, with a pinhole vaginal opening appearing approximately 4 days earlier than CON females (Figure 8A). However, MS did not affect the age of full puberty in males or females (Figure 8B). Initiation of male puberty, assessed as a partial preputial separation, was also not affected by MS. Therefore, there was a Sex × Group interaction found for the effects of MS on puberty initiation (F1,56=32.88; p<0.0001), but not full puberty achievement.
Figure 8.
Effects of MS on pubertal development and testosterone. A) Means ± SEM age at which preputial separation (males) or vaginal opening (females) initiated. *p<0.05 difference from CON. B) Means ± SEM age at which preputial separation (males) or vaginal opening (females) was fully completed; n=12–15/group/sex. C) Means ± SEM circulating testosterone at P35. *p<0.05 difference from CON.
3.8.Effects of MS on Adolescent Testosterone Levels
MS did affect male testosterone levels at P35, but not female testosterone levels (Group × Sex interaction: F1,28=7.81; p=0.0093). Specifically, MS-exposed males expressed significantly lower levels of circulating testosterone at P35, compared to CON Males (p=0.0082; Figure 8C). Female levels of testosterone were expectedly low at P35 and were unaffected by MS.
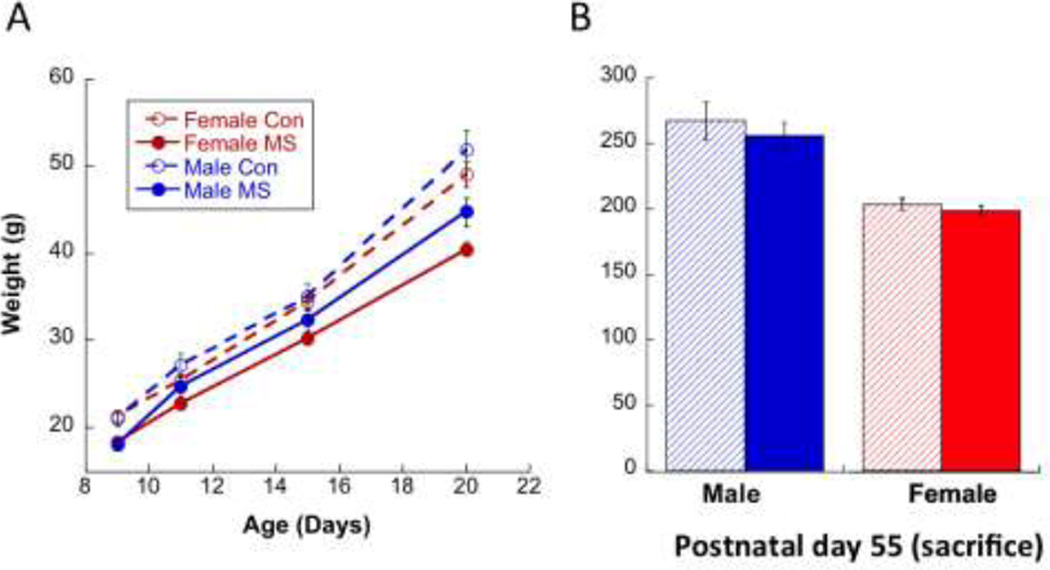
3.9.Effects of MS on Body Weight
During MS, pups were weighed on P9, P11, P15, and P20. A Group × Age (F1,51=15.3; p<0.001) but not Sex × Age (p=0.055) interaction was noted (Figure 9), whereby MS animals gained less weight over early development than CON. An expected Main Effect of Sex (F1,51=5.29; p=0.025) was also observed, with females weighing consistently less than males. By P55, MS subjects were comparable in weight to CON subjects [no Group × Sex differences (p=0.215, with only a Main Effect of Sex (p<0.0001); Figure 9].
Figure 9.
(A) A Group × Age (F1,51=15.3; p<0.001) but not Sex × Age (p=0.055) interaction illustrated that MS animals gained less weight over early development than CON. A Main Effect of Sex (F1,51=5.29; p=0.025) was also observed, with females weighing consistently less than males. (B) By P55, MS subjects were comparable in weight to CON subjects (no Group × Sex differences (p=0.215), with only a Main Effect of Sex (p<0.0001).
4. Discussion
While previous studies have largely measured immune responses to subsequent challenges after early adversity (Ganguly and Brenhouse, 2015), the current report assessed whether MS-exposed animals had an increased baseline pro-inflammatory profile. We observed here that males and females follow distinct developmental patterns of immune alterations. Specifically, while baseline-circulating levels of IL-1β and IL-4 were unaffected by MS regardless of sex, MS-exposed males displayed a drop in IL-10 in early adolescence (P35). Baseline circulating levels of pro- and anti-inflammatory cytokines can affect brain development and function in both healthy and pathological states (Banks, 2015), which is supported by our observations that perturbations of baseline immune environment affects the development of cognitive function.
Both male and female adolescent rats displayed significant deficits in the win-shift paradigm after MS, consistent with previous reports of early adversity induced cognitive dysfunction in both rodents (Brenhouse and Andersen, 2011; Garcia et al., 2013) and humans (Aas et al., 2012; Mothes et al., 2015; Mueller et al., 2012; Pechtel and Pizzagalli, 2011; Pesonen et al., 2013; Viola et al., 2013). While the number of days to reach win-shift criterion (task-learning) was predicted by several cytokines, only P35 IL-10 predicted the number of errors committed by males on the win-shift task. The ability to learn the win-shift task, and subsequent accuracy during testing after a 3-hr delay, likely reflect two different but related cognitive functions (Enomoto and Floresco, 2009). MS itself did not affect task-learning, however MS mediated the relationships between early cytokine expression and task-learning two weeks later. In contrast, MS had a robust effect on win-shift performance after a 3-hr delay between Phase 1 and Phase 2, which was predicted by lower P35 IL-10 in MS-exposed males.
Due to its profound antagonistic effects on Th1 responses, IL-10 was considered only a Th2 marker for quite some time, however increasing evidence from animal models of allergic diseases suggests that IL-10 acts as an inhibitor of Th2 responses as well (Cottrez et al., 2000; Trinchieri, 2007). The Th1/Th2 paradigm in which Th1 cells were considered proinflammatory and Th2 cells were considered anti-inflammatory has undergone a major change since both Th1 and Th2 cells are known to mediate inflammation and tissue damage as well as pathogen killing (Couper et al., 2008). On the other hand, anti-inflammatory functions are known to rest with populations of regulatory myeloid or lymphoid cells, including fully mature classical Th1 or Th2 cells that produce IL-10 as a negative feedback mechanism to limit their own response (Couper et al., 2008). Therefore, the lower levels of peripheral IL-10 in early adolescence among MS-exposed males can be understood as a reduction of an essential component of the immune regulatory response needed to inhibit pro-inflammatory cytokine synthesis, down-regulate the release of reactive oxygen species, and suppress proliferative and cytotoxic T-cell responses (Couper et al., 2008). Similarly, the observation that low juvenile levels of IL-1β correlated with win-shift deficits in adolescent females and control males suggests that sufficiently balanced immune activity of both pro- and anti-inflammatory regulators is necessary for proper neuronal development and behavior.
These findings also corroborate previous evidence that IL-10−/− male mice exhibited higher peripheral levels of IL-1β, IL-6 and TNFα and more pronounced learning and memory deficits after LPS treatment compared to wild type mice (Richwine et al., 2009). Conversely, intracerebroventricular administration of different doses of IL-1β or IL-1 receptor antagonists in adult male mice following learning revealed that a slight increase in brain IL-1β levels can improve memory, whereas any deviation from the physiological range, either by excess elevation in IL-1β levels or by blockade of IL-1 signaling, results in impaired memory (Goshen et al., 2007). Since the majority of evidence of IL-1β impact on memory performance has come from male animal studies, the observations reported here are interesting as they show a different pattern in females. Moreover, our observation that juvenile females with lower levels of IL-4 show higher cognitive deficits in adolescence corroborates evidence that meningeal T cell–derived IL-4 plays a role in the maintenance of cognitive function by antagonizing the deleterious effects of proinflammatory cytokines on astrocytes and neurons [(Derecki et al., 2010), reviewed in (Gadani et al., 2012)].
The interactive effects of sex and age on the relationship of cytokines with behavior evoke two interpretations. First, immune influences on neurocircuitry largely depend on developmental stage. Appropriate levels of cytokines are necessary for healthy neurodevelopment, with the highest levels of circulating cytokines observed in the neonatal brain (Bilbo and Schwarz, 2012). Therefore, lower levels of pro-inflammatory cytokines during juvenility may have a deleterious effect on brain development in vulnerable individuals. Second, the discrete vulnerability of females to juvenile cytokine levels suggests that cytokines during juvenility are particularly important for the development of prefrontal and cognitive circuits, which have been shown to develop earlier in females than in males (Markham et al., 2013; Roalf et al., 2014). Taken together, the predictability of adolescent cognitive function by developmental circulating cytokine levels observed here supports the theory that the appropriate balance of pro- and anti-inflammatory activity may depend on sex and early life experience, and may be particularly important during juvenility in females and during the peri-pubertal period in males.
During puberty, testosterone has been shown to promote IL-10 expression in males (Zhang et al., 2007); therefore the observed drop in IL-10 in MS males during puberty suggests that the reduced testosterone reported here, and observed by others (Bodensteiner et al., 2014), in adolescent MS males could have a modulatory role of IL-10 levels in these rats. Notably, we did not observe an effect of MS on the timing of puberty in males as measured by balano-preputial separation; therefore it does not appear that decreased testosterone levels affected the timing of pubertal development. Females also completed vaginal opening at the same time regardless of MS experience. However the initiation of puberty in MS-exposed females was seen at ~P29 compared to ~P33 in controls, which may be due to increased catch-up weight after MS exposure as has been reported after neonatal immune challenge (Sominsky et al., 2012). Taken together, these data suggest that MS confers vulnerability to immune-mediated disruption of the development of cognitive systems in males that may be related to lower peri-pubertal testosterone levels. Moreover, less pro-inflammatory activity during early adolescence may indicate resilience in MS-exposed males.
Previous studies have shown that the developmental maturation of executive function correlates with the maturation of PVB-interneuronal networks throughout childhood and adolescence (Doischer et al., 2008; Rao et al., 2000; Uhlhaas et al., 2009; Wilson et al., 1994). Therefore, environmental insults affecting the development of this inhibitory network might lead to abnormal cognitive function, as we observed in adolescence following MS. We found that adolescent PVB levels were decreased in MS-exposed males but not females, which supports previous work demonstrating an earlier effect of MS on PVB in juvenile, but not adolescent females (do Prado et al., 2015). However, adolescent PVB levels were significantly correlated with win-shift performance, providing compelling support to evidence suggesting that PVB interneuron functional integrity is critical for cognitive functions such as working memory (Lewis et al., 2012). We also observed that subjects trained and tested in the win-shift paradigm and sacrificed at P55 displayed higher PFC PVB levels compared to subjects without any win-shift experience that were sacrificed at P40. Since these two cohorts differed in both age and experience, it is difficult to interpret these findings. However, an age-related increase in PVB between P40 and P55 could be interpreted in the context of earlier findings in monkeys showing an increase in PFC basket cell PVB expression between adolescence and adulthood (Fish et al., 2011); while our analysis did not distinguish between PVB interneuron subtypes, it is possible that the developmental increase of these neurons accounted for the increase we observed. Alternatively, it is possible that training in the win-shift paradigm increased PVB expression from sub-threshold levels to detectable levels in some neurons, since PVB-expressing interneurons have been shown to exhibit experience-dependent plasticity with increased PVB intensity during memory consolidation and retrieval (Donato et al., 2013).
This study should be interpreted in light of some limitations and perspectives for future studies. First, juvenile cognitive function was not assessed. It is therefore unclear whether the association between juvenile cytokine levels and adult cognitive function are indeed predictive of future cognitive deterioration or an inherent characteristic of the response to MS that exists throughout life. Second, since IL-10 can modulate other pro-inflammatory cytokines such as TNF-alpha and IL-6, future studies should include these markers to strengthen the hypothesis that a pro-inflammatory/ anti-inflamatory cytokines imbalance leads to cognitive dysfunction after MS. Additionally, since peripheral cytokines do not necessarily reflect cytokine levels in the brain, future investigations should determine how MS affects the timing and expression of immune mediators in the brain. Indeed, peripheral cytokines can and do access the brain though blood-brain-barrier active transport and direct actions on brain endothelial cells (Banks, 2015).
In summary, experience with MS influences the predictive relationship between circulating cytokines and adolescent cognitive function, which is also related to changes in the PFC interneuron population. These data contribute to understanding the nuanced role of circulating cytokines during development as potential biomarkers of enduring PFC-related cognitive impairment associated with early life adversity, and therefore provide insights and putative targets for possible clinical assessments and interventions.
Supplementary Material
Highlights.
We observed sex- and age-dependent effects of MS on circulating cytokines.
MS also yielded adolescent cognitive deficits and decreases in mPFC PVB.
Win-shift deficits were predicted by early cytokine expression in a sex- and experience-dependent manner.
Circulating cytokines and PVB levels predicted adolescent cognitive deficits
Acknowledgments
We would like to thank Courtney Brown, Thomas Baccus, Andrea Wieck, Carine Prado and Mateus Levandowski for their technical assistance.
Role of funding
This work was supported by NIMH 5R21MH097182-01 (HCB), as well as BBRF and the SHINE Foundation (HCB). The funding sources had no such involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: none
Contributors
All authors participated in the research and article preparation, including design, analysis and writing.
References
- Aas M, Steen NE, Agartz I, Aminoff SR, Lorentzen S, Sundet K, Andreassen OA, Melle I. Is cognitive impairment following early life stress in severe mental disorders based on specific or general cognitive functioning? Psych Res. 2012;198:495–500. doi: 10.1016/j.psychres.2011.12.045. [DOI] [PubMed] [Google Scholar]
- Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, Troudart T, Bloch M, Heresco-Levy U, Lerer B. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psych. 1999;4:163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- Aisa B, Elizalde N, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Effects of neonatal stress on markers of synaptic plasticity in the hippocampus: Implications for spatial memory. Hippocampus. 2009;19:1222–1231. doi: 10.1002/hipo.20586. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Maayan R, Weizman A. Neonatal stress modulates sickness behavior: role for proinflammatory cytokines. J Neuroimmun. 2013;257:59–66. doi: 10.1016/j.jneuroim.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Powell N, Padgett DA, Sheridan JF. Social interactions, stress, and immunity. Immun Allergy Clin N Amer. 2009;29:285–293. doi: 10.1016/j.iac.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Banks WA. The blood-brain barrier in neuroimmunology: Tales of separation and assimilation. Brain Beh Immun. 2015;44:1–8. doi: 10.1016/j.bbi.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. The immune system and developmental programming of brain and behavior. Front Neuroendocrin. 2012;33:267–286. doi: 10.1016/j.yfrne.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodensteiner KJ, Christianson N, Siltumens A, Krzykowski J. Effects of early maternal separation on subsequent reproductive and behavioral outcomes in male rats. J Gen Psychol. 2014;141:228–246. doi: 10.1080/00221309.2014.897215. [DOI] [PubMed] [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosc. 2004;19:1863–1874. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Nonsteroidal anti-inflammatory treatment prevents delayed effects of early life stress in rats. Biol Psychiatry. 2011;70:434–440. doi: 10.1016/j.biopsych.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton PJ. Programming the brain and behaviour by early life stress: A focus on neuroactive steroids. J Neuroendocrin. 2015;27(6):468–480. doi: 10.1111/jne.12265. [DOI] [PubMed] [Google Scholar]
- Cottrez F, Hurst SD, Coffman RL, Groux H. T regulatory cells 1 inhibit a Th2-specific response in vivo. J Immunol (Baltimore, Md. : 1950) 2000;165:4848–4853. doi: 10.4049/jimmunol.165.9.4848. [DOI] [PubMed] [Google Scholar]
- Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol (Baltimore, Md. : 1950) 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison U, McKernan D, Cryan J, Dinan T. Schizophrenia patients with a history of childhood trauma have a pro-inflammatory phenotype. Psych Med. 2012;42:1865–1871. doi: 10.1017/S0033291712000074. [DOI] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimatelis JJ, Pillay NS, Mutyaba AK, Russell VA, Daniels WM, Stein DJ. Early maternal separation leads to down-regulation of cytokine gene expression. Metabolic brain disease. 2012;27:393–397. doi: 10.1007/s11011-012-9304-z. [DOI] [PubMed] [Google Scholar]
- do Prado CH, Narahari T, Holland FH, Lee HN, Murthy SK, Brenhouse HC. Effects of early adolescent environmental enrichment on cognitive dysfunction, prefrontal cortex development, and inflammatory cytokines after early life stress. Dev Psychobio. 2016;58:482–491. doi: 10.1002/dev.21390. [DOI] [PubMed] [Google Scholar]
- Doischer D, Hosp JA, Yanagawa Y, Obata K, Jonas P, Vida I, Bartos M. Postnatal differentiation of basket cells from slow to fast signaling devices. J Neurosci. 2008;28:12956–12968. doi: 10.1523/JNEUROSCI.2890-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato F, Rompani SB, Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature. 2013;504:272–276. doi: 10.1038/nature12866. [DOI] [PubMed] [Google Scholar]
- Enomoto T, Floresco SB. Disruptions in spatial working memory, but not short-term memory, induced by repeated ketamine exposure. Prog Neuropsychopharm Biol Psychiatry. 2009;33:668–675. doi: 10.1016/j.pnpbp.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Fish KN, Sweet RA, Lewis DA. Differential distribution of proteins regulating GABA synthesis and reuptake in axon boutons of subpopulations of cortical interneurons. Cereb Cortex. 2011;21:2450–2460. doi: 10.1093/cercor/bhr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani SP, Cronk JC, Norris GT, Kipnis J. IL-4 in the brain: a cytokine to remember. J Immunol (Baltimore, Md. : 1950) 2012;189:4213–4219. doi: 10.4049/jimmunol.1202246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly P, Brenhouse HC. Broken or maladaptive? Altered trajectories in neuroinflammation and behavior after early life adversity. Dev Cog Neurosc. 2015;11:18–30. doi: 10.1016/j.dcn.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia VA, Hirotsu C, Matos G, Alvarenga T, Pires GN, Kapczinski F, Schroder N, Tufik S, Andersen ML. Modafinil ameliorates cognitive deficits induced by maternal separation and sleep deprivation. Behav Brain Res. 2013;253:274–279. doi: 10.1016/j.bbr.2013.07.029. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Hays SL, McPherson RJ, Juul SE, Wallace G, Schindler AG, Chavkin C, Gleason CA. Long-term effects of neonatal stress on adult conditioned place preference (CPP) and hippocampal neurogenesis. Behav Brain Res. 2012;227:7–11. doi: 10.1016/j.bbr.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Fitch C, Jacobs S, Deak T, Schiml PA. Behavioral effects of peripheral corticotropin-releasing factor during maternal separation may be mediated by proinflammatory activity. Psychoneuroendocrinology. 2011;36:996–1004. doi: 10.1016/j.psyneuen.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland FH, Ganguly P, Potter DN, Chartoff EH, Brenhouse HC. Early life stress disrupts social behavior and prefrontal cortex parvalbumin interneurons at an earlier time-point in females than in males. Neurosci Lett. 2014;566C:131–136. doi: 10.1016/j.neulet.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R, Davis C, Kendler K. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psych Med. 1997;27:1101–1119. doi: 10.1017/s0033291797005588. [DOI] [PubMed] [Google Scholar]
- Kohut S, Roma P, Davis C, Zernig G, Saria A, Dominguez J, Rice K, Riley A. The impact of early environmental rearing condition on the discriminative stimulus effects and Fos expression induced by cocaine in adult male and female rats. Psychopharmacology. 2009;203:383–397. doi: 10.1007/s00213-008-1368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Xue X, Shao S, Shao F, Wang W. Cognitive, emotional and neurochemical effects of repeated maternal separation in adolescent rats. Brain Res. 2013;1518:82–90. doi: 10.1016/j.brainres.2013.04.026. [DOI] [PubMed] [Google Scholar]
- Markham JA, Mullins SE, Koenig JI. Periadolescent maturation of the prefrontal cortex is sex-specific and is disrupted by prenatal stress. J Comp Neurol. 2013;521:1828–1843. doi: 10.1002/cne.23262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher MW, Sieve AN, Johnson RR, Satterlee D, Belyavskyi M, Mi W, Prentice TW, Welsh TH, Jr, Welsh CJ. Neonatal maternal separation alters immune, endocrine, and behavioral responses to acute Theiler's virus infection in adult mice. Beh Gen. 2010;40:233–249. doi: 10.1007/s10519-010-9333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cole SW. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biol Psychiatry. 2012;72:34–40. doi: 10.1016/j.biopsych.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothes L, Kristensen CH, Grassi-Oliveira R, Fonseca RP, Lima Argimon II, Irigaray TQ. Childhood maltreatment and executive functions in adolescents. Child Adol Mental Health. 2015;20:56–62. doi: 10.1111/camh.12068. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Hardin MG, Korelitz K, Daniele T, Bemis J, Dozier M, Peloso E, Maheu FS, Pine DS, Ernst M. Incentive effect on inhibitory control in adolescents with early-life stress: an antisaccade study. Child abuse & neglect. 2012;36:217–225. doi: 10.1016/j.chiabu.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina V, Widom CS. Child maltreatment and executive functioning in middle adulthood: a prospective examination. Neuropsychology. 2013;27:417–427. doi: 10.1037/a0032811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor RM, Moloney RD, Glennon J, Vlachou S, Cryan JF. Enhancing glutamatergic transmission during adolescence reverses early-life stress-induced deficits in the rewarding effects of cocaine in rats. Neuropharmacology. 2015;99:168–176. doi: 10.1016/j.neuropharm.2015.07.012. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology. 2011;214:55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen AK, Eriksson JG, Heinonen K, Kajantie E, Tuovinen S, Alastalo H, Henriksson M, Leskinen J, Osmond C, Barker DJ, Raikkonen K. Cognitive ability and decline after early life stress exposure. Neurobiol Aging. 2013;34:1674–1679. doi: 10.1016/j.neurobiolaging.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Pinheiro RM, de Lima MN, Portal BC, Busato SB, Falavigna L, Ferreira RD, Paz AC, de Aguiar BW, Kapczinski F, Schroder N. Long-lasting recognition memory impairment and alterations in brain levels of cytokines and BDNF induced by maternal deprivation: effects of valproic acid and topiramate. J Neural Transm (Vienna) 2015;122:709–719. doi: 10.1007/s00702-014-1303-2. [DOI] [PubMed] [Google Scholar]
- Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Rana SA, Aavani T, Pittman QJ. Sex effects on neurodevelopmental outcomes of innate immune activation during prenatal and neonatal life. Horm Behav. 2012;62:228–236. doi: 10.1016/j.yhbeh.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richwine AF, Sparkman NL, Dilger RN, Buchanan JB, Johnson RW. Cognitive deficits in interleukin-10-deficient mice after peripheral injection of lipopolysaccharide. Brain Behav Immun. 2009;23:794–802. doi: 10.1016/j.bbi.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf DR, Gur RE, Ruparel K, Calkins ME, Satterthwaite TD, Bilker WB, Hakonarson H, Harris LJ, Gur RC. Within-individual variability in neurocognitive performance: age- and sex-related differences in children and youths from ages 8 to 21. Neuropsychology. 2014;28:506–518. doi: 10.1037/neu0000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque A, Ochoa-Zarzosa A, Torner L. Maternal separation activates microglial cells and induces an inflammatory response in the hippocampus of male rat pups, independently of hypothalamic and peripheral cytokine levels. Brain Behav Immun. 2015 doi: 10.1016/j.bbi.2015.09.017. [DOI] [PubMed] [Google Scholar]
- Slopen N, Kubzansky LD, Koenen KC. Internalizing and externalizing behaviors predict elevated inflammatory markers in childhood. Psychoneuroendocrinology. 2013;38:2854–2862. doi: 10.1016/j.psyneuen.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Sominsky L, Meehan CL, Walker AK, Bobrovskaya L, McLaughlin EA, Hodgson DM. Neonatal immune challenge alters reproductive development in the female rat. Horm Behav. 2012;62:345–355. doi: 10.1016/j.yhbeh.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Polcari A, Andersen SL. Length of time between onset of childhood sexual abuse and emergence of depression in a young adult sample: a retrospective clinical report. J Clin Psychiatry. 2009;70:684–691. doi: 10.4088/jcp.08m04235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Tomoda A, Andersen SL. Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Annals of the NY Acad Sci. 2006;1071:313–323. doi: 10.1196/annals.1364.024. [DOI] [PubMed] [Google Scholar]
- Terrando N, Rei Fidalgo A, Vizcaychipi M, Cibelli M, Ma D, Monaco C, Feldmann M, Maze M. The impact of IL-1 modulation on the development of lipopolysaccharide-induced cognitive dysfunction. Crit Care. 2010;14:R88. doi: 10.1186/cc9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AW, Caporale N, Wu C, Wilbrecht L. Early maternal separation impacts cognitive flexibility at the age of first independence in mice. Dev Cog Neurosci. 2015;18:49–56. doi: 10.1016/j.dcn.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J Exp Med. 2007;204:239–243. doi: 10.1084/jem.20070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Singer W, Haenschel C, Sireteanu R, Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc Natl Acad Sci USA. 2009;106:9866–9871. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Reber SO, Selch S, Obermeier F, Neumann ID. Early life stress enhances the vulnerability to chronic psychosocial stress and experimental colitis in adult mice. Endocrinology. 2008;149:2727–2736. doi: 10.1210/en.2007-1469. [DOI] [PubMed] [Google Scholar]
- Viola TW, Tractenberg SG, Pezzi JC, Kristensen CH, Grassi-Oliveira R. Childhood physical neglect associated with executive functions impairments in crack cocaine-dependent women. Drug Alcohol Depend. 2013;132:271–276. doi: 10.1016/j.drugalcdep.2013.02.014. [DOI] [PubMed] [Google Scholar]
- Wieck A, Andersen SL, Brenhouse HC. Evidence for a neuroinflammatory mechanism in delayed effects of early life adversity in rats: relationship to cortical NMDA receptor expression. Brain Beh Immun. 2013;28:218–226. doi: 10.1016/j.bbi.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Wilson FA, O'Scalaidhe SP, Goldman-Rakic PS. Functional synergism between putative gamma-aminobutyrate-containing neurons and pyramidal neurons in prefrontal cortex. Proc Natl Acad Sci USA. 1994;91:4009–4013. doi: 10.1073/pnas.91.9.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X, Shao S, Li M, Shao F, Wang W. Maternal separation induces alterations of serotonergic system in different aged rats. Brain Res Bull. 2013;95:15–20. doi: 10.1016/j.brainresbull.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Zhang YZ, Xing XW, He B, Wang LX. Effects of testosterone on cytokines and left ventricular remodeling following heart failure. Cell Phys Biochem : Intern J Exp Cell Phys Biochem Pharmacol. 2007;20:847–852. doi: 10.1159/000110444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.