Abstract
Cancer survival rates are generally increasing in the United States. These trends have been partially attributed to improvement in therapeutic strategies. Cancer immunotherapy is an example of one of the newer strategies used to fight cancer, which primes or activates the immune system to produce antitumor effects. The first half of this review paper concisely describes the cell mechanisms that control antitumor immunity and the major immunotherapeutic strategies developed to target these mechanisms. The second half of the review discusses in greater depth immune checkpoint inhibitors that have recently demonstrated tremendous promise for the treatment of diverse solid tumor types, including melanoma, non-small cell lung cancer, and others. More specifically, the mechanisms of action, side effects, and patient and family management and education concerns are discussed to provide oncology nurses up-to-date information relevant to caring for cancer-affected patients treated with immune checkpoint inhibitors. Future directions for cancer immunotherapy are considered.
Keywords: Cancer immunotherapy, immune checkpoint inhibitor, oncology nursing, symptom management

Introduction
According to the National Cancer Institute, approximately 39% of all Americans will develop cancer at some point in their lifetime.[1] However, the overall mortality rate of those diagnosed with cancer has declined, in part due to improvements in therapeutic approaches. The development of immunotherapies reflects a promising new approach to cancer treatment involving activation of the immune system against cancer.[2,3]
The use of immunotherapy for cancer has become widespread in recent decades and is used to treat both solid and hematological malignances.[4] Immune checkpoint inhibitors, in particular, have demonstrated considerable promise in their recent approval for the treatment of melanoma, non-small cell lung cancer, and other cancers.[5] Thus, with the inclusion of immunotherapy in cancer treatment regimens, it is imperative that oncology nurses are knowledgeable about the mechanisms of action, treatment regimens, and symptoms associated with these new agents to optimally educate and manage patients and families. The purpose of this manuscript is to provide an overview of immunotherapies, including a review of the cancer-immunity cycle, and an update on immune checkpoint inhibitors and their associated toxicity, management, and patient and family education concerns for the oncology nurse.
Defining Immunotherapy
Cancer immunotherapy involves the utilization of naturally derived or synthetically generated components to stimulate or enhance the immune system to fight cancer.[6] In 1891, Dr. William B. Coley developed the first known immunotherapeutic strategy for humans by inoculating patients affected by sarcoma with bacteria that stimulated a sustained antitumor immune response.[7] This seminal observation laid the foundation for investigating the interplay between a person's immune defenses and malignant cells.
The immune system engages in a complex balance wherein identification and eradication of foreign antigens is counterbalanced with processes necessary for suppressing an uncontrolled immune response. Regulation of the T-cell-mediated response to antigen presenting cells (APCs), including foreign and the body's own dendritic cells, is critical to eliminating cancer cells.[8,9] More specifically, CD8+ effector T-cells, or cytotoxic T-cells, can recognize “self” and “nonself” antigens bound to major histocompatibility class I complexes that are expressed on APCs. Dendritic cells process and present the antigen to T-cells, which allows them to recognize the foreign cells that express the antigen.[10] Due to genetic mutation and aberrant cellular processes,[11] cancer cells will produce neoantigens that normal host defenses will not recognize as “self.” This results in the production of cytotoxic T-cells that will be able to identify the neoantigen-presenting cells (i.e., cancer cells) for elimination. However, an immune response is rarely mounted against the cancer cell, which has been hypothesized to be due, in part, to immunosuppressive mechanisms that are physiologically necessary to prevent an exaggerated and damaging immune response.[12] These findings raise questions concerning the inhibitory and stimulatory processes requisite for robust anticancer immunity while minimizing potentially harmful side effects.[5,13]
The Cancer-Immunity Cycle
Recently, studies have shown that inhibitory signaling pathways, or immune checkpoints, may prevent the mounting of an immune response.[14] While the ability to evade immune surveillance has been identified as an important characteristic of cancer,[15] the cellular mechanisms that govern anticancer immunity are still being elucidated. To provide a cohesive model of the processes involved in fostering anticancer immunity, Chen and Mellman described the cancer-immunity cycle, which sequentially links together events that must be allowed to recursively occur to mount an effective immune response against cancer [Box 1].[13] In order for the cycle to proceed, inhibitory processes, including the activation of immune checkpoint inhibitors that suppress cytotoxic T-cell recognition of cancer, must be overridden. The identification of these inhibitory processes in the cycle has since led to the development of therapeutic strategies that have been designed to overcome them and fight cancer.[13]
Box 1.
Steps in the cancer-immunity cycle
| Step 1: Dead cancer cells release neoantigens that are captured by dendritic cells |
| Step 2: Dendritic cells process and present the major histocompatibility class I (MHCI) or major histocompatibility class II (MHCII)-bound neoantigen to T cells |
| Step 3: Effector T cells become primed and activated, including CD8+ cytotoxic T cells and CD4+ helper T cells that recognize the neoantigen/MHCI complex and neoantigen/MHCII complex, respectively |
| Step 4: Cytotoxic T cells travel to the tumor site |
| Step 5: Cytotoxic T cells infiltrate the tumor bed |
| Step 6: Cytotoxic T cells recognize cancer cells via the interaction between the antigen/MHCI complex and receptors |
| Step 7: Cytotoxic T cells destroy the cancer cells through signaling mechanisms. More neoantigens are released, which amplifies the T cell response in the next the cycle |
Immunotherapeutic Strategies
Cancer immunotherapies possess distinct mechanisms of action and primarily fall into the following categories: Adoptive T-cell transfer, oncolytic viruses, cancer vaccines, and monoclonal antibodies. The following section will provide a brief description of the aforementioned immunotherapeutic strategies.
Adoptive T-cell transfer involves the genetic engineering of patients’ own T-cells to recognize cancer cells. T-cells are grown in large volumes and modified to express receptors that allow them to recognize cancer cells. They are then infused back into the patient to fight cancer and are associated with lasting benefits. This type of strategy is also sometimes called chimeric antigen receptor T-cell therapy and currently is in clinical trial testing.[16,17]
Oncolytic viruses utilize specially modified viruses to infect and destroy cancer cells. These special viruses, which are designed to avoid normal tissues, will recognize a specific antigen on the surface of cancer cells. The virus will then infect the cancer cell, replicate inside it, and eventually rupture the cell. When the cell dies, antigens are released, which activates the immune system to seek out and destroy more cancer cells. Currently, talimogene laherparepvec, or T-VEC, for melanoma is the only vaccine approved for cancer treatment.[16]
Cancer vaccines involve exposing the immune system to an antigen for prevention or cure. In both instances, the immune system will recognize antigens expressed on cancer cells to facilitate their elimination. Currently, there are more preventive vaccines available than curative, including two vaccines that foster immunity against forms of the human papillomavirus that are associated with cancer development. Only one curative vaccine, sipuleucel-T, is available for the treatment of metastatic prostate cancer. This therapy involves exposing patient-derived APCs to prostatic acidic phosphatase, which is an antigen widely expressed on prostate cancer cells, and infusing these cells back into the patient to prime and activate other immune cells for recognizing and eliminating cancer.[16,18]
Finally, monoclonal antibodies are proteins manufactured by immune cells to specifically recognize a cell target. In the context of cancer treatment, monoclonal antibodies can suppress the activity of a specific protein in the cancer cell to kill the cell or prevent it from growing. Monoclonal antibodies are also used for cancer immunotherapy, most notably in the context of immune checkpoint inhibitors.[16] The immune checkpoint proteins, cytotoxic T lymphocyte-associated 4 (CTLA-4), and programmed cell death protein 1 (PD-1), are receptors expressed on the surface of cytotoxic T-cells that interact with their ligands cluster differential 80 (CD80)/cluster differential 86 (CD86) and programmed death ligand-1 (PDL-1) on APCs, which helps the cancer cell evade T-cell-mediated death. Immune checkpoint inhibitors prevent the receptors and ligands from binding to each other, thereby disrupting signaling [Figure 1].[4,5,10,12] These agents have recently demonstrated improved survival outcomes for adults with solid tumors in clinical trials and have subsequently been approved to treat several disease types, including melanoma. Approved immune checkpoint inhibitors include the anti-CTLA-4 agent, ipilimumab; anti-PD-1 agents, nivolumab and pembrolizumab; and anti-PDL-1 agent, atezolizumab [Table 1].[5]
Figure 1.
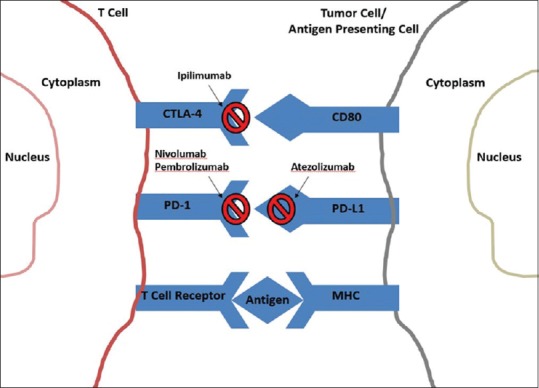
Therapeutic targets of immune checkpoint inhibitors
Table 1.
Food and Drug Administration approved immune checkpoint inhibitors
| Drug | Immune checkpoint target | Date of approval and indication | Combinatorial therapy |
|---|---|---|---|
| Ipilimumab | CTLA-4 | Approved in 2011 Unresectable metastatic melanoma Approved in 2015 Adjuvant therapy with Stage III melanoma |
Approved in 2015 In combination with nivolumab for unresectable or metastatic melanoma |
| Pembrolizumab | PD-1 | Approved in 2014 Advanced or unresectable melanoma Approved in 2015 Metastatic NSCLC with PDL-1 expression and progression on or after platinum therapy Approved in 2016 Recurrent SCCHN |
None |
| Nivolumab | PD-1 | Approved in 2014 Unresectable or metastatic melanoma with progression after ipilimumab or BRAF inhibitor if BRAF V600 mutant Approved in 2015 NSCLC with progression after or on platinum therapy Metastatic RCC after prior anti-angiogenic therapy |
See ipilimumab combinatorial therapy |
| Atezolizumab | PDL-1 | Approved in 2015 NSCLC with progression after or on platinum therapy Approved in 2016 Urolthelial carcinoma with progression on or after platinum therapy |
None |
NSCLC: Nonsmall cell lung cancer, RCC: Renal cell carcinoma, SCCHN: Squamous cell carcinoma of the head and neck, PDL-1: Programmed death ligand-1, CTLA-4: Cytotoxic T lymphocyte associated-4, PD-1: Programmed cell death protein 1
Nursing Care and Management of Patients Treated with Immune Checkpoint Inhibitors
The successful use of immune checkpoint inhibitors in recent years has brought hope for cure and survival for those suffering from various cancers. To address the unique immune-related side effects of checkpoint inhibitors, this section of the paper addresses the associated toxicities, patient management, and nursing care considerations.
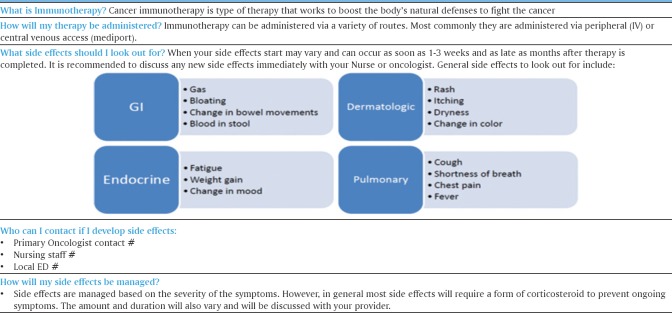
Nurses are instrumental in providing patients a fundamental understanding of immunotherapy that helps them understand the need for prompt identification and careful surveillance during and after therapy. Patient management and nursing care strategies can improve a patient's quality of life, while minimizing treatment delays or early discontinuation of therapy.[19] Managing patients on immune checkpoint inhibitors involves ongoing education that addresses how these agents work, their side effects, and patient management and nursing care strategies. A sample patient education tool is provided to exemplify critical immunotherapy-related information that needs to be conveyed to patients and families [Figure 2].[20] Many patients with previous chemotherapy experience may have preconceived notions about what their new treatment experiences will be like. Patient education should include a discussion of immune activation and how responses to immunotherapy differ from that of chemotherapy.[21]
Figure 2.
Sample immunotherapy education tool for patients
Specifically, immunotherapy can take longer to elicit a response than conventional chemotherapy, and patients may experience stable disease or even progression after initial treatment before observing improvement. Furthermore, side effects tend to be characterized by inflammation and require vigilance in observing and reporting to providers to facilitate a timely intervention. Patients need to be educated on these unique responses attributed to immunotherapy since they may be unexpected.[22]
Gastrointestinal Side Effects
Immune-mediated colitis is one of the most prevalent side effects associated with checkpoint blockade. In patients treated with ipilimumab, the overall incidence of diarrhea and colitis has been reported as 32.8%.[23] Ipilimumab-induced diarrhea has been associated with bowel perforation and subsequent death.[24] This side effect has also been seen in patients treated with anti-PD-1 therapy. The onset of symptoms has been observed within 6–7 weeks following the initiation of ipilimumab treatment, and 6–18 weeks in patients treated with PD-1 blockade.[25] Clinical presentation includes watery bowel movements, blood or mucus in stool, flatulence, and abdominal cramping. Microscopic abnormalities included erythema, edema, bleeding, erosions, and neutrophil invasion.[24]
Autoimmune hepatitis has been reported in a small number of patients treated with ipilimumab and anti-PD-1 therapy.[24] Autoimmune hepatitis presents as an asymptomatic increase in aspartate transaminase, alanine transaminase, and total bilirubin; fatigue and low-grade fevers have been observed.[23] Time of onset for this side effect was variable, ranging from 1 to 23 weeks in patients with metastatic melanoma.[25] Radiographic findings include hepatomegaly, periportal lymphadenopathy, and periportal edema.[23] Hepatic profile should be obtained at baseline and before each cycle of therapy to assure early recognition of any of these deleterious side effects.
To ensure patients receive a timely intervention for these gastrointestinal side effects of immune checkpoint inhibitors, nursing care will include assessing for and educating patients and family members on reporting changes in bowel habits and symptoms including blood or mucus in stool, abdominal pain, and diarrhea. While mild gastrointestinal symptoms may be resolved with dietary changes or the administration of loperamide, severe symptoms, including having >7 stools over initial assessment in 1 day, may require withholding the agent and starting corticosteroid therapy.[26]
Pulmonary Side Effects
Immune-mediated pneumonitis is described as a noninfectious inflammation of the lining of the lung with associated interstitial or alveolar infiltrations.[25] This side effect is not as prevalent as colitis or hepatitis, but it is associated with morbidity and mortality and often leads to discontinuation of treatment.
Pulmonary toxicity is uncommon in patients treated with ipilimumab. For patients treated with anti-PD-1 therapy, the overall incidence rate of pneumonitis is 9%.[24] Pneumonitis may occur at any point during and after treatment. Reduced lung reserve due to preexisting lung disease and chest radiotherapy may increase the risk of developing pneumonitis.[24] The diagnosis of pneumonitis is based on clinical presentation and radiographic imaging. Clinical signs and symptoms include dyspnea, cough, fatigue, hypoxia, chest pain, and hemoptysis. The severity of symptoms varies among patients and frequently mimics other common respiratory illnesses. Radiographic findings of diffuse infiltrates, lobular nodularity with air trapping, and interstitial fibrosis support the diagnosis. In patients treated with anti-PD-1 therapy, microscopic findings include diffuse lymphocytic infiltrates,[27] whereas in limited reports of ipilimumab-induced pneumonitis, histologic findings were described as sarcoid-like granulomatous reactions with macrophages surrounded by inflammation.[28] Nursing care considerations include the assessment of and education of patients and families to report on changes in pulmonary function, including shortness of breath, coughing, chest pain, and fever. In severe cases, corticosteroid or oxygen therapy may be required.[21]
Dermatological Side Effects
The most common cutaneous toxicities include pruritus and maculopapular rash. Cases of immune-mediated cutaneous toxicities include vitiligo, Stevens–Johnson syndrome, Sweet's syndrome, toxic epidermal necrolysis, bullous pemphigoid, and lichen sclerosus. Rash and pruritus occur early and are observed in nearly 50% of all patients treated with ipilimumab as compared to 28%–37% in patients treated with nivolumab and pembrolizumab, respectively.[23] Patients with suspected Stevens–Johnson syndrome or toxic epidermal necrolysis require immediate hospitalization.[29] The offending agent should be discontinued in these instances.
Nursing care of patients with dermatological side effects depends on the nature and severity of the reported symptom. Nurses should assess for and educate patients and families to report any of the aforementioned symptoms. Fragrance-free creams, lukewarm showers, and oatmeal baths may be recommended, in addition to antihistamines to address pruritus.[22] In addition to the sample educational tool provided in this article [Figure 2], other educational materials are available, including Skin Reactions to Targeted Therapies and Immunotherapy by the American Society of Clinical Oncology,[30] available at http://www.cancer.net/navigating-cancer-care/side-effects/skin-reactions-targeted-therapy-and-immunotherapy. Educational tools for dermatological symptom management may also be provided by organizations and institutions, such as the one found at https://www.mskcc.org/cancer-care/patient-education/skin-care during-treatment-targeted-therapies.[31] These may serve as references for patients in their self-management of dermatological toxicities.
Endocrine-related Side Effects
Immune-mediated endocrine toxicities include hypophysitis (pituitary gland inflammation), thyroiditis, hypothyroidism, and adrenal insufficiency. The incidence of hypophysitis is mostly associated with CTLA-4 blockade. In patients treated with ipilimumab alone or in combination with nivolumab, the incidence of hypophysitis was 11%–17%[32] as compared to < 1% in patients treated with PD-1 monotherapy.[33]
The clinical manifestation of hypophysitis includes headaches, dizziness, diplopia, loss of peripheral vision, extreme fatigue, irritability, cold intolerance, nausea, or vomiting.[29] Diagnosis is based on clinical presentation, laboratory evaluation of hypopituitarism, and radiographic imaging with a pituitary magnetic resonance imaging. In most cases, hypophysitis is associated with hypothyroidism, adrenal insufficiency, or adrenal crisis, which is a potentially life-threatening condition requiring immediate medical attention.[34]
Immune-mediated thyroid dysfunction can present as hyperthyroidism, destructive thyroiditis, or hypothyroidism.[35] The incidence of thyroid disorders is similar for both CTLA-4 blockage and anti-PD-1 monotherapy but increases significantly when combined or in combination.[33] The symptoms of thyroid dysfunction may include palpitations, irritability, fatigue, changes in weight, heat or cold sensitivity, alopecia, or constipation.[25] Thyroid functions test should be obtained at baseline and periodically throughout the treatment.[25] Elevated thyroid-stimulating hormone (TSH) levels with low thyroxine triiodothyronine (TTT) is indicative of hypothyroidism whereas a low TSH level and elevated TTT indicate thyroiditis/hyperthyroidism.
Nursing care to guarantee patients receive swift intervention for endocrine-related side effects includes assessing for changes in the aforementioned symptoms as well as changes in mental status, low blood pressure, headaches, atypical bowel habits, and fatigue. Patients and family members must also be taught to recognize these symptoms and to promptly report them. The offending agent may be withheld until the resolution of the symptoms, and in some cases, patients may undergo hormone treatment or corticosteroid therapy or have the agent discontinued.[34] Additional information pertaining to the management of immune-related toxicities is shown in Table 2.[36]
Table 2.
Management of immune related toxicities
| Common side effects | Work up for alternative/noninflammatory etiologies | Grade of toxicity | Recommended management of immune-mediated AEs |
|---|---|---|---|
| Gastrointestinal Diarrhea/colitis | Rule out infectious etiology (Clostridium difficile) | Mild | Symptom management |
| Consider budesonide 9 mg daily | |||
| Continue I-O therapy | |||
| Moderate | Delay immunotherapy therapy | ||
| Methylprednisolone IV or oral equivalent 0.5-1 mg/kg/day | |||
| Consider GI consult and colonoscopy | |||
| When improve to Grade 1 or less, taper over at least 4 weeks | |||
| Severe | Discontinue immunotherapy | ||
| Methylprednisolone IV 1-2 mg/kg/day | |||
| When improve to Grade 1 or less, taper steroids over at least 4 weeks | |||
| No improvement in symptoms within 48-72 h consider second line immunosuppression (Infliximab) | |||
| Hepatitis | Evaluate for EtOH intake Concomitant medications with hepatotoxic potential Rule out biliary disease/obstruction | Mild | Continue I-O therapy l |
| Repeat LFTs in 1 week | |||
| Moderate | Delay I-O therapy | ||
| Repeat LFTs every 3-5 days | |||
| Methylprednisolone 0.5-1 mg/kg/day or oral equivalent | |||
| Monitor LFTs every 3 days. When improves to mild or baseline, taper steroids over at least 4 weeks | |||
| Severe | Discontinue therapy | ||
| Increase frequency of LFT monitoring to 1-2 days | |||
| Methylprednisolone IV 12 mg/kg/day | |||
| Consult GI | |||
| No improvement in 48-72 h consider second line immunosuppression | |||
| Pneumonitis | Evaluate for Pulmonary embolism Cardiac causes Infectious etiology COPD Seasonal allergies/cough from postnasal drip | Mild | Delay immunotherapy |
| Monitor for symptoms | |||
| Repeat chest radiograph in 2-4 weeks | |||
| Moderate | Delay therapy | ||
| Monitor symptoms closely, consider hospitalization | |||
| Re-image every 1-3 days | |||
| Pulmonary and ID consults, consider bronchoscopy | |||
| Methylprednisolone IV or oral equivalent 1-2 mg/kg/day | |||
| When symptoms improve, taper steroids over at least 4 weeks | |||
| Severe | Discontinue immunotherapy | ||
| Methylprednisolone IV 2-4 mg/kg/day, taper steroids over at least 6 weeks | |||
| No improvement in symptoms, consider second line immunosuppression (Infliximab, CellCept, IVIG) | |||
| Dermatological toxicities | Rule out noninflammatory causes (allergic reaction to other medications, photosensitivity, etc.) | Mild | Continue immunotherapy |
| Supportive management emollients, low potency topical steroids, antihistamines | |||
| Moderate | Continue immunotherapy | ||
| Moderate-high potency topical steroids | |||
| If persistent despite optimal topical management, consider methylprednisolone 0.5-1 mg/kg/day or oral equivalent | |||
| If improved to mild or resolves – taper steroids over 4 weeks | |||
| Consider dermatology evaluation and skin biopsy | |||
| Severe | Delay immunotherapy | ||
| Methylprednisolone IV 1-2 mg/kg/day or oral equivalent | |||
| If improves to mild or resolves, taper steroids over at least 4 weeks | |||
| Consider skin biopsy | |||
| Endocrinopathy | Rule out noninflammatory etiology of symptoms | Mild | Continue immunotherapy |
| If abnormal TSH, add free T4 and T3 | |||
| Consider am cortisol, ACTH | |||
| Moderate | TSH, free T4, am cortisol, ACTH | ||
| Consider pituitary MRI | |||
| Methylprednisolone 1-2 mg/kg/day or oral equivalent | |||
| If improved, taper steroids over at least 4 weeks | |||
| Hormone replacement therapy if indicated | |||
| Endocrine consult | |||
| Severe | Delay or discontinue immunotherapy | ||
| Concerned for adrenal crisis - rule out infection/sepsis, BP support | |||
| Stress doses of mineralocorticosteroid |
BP: Blood pressure, MRI: Magnetic resonance imaging, ACTH: Adrenocorticotropic hormone, TSH: Thyroidstimulating hormone, T4: Thyroxine, T3: Triiodothyronine, IV: Intravenous, IVIG: Intravenous immunoglobulin, LFTs: Liver function tests, GI: Gastrointestinal, COPD: Chronic obstructive pulmonary disease, AEs: Adverse events
Future Directions of Cancer Immunotherapy
The early success of immune checkpoint inhibitors in solid tumors and cancer immunotherapies in general has generated a tremendous interest in further developing and exploring these strategies across the oncology disease spectrum. In light of the sometimes extended time period required for observing an antitumor response and need to ensure potential candidates are likely to benefit from a specific type of immunotherapy,[37] the identification of biomarkers that can aid with predicting response and ultimately in helping make informed decisions about selecting patients for treatment is one of the most important future steps in immunotherapy. For example, PDL-1 expression has been correlated with response to anti-PD-1/PD-L1 therapeutic agents in patients,[38,39] suggesting that higher levels of PDL-1 expression better predict responses to this type of treatment. However, CTLA-4 expression does not help predict response to PD-1/PDL-1 inhibition, which illustrates the potentially high specificity of biomarkers.
While observations, such as the aforementioned, are important first steps in identifying potentially useful predictive biomarkers, there is also a need for additional work in the development of clinical assays that standardize the evaluation of patient samples so that biomarker identification is consistent and valid without requiring substantial additional effort on behalf of patients in providing samples.[37] Oncology nurses need to be up-to-date with respect to biomarker identification for immunotherapies to be able to educate patients on the complexities surrounding the development of a truly personalized therapeutic strategy for their cancer treatment.
Conclusion
Cancer immunotherapy has created a new and exciting avenue for treatment and potentially cure for patients with cancer. Immune checkpoint inhibitors have recently provided major advances in the care of individuals with a variety of advanced solid tumors, and their ongoing testing in clinical trials creates new hope for patients affected by other types of disease. Oncology nurses are at the forefront of patient care and so must be knowledgeable of the unique treatment, side effect, and patient and family learning and management considerations associated with these agents to ensure the best quality of life and minimization of symptoms in patients treated with immune checkpoint inhibitors and other immunotherapies.
Financial support and sponsorship
This work was supported by NIH/NCI Cancer Center Support Grant P30 CA008748.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to acknowledge Sean Burke, B.S. for her contributions in assisting with identifying manuscripts for this review.
References
- 1.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, et al., editors. SEER Cancer Statistics Review, 1975-2013, Based on November 2015 SEER Data Submission, Posted to the SEER. Bethesda, MD: National Cancer Institute; 2016. Apr, [Last accessed on 2017 Jan 03]. Available from: http://www.seer.cancer.gov/csr/1975_2013/ [Google Scholar]
- 2.Zeng C, Wen W, Morgans AK, Pao W, Shu XO, Zheng W. Disparities by race, age, and sex in the improvement of survival for major cancers: Results From the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program in the United States, 1990 to 2010. JAMA Oncol. 2015;1:88–96. doi: 10.1001/jamaoncol.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel JD, Krilov L, Adams S, Aghajanian C, Basch E, Brose MS, et al. Clinical cancer advances 2013: Annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2014;32:129–60. doi: 10.1200/JCO.2013.53.7076. [DOI] [PubMed] [Google Scholar]
- 4.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shih K, Arkenau HT, Infante JR. Clinical impact of checkpoint inhibitors as novel cancer therapies. Drugs. 2014;74:1993–2013. doi: 10.1007/s40265-014-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Cancer Institute. Biological Therapies for Cancer. 2013. [Last accessed on 2016 Dec 20]. Available from: https://www.cancer.gov/aboutcancer/treatment/types/immunotherapy/bio-therapies-factsheet#q1 .
- 7.Cancer Research Institute. Timeline of Progress. 2016. [Last accessed on 2016 Dec 20]. Available from: http://www.cancerresearch.org/our-strategy-impact/timeline-of-progress/timeline-detail .
- 8.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208:2005–16. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–77. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian T, Olson S, Whitacre JM, Harding A. The origins of cancer robustness and evolvability. Integr Biol (Camb) 2011;3:17–30. doi: 10.1039/c0ib00046a. [DOI] [PubMed] [Google Scholar]
- 12.Melero I, Berman DM, Aznar MA, Korman AJ, Pérez Gracia JL, Haanen J. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer. 2015;15:457–72. doi: 10.1038/nrc3973. [DOI] [PubMed] [Google Scholar]
- 13.Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Mullard A. New checkpoint inhibitors ride the immunotherapy tsunami. Nat Rev Drug Discov. 2013;12:489–92. doi: 10.1038/nrd4066. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 16.American Society of Clinical Oncology. Understanding Immunotherapy. 2017. [Last accessed on 2017 Jan 03]. Available from: http://www.cancer.net/navigating-cancer-care/how-cancertreated/immunotherapy-and-vaccines/understandingimmunotherapy .
- 17.Leukemia and Lymphoma Society. Chimeric Antigen Receptor (CAR)-T Cell Therapy. 2015. [Last accessed on 2017 Jan 03]. Available from: https://www.lls.org/treatment/types-of-treatment/immunotherapy/chimeric-antigen-receptor-car-t-cell-therapy .
- 18.American Society of Clinical Oncology. What are Cancer Vaccines? 2017. [Last accessed on 2017 Jan 03]. Available from: http://www.cancer.net/navigating-cancer-care/how-cancer-treated/immunotherapy-and-vaccines/what-are-cancer-vaccines .
- 19.Ledezma B, Heng A. Real-world impact of education: Treating patients with ipilimumab in a community practice setting. Cancer Manag Res. 2013;6:5–14. doi: 10.2147/CMAR.S52543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Cancer Institute. Immunotherapy. 2017. [Last accessed on 2017 Jan 01]. Availabe from: https://www.cancer.gov/about-cancer/treatment/types/immunotherapy#4 .
- 21.Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol. 2015;33:3541–3. doi: 10.1200/JCO.2015.61.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kannan R, Madden K, Andrews S. Primer on immuno-oncology and immune response. Clin J Oncol Nurs. 2014;18:311–7. doi: 10.1188/14.CJON.311-317. 326. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann L, Forschner A, Loquai C, Goldinger SM, Zimmer L, Ugurel S, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190–209. doi: 10.1016/j.ejca.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 24.Howell M, Lee R, Bowyer S, Fusi A, Lorigan P. Optimal management of immune-related toxicities associated with checkpoint inhibitors in lung cancer. Lung Cancer. 2015;88:117–23. doi: 10.1016/j.lungcan.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Eigentler TK, Hassel JC, Berking C, Aberle J, Bachmann O, Grünwald V, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev. 2016;45:7–18. doi: 10.1016/j.ctrv.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Rizvi NA, Mazières J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial. Lancet Oncol. 2015;16:257–65. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–84. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 28.Tabchi S, Messier C, Blais N. Immune-mediated respiratory adverse events of checkpoint inhibitors. Curr Opin Oncol. 2016;28:269–77. doi: 10.1097/CCO.0000000000000291. [DOI] [PubMed] [Google Scholar]
- 29.Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: A multidisciplinary approach. Oncologist. 2013;18:733–43. doi: 10.1634/theoncologist.2012-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Society of Clinical Oncology. Skin Reactions to Targeted Therapy and Immunotherapy. 2017. [Last accessed on 2017 Jan 03]. Availabe from: http://www.cancer.net/navigating-cancer-care/side-effects/skin-reactions-targeted-therapy-and-immunotherapy .
- 31.Barton-Burke M, Ciccolini K, Mekas M, Burke S. Dermatologic reactions to targeted therapy: A focus on epidermal growth factor receptor inhibitors and nursing care. Nurs Clin N Am. 2017;52:83–113. doi: 10.1016/j.cnur.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder PJ. Causes of Hypopituitarism. 2016. [Last accessed on 2016 Oct 18]. Available from: http://www.uptodate.com/contents/causes-ofhypopituitarism .
- 33.Joshi MN, Whitelaw BC, Palomar MT, Wu Y, Carroll PV. Immune checkpoint inhibitor-related hypophysitis and endocrine dysfunction: Clinical review. Clin Endocrinol (Oxf) 2016;85:331–9. doi: 10.1111/cen.13063. [DOI] [PubMed] [Google Scholar]
- 34.Bristol-Myers Squibb Company. Human Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) Blocking Monoclonal Antibody Risk Evaluation and Mitigation Strategy. 2012. [Last accessed on 2017 Jan 03]. Available from: http://www.fda.gov/downloads/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm249435.pdf .
- 35.Postow M, Wolchok J. Toxicities Associated with Checkpoint Inhibitor Immunotherapy. 2016. [Last accessed on 2016 Oct 18]. Available from: http://www.uptodate.com/contents/toxicities-associated-withcheckpoint-inhibitor-immunotherapy .
- 36.Weber JS, Postow M, Lao CD, Schadendorf D. Management of adverse events following treatment with anti-programmed death-1 agents. Oncologist. 2016;21:1230–40. doi: 10.1634/theoncologist.2016-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan J, Hegde PS, Clynes R, Foukas PG, Harari A, Kleen TO, et al. Novel technologies and emerging biomarkers for personalized cancer immunotherapy. J Immunother Cancer. 2016;4:3. doi: 10.1186/s40425-016-0107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–74. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]