Abstract
Background:
The objective of this study was to investigate the fatty acids profiling in diabetic rats induced by sterptozocine (STZ) and their response to administration of lutein and carnitine.
Materials and methods:
Ninety male albino rats were divided into 6 groups as follows: Normal control. The remaining rats were injected i.p a single dose of STZ (65 mg /kg bw) for induction of diabetes. Diabetic rats were grouped as: GP II: (Untreated): GP III: Rats were given orally with L-lutein (100 mg/kg bw).GP IV: Rats were given carnitine (30 μg/kg) i.p. GP V: Rats were given carnitine and lutein GP VI were given metformin (100mg/kg bw/d) for 6 weeks.
Results:
Treatment of diabetic rats with lutein, L-carnitine, combined decreased the levels of glucose, HA1C compared with untreated diabetic (p<0.001). Administration of L-lutein, carnitine, combined to normal rats significantly decreased the levels of myristic, palmitice, palmitoleic, stearic, linoleic, α-linolenic, arachidic and eicosadienoic when compared with control normal rats (p<0.001).
Conclusion:
Abnormalities of fatty acids composition was observed in diabetic rats. Combination treatment with lutein and carnitine could ameliorate deleterious effect induced by STZ and attenuate the changed fatty acid composition
Keywords: Fatty acids profiling- lutein-carnitine-rats
Introduction
Diabetic is considered the most medical problem the world over. Most research focuses on lowering the prevalence of disease by early predication or minimizes its complications that increase morbidity and mortality [Ridderstråle and Groop, 2009]. Metabolomics is a new research trend deployed to discover various metabolites in different cases (normal and diseases) in biological fluids can be used as diagnostic or prognostic biomarkers or therapeutic protocol [Muoio and Newgard, 2008]. This allows for accurate and rapid identification of that disease. Metabolomic strategies present several practical advantages, including cost effectiveness per sample, high throughput and fully automated [Castle et al.,2006]. Metabolomics profiling might be an early diagnostic and prognostic biomarkers for diabetic and we will see the basis of biomarker for the disease and detect were most deregulated that might be the basis of biomarker for the disease therapies that intend to correct metabolic deregulation [German etal.,2005]. Life styles and feeding could affect the resulting metablomic deregulation; hence affects the nature and aggressiveness of the disease by doing this research we will show which differences in the metablomic profiling [Matthews etal.,1985]. However, we do not know whether the biomarkers found from these metabolomics research could also be applied on Saudi patients due to differences in life styles and feeding. This could be said to have justified obtaining serum as a tool for early detection of diabetes. But we will focus on the markers that has been shown to be most deregulated in diabetic rats [Hollywood et al., 2006]. L-Carnitine is an important fatty acyl carrier from cytosol to mitochondrial matrix for β oxidation. It obtained from diet (animals sources) or be synthesized from lysine and methionine. Deficiency of carnitine resulted in decrease oxidation of fatty acids and decreased energy supplied to vital organs as heart, nervous system and skeletal muscles [Lindon et al., 2007].
Lutein is a xanthophyll member of carotenoids, which is synthesized in green leaf plants. It was recorded as safe when supplemented to foodstuff [Han and Gross, 2003]. Lutein is absorbed via lipoprotein in the intestinal tract. Tissue content of lutein is related to dietary intake. After intake, lutein concentration is approximately 0.2 μΜ in circulation to different tissues and play important role in maintenance of tissue homeostasis [Watson, 2006]. Reactive oxygen species (ROS) was considered the most common free radical in the cell. It is generated as byproducts in metabolism of drugs. The accumulation of these radicals has been reported as a mechanism for apoptosis. Luteins plays an effective role in the removal of free radicals [Lin et al., 2011].
The rational behind this study is to evaluate the importance of lutein and carnitine in enhancing the metabolism and management of diabetic complications. In this study we will focus on the changes in free fatty acids metabolites that have been secondarily affected and could serve as a biomarker for the disease or substantially for those which are behind but associated with the disease. This will enhance the understanding the pathogenesis of the disease from a metabolomics. The main objective of this study is to identify free fatty acids metabolites in diabetic rats and their response to administration of lutein and carnitine. This will help to know the biological role of lutein and L-carnitine in modulation the metabolism in diabetes and consequently the complications and severity of the diseases.
Materials and Methods
Animals
Ninety male albino rats weighting between 200±20 g were included in this study. Rats were divided into 6 groups (each 15 rats) as follows: Group I: Control group which was received a single dose of 0.1 μmοΙΛ citrate buffer only. The remaining rats were injected with a single dose of streptozocine (STZ) (65 mg /kg b.w) i.p for induction of diabetes. These animals developed diabetes within three days. Blood sugar more than 250 mg/dl was considered diabetic. Diabetic rats were divided into five groups as follows: GP II: (Untreated diabetic): GP III: Rats were given orally with L-lutein (100 mg/kg bw).GP IV: Rats were given with L-carnitine (30 μg/kg b w) ip. GP V: Rats were given L-carnitine and lutein. GP IV: rats were given metamorfin (10 mg/kg bw) daily. At the end of the experiments (6 weeks). Blood was collected directly from all groups. Serum was used for the determination of glucose, glycated hemoglobin, urea, creatinine and nitric oxide, malondilaldhyde [Shearer etal.,2008], total antioxidant activity. Superoxide dismutase, interleukin-6, tumor necrosis factor (TNF-α) by using ELISA kit from BIORAD, ENGLAND.
Profiling of serum fatty acids
Serum fatty acids were extracted with mixture of chloroform/methanol (2:1). Methyl ester of fatty acids (FAME) were prepared with by using 20% triflouroboron with methanol 110°C. Analyses of the ester were done by GC using from Agilent Company. The GC was equipped with capillary column 60m, 0,25mm) and flame ionization detector at 260°C and injector at 250°C. The carrier gas used was Helium with flow rate at 43cm/sec. The retention times of standards fatty acids were used. For all fatty acids the mean value in percent was calculated [Rebouche and Chenard,1991].
Statistical analysis
The data were calculated as mean ± SD using SPSS, version 22. Any significant differences between different treated groups using ANOVA test.
Results
The body weight of the rats at the beginning of the study were similar in all groups. At the end of the experiment, diabetic animals presented a significant weight loss (p <0.001). The weights of the rats at the beginning of the study were similar in all groups. At the end of the experiment, diabetic animals presented a significant weight loss. Injection of rats with STZ resulted in a significant increase in blood glucose levels in the diabetic group compared with the control group (p <0.01), while treatment with lutein or carnitine or combined resulted in a significance decrease in blood glucose compared with the untreated diabetic animals (p <0.05, table 1). As a result, diabetes, HbA1c was significantly increased (p<0.05) in the diabetic untreated group. Treatment of animals with lutein or carnitine or combined improves the two parameters in a dose dependent manner (table 1). The HCA exert hypoglycemic action but less potent than insulin (p<0.05).
Table 1.
Body weight and blood glucose and glycated hemoglobin levels in all groups
| Group 1 | Group IIa | Group IIb | Group IIc | Group IId | |
|---|---|---|---|---|---|
| Initial body weight(gm) | 120.13±9.5 | 125.44±7 | 129.19±6 | 122±7.2 | 131.33±6.5 |
| Final body weight(gm) | 199.5±8.2 | 150.8±7.2a | 167.3±16.5a,b | 165.52±8.9a,b | 210±12.3a,b |
| Glucose (mg/dL) | 92.78±0.45 | 275.35±1.45a | 145.92±1.4a,b | 137.71±1.23b | 102.21±0.57a,b |
| HbA1c(%) | 5.54±0.41 | 8.42±0.34a | 6.8±0.54 b | 6.8±0.38 b | 5. 8±0.52 b |
The antioxidant activities like GSH reduced, catalase, SOD in retina of diabetic animals were significantly reduced as a result of STZ injection. Supplementation of lutein or L-carnitine or combined resulted in a significant elevation in GSH (p<0.05 for each) level and the activities of catalase and SOD (p<0.001, <0.01 and <0.05)) respectively (table 1). The elevation of the antioxidant enzymes was found to be dose dependent. The degree of lipid peroxidation in the retina was significantly elevated as a result of diabetes. Administration of the combined dose resulted in a dose dependent decrease of MDA levels, table 1.
Figures (1, 2) showed that, serum aldolase reductase and total antioxidant activities were significantly decreased in diabetic rats when compared with the controlled ones (p<0.01 and 0.001) respectively. Lutein or L-carnitine or combined showed an elevation in these activities with dose dependent. The enhancement in insulin injection is better than alone (p<0.05).
Figure 1.
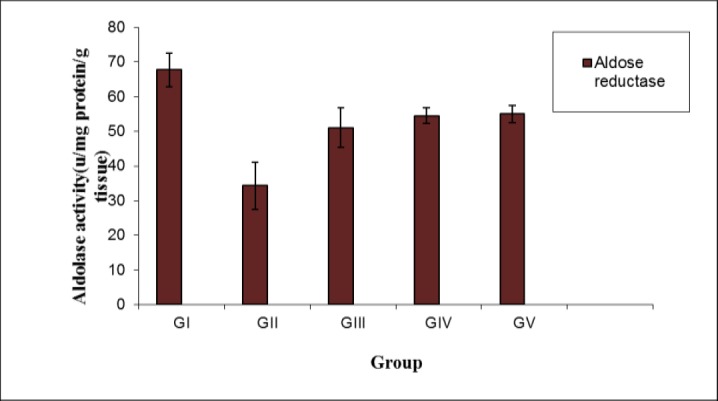
Serum aldose reductase activity in all studied groups (Mean SD).
Figure 2.
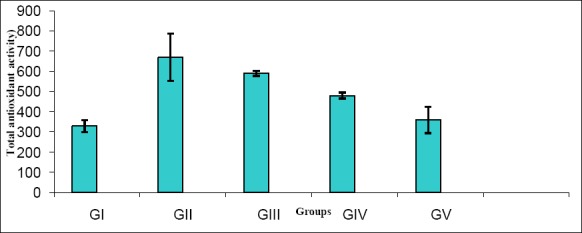
Serum total antioxidant activity in all studied groups (Mean SD).
Figure 3 showed that, both TNFα and IL-1 play an important role in the pathogenesis of diabetic retinopathy. As a result of STZ injection the retinal levels of TNFα and IL-1 were significantly elevated (p<0.001) for each indicating a considerable level of inflammation compared with the normal control rats. Administration of lutein or carnitine or combined results in a significant and a dose dependent reduction of these elevated levels (p<0.01). As a result of diabetes and enhanced formation of AGEs was marked and activated in the diabetic untreated group compared with the normal control group. HCA supplementation lutein or L-carnitine or combined resulted in a significant reduction of AGEs in a dose dependent manner as indicated in Figure 3. HCA attenuated AGE production compared with insulin (p<0.001).
Figure 3.
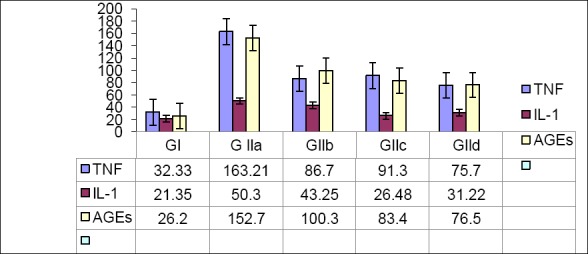
Serum (TNF- α), interleukine-1, and advanced glycated end products (AGEs) levels
Serum glucose, HA1C levels in STZ-induced diabetic rats showed about four-fold elevation compared with control group (p<0.001). Treatment of diabetic rats with L-lutein, carnitine, combined were statistically significant (p<0.01,<0.001), (p<0.01,<0.01),(p<0.001,<0.001) respectively decreased in the levels of glucose, HA1C compared with untreated diabetic. The combined effect was similar to metformin treatment.
The results in table (2) revealed that, a statistical significant elevation in the levels of saturated palmitic acid, monounsaturated fatty acids (palmitoleic oleic and arachidic) in diabetic rats.
Table 2.
Serum malondialdhyde (MDA) level and reduced glutathione (GSH) levels, catalase, and superoxide dismutase (SOD) activity in retina of different groups (Mean ± S.D)
| Groups | Serum | |||
|---|---|---|---|---|
| MDA μmol/mg protein | GSH μg /mg protein | Catalase U/mg protein | SOD U/mg protein | |
| Group I Range Mean ±S.D. |
98-196 66.7 ± 28 |
104 – 546 284 ±52 |
992-2349 1249 ±140 |
956 – 2623 1545±191 |
| Group II Range Mean±S.E. p* value |
190-261 188 ± 37 N. S. |
68.7-221.5 123.6 ±19.3 < 0.01 |
109.5 – 642 358 ±65 <0.001 |
453-1342 760±78 <0.001 |
| Group III Range Mean ± S.E. p* value |
149 – 213 142 ± 32 <0.001 |
121 – 496 259 ±50 <0.001 |
706 – 2002 843±134 <0.001 |
876 -1987 1021 ±163 <0.001 |
| Group IV Range Mean ± S.E. p** value |
136-261 133± 25 <0.001 |
69.6- 213.9 128 ± 16.4 p< 0.05 |
456 -1632 987 ±56 <0.001 |
962-1316 836±53 <0.001 |
| Group V Range Mean ± S.E. p** value |
156-261 121± 25 N.S. |
69.6- 213.9 128 ± 16.4 p< 0.05 |
546 -1756 1112 ± 86 <0.001 |
624-2116 1233 ± 153 <0.001 |
P value, all groups vs control
P value, treated Vs untreated
Table (2).
Fatty acids levels in different studied groups (Mean ± SD).
| Animals groups Fatty acids |
Control group | Diabetic | Diabetic+Lutein | Diabetic+carnitine | Diabetic+lutein+carnitine | Diabetic+metformin |
|---|---|---|---|---|---|---|
| Myristic 14:0 | 0.99±0.04 | 1.1±0.24 | 1.0±0.11 | 1.0±0.12 | 1.0±0.21 | 1.1±0.20 |
| Palmitic 16:0 | 1.3±0.11 | 1.41±0.20 | 1.44±0.19 | 1.43±0.16 | 1.37±0.15 | 1.30±0.31 |
| Palmitoleic 16:1 | 0.45±0.12 | 0.49±0.09 | 0.48±0.07 | 0.43±0.05 | 0.40±0.1 | 0.40±0.08 |
| Stearic 18:0 | 2.7±0.63 | 2.88±0.83 | 2.93±0.93 | 2.81±0.73 | 2.99±0.89 | 2.62±0.63 |
| Oleic 18:1 | 1.3±0.06 | 1.56±0.09 | 1.65±0.11 | 1.77±0.12 | 1.52±0.32 | 1.81±0.41 |
| α-Linolenic 18:3 | 1.89±0.09 | 1.49±0.29 | 1.55±0.39 | 1.61±0.41 | 1.71±0.53 | 1.91±0.32 |
| Linolenic 18:3 | 1.44±0.07 | 1.14±0.17 | 1.14±0.17 | 1.14±0.17 | 1. 14±0. 17 | 1.14±0.17 |
| Arachidic acid 20:0 | 0.92±0.05 | 1.3±0.35 | 1.1±0.25 | 1.2±0.33 | 1.0±0.41 | 0.94±0.44 |
| Ecosadienoic acid 20:2 | 0.98±0.55 | 1.2±0.65 | 1.3±0.45 | 1. 1 ±0. 3 5 | 1.4±0.71 | 1.2±0.56 |
Mean values with different superscript letters in the same column are significantly different at p≤0.05
Treatment of diabetic rats with L-lutein or L-carnitine or combined significantly reduction of saturated palmitic acid (p<0.01), monounsaturated fatty acids (palmitoleic oleic and arachidic) (p<0.001). The effect of combined treatment nearly similar to metformin treatment.
In diabetes rats, linoleic acid level was above normal, whereas arachidonic acid was below normal. However, diabetics rats treated with metformin arachidonic acids elevate significantly (p<0.05).
Administration of L-lutein, carnitine, combined to normal rats significantly decreased the levels of myristic, palmitice, palmitoleic, stearic, linoleic, 0 -linolenic, arachidic and eicosadienoic when compared with control normal rats. Diabetic rats treated with L-lutein, L-carnitine, combined significantly (p<0.01) decreased the percentage of palmitic, stearic, oleic, arachidic compared with untreated rats.
Discussion
Hyperglycemia plays an important role in the development and progression of diabetic complications [Kim et al., 2011]. The oxidative stress and AGEs can enhance inflammatory processes and diabetic complications [Trumbo and Ellwood,2006]. The elevation in serum glucose and HA1c levels in diabetic rats are in according to results obtained [Alves-Rodrigues and Shao 2004], who reported that, STZ-induced showed 4-5fold increase in the blood glucose levels after STZ administration.
The hyperglycemia due to the toxic effects of STZ through glucose transporter-2, these lead to organ damage through activation accumulation of sorbitol in vital organs [Griffin et al.,1999]. Treatment with lutein or carnitine and their combination significantly reduced elevati glucose This is in accordance to [Girard et al.,2005]. The combined effect is nearly similar to metformin action
These effects of these supplements can be attributed to the antioxidant of lutein and combination with carnitine to reduce the glucose in t and stimulate the activity of pancreatic islets and insulin production in diabetic rats. The observations were consistent with the overall improveme complications associated with diabetes with insulin [Evans etal.,2001]. Treatment with lutein combined with carnitine or with metformin were sigi lowered the levels of myristic, palmitic, palmitoleic and elevates the level of stearic acids when compared with the untreated dietiic rats.
This is in accordance to results of [Gupta et al., 2013], who stated that, total saturated fatty acids were higher in the non-diabetic rats treated with lu untreated. Level of arachidonic may be decreased in diabetes and some degenerative diseases. Desaturase activities may be increased by insulin restriction [Na et al., 2011]. Insulin therapy can correct this defect by influencing the desaturase level. The results obtained confirm that treatn these supplements have beneficial effects on the D-6 desaturase system and unsaturated fatty acid levels and protective effects on unsaturated fa and [Neyrinck et al., 2013].
Conclusion
It could be concluded that, abnormalities of fatty acids composition was observed in rats. It may be possible that, treatment with lutein alone or combined with L-carnitine could ameliorate toxicity induced by STZ and attenuate the changed fatty acid composition.
Acknowledgment
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. (G/58/130/1437). The author, therefore, acknowledge with thanks DSR technical and financial support.
Footnotes
Conflict of Interests The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Alves-Rodrigues A, Shao A. The science behind lutein. Toxicol. Lett. 2004;150:57–83. doi: 10.1016/j.toxlet.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 2.Castle AL, Fiehn O, Kaddurah-Daouk R, Lindon JC. Metabolomics Standards Workshop and the development of international standards for reporting metabolomics experimental results. Brief Bioinform. 2006;7:159–165. doi: 10.1093/bib/bbl008. [DOI] [PubMed] [Google Scholar]
- 3.De Fronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 4.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. PMID: 18. Araújo CC, Leon LL. Biological activities of Curcuma longa L. Mem. Inst Oswaldo Cruz. 2001; 96: 723–728. [DOI] [PubMed] [Google Scholar]
- 5.German JB, Hammock BD, Watkins SM. Metabolomics: building on a century of biochemistry to guide human health. Metabolomics. 2005;1:3–9. doi: 10.1007/s11306-005-1102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 7.Girard A, Madani S, El Boustani ES, Belleville J, Prost J. Changes in lipid metabolism and antioxidant defense status in spontaneously hypertensive rats and Wistar rats fed a diet enriched with fructose and saturated fatty acids. Nutrition. 2005;21:240–248. doi: 10.1016/j.nut.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 8.Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han X, Gross RW. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: a bridge to lipidomics. J Lipid Res. 2003;44:1071–1079. doi: 10.1194/jlr.R300004-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Hollywood K, Brison DR, Goodacre R. Metabolomics: current technologies and future trends. Proteomics. 2006;6:4716–4723. doi: 10.1002/pmic.200600106. [DOI] [PubMed] [Google Scholar]
- 11.Kim HJ, Kim JH, Noh S, Hur HJ, Sung MJ, Hwang JT, Jae HP, Hye JY, Myung SK, Dae YK, SUK HY. Metabolomic analysis of livers and serum from high-fat diet induced obese mice. J Proteome Res. 2011;10:722–731. doi: 10.1021/pr100892r. [DOI] [PubMed] [Google Scholar]
- 12.Lin S, Yang Z, Liu H, Tang L, Cai Z. Beyond glucose: metabolic shifts in responses to the effects of the oral glucose tolerance test and the high-fructose diet in rats. Mol BioSyst. 2011;7:1537–1548. doi: 10.1039/c0mb00246a. [DOI] [PubMed] [Google Scholar]
- 13.Lindon JC, Holmes E, Nicholson JK. Metabonomics in pharmaceutical R&D. FEBS J. 2007;274:1140–1151. doi: 10.1111/j.1742-4658.2007.05673.x. Muoio DM, Newgard CB. Molecular and metabolic mechanisms of insulin resistance and _-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 2008;9:193–205. [DOI] [PubMed] [Google Scholar]
- 14.Palmer JP, Walter RM, Ensinck JW. Lutein- stimulated acute phase of insulin and glucagon secretion in normal men. Diabetes. 1975;24:735–740. doi: 10.2337/diab.24.8.735. [DOI] [PubMed] [Google Scholar]
- 15.Rebouche CJ, Chenard CA. Metabolic fate of dietary carnitine in human adults: identification and quantification of urinary and fecalmetabolites. Nutr J. 1991;121:539–546. doi: 10.1093/jn/121.4.539. [DOI] [PubMed] [Google Scholar]
- 16.Ridderstråle M, Groop L. Genetic dissection of type 2 diabetes. Mol Cell Endocrinol. 2009;297:10–17. doi: 10.1016/j.mce.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Trumbo P.R, Ellwood K.C. Lutein and zeaxanthin intakes and risk of age-related macular degeneration and cataracts: An evaluation using the Food and Drug Administration’s evidence-based review system for health claims. Am Clin J. Nutr. 2006;84:971–974. doi: 10.1093/ajcn/84.5.971. [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Na LX, Zhang YL, Li Y, Liu LY, Li R, Kong T, Sun HS. Curcumin improves insulin resistance in skeletal muscle of rats. Nutr Metab Cardiovasc Dis. 2011;21:526–533. doi: 10.1016/j.numecd.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Neyrinck AM, Alligier M, Memvanga PB, Névraumont E, Larondelle Y, Préat V, Cani PP, Delzenne NM. Curcuma longa extract associated with white pepper lessens high fat diet-induced inflammation in subcutaneous adipose tissue. PloS one. 2013;8(11):e81252. doi: 10.1371/journal.pone.0081252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shearer J, Duggan G, Weljie A, Hittel DS, Wasserman DH, Vogel HJ. Metabolomic profiling of dietaryinduced insulin resistance in the high fat-fed C57BL/6J mouse. Diabetes Obes Metab. 2008;10:950–958. doi: 10.1111/j.1463-1326.2007.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson AD. Thematic review series: systems biology approaches to metabolic and cardiovascular disorders: lipidomics: a global approach to lipid analysis in biological systems. J Lipid Res. 2006;47:2101–2111. doi: 10.1194/jlr.R600022-JLR200. [DOI] [PubMed] [Google Scholar]


