Abstract
Background:
The present study has evaluated the Emodin efficacy on the Akt, MAPK, ERK and DNMT expression pattern during 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinoma in golden Syrian hamsters, in order to explore its antitumor potential.
Materials and methods:
Oral tumors were developed in the buccal pouches of golden Syrian hamsters using the carcinogen, DMBA.
Results:
While the incidence of tumor formation was 100% in hamsters treated with DMBA alone, the tumor formation was not noticed in DMBA+ Emodin treated hamsters. Also, Emodin reduced the severity of precancerous pathological lesions such as dysplasia, in the hamsters treated with DMBA. Emodin administration corrected the abnormalities in the expression pattern of Akt, MAPK, ERK and DNMT in the buccal mucosa of hamsters treated with DMBA.
Conclusions:
The present study thus suggests that the tumor preventive potential of Emodin is partly related to its modulating effect on the Akt, MAPK, ERK and DNMT expression pattern, as these molecular markers have a pivotal role in the process of cell proliferation, inflammation, invasion, and apoptosis.
Keywords: Oral carcinoma, Akt, MAPK, DNMT
Introduction
Oral squamous cell carcinoma, a cancer of the oral cavity, is one of the ten most common malignant neoplasms and is the 5th most frequent cancer worldwide (Markopoulos, 2012). The development of oral cancer is usually preceded by distinct precancerous lesions such as leukoplakia, erythroplakia and oral submucous fibrosis. However, the potential or degree of malignant transformation varies from one another (Carnelio et al., 2011). Oral carcinoma has a spectrum of risk factors, which include tobacco, alcohol, diet and nutrition, betel quid, viruses, immune deficiency and poor oral hygiene (Khyani et al., 2015). Tobacco chewing together with tobacco smoking, betel quid chewing and alcohol abuse is recognized as the strongest risk factor of oral cancer (Sridharan, 2014). Several epidemiological and aetiological studies pointed out the synergistic efficacy of tobacco and alcohol in the development of oral carcinoma (Patil et al., 2013). Although oral cancer is common in several countries, the highest incidence is reported every year from India, Pakistan, Sri Lanka, France and Hungary (Vigneswaran and Williams, 2014). The behavioral risk factors, tobacco and alcohol, accounts for 75-95% of all oral cancers in India (Gupta et al., 2013). Creating awareness on the risk factors of oral cancer to the public could help to reduce the annual incidence, since the risk factors are avoidable one (El Rhazi et al., 2014). It has also been pointed out that early diagnosis will improve the survival outcome of the patients (80%) and quality of life than diagnosis at late stage [30%] (Baykul et al., 2010).
Golden Syrian hamsters serve as an ideal model to study the tumor inhibitory potential of natural products, especially in oral carcinoma (Steele and Lubet, 2010). Hamsters possess buccal pouches (a pocket like anatomy) on their oral cavity, which can retain the carcinogen for the longest time when applied topically and thus tumors develop within a shorter duration (Chen et al., 2012). DMBA induces buccal pouch carcinogenesis in the hamsters through inducing chronic inflammation, causing extensive oxidative DNA damage and by inducing DNA mutations (Ismail et al., 2016). Oral cancer chemoprevention researchers prefer DMBA induced hamster buccal pouch carcinogenesis model, as this model exhibits close resemblance and similarities with human oral tumor in many aspects, including at histopathological and molecular levels (Manoharan et al., 2016; Casto et al., 2013).
P13K/Akt and ERK/MAPK pathways perform a pivotal role in the regulation of tumor growth, progression, invasion and metastasis (Gan et al., 2010). As P13K/Akt pathways play crucial and critical role in both cell survival and apoptotic pathway, their abnormal regulation or expression could mediate the neoplastic transformation in various cancers including oral carcinoma (Chang et al., 2013). The status of p-Akt has been focused as an independent prognostic marker in various cancers. Inhibition of P13K/Akt pathway was resulted in the induction of apoptotic cascade in various cancers (Matsuoka and Yashiro, 2014). P13K/Akt pathway was significantly activated during oral carcinogenesis. Tumor growth inhibition could thus be achieved by blocking the activation of Akt or ERK signaling pathways (Chappell et al., 2011).
MAPK family plays an important role in cell differentiation, proliferation, inflammation and apoptosis. This family also consists of ERK and P38. Profound studies pointed out that P38 MAPK are activated by a bundle of stimuli such as growth factors, cytokines and chemical stresses, resulting in cell proliferation, migration and apoptosis (Gerthoffer et al., 2012). MAPK has also a pivotal role in the regulation of angiogenesis. ERK, a subfamily of the MAPK family, plays an important role in the regulation of cell differentiation and proliferation (Munshi and Ramesh, 2013). ERK has been identified as a positive regulator of cell cycle and it stimulates cyclic dependent kinases - cyclin D complex, which is essential for the progression of the cell cycle (Villanueva et al., 2007). Several studies have pointed out that the accumulation of activated ERK (pERK) in the nucleus could result in genomic instability as well as promotes tumorigenesis. ERK also has a role in the stimulation of tumor suppressor pathways (Bobrovnikova et al., 2010). DNA methylation, a major factor in epigenetic phenomenon, plays prominent role in the process of carcinogenesis. The enzyme DNA methyl transferease (DNMT) regulates the process of DNA methylation (Jin et al., 2011). Profound studies on various cancers documented the over expression of DNMT (Estève et al., 2016). Thus, DNMT has been utilised as a major target for cancer treatment.
Emodin, an anthraquinone derivative, is present in the root and rhizome of several Chinese medicinal plants and used in traditional Chinese medicine to treat numerous illnesses (Yang et al., 2014). Profound studies documented the pharmacological, biochemical and therapeutic effects of Emodin using experimental animal models or via in vitro approach. Emodin explored its cytotoxic potential against various tumor cell lines including human lung squamous carcinoma cells, human cervical cancer cells, human hepatoma cells, human breast cancer cells and human cervix epithelioid carcinoma cells (Zu et al., 2015; Yaoxian et al., 2013; Huang et al., 2013; Huang et al., 2013). Emodin exerted apoptotic potential in various cancer cell lines. In vivo studies demonstrated its antidiabetic, hepatoprotective and anticancer properties (Zhao et al., 2009; Lin et al., 2012). The present study explores the Emodin efficacy on the Akt, MAPK, ERK and DNMT expression pattern during 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinoma.
Materials and methods
Animals
We procured forty golden Syrian hamsters, (male; 80-120g) from National Institute of Nutrition, Hyderabad, India and were maintained according to the Institutional ethical committee guidelines in the Central Animal House, Annamalai University (Registration Number 160/1999/ CPCSEA).
Tumor induction
The present study utilized DMBA, the site specific and organ specific carcinogen to produce tumors in the buccal pouches of golden Syrian hamsters. Topical application of this carcinogen (0.5% in liquid paraffin) three times a week for 14 weeks developed well differentiated squamous cell carcinoma (confirmed by oral pathologist, Annamalai University)
Experimental design
The experimental hamsters were divided into four groups of ten in each and housed in the polypropylene animal cages (5 animals/cage). The experimental hamsters were provided with pellet diet and water ad libitum. The experimental protocol followed in the present study is given in the figure 1.
Figure 1.

Experimental protocol
Group I hamsters received liquid paraffin alone on their buccal pouches (topical application, three times a week for 14 weeks) and served as a vehicle treated control. The buccal pouches of group II hamsters were exposed to 0.5% DMBA in liquid paraffin alone (Topical application; three times a week for 14 weeks) and served as carcinogen treated control. Group III hamsters were exposed to DMBA, as in group II, and were orally administered with Emodin (50mg/kg b.w), three times a week for 14 weeks; on alternate days of DMBA painting. Group IV hamsters received Emodin alone orally (three times a week for 14 weeks). After the experimental period, the buccal mucosa was excised from all the experimental hamsters and subjected to Western blotting to assess the expression pattern of p-Akt, p-ERK, p-P38 MAPK, DNMT1, DNMT3a and DNMT3b
Western blotting
The proteins were extracted from the buccal mucosa tissues and quantified (Bradford, 1976). The tissue extract was then subjected to polyacrylamide gel electrophoresis (PAGE) to separate the various proteins. Electroblotting was employed to transfer the separated protein bands onto PVDF membrane. The membrane containing protein blots were then incubated with the corresponding primary antibodies (p-Akt, p-ERK, p-P38 MAPK, DNMT1, DNMT3a and DNMT3b, Cell Signaling Technology, Danvers, MA, USA). After the incubation period the blot was treated with the secondary antibodies labelled with horseradish peroxidase (Santa Cruz Biotechnology, USA). The immune complex formed was then treated with diaminobenzidine, and the bands were scanned and analysed densitometrically (Bio-Rad Image Lab™ software version 4.1 software).
Statistical analysis
The statistical significance between the groups was assessed using One way analysis of variance (ANOVA) followed by Duncan’s Multiple Range Test (DMRT). The results obtained were considered statistically significant if the p value was less than 0.05 between the two groups.
Results
Buccal mucosa p-Akt, p-ERK, p-P38 MAPK, DNMT1, DNMT3a and DNMT3b expression pattern obtained using Western blotting and their densitometry analysis in the control and experimental hamsters are given in the figures 2-5. Western blot analysis revealed over-expression of the above said molecular markers in the hamsters treated with DMBA alone when compared to control hamsters. Emodin administration orally at the dose of 50mg/kg b.w to the hamsters treated with DMBA brought back the expression pattern of all the markers to near normal range.
Figure 2.
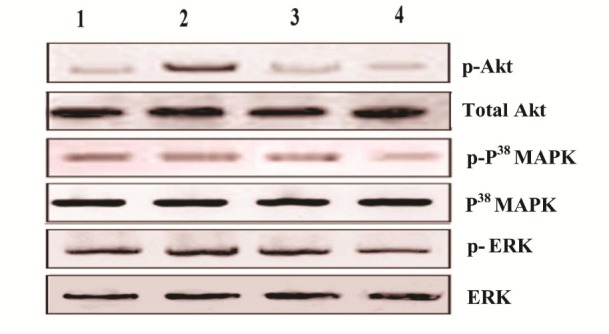
Expression pattern of p-Akt, p-P38 MAPK and p-ERK in the buccal pouch tissues of control and experimental animals in each group. Lane 1: Control, Lane 2: DMBA alone, Lane 3: DMBA + Emodin, Lane 4: Emodin alone.
Figure 3.
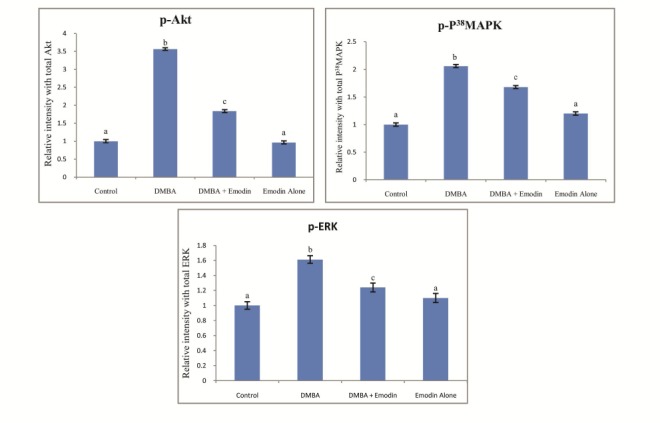
Densitometric analysis for p-Akt, p-P38 MAPK and p-ERK expression pattern in control and experimental animals in each group. Data presented are the mean ± SD (n=10). Common superscripts between two groups - not significant. Different superscripts between two groups - significant p<0.05.
Figure 4.
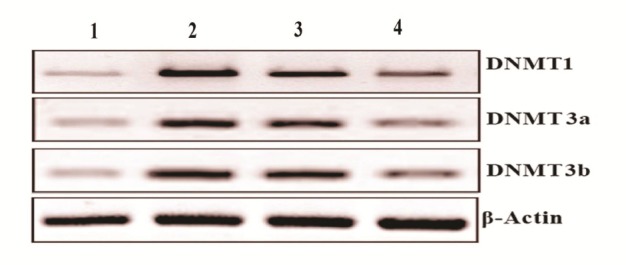
Expression pattern of DNMT1, DNMT3a and DNMT3b in the buccal pouch tissues of control and experimental animals. Lane 1: Control, Lane 2: DMBA alone, Lane 3: DMBA + Emodin, Lane 4: Emodin alone.
Figure 5.

Densitometric analysis for DNMT1, DNMT3a and DNMT3b expression pattern in control and experimental animals in each group. Data presented are the mean ± SD (n=10). Common superscripts between two groups - not significant. Different superscripts between two groups - significant p<0.05.
Discussion
Oral cancer arises due to the abnormalities in multiple molecular pathways, which include pathways of cell proliferation, apoptosis, inflammation and angiogenesis (Manoharan et al., 2015). Extensive studies utilized these molecular pathways to evaluate the antitumor potential of the medicinal plants, their active constituents and synthetic agents (Schetter et al., 2010). The present study has utilized the Akt, MAPK, ERK and DNMT pathways to explore the Emodin efficacy in the inhibition of tumor formation during 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinoma.
Akt and MAPK pathways are involved in the regulation of genes that play pivotal role in cell proliferation, inflammation, invasion, autophagy and apoptosis. Apoptosis and arrest of cell growth can be achieved by blocking Akt activation in tumor cells (Jiang and Liu, 2009). The Akt pathway plays an important role in the regulation of cell-cycle progression and tumor cell growth (Antico Arciuch et al., 2012). A large number of studies showed increased activity of Akt, a well known serine/ threonine kinase, in various types of malignancies including oral cancer (Lee, 2014). Upregulation of Akt/P38 MAPK signaling pathway has been reported in oral carcinoma HSC-3 cells. Akt mediates its self proliferative role via inhibiting pro-apoptotic proteins or promoting anti-apoptotic genes (Kalimuthu and Se-Kwon, 2013). Akt pathway was up-regulated in 30 to 60% of prostate carcinogenesis (Shtivelman et al., 2014).
A pro-apoptotic as well as anti-apoptotic role for P38 MAPK has been reported in tumor cells. Multiple evidences explored deregulation in P38MAPK signaling pathway, which has been associated with malignant transformation (Wagner and Nebreda, 2009). Deregulation of MAPK pathway has been reported in several cancers, particularly in more than 50% of hepatocellular carcinomas. It has been reported that the mammary cancer metastasis can be inhibited by inhibiting P38 expression (Dhillon et al., 2007).
ERKs occupy the central position of diverse cellular signaling pathways that are involved in cell growth, proliferation and survival (Hong et al., 2015). Deregulation in the ERK / MAPK pathway is associated with the poor prognosis of oral cancer. ERK1/2 and P38, the subfamilies of MAPK, were deregulated in various carcinogenesis (Mebratu and Tesfaigzi, 2009). Aberrant ERK signaling could therefore lead to malignant transformation. ERK activation resulted in the activation of the spectrum of genes including PCNA, which in turn favors malignant transformation (Jin and Robertson, 2013).
There are three classes of DNMTs in the mammalian cells, DNMT1, DNMT3a and DNMT3b. DNMT1 is a maintenance methyltransferase and DNMT3a and DNMT3b are denovo methyltransferases (Liang et al., 2002). All three types of DNMT were abnormally expressed in cancerous conditions (Skowronski et al., 2010). DNMT1 plays prominent and pertinent role for DNA methylation during DNA replication. DNMT1 over expression was reported in lung, colorectal, prostate, breast, gastric and oral cavity cancers (Delpu et al., 2013). It has been pointed out that DNMT inhibition might lead to reactivation of the silenced genes. Down-regulation of p53 has been associated with up-regulation of DNMT1 (Au Yeung et al., 2010).
Extensive studies reported the effect of Emodin on the Akt, MAPK, ERK and DNMT expression pattern in various cancer cell lines. Lin et al., (2016) pointed out that Emodin induced apoptotic process in the hepatic cancer cell lines via P13K/Akt and MAPK signaling pathways. Liu et al., (2015) explored the inhibitory effect of Emodin on the expression of P13K/Akt in the mechanical stress induced hypertrophic scarring. Emodin prevented the mammary cancer cell proliferation via down-regulating P13K/Akt protein expression (Sui et al., 2014). It has been reported that Akt down-regulation by Emodin is due to the inhibition of the components of the P13K pathway (Olsen et al., 2007). Zheng et al., (2015) observed that Emodin downregulated Akt expression in K562/Adr cells. Way et al., (2014) demonstrated that Emodin effectively inhibited the epithelial-mesenchymal transition in head and neck cancer cells via inhibiting Akt pathways. Inhibition of Akt activation has been suggested as a major mechanism of antitumor effect of Emodin against pancreatic cancer in mice (Wei et al., 2011). It has been pointed out that Emodin attenuated the phosphorylation of P13K/Akt and ERK in HPG2 cells (Cui et al., 2016). Emodin exerted its antiinvasive property via down-regulating ERK1/2 and Akt/PKB activation in glioma cells (Kim et al., 2005). Inactivation of ERK and Akt has been demonstrated in human lung adenocarcinoma cells (Su et al., 2005). Emodin downregulated the expression of DNMT1 and DNMT3 in pancreatic cancer cells. (Pan et al., 2016; Zhang et al., 2015). We, for the first time, demonstrated the modulating effect of emodin on the Akt, MAPK, ERK and DNMT expression pattern in experimental oral carcinogenesis.
In the present study, we have explored the activation of Akt, ERK, P38 MAPK and DNMT, which have a crucial role in the process of cell proliferation, apoptosis and cell differentiation in the tumor bearing hamsters (DMBA alone treated hamsters). Emodin administration at a dose of 50mg/kg b.w to hamsters treated with DMBA inhibited the activation of all these molecular markers, as evidenced by the down regulation of these markers (Western blotting). The present study thus suggests that Emodin might have modulated the Akt, ERK, P38 MAPK and DNMT pathways towards suppression of tumor formation during DMBA induced oral carcinogenesis.
Conclusion
The present study concludes that the antitumor potential of Emodin might have partly attributed to its inhibitory effect on the activation of Akt, ERK, P38 MAPK and DNMT pathways during DMBA induced hamster buccal pouch carcinogenesis.
Acknowledgements
Mr.A. Manimaran extends sincere thanks to ICMR, New Delhi for providing financial assistance to carry out this research work.
References
- 1.Antico Arciuch V.G, Elguero M.E, Poderoso J.J, Carreras M.C. Mitochondrial regulation of cell cycle and proliferation. Antioxid Redox Signal. 2012;16(10):1150–1180. doi: 10.1089/ars.2011.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Au Yeung C.L, Tsang W.P, Tsang T.Y, Co N.N, Yau P.L, Kwok T.T. HPV-16 E6 upregulation of DNMT1 through repression of tumor suppressor p53. Oncol Rep. 2010;24:1599–1604. doi: 10.3892/or_00001023. [DOI] [PubMed] [Google Scholar]
- 3.Baykul T, Yilmaz H.H, Aydin U, Aydin M.A, Aksoy M, Yildirim D. Early diagnosis of oral cancer. J Int Med Res. 2010;38(3):737–49. doi: 10.1177/147323001003800302. [DOI] [PubMed] [Google Scholar]
- 4.Bobrovnikova M.E, Grigoriadou C, Pytel D, Zhang F, Ye J, Koumenis C, Diehl J.A. PERK promotes cancer cell proliferation and tumor growth by limiting oxidative DNA damage. Oncogene. 2010;29(27):3881–3895. doi: 10.1038/onc.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradford MM. A rapid and sensitive for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1):248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 6.Carnelio S, Rodrigues G.S, Shenoy R, Fernandes D. A brief review of common oral premalignant lesions with emphasis on their management and cancer prevention. Indian J Surg. 2011;73(4):256–261. doi: 10.1007/s12262-011-0286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casto B.C, Knobloch T.J, Galioto R.L, Yu Z, Accurso B.T, Warner B.M. Chemoprevention of oral cancer by lyophilized strawberries. Anticancer Res. 2013;33(11):4757–4766. [PMC free article] [PubMed] [Google Scholar]
- 8.Chang K.Y, Tsai S.Y, Chen S.H, Tsou H.H, Yen C.J, Liu K.J, Chang J.Y. Dissecting the EGFR-PI3K-AKT pathway in oral cancer highlights the role of the EGFR variant III and its clinical relevance. J Biomed Science. 2013;20(1):43. doi: 10.1186/1423-0127-20-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chappell W.H, Steelman L.S, Long J.M, Kempf R.C, Abrams S.L, Franklin R.A, McCubrey J.A. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget. 2011;2(3):135–164. doi: 10.18632/oncotarget.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Zhang X, Lu Y, Shim J.Y, Sang S, Sun Z, Chen X. Chemoprevention of 7,12-dimethylbenz[a]anthracene (DMBA)-induced Hamster Cheek Pouch Carcinogenesis by a 5-Lipoxygenase Inhibitor. Garcinol. Nutr Cancer. 2012;64(8):1211–1218. doi: 10.1080/01635581.2012.718032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui Y, Lu P, Song G, Liu Q, Zhu D, Liu X. Involvement of PI3K/Akt, ERK and p38 signaling pathways in emodin-mediated extrinsic and intrinsic human hepatoblastoma cell apoptosis. Food Chem Toxicol. 2016;92:26–37. doi: 10.1016/j.fct.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Delpu Y, Cordelier P, Cho W.C, Torrisani J. DNA methylation and cancer diagnosis. Int J Mol Sci. 2013;14(7):15029–15058. doi: 10.3390/ijms140715029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhillon A.S, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26(22):3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 14.El Rhazi K, Bennani B, El Fakir S, Boly A, Bekkali R, Zidouh A, Nejjari C. Public awareness of cancer risk factors in the Moroccan population: a population-based cross-sectional study. BMC Cancer. 2014;14:695. doi: 10.1186/1471-2407-14-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estève P.O, Zhang G, Ponnaluri V.K.C, Deepti K, Chin H.G, Dai N, Pradhan S. Binding of 14-3-3 reader proteins to phosphorylated DNMT1 facilitates aberrant DNA methylation and gene expression. Nuc Acids Res. 2016;44(4):1642–1656. doi: 10.1093/nar/gkv1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gan Y, Shi C, Inge L, Hibner M, Balducci J, Huang Y. Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells. Oncogene. 2010;29(35):4947–58. doi: 10.1038/onc.2010.240. [DOI] [PubMed] [Google Scholar]
- 17.Gerthoffer W.T, Schaafsma D, Sharma P, Ghavami S, Halayko A.J. year not correspond Motility, survival and proliferation. Compr Physiol. 2012;2(1):255–281. doi: 10.1002/cphy.c110018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta B, Ariyawardana A, Johnson N.W. Oral cancer in India continues in epidemic proportions: evidence base and policy initiatives. Int Dent J. 2013;63(1):12–25. doi: 10.1111/j.1875-595x.2012.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong B, Li H, Zhang M, Xu J, Lu Y, Zheng Y, Yi Q. p38 MAPK inhibits breast cancer metastasis through regulation of stromal expansion. International Journal of Cancer. J Int Du Cancer. 2015;136(1):34–43. doi: 10.1002/ijc.28958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang F.J, Hsuuw Y.D, Chan W.H. Characterization of Apoptosis Induced by Emodin and Related Regulatory Mechanisms in Human Neuroblastoma Cells. Int J Mol Sci. 2013;14(10):20139–20156. doi: 10.3390/ijms141020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang P.H, Huang C.Y, Chen M.C, Lee Y.T, Yue C.H, Wang H-Y, Lin H. Emodin and aloe-emodin suppress breast cancer cell proliferation through ERa inhibition. Evid Based Complement Alternat Med. 2013:e376123. doi: 10.1155/2013/376123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ismail T, Calcabrini C, Diaz A. R, Fimognari C, Turrini E, Catanzaro E, Sestili P. Ellagitannins in cancer chemoprevention and therapy. Toxins. 2016;8(5):e151. doi: 10.3390/toxins8050151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang B.H, Liu L.Z. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv Cancer Res. 2009;102:19–65. doi: 10.1016/S0065-230X(09)02002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin B, Robertson K. D. DNA Methyltransferases (DNMTs), DNA damage repair, and cancer. Adv Exp Med Biol. 2013;754:3–29. doi: 10.1007/978-1-4419-9967-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin B, Li Y, Robertson K.D. DNA methylation: superior or subordinate in the epigenetic hierarchy? Genes Cancer. 2011;2(6):07–617. doi: 10.1177/1947601910393957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalimuthu S, Se-Kwon K. Cell survival and apoptosis signaling as therapeutic target for cancer: Marine bioactive compounds. Int J Mol Sci. 2013;14(2):2334–2354. doi: 10.3390/ijms14022334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khyani I.A.M, Qureshi M.A, Mirza T, Farooq M.U. Salivary detection of human Papilloma virus 16 &18 in pre-malignant and malignant lesions of oral cavity: Is it feasible in Pakistani context of socio-cultural taboos. PaK J Med Sci. 2015;31(5):1104–1109. doi: 10.12669/pjms.315.7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim M.S, Park M.J, Kim S.J, Lee C.H, Yoo H, Shin SH, Song E.S, Lee SH. Emodin suppresses hyaluronic acid-induced MMP-9 secretion and invasion of glioma cells. Int J Oncol. 2005;27(3):839–846. [PubMed] [Google Scholar]
- 29.Lee A.S. Glucose regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nature Rev. Cancer. 2014;14(4):263–276. doi: 10.1038/nrc3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang G, Chan M.F, Tomigahara Y, Tsai Y.C, Gonzales F.A, Li E, Jones P.A. Cooperativity between DNA methyltransferases in the maintenance methylation of repetitive elements. Mol Cell Biol. 2002;22(2):480–491. doi: 10.1128/MCB.22.2.480-491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin S.Z, Wei W.T, Chen H, Chen K.J, Tong H.F, Wang Z.H, Liu D.L. Antitumor activity of emodin against pancreatic cancer depends on its dual role: promotion of apoptosis and suppression of angiogenesis. PLoS ONE. 2012;7(8):e42146. doi: 10.1371/journal.pone.0042146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin W, Zhong M, Yin H, Chen Y, Cao Q, Wang C, Ling C. Emodin induces hepatocellular carcinoma cell apoptosis through MAPK and PI3K/AKT signaling pathways in vitro and in vivo. Onco Rep. 2016;36(1):961–967. doi: 10.3892/or.2016.4861. [DOI] [PubMed] [Google Scholar]
- 33.Liu C. Inhibition of mechanical stress-induced hypertrophic scar inflammation by emodin. Mol Med Rep. 2015;11(6):4087–4092. doi: 10.3892/mmr.2015.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manoharan S, Karthikeyan S, Essa M.M, Manimaran A, Selvasundram R. An overview of oral carcinogenesis. Int J Nutr Pharmacol Neurol Dis. 2016;6:51–62. [Google Scholar]
- 35.Manoharan S, Rajasekaran D, Prabhakar M. M, Karthikeyan S, Manimaran A. Modulating Effect of Enicostemma littorale on the Expression Pattern of Apoptotic, Cell Proliferative, Inflammatory and Angiogenic Markers During 7, 12-Dimethylbenz (a) Anthracene Induced Hamster Buccal Pouch Carcinogenesis. Toxicol Int. 2015;22(1):130–140. doi: 10.4103/0971-6580.172276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Markopoulos A.K. Current aspects on oral squamous cell carcinoma. Open Dent J. 2012;6:126–130. doi: 10.2174/1874210601206010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuoka T, Yashiro M. The role of PI3K/Akt/mTOR signaling in gastric carcinoma. Cancers. 2014;6(3):1441–1463. doi: 10.3390/cancers6031441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death is subcellular localization the answer? Cell Cycle. 2009;8(8):1168–1175. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munshi A, Ramesh R. Mitogen-activated protein kinases and their role in radiation response. Genes Cancer. 2013;4(9-10):401–408. doi: 10.1177/1947601913485414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olsen B.B, Bjørling-Poulsen M, Guerra B. Emodin negatively affects the phosphoinositide 3-kinase/AKT signalling pathway: a study on its mechanism of action. Int J Biochem Cell Biol. 2007;39(1):227–237. doi: 10.1016/j.biocel.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Pan F.P, Zhou H.K, Bu H.Q, Chen Z.Q, Zhang H, Xu L.P, Chen L. Emodin enhances the demethylation by 5-Aza-CdR of pancreatic cancer cell tumor-suppressor genes P16, RASSF1A and ppENK. Oncol Rep. 2016;35(4):1941–1949. doi: 10.3892/or.2016.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patil P.B, Bathi R, Chaudhari S. Prevalence of oral mucosal lesions in dental patients with tobacco smoking, chewing, and mixed habits: A cross-sectional study in South India. J Family Community Med. 2013;20(2):130–135. doi: 10.4103/2230-8229.114777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schetter A.J, Heegaard N. H. H, Harris C. C. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31(1):37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shtivelman E, Beer T. M, Evans C. P. Molecular pathways and targets in prostate cancer. Oncotarget. 2014;5(17):7217–7259. doi: 10.18632/oncotarget.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skowronski K, Dubey S, Rodenhiser D, Coomber B.L. Ischemia dysregulates DNA methyltransferases and p16INK4a methylation in human colorectal cancer cells. Epigenetics. 2010;5(6):547–556. doi: 10.4161/epi.5.6.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sridharan G. Epidemiology, control and prevention of tobacco induced oral mucosal lesions in India. Indian J Cancer. 2014;51(1):80–85. doi: 10.4103/0019-509X.134651. [DOI] [PubMed] [Google Scholar]
- 47.Steele V.E, Lubet R.A. The use of animal models for cancer chemoprevention drug development. Semin Oncol. 2010;37(4):327–338. doi: 10.1053/j.seminoncol.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su Y.T, Chang H.L, Shyue S.K, Hsu S.L. Emodin induces apoptosis in human lung adenocarcinoma cells through a reactive oxygen species-dependent mitochondrial signaling pathway. Biochem Pharmacol. 2005;70(2):229–41. doi: 10.1016/j.bcp.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 49.Sui J.Q, Xie K.P, Zou W, Xie M.J. Emodin inhibits breast cancer cell proliferation through the ERa-MAPK/Akt-cyclin D1/Bcl-2 signaling pathway. Asian Pac J Cancer Prev. 2014;15(15):6247–6251. doi: 10.7314/apjcp.2014.15.15.6247. [DOI] [PubMed] [Google Scholar]
- 50.Vigneswaran N, Williams M.D. Epidemiological trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26(2):123–141. doi: 10.1016/j.coms.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Villanueva J, Yung Y, Walker J.L, Assoian R. K. ERK activity and g1 phase progression: Identifying dispensable versus essential activities and primary versus secondary targets. Mol Biol Cell. 2007;18(4):1457–1463. doi: 10.1091/mbc.E06-10-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wagner E.F, Nebreda A.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9(8):537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 53.Way T.D, Huang J.T, Chou C.H, Huang C.H, Yang M.H, Ho C.T. Emodin represses TWIST1-induced epithelial-mesenchymal transitions in head and neck squamous cell carcinoma cells by inhibiting the β-catenin and Akt pathways. Eur J Cancer. 2014;50(2):366–378. doi: 10.1016/j.ejca.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 54.Wei W.T, Chen H, Ni Z.L, Liu H.B, Tong H.F, Fan L, Liu A, Qiu M.X, Liu D.L, Guo H.C, Wang Z.H, Lin S.Z. Antitumor and apoptosis-promoting properties of emodin, an anthraquinone derivative from Rheum officinale Baill, against pancreatic cancer in mice via inhibition of Akt activation. Int J Oncol. 2011;39(6):1381–1390. doi: 10.3892/ijo.2011.1147. [DOI] [PubMed] [Google Scholar]
- 55.Yang F, Yuan P, Hao Y.Q, Lu Z.M. Emodin enhances osteogenesis and inhibits adipogenesis. BMC Complement Altern Med. 2014;14:e74. doi: 10.1186/1472-6882-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yaoxian W, Hui Y, Yunyan Z, Yanqin L, Xin G, Xiaoke W. Emodin induces apoptosis of human cervical cancer hela cells via intrinsic mitochondrial and extrinsic death receptor pathway. Cancer Cell Int. 2013;13:e71. doi: 10.1186/1475-2867-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang H, Chen L, Bu H.Q, Yu Q.J, Jiang D.D, Pan F.P, Wang Y, Liu D.L, Lin S.Z. Effects of emodin on the demethylation of tumor-suppressor genes in pancreatic cancer PANC-1 cells. Oncol Rep. 2015;33(6):3015–3023. doi: 10.3892/or.2015.3914. [DOI] [PubMed] [Google Scholar]
- 58.Zhao X.Y, Qiao G.F, Li B.X, Chai L.M, Li Z, Lu Y.J, Yang B.F. Hypoglycaemic and hypolipidaemic effects of emodin and its effect on L-type calcium channels in dyslipidaemic-diabetic rats. Clin Exp Pharmacol Physiol. 2009;36(1):29–34. doi: 10.1111/j.1440-1681.2008.05051.x. [DOI] [PubMed] [Google Scholar]
- 59.Zheng H.Y, Lin W.Q, Hu J.D, Lin M.H, Xie L.J. Emodin Induces Apoptosis of K562/Adr Cells Probably through Akt-Caspase 3 Signal Pathway. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2015;23(6):1556–1559. doi: 10.7534/j.issn.1009-2137.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 60.Zu C, Zhang M, Xue H, Cai X, Zhao L, He A, Zheng X. Emodin induces apoptosis of human breast cancer cells by modulating the expression of apoptosis-related genes. Oncol Lett. 2015;10(5):2919–2924. doi: 10.3892/ol.2015.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]


