Abstract
Background and objective:
The purpose of the current investigation was to study the influences, preventive and diuretic, of Nigella sativa L. seeds oil (NSSO) on calcium oxalate (CaOx) urolithiasis induced in Wistar male rats.
Methodology:
Seeds of Nigella sativa L. (N.S) were analysed for the evaluation of the concentration of oxalate and calcium. Nigella sativa L. seeds oil is obtained by hydrodistillation and HPTLC densitometric method was adopted to determine the amount of thymoquinone (TQ) present.
Thirty male Wistar rats were divided into 5 groups (N=6). Group I, negative control, drank tap water. The other groups were II Positive control, III, IV and V received a treatment model inducing calcium oxalate urolithiasis for 28 days, using an aqueous solution involve 0.75% (EG) ethylene glycol and 1.0 % (AC) chloride ammonium. Rats in group III received in addition, 750 mg/kg Cystone from the beginning to the end of calculi induction experimentation.
However, rats in Groups IV and V received (NSSO) at 5 ml/kg b.w by gavage on days 1st to 28th and 15th to 28th days, respectively. On days 0, 7, 14, 21 and 28, body weights were measured and the 24-hour urine samples were accumulated and analysed for biochemical elements. On the 28th day, blood samples were collected for the estimation of serum parameters including creatinine, BUN and uric acid.
All animals were sacrificed at the end of the experiment and the kidneys were detached for histopathological examination.
Results:
Administration of (NSSO) at 5 ml/kg body weight/dose/day for 28 days exerts a protective effect by reducing significantly (p <0.01) urinary and serum rates of calcium, phosphate and oxalate. This preventive diet could increase the volume of urine excreted.
Conclusion:
The nephroprotectrice and diuretic activity demonstrated by Nigella sativa L. gives a scientific basis that approves their traditional use like a remedy against urolithiasis.
List of Abbreviations: NSSO: Nigella sativa L. Seeds oil ; CaOx: Calcium Oxalate; N.S: Nigella sativa L.; HPTLC: High performance thin layer chromatography; TQ: Thymoquinone; N: Number; EG: Ethylene Glycol; AC: Chloride Ammonium BUN: Blood Urea Nitrogen; LD50: Lethal Dose 50; b.w: body weight; H & E: Haematoxyline and Eosin; HPLC-UV:;Caph: calcium phosphate; FR: glomerular filtration rate
Keywords: Renal stones, Nigella sativa L, Ethylene glycol, Calcium oxalate, Wistar rat
Introduction
Urolithiasis has been known since ancient times and that appear inseparable from the history of Humanity (Daudon et al. 2008). However, over the 25 years past, significant changes have been felt in developing countries, so that today, stones became essentially kidney and calcium oxalate became the main component of a most calculations in most countries of the world (Daudon et al. 2004). This condition affects 13% of the male population and 6% of women, 50% of this population are exposed to recurrent lithiasic (Bihl and Meyers 2001). Thus, the non-surgical therapeutic indications are increasingly being used to prevent recurrence of urinary stones.
Several products from medicinal plants have been tested in urinary calculi therapy for centuries (Laroubi et al. 2007; Atmani et al. 2004). Historically, most therapies are derived from natural products, but there has been an abandonment of their use with the progress and before dominance of molecular approaches for the benefit of drug discovery (Harvey 1999). Among natural sources most used in chemotherapy or chemoprevention include Nigella sativa L. (Ranunculaceae) (Vihan and Panwar 1987). It is a picturesque plant planted in different places of the world. There seeds, also known as black cumin, are generally used in the Middle East, India and North Africa for medicinal purposes as a natural remedy against a number of diseases including bronchial asthma, rheumatism, hypertension, diabetes, inflammation, cough, headaches, eczema, fever and flu (Burits and Bucar 2000; Salem 2005). Thymoquinone (TQ) is the most important component pharmacologically present in the essential oil of these seeds (Amm et al. 2011), with an important antioxidant power (Rifaioglu et al. 2013) which allows it to keep many organs opposed to oxidative damage beget by various free radical (Yaman and Balikci 2010). Having regard to the low toxicity of its oil highlighted by high values LD50 = 28.8 ml/kg (Ghedira and Le jeune 2010), this suggests a significant therapeutic range. Many in vitro and in vivo studies evaluating its biological activity, including immune-potentiation, anti-tumor, anti-inflammatory, analgesic, anti-hypertensive, anti-diabetic stimulation, anti-ulcerogenic, antibacterial, antifungal have been reported (Kumar and Huat 2001; Al-Naggar et al. 2003).
The current investigation focuses on the evaluation of beneficial effects; preventive and diuretic of Nigella sativa L. seeds oil (NSSO) administered by gavage to male Wistar rats on calcium oxalate stones induced by ethylene glycol (EG) and ammonium chloride (AC).
Materials and methods
Plant material
We have used Nigella sativa L. (voucher number MPS2012/22) seeds that were collected in 2012 from the region of Slatna (Bordj Bouareridj, west of Algeria). The plant species is identified by Dr. A. Mokhebi and a sample was deposited at the herbarium of the Department of Biological Sciences of the University of Abdelhamid Benbadis Mostaganem, Algeria. Seeds were kept in the dark at 4 °C.
Preparation of extract of Nigella sativa L seeds
Dried seeds of Nigella sativa L. 100 g were ground and extracted by hydrodistillation at atmospheric pressure (4 h) and the essential oil obtained was dried by Na2SO4 (Hanen et al. 2007) and stored at 4 °C.
Analysis of Nigella sativa L seeds
Determination of the levels of calcium and oxalate:
The calcium level was determined by atomic absorption after mineralisation of N.S powder at 550 °C/12h. The ash obtained was dissolved in HNO3 (Falade et al. 2005). The oxalate level was analysed by the technique reported by Roswitha. S (Roswitha et al. 2006).
Quantitative determination of thymoquinone by HPTLC
The amount of thymoquinone in our sample was determined by densitometric method HPTLC (Prawez et al. 2013).
Animals
Thirty male Wistar rats healthy adults of approximately the same age weighing between 120 and 130 g, were obtained from Algiers Pasteur Institute, Algeria. Rats were acclimated in polypropylene cages with aseptic conditions and feeded with standard animal and water ad libitum, they were housed in a single temperature controlled 20-25 °C cage, at relative humidity of 50-55%, in 12h/12h dark/light cycle. All procedures were performed according with ethical guidelines for care and use of the animals laboratory, and they were allowed by the care of Experimental Animals Committee (MESRS/IVST/CEA/12/27). Rats were arbitrarily sectioned into five groups of six rats each.
Ethylene glycol and ammonium chloride induced urolithiasis Model
Kidney stones were induced by ethylene glycol and ammonium chloride in rats (Fan et al., 1999). Group I served as control group (negative control), received regular rat food and drinking water ad libitum. All the remaining groups (II -V) received 0.75 % ethylene glycol (EG) (E-Merk, Germany) and 1.0 % ammonium chloride (CA) (E-Merk, Germany) in drinking water for 28 days, of which for 3 days comprised of (EG + AC) to accelerate lithiasis followed by only (EG) for 25 days. Group III received in addition Cystone at 750 mg/kg b.w during the experimental test period (Mitra et al. 1998). Rats in Groups IV and V have received NSSO daily by gavage at dose equivalent to 5 ml/kg b.w (El-Abhar et al. 2003), from 1st and 15th to 28th day, respectively.
Body Weight
Body weights were measured on days 0, 7, 14, 21 and 28. Evaluations of body weights (BWe) were determined using the formula (Bouanani et al. 2010):
BWe = (BWd0 – BWds) / BWd0
BWe: Body weight evaluation on days (0, 7, 14, 21 and 28)
BWd0: Body weight on the first day (day o)
BWds: Body weight on days (0, 7, 14, 21 and 28)
Collection and urinalysis:
Twenty-four hours urine samples were collected and measured on days 0, 7, 14, 21 and 28 using individual metabolic cages. At the same time, oxalate, phosphate, uric acid, calcium and magnesium were determined using commercially accessible test kits (Sigma-Aldrich, USA) (Rajagopal 1984; Sriboonlue et al. 1998).
Serum analysis
On day 28, sanguine fluid was accumulated from the retro-orbital sinus under anaesthetised condition and the serum was recovered by centrifugation at 10,000 g /10 minutes and analysed for uric acid, BUN and creatinine using an automated analyser (Model 705, Hitachi, Tokyo, Japan).
Renal Histology
At the experiment termination, the right and left kidneys were removed and used as a support for histological and biochemical examination respectively. The right renal (excretory organ) was put in formalin (10%), then in a range of ranked alcohol and xylene concentration, inserted in paraffin and cut to section of 5 micrometer in thickness, treated with Haematoxyline and Eosin (H & E) for histopathological microscope examination. Slides were analysed under optical microscope for calcium oxalate deposits (Hadjzadeh et al. 2007). The left renal (excretory organ) was chopped and 20% of mixed was prepared in Tris-HCl buffer (0.02 mol/l, pH=7.4). Left renal mixed was used to determine the rate of calcium (Chow et al. 1975), phosphate (Medeiros and Mustafa 1985) and oxalate (Hodgkinson and Williams 1972).
Statistical analysis
All results were expressed as mean ± standard deviation. Statistical analysis of data was performed by ANOVA, followed by Dunnett’s Comparison Test, (p <0.05) was considered significant. Statistical package for Social Sciences (SPSS 12.0) was used for this analysis.
Results
Seeds Analysis of Nigella sativa L
The oil yield and concentration of calcium and oxalate existing in Nigella sativa L. seeds are presented in Table 1. All results are shown by an average of three measurements for each sample.
Table 1.
Chemical characteristics of Nigella sativa seeds
The values are expressed as mean±S.E.M of three determinations.
In (%) dry matter.;
(mg/Kg) dry matter.
Determination of the amount of thymoquinone by HPTLC
After the hydrodistillation of Nigella sativa L. seeds, a yellowish volatile oil was obtained and characterised by a spicy odour. The oil extract was the subject of the analysis. The method of HPTLC revealed thymoquinone (TQ) (Fig. 1) rate of 2.70 %, corresponding to an RF value of 0.48 (Fig. 2).
Figure 1.
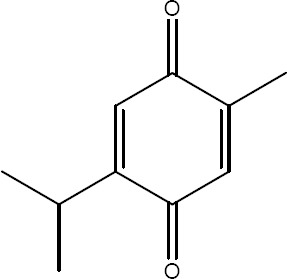
Chemical structure of thymoquinone (TQ)
Figure 2.
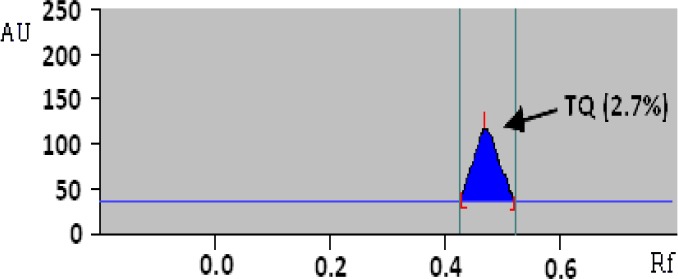
HPTLC chromatogram of Nigella sativa seeds essential oil
Body weight
Initially before the test start, the body weight of different rats in all groups have no significant difference (from 124.66 ± 3.14 g to 128.33 ± 2.94 g) Table 2. From the first week of the test, rats of Groups II and V have shown a significant decrease (p <0.01) with loss rates of 7.19 % and 10.95 % respectively. However, during the four weeks of experiment, there was an increase in body weight of the rats in Groups III and IV treated with Cystone and NSSO (p <0.01) with a growth rate of 59.22 % and 32.53 % respectively.
Table 2.
Effect of Nigella Sativa seeds oil on the body weight of wistar rats (g).
| days | group I | group II | group III | group IV | group V |
|---|---|---|---|---|---|
| 0 | 126,33 ± 4,03 | 127,33 ± 3,50 | 128,33 ± 2,94 | 125,50 ± 3,93 | 124,66 ± 3,14 |
| 7 | 163,00 ± 5,83 | 118,17 ± 1,94 a** | 149,00 ± 5,44 a*b** | 124,50 ± 3,51 a** | 111,00 ± 3,03 a** |
| 14 | 186,00 ± 5,73 | 110,17 ± 2,99 a** | 169,83 ± 6,58 a**b** | 127,17 ± 4,44 a**b* | 102,33 ± 2,80 a** |
| 21 | 207,67 ± 5,00 | 100,83± 2,04 a** | 188,33 ± 5,82 a**b** | 145,67 ± 4,54 a**b** | 102,17 ± 3,49 a** |
| 28 | 234,17 ± 7,98 | 94,67 ± 2,87 a** | 204,33 ± 3,77 a**b** | 166,33 ± 4,03 a**b** | 109,33 ± 1,86 a**b* |
The values are gived as mean±S.E.M. (n = 6 rats/group).
Comparisons are made with group I.;
Comparisons are made with group II.
p < 0.05.;
p < 0.01.;
***p < 0.001.
Urinary output
At 28th days, Curative group (IV) recorded the higher volume 27.83 ± 2.31 ml (p <0.01) compared with the control (-) group I. However, these animals (group I) have excreted a volume of 9.33 ± 1.0 ml/day on the last test day (Table 3) and which has eleveted significantly (p <0.01) to 19.67 ± 0 81 ml/day in the group (II) where rats are rendered lithiasic with a diet of EG + AC.
Table 3.
Effect of Nigella Sativa seeds oil on urinary output in different groups of Wistar rats (ml). daysgroup Igroup IIgroup IIIgroup IVgroup V
| days | group I | group II | group III | group IV | group V |
|---|---|---|---|---|---|
| 0 | 08,00 ± 1,09 | 07,00 ± 0,89 | 07,83 ± 0,75 | 07,17 ± 0,75 | 06,83 ± 0,75 |
| 7 | 07,83 ± 1,17 | 12,33 ± 0,82a** | 17,00 ± 0,89 a**b* | 14,50 ± 2,59 a** | 12,33 ± 0,51 a** |
| 14 | 09,00 ± 0,63 | 17,00 ± 0,55 a** | 19,16 ± 0,75 a** | 18,67 ± 2,16 a** | 18,00 ± 0,89 a** |
| 21 | 09,83 ± 0,75 | 18,50 ± 1,05 a** | 21,83 ± 0,75 a** | 24,33 ± 2,42 a** | 20,00 ± 2,28 a** |
| 28 | 09,33 ± 1,03 | 19,67 ± 0,81 a** | 23,33 ± 1,03 a** | 27,83 ± 2,31 a**b** | 21,83 ± 2,31 a** |
The values are gived as mean±S.E.M. (n = 6 rats/group).
Comparisons are made with group I.;
Comparisons are made with group II.
Asterisks: significant;
p < 0.05.;
p < 0.01.;
*** p < 0.001.
Biochemical analysis of urine
Table 4 shows the details of 24h-urine excreted and its oxalate, calcium, phosphate, magnesium and uric acid content in different rats of different groups. Rats in group II, which received for four weeks ethylene glycol 0.75 % regime were marked by a significant hyperoxaluria (P <0.01) compared to rats in control group (-). Similarly, phosphate and uric acid levels present in 24h-urine excreted increased significantly (P <0.01) while urinary calcium and magnesium have dropped as compared to group I. However, for a curative and preventive treatment with NSSO, oxalate, phosphate and uric acid urinary levels excreted were significantly reduced (P< 0.01). These results are comparable to those recorded in rats of group treated with Cystone (group III).
Table 4.
Effect of Nigella Sativa seeds oil on urinary Magnesium, Uric acid, oxalate, calcium and phosphate levels in rats with induced urolithiasis (mg/24 h).
| days | group I | group II | group III | group IV | group V |
|---|---|---|---|---|---|
| Oxalate | |||||
| 0 | 05,21 ± 0,15 | 05,08 ± 0,08 | 05,03 ± 0,10 | 05,11 ± 0,09 | 05,06 ± 0,08 |
| 7 | 05,24 ± 0,06 | 06,75 ± 0,06 a** | 05,36 ± 0,06 b** | 05,49 ± 0,10 b** | 06,68 ± 0,09 a** |
| 14 | 05,26 ± 0,10 | 08,25 ± 0,07 a** | 05,62 ± 0,09 b** | 05,88 ± 0,08 a*b** | 08,16 ± 0,11 a** |
| 21 | 05,22 ± 0,03 | 10,17 ± 0,12 a** | 05,92 ± 0,08 a*b** | 06,42 ± 0,09 a**b** | 08,87 ± 0,06 a**b** |
| 28 | 05,23 ± 0,08 | 11,82 ± 0,07 a** | 06,30 ± 0,10 a**b** | 06,98 ± 0,07 a**b** | 09,27 ± 0,12 a**b** |
| Phosphate | |||||
| 0 | 05,87 ± 0,12 | 05,99 ± 0,06 | 05,87 ± 0,08 | 05,88 ± 0,09 | 05,91 ± 0,09 |
| 7 | 05,85 ± 0,16 | 06,38 ± 0,06 a** | 06,02 ± 0,07 b* | 05,96 ± 0,08 b** | 06,28 ± 0,03 a* |
| 14 | 05,93 ± 0,06 | 06,81 ± 0,07 a** | 05,93 ± 0,06 b** | 06,05 ± 0,11 b** | 06,81 ± 0,05 a** |
| 21 | 05,95 ± 0,08 | 07,37 ± 0,11 a** | 05,90 ± 0,11 b** | 06,07 ± 0,08 b** | 06,96 ± 0,06 a**b** |
| 28 | 05,87 ± 0,09 | 07,99 ± 0,09 a** | 05,92 ± 0,06 b** | 06,16 ± 0,10 a*b** | 07,00 ± 0,07 a**b** |
| Calcium | |||||
| 0 | 00,69 ± 0,03 | 00,56 ± 0,05 | 00,68 ± 0,03 | 00,61 ± 0,08 | 00,53 ± 0,05 |
| 7 | 00,71 ± 0,03 | 00,34 ± 0,07 a** | 00,55 ± 0,02 a*b* | 00,48 ± 0,06 a**b* | 00,32 ± 0,09 a** |
| 14 | 00,67 ± 0,03 | 00,24 ± 0,08 a** | 00,56 ± 0,07 a*b** | 00,41 ± 0,06 a**b** | 00,28 ± 0,05 a** |
| 21 | 00,60 ± 0,07 | 00,21 ± 0,04 a** | 00,60 ± 0,10 b** | 00,39 ± 0,07 a**b** | 00,24 ± 0,08 a** |
| 28 | 00,66 ± 0,08 | 00,13 ± 0,04 a** | 00,61 ± 0,11 b** | 00,34 ± 0,08 a**b** | 00,21 ± 0,04 a**b* |
| Uric acid | |||||
| 0 | 01,39 ± 0,03 | 01,34 ± 0,06 | 01,41 ± 0,07 | 01,40 ± 0,04 | 01,41 ± 0,04 |
| 7 | 01,41 ± 0,04 | 01,68 ± 0,08 a* | 01,49 ± 0,08 | 01,52 ± 0,04 b* | 01,63 ± 0,05 a* |
| 14 | 01,40 ± 0,02 | 02,16 ± 0,06 a** | 01,62 ± 0,10 a*b** | 01,65 ± 0,03 a*b** | 01,82 ± 0,06 a**b** |
| 21 | 01,40 ± 0,05 | 02,47 ± 0,06 a** | 01,72 ± 0,07 a*b** | 01,78 ± 0,06 a*b** | 02,16 ± 0,07 a**b** |
| 28 | 01,42 ± 0,04 | 02,79 ± 0,09 a** | 01,79 ± 0,13 a*b** | 01,94 ± 0,08 a**b** | 02,02 ± 0,07 a**b** |
| Magnesium | |||||
| 0 | 02,77 ± 0,08 | 02,74 ± 0,12 | 02,88 ± 0,06 | 02,85 ± 0,05 | 02,81 ± 0,10 |
| 7 | 02,80 ± 0,07 | 02,36 ± 0,07 a** | 02,76 ± 0,04 b* | 02,71 ± 0,07 b** | 02,48 ± 0,09 a* |
| 14 | 02,81 ± 0,07 | 02,06 ± 0,06 a** | 02,69 ± 0,05 b** | 02,51 ± 0,08 a*b** | 02,35 ± 0,05 a**b* |
| 21 | 02,72 ± 0,07 | 01,80 ± 0,07 a** | 02,52 ± 0,06 a*b** | 02,37 ± 0,04 a**b** | 02,23 ± 0,04 a**b** |
| 28 | 02,81 ± 0,08 | 01,56 ± 0,05 a** | 02,40 ± 0,06 a**b** | 02,23 ± 0,06 a**b** | 02,11 ± 0,04 a**b** |
The values are gived as mean±S.E.M. (n = 6 rats/group).
Comparisons are made with group I.;
Comparisons are made with group II.
Asterisks: significant;
p < 0.05.;
p < 0.01.;
*** p < 0.001.
Serum analysis
Creatinine, uric acid and BUN serum rates are functioning kidney parameters. From this we evaluated the effect of NSSO on rats made lithiasic with EG and AC. Serum analysis on 28th day showed significant increase in these parameters (P <0.01) in rats with urolithiasis (Table 5. Group II) compared with control (-) group I while both groups preventive IV and curative V treated with NSSO reduced exhibited significant (P <0.01) decrease in serum BUN, uric acid and creatinine. Similarly, the results of group III are comparable to those of Groups IV and V.
Table 5.
Effect of Nigella Sativa seeds oil on serum parameters (creatinine, uric acid, BUN) in experimental rats (mg/dl).
| Parameters | group I | group II | group III | group IV | group V |
|---|---|---|---|---|---|
| Creatinine | 00,69 ± 0,04 | 01,10 ± 0,02 a** | 00,84 ± 0,04 a*b** | 00,94 ± 0,01 a*b* | 01,00 ± 0,02 a** |
| Uric acid | 0 1, 73 ± 0,05 | 03,81 ± 0,08 a** | 01,97 ± 0,04 a*b** | 02,13 ± 0,03 a** b** | 02,23 ± 0,03 a** b** |
| BUN | 33,21 ± 0,06 | 45,65 ± 0,29 a** | 36,72 ± 0,11 a*b** | 39,04 ± 0,12 a** b** | 41,23 ± 0,09 a** |
Biochemical analysis of renal homogenate
In group II, phosphate, calcium and oxalate rates deposited in renal tissue were notably elevated (P <0.01) in comparison to group I (Table 6). However, cure diet with 750 mg/kg Cystone and 5 ml/kg Nigella essential oil in curative as in preventive system reduced significantly (P <0.01) increased rates of phosphate, calcium and oxalate stored in renal tissue.
Table 6.
Effect of Nigella Sativa seeds oil on kidney parameters in differents groups of wistar rats (mg/g).
| Crystalline component | group I | group II | group III | group IV | group V |
|---|---|---|---|---|---|
| Oxalate | 01,41 ± 0,02 | 05,79 ± 0,11 a** | 01,69 ± 0,08 b** | 02,07 ± 0,09 a**b** | 02,25 ± 0,06 a**b** |
| Phosphates | 02,33 ± 0,03 | 03,76 ± 0,08 a** | 02,58 ± 0,07 b** | 02,80 ± 0,10 a*b* | 03,00 ± 0,05 a**b* |
| Calcium | 00,20 ± 0,02 | 00,45 ± 0,03 a** | 00,25 ± 0,05 b** | 00,29 ± 0,04 a* b** | 00,33 ± 0,10 a*b* |
The values are gived as mean±S.E.M. (n = 6 rats/group).
Comparisons are made with group I.;
Comparisons are made with group II.
Asterisks: significant;
p < 0.05.;
p < 0.01.;
*** p < 0.001.
Histopathology
Histopathological microscopique analysis of renal sections also reinforces the above results. Optical microscope has revealed to us the deposition of calcium oxalate crystals (CaOx) and consequently the resulting damage on the glomeruli and tubules with interstitial inflammation compared to renal texture of control rats (-) (Fig 3 and 4). Similarly, tubules exhibited accumulation of mature lymphocytes (Fig. 5) and the epithelium is flattened with focal vacuolar degeneration and necrosis of the epithelial lining of tubules, irregular crystals were present within the tubules, along the nephron and papillary level (Fig. 6). All these histological changes were restored with Cystone (Fig. 8) and with applying preventative regime with NSSO, these groups have shown a relatively normal renal histology with normal glomeruli and tubules compared to Group I, but with a slight edema of the tubular cells (Fig. 7) and a number of evidence of small-sized crystals in the tubules but lower than group II.
Figure 3.
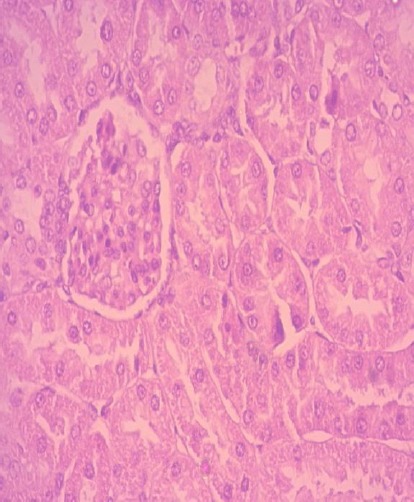
Normal renal parenchyma in negative control rats; (H&E x40).
Figure 4.
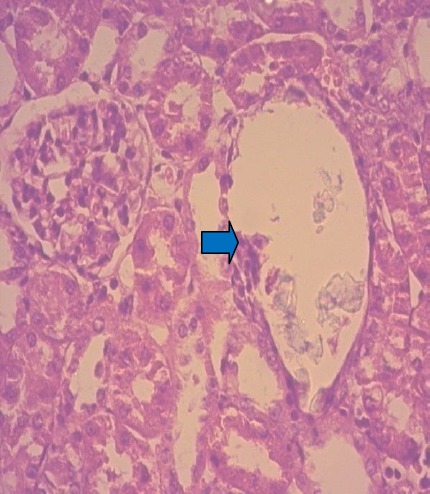
Damage of renal tubules and collecting system (blue arrowhead) of positive control rats; (H&E x40).
Figure 5.
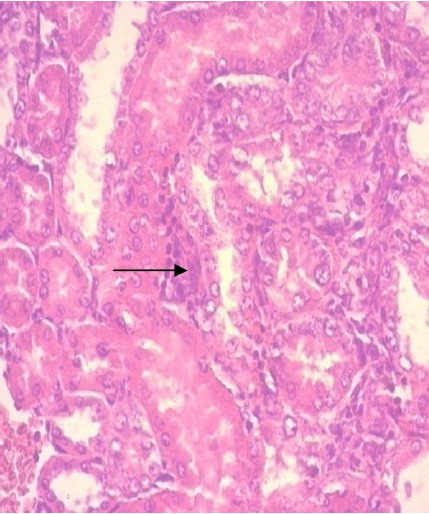
Tubules surrounded by inflammatory infiltration mainly lymphocytes (black arrow); (H&E x40).
Figure 6.
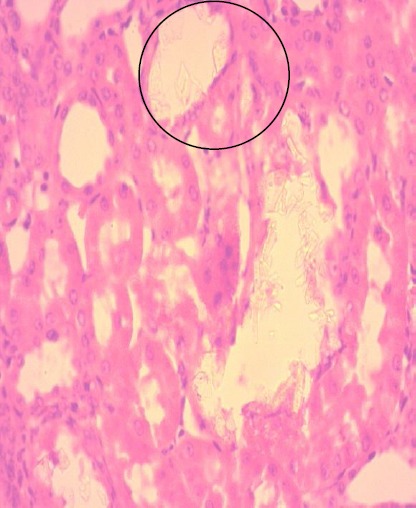
Flattened epithelium with focal vacuolar degeneration; (H&E x40).
Figure 8.
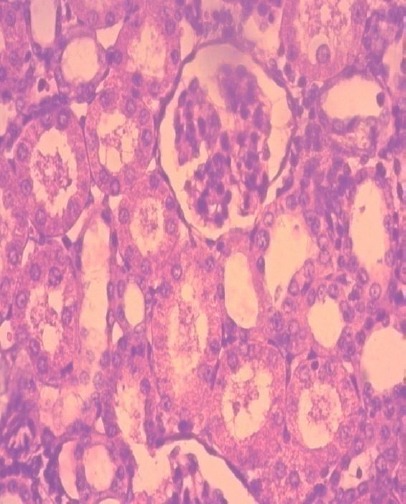
Almost recovery of renal parenchyma in rats treated with cystone; (H&E x40).
Figure 7.
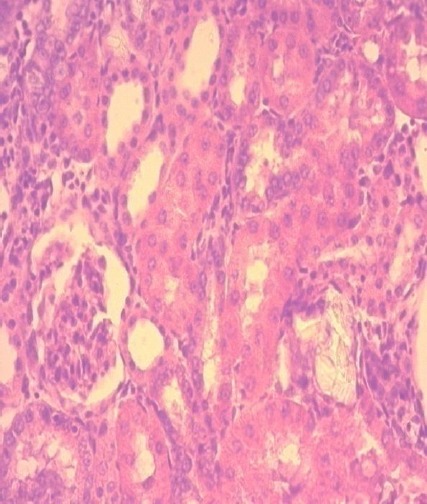
Normal renal histology with normal glomeruli and slight edema of tubular cells in rats treated with N. Sativa seeds oil; (H&E x40).
Discussion
In Algeria as in many other countries, many herbs have proven useful in traditional medicine for the prophylaxis and treatment of many diseases including urinary lithiasis. Nigella sativa L. (NS) is one of these herbs with a rich historical and religious background. There seeds are the source of active substances (Goreja 2003). The most challenging aspect is to provide base trace elements of plant material and obtain sufficient concentration that the supplement be ingested without consuming large amounts of plant tissue. As for minerals, N. sativa L. seeds, which we reported in (Table 1), appeared to involve useful concentration of calcium (536 mg/Kg); this amounts being comparable to that obtained with Tunisian (572 mg/Kg) and Iranian (564mg/Kg) varieties (Salma et al. 2007).
Many people from Indian subcontinent and Mediterranean region use N. sativa seeds oil constantly as protective and curative natural treatment. Many analyses to quantify N. sativa seeds composition approved that are very rich in volatile and fixed oils, proteins, amino acids, carbohydrates (Abdel-Aal and Attia 1993; Salem 2001; Takruri and Dameh 1998). Many of the pharmacological activities of this miraculous herb were attributed to thymoquinone (TQ). This one is the essential effective component of black cumin volatile oil. Recently, the presence of TQ in N. sativa L. seeds was confirmed using highly advanced methods; HPLC-UV (Goreja 2003) and HTPLC (Velho-Pereira et al. 2011). HPTLC is among the most recently employed methods described and used to quantify the most pharmacologically active component (TQ) (Prawez et al. 2013). The total volatile constituents extracted show the presence of a low percentage of thymoquinone (2.70 ± 0.05) %. These results are quite similar to those obtained by Farid for Algerian N. sativa L. (Farid et al. 2013) and Nickavar to that from Iran (Nickavar et al. 2003).
Since the urinary system of humans is similar to that of rats, they were chosen to induce urolithiasis (Liu et al. 2007), thus, recent studies have shown that the amount of calculations deposition in female rats was less significant than in male (Karadi et al. 2006). This model was approved to trigger urolithiasis kind of CaOx kind by calcium oxalate crystallisation and the high oxalate rate in the nephron damage the epithelial cells. However, crystals aggregation can begin after heterogeneous crystal nucleation (Scheid et al. 2004; Thamilselvan et al. 2003). Therefore, we investigated the effect of Nigella sativa L. seeds oil against EG and AC generates CaOx urinary calculi formation in Wistar male rats. Urinary stone formation takes place because of the changes in urine chemical composition, as hypercalciuria and hyperoxaluria that lead to concentrated urine, which subsequently finish up in calculi formation (Pak and Resnick 2000). Evidences in previous studies have shown that, in response to 14 days period of (0.75 % v/v) ethylene glycol and (1.0 %) of ammonium chloride administration, young albino rats form urinary stones composed mainly of CaOx. Generally, in mammals and by enzymatic medium, ethylene glycol is decomposed in vivo into four organic acids; glycolaldehyde, glycolic acid, glycooxalique acid and oxalic acid beginning to hyperoxaluria which is the essential factor of urolithiasis formation, composed mainly of oxalate (Leth and Gregersen 2005). The enzyme disturbances are the cause factors for the idiopathic hyperoxaluria; while, the defective intestinal absorption of oxalate plays an vital role in enteric hyperoxaluria and leads to an increase in urinary oxalate concentration (Williams and Wandzilak 1989). In the present study, chronic administration of 0.75% ethylene glycol and 1.0% ammonium chloride dissolved in drinking water to Wistar male rats resulted in hyperoxaluria. However, oxalate and calcium excretion in urine has significantly increased. It is accepted that high level in oxalate is more markedly risk parameter in the induction of kidney stones than hypercalciuria, so levels of urinary oxalate are comparatively more important than calcium (Divakar et al. 2010). Oxalate has a crucial role in calculi formation and has about 15 fold higher effect than urinary calcium (Borghi et al. 1996).
Urinary calcium levels increases are promoter factor of nucleation and precipitation of CaOx or apatite (Caph) and later crystals aggregation (Leemann et al. 1991). Deficient renal tubular reabsorption system may be caused by the elevation in calcium urinary excretion and its deposition in kidney (Hautmann and Lehamnn 1980) or an increase in absorption from the intestine as in patients with kidney stones calcium are reported to have a calcium hyperabsorption (Pak et al. 1974). However, Nigella sativa L. seeds oil decreased urinary levels of oxalate and calcium and even their retention in kidneys.
During the whole treatment period, body weight of control rats (-), treated with Cystone and who have undergone preventive regime with Nigella sativa L. seeds oil has increased. However, a decrease down of body weight gain was detected in group IV rats, in comparison with Groups I and III rats. The action of Nigella sativa L. on lipid metabolism can explain these acts and influences. Oral and intraperitoneal administration of Nigella sativa L. fixed oil give the same results as previous (Zaoui et al. 2002; Alsaif 2008). Rats in group II showed a significant elevation in urinary phosphate was observed in. The urinary excretion growth of phosphate and oxalate seems to give an area adapted for stone formation by composing Caph crystals, which increase epitaxial CaOx retention (Karadi et al. 2006; Divakar et al. 2010). Treatment with N. sativa L. seeds oil restores urinary phosphate rate, thereby decreasing the risk of calculi formation. Increase in urinary uric acid elimination was also watched in urolithiasic rats. Literature reports that the high rate of uric acid excretion has been affirmed in urolithiasic rats. However, calcium oxalate solubility can react with uric acid and lowers the inhibitory activity of glycosaminoglycans (Selvam et al. 2001). Magnesium is one such well known inhibitor in stones formers. Magnesium can reduce the supersaturation of calcium oxalate and it can also decrease CaOx nucleation and crystallization (Selvam et al. 2001; Soundararajan et al. 2006). Urinary magnesium was significantly decreased in urolithiasic rats. Treatment with N. sativa L. seeds oil has restored magnesium excretion which has decreased the development of CaOx crystals in urolithiasic rats.
In urolithiasis, the glomerular filtration rate (GFR) reduces, due to the blockage to the excretion of urine by calculi in renal (excretory organ); Thus products, which contains nitrogen materials in particularly creatinine, uric acid and urea are stocked in blood (Ghodkar 1994). Moreover, lipid peroxidation and diminution in rates of antioxidant potential have been reported in the renal of rats enriched with diet inducing stones. For this, oxalate has been described to cause lipid peroxidation and kidney deterioration by operating with polyunsaturated fatty acids in cells tissus (Karadi et al. 2006; Ghodasara et al. 2010).
In stones induced rats, high serum levels of creatinine, uric acid and BUN indicate marked renal damage (Anil and Niraj 2015). Treatment with NSSO showed significant reduction of serum rates of these parameters and prevented lipid peroxidation (Badary et al. 2003).
However, in traditional medicine, NSSO is used as a diuretic (Sathish et al. 2010), hence, it accelerates the process of dissolving the preformed stones by curing and preventing the formation of new stones in the urinary system (Anil et al. 2012). Moreover, the histopathological examination of renal tissues confirmed the biochemical results reported, where treatment with N. sativa L. seeds oil prevented the degenerative changes in renal tissues induced by EG (Sayed-Ahmed and Nagi 2007). The markedly hight serum rates of BUN, uric acid and creatinine in urolithiasic rats were parameters indicative of marked necrosis of kidney epithelium and dilatation of proximal tubules with interstitial inflammation. In addition, in stones induced rats, there was damage in the last part of nephron and collecting system. High rates of urinary oxalate and even its deposition in renal (excretory organ) can cause the peroxidative deterioration of the kidney epithelium (Rathod et al. 2012), however, preventive and curative treatment with NSSO prevented lipid peroxidation induced by oxalate and caused regeneration of renal epithelium. Earlier studies described a large antioxidant and anti- inflammatory power of NSSO (Ragheb et al. 2009; Nagi and Mansour 2000; Mansour and Tornhamre 2004). This is why oil extract from Nigella may protect against CaOx crystal retention in kidneys and protecting hyperoxaluria engendered peroxidative deterioration to kidney tubular membranes.
Conclusion
In conclusion, the present data agree with the popular use of the oil isolated from Nigella sativa L. seeds for the treatment of urolithiasis. Thymoquinone is the main active compound found in Nigella sativa L. seeds, one of the most promising medicinal plants with many therapeutic effects. Among these effects, a potential treatment was confirmed by its ability to maintain kidney function, prevent the formation of urinary stones, reduce retention crystals in the kidney tissue and stimulate their excretion in urine. It also appears that the cure effect is less effective than preventive. The mechanism underlying this effect is probably controlled by an antioxidant.
Acknowledgments
This research was supported by STEVA research laboratory, MESRS, Algeria. The authors would like to acknowledge and give a big thank to the Department of Biology, Natural and Life Sciences Faculty, Abdelhamid Ben Badis University, Mostaganem, Algeria; Dr. A. Mokhebi for his help in the identification of plant material and Dr. D. Ait Saada and R. Chadli. We also thanks Dr. T. Benmahdi, from Regional Veterinary Laboratory, Mostaganem, Algeria, for helping in the preparation of histopathological slides.
References
- 1.Abdel-Aal E.S.M, Attia R.S. Characterization of black cumin (Nigella sativa)seeds. 2-Proteins. Alexandria Science Exchange. 1993;14:483–496. [Google Scholar]
- 2.Al-Naggar T.B, Gomez-Serranillos M.P, Carreto M.E, Villar A.M. Neuropharmacological activity of Nigella sativa L. extracts. Journal of Ethnopharmacology. 2003;88:63–68. doi: 10.1016/s0378-8741(03)00157-0. [DOI] [PubMed] [Google Scholar]
- 3.Alsaif M.A. Effect of Nigella sativa oil on impaired glucose tolerance and insulin insensitivity induced by high-fat-diet and turpentine induced-trauma. Pakistan Journal of Biological Sciences. 2008;11:1093–1098. doi: 10.3923/pjbs.2008.1093.1099. [DOI] [PubMed] [Google Scholar]
- 4.Ammel S.M, Gameil N.M, Shawky N.M, Nader M.A. Comparative evaluation of anti-inflammatory properties of thymoquinone and curcumin using an asthmatic murine model. Int. Immunopharmacology Journal. 2011;11:2232–2236. doi: 10.1016/j.intimp.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Anil T. Pawar, Gayatri D. Gaikwad, Kavita S. Metkari, Kiran A. Tijore, Jaydip V. Ghodasara, Bhanudas S. Kuchekar. Effect of Terminalia chebula fruit extract on ethylene glycol induced urolithiasis in rats. Biomedicine & Aging Pathology. 2012;2:99–103. [Google Scholar]
- 6.Anil T. Pawar, Niraj S. Vyawahare. Protective effect of standardized extract of Biophytum sensitivum against calcium oxalate urolithiasis in rats. Bulletin of Faculty of Pharmacy. 2015;53:161–172. [Google Scholar]
- 7.Atmani F, Slimani Y, Mimouni M, Aziz M, Hacht B, Ziyyat A. Effect of aqueous extract from Herniaria hirsuta L. on experimentally nephrolithiasic rats. Journal of Ethnopharmacology. 2004;95:87–93. doi: 10.1016/j.jep.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 8.Badary O.A, Taha R.A, Gamal el-Din A.M, Abdel-Wahab M.H. Thymoquinone is a potent superoxide anion scavenger. Drug Chem Toxicol. 2003;26:87–98. doi: 10.1081/dct-120020404. [DOI] [PubMed] [Google Scholar]
- 9.Bihl G, Meyers A. Recurrent renal stone disease. Advances in pathogenesis and clinical management. 2001;358:651–656. doi: 10.1016/S0140-6736(01)05782-8. [DOI] [PubMed] [Google Scholar]
- 10.Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A. Urinary volume, water and recurrence in idiopathic calcium nephrolithiasis. A 5 year randomized prospective study. Urol. J. 1996;155:839–843. [PubMed] [Google Scholar]
- 11.Bouanani S, Henchiri C, Migianu-Griffoni E, Aoufa N, Lecouvey M. Pharmacological and toxicological effects of Paronychia argentea in experimental calcium oxalate nephrolithiasis in rats. Journal of Ethnopharmacology. 2010;129:38–45. doi: 10.1016/j.jep.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 12.Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytotherapy Research. 2000;14:323–328. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 13.Chow F.C, Dysent I.M, Hamer D.W, Udall H.R. Control of oxalate urolithiasis by DL-alanine. Investigative Urology. 1975;13:113–116. [PubMed] [Google Scholar]
- 14.Daudon M, Bounxouei B, Santa Cruz F, Leite D.A, Silva S, Diouf B. Composition des calculs observes aujourd’hui dans les pays non industrialises. Progrès en urologie. 2004;14:1151–1161. [PubMed] [Google Scholar]
- 15.Daudon M, Traxer O, Lechevallier E, Saussine C. Epidemiologie des lithiases Urinaires. Progrès en urologie. 2008;18:802–814. doi: 10.1016/j.purol.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 16.Divakar K, Pawar A.T, Chandrasekhar S.B, Dighe S.B, Divakar G. Protective effect of the hydro-alcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rats. Food Chemistry and Toxicology. 2010;48:1013–1018. doi: 10.1016/j.fct.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 17.El-Abhar H.S, Abdallah D.M, Saleh S. Gastroprotective activity of Nigella sativa oil and its constituent, thymoquinone, against gastric mucosal injury induced by ischaemia/ reperfusion in rats. Journal of Ethnopharmacology. 2003;84:251–258. doi: 10.1016/s0378-8741(02)00324-0. [DOI] [PubMed] [Google Scholar]
- 18.Falade O.S, Otemuyiwa I.O, Oladipo A, Oyedapo O.O, Akinpelu B.A, Adewusi S.R.A. The chemical composition and membrane stability activity of some herbs used in local therapy for anemia. Journal of Ethnopharmacology. 2005;102:15–22. doi: 10.1016/j.jep.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 19.Fan J, Micheal A, Paramjit G, Chandhoke S. Impact of Ammonium chloride administration on rat ethylene glycol urolithiasis models. Scanning Microscopy. 1999;13:299–306. [Google Scholar]
- 20.Farid Benkaci-Ali, Rym Akloul, Asma Boukenouche, Edwin De, Pauw Chemical Composition of the Essential Oil of Nigella sativa Seeds Extracted by Microwave Steam Distillation. Journal of Essential Oil Bearing Plants. 2013;16:784–791. [Google Scholar]
- 21.Ghedira K, Le jeune R. Huile de nigelle cultivee Nigella sativa L. (Renonculacees) Phytotherapie. 2010;8:124–128. [Google Scholar]
- 22.Ghodasara J, Pawar A, Deshmukh C, Kuchekar B. Inhibitory effect of rutin and curcumin on experimentally-induced calcium oxalate urolithiasis in rats. Pharmacogn. Res. 2010;2:388–392. doi: 10.4103/0974-8490.75462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghodkar P.B. Text book of medical laboratory technology. Mumbai: Bhalani Publishing House; 1994. [Google Scholar]
- 24.Goreja W.G. Black Seed: Natural Medical Remedy. New York: Amazing Herbs Press; 2003. Ref type: Generic. [Google Scholar]
- 25.Hadjzadeh M.A, Khoei A, Hadjzadeh Z, Parizady M. Ethanolic extract of Nigella sativa L. seeds on ethylene glycol induced kidney calculi in rats. Urol. J. 2007;4:86–90. [PubMed] [Google Scholar]
- 26.Hanen Najjaa, Mohamed Neffati, Sami Zouari, Emna Ammar. Essential oil composition and antibacterial activity of different extracts of Allium roseum L, az North African endemic species. Compte Rendus Chimie. 2007;10:820–826. [Google Scholar]
- 27.Harvey AL. Medicines from nature: are natural products still relevant to drug Discovery? Trends in Pharmacological Sciences. 1999;20:196–198. doi: 10.1016/s0165-6147(99)01346-2. [DOI] [PubMed] [Google Scholar]
- 28.Hautmann R, Lehamnn A. Intrarenal distribution of oxalic acid, calcium, sodium and potassium in man. Eur. J. Clin. Invest. 1980;10:173–176. doi: 10.1111/j.1365-2362.1980.tb02077.x. [DOI] [PubMed] [Google Scholar]
- 29.Hodgkinson A, Williams A. sAn improved colorimetric procedure for urine oxalate. Clinica Chimica Acta. 1972;36:127–132. doi: 10.1016/0009-8981(72)90167-2. [DOI] [PubMed] [Google Scholar]
- 30.Karadi R.V, Gadge N.B, Alagawadi K.R, Savadi R.V. Effect of Moringa oleifera Lam. root-wood on ethylene glycol induced urolithiasis in rats. Journal Ethnopharmacology. 2006;105:306–311. doi: 10.1016/j.jep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Kumar S.S, Huat B.T. Extraction, isolation and characterization of antitumor Principle, alpha-hederin from the seeds of Nigella sativa. Planta Medica. 2001;67:29–32. doi: 10.1055/s-2001-10628. [DOI] [PubMed] [Google Scholar]
- 32.Laroubi A, Touhami M, Farouk L, Zrara I, Aboufatima R, Benharref A, Chait A. Prophylaxis effect of Trigonella foenum graecum L. seeds on renal stone formation in rats. Phytotherapy Research. 2007;21:921–925. doi: 10.1002/ptr.2190. [DOI] [PubMed] [Google Scholar]
- 33.Leemann J, Worcestor E.M, Gray R.W. Hypercalciuria and stones. Am. J. Kidney Dis. 1991;17:386–391. doi: 10.1016/s0272-6386(12)80628-7. [DOI] [PubMed] [Google Scholar]
- 34.Leth P.M, Gregersen M. Ethylene glycol poisoning. Forensic Sci Int. 2005;155:179–184. doi: 10.1016/j.forsciint.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Cao Z, Zhang Z, Zhou S, Ye Z. A comparative study on several models of experimental renal calcium oxalate stones formation in rats. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2007;27:83–87. doi: 10.1007/s11596-007-0124-z. [DOI] [PubMed] [Google Scholar]
- 36.Mansour M, Tornhamre S. Inhibition of 5-lipoxygenase and leukotriene C4 synthase in human blood cells by thymoquinone. J. Enzyme Inhib. Med. Chem. 2004;19:431–436. doi: 10.1080/14756360400002072. [DOI] [PubMed] [Google Scholar]
- 37.McHarg T, Rodgers A, Charlton K. Influence of Cranberry juice on the urinary risk factors for calcium oxalate kidney stone formation. B.J.U international. 2003;92:765–768. doi: 10.1046/j.1464-410x.2003.04472.x. [DOI] [PubMed] [Google Scholar]
- 38.Medeiros D.M, Mustafa M.A. Proximate composition, mineral content and fatty acids of cat fish (Ictalurus punctatus rafinesque) for different seasons and cooking methods. Journal of Food Science. 1985;50:585–588. [Google Scholar]
- 39.Mitra S.K, Gopumadhavan S, Venkatarangannna M.V, Sundaram R. Effect of cystone, an herbal formulation on glycolic acid induced urolithiasis in rats Phytotherapy Research. 1998;12:372–374. [Google Scholar]
- 40.Nagi M.N, Mansour M.A. Protective effect of thymoquinone against doxorubicin induced cardiotoxicity in rats: a possible mechanism of protection. Pharmacol. Res. 2000;41:283–289. doi: 10.1006/phrs.1999.0585. [DOI] [PubMed] [Google Scholar]
- 41.Nickavar B, Mojab F, Javidnia K, Amoli M.A.R. Chemical composition of the fixed and volatile oils of Nigella sativa from Iran. Z. Naturforsch. 2003;58:629–631. doi: 10.1515/znc-2003-9-1004. [DOI] [PubMed] [Google Scholar]
- 42.Pak C.Y.C, Delea C.S, Bartler F. Successful treatment of recurrent nephrolithiasis (calcium stones) with cellulose phosphate. N. Engl. J. Med. 1974;290:175–180. doi: 10.1056/NEJM197401242900401. [DOI] [PubMed] [Google Scholar]
- 43.Pak C.Y.C, Resnick M.I. Medical therapy and new approaches to management of urolithiasis. Urol. Clin. North Am. 2000;27:243–253. doi: 10.1016/s0094-0143(05)70254-8. [DOI] [PubMed] [Google Scholar]
- 44.Prawez Alam, Hasan Yusufoglu, Aftab Alam. HPTLC densitometric method for analysis of thymoquinone in Nigella sativa extracts and marketed formulations. Asian Pac. J. Trop. Dis. 2013;3:467–471. [Google Scholar]
- 45.Ragheb A, Attia A, Eldin W.S, Elbarbry F, Gazarin S, Shoker A. The protective effect of thymoquinone, an anti-oxidant and anti-inflammatory agent, against renal injury: A review. Saudi J. Kidney Dis. Transpl. 2009;20:741–752. [PubMed] [Google Scholar]
- 46.Rajagopal G. A simple colorimetric procedure for estimation of citric acid in urine. Indian J. Exp. Biol. 1984;22:391–392. [PubMed] [Google Scholar]
- 47.Rathod N.R, Dipak Biswas, Chitme H.R, Sanjeev Ratna, Muchandi I.S, Ramesh Chandra. Anti-urolithiatic effects of Punica granatum in male rats. Journal of Ethnopharmacology. 2012;140:234–238. doi: 10.1016/j.jep.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 48.Rifaioglu M.M, Nacar A, Yuksel R, Yonden Z, Karcioglu M, Zorba O.U, Davarci I, Sefil N.K. Antioxidative and anti-inflammatory effect of thymoquinone in an acute Pseudomonas prostatitis rat model. Urol. Int. 2013;91:474–481. doi: 10.1159/000351261. [DOI] [PubMed] [Google Scholar]
- 49.Roswitha Siener, Ruth Hönow, Ana Seidler, Susanne Voss, Albrecht Hesse. Oxalate contents of species of the Polygonaceae, Amaranthaceae and Chenopodiaceae Families. Food Chemistry. 2006;98:220–224. [Google Scholar]
- 50.Salem M.A. Effect of some heat treatment on nigella seeds characteristics. 1-Some physical and chemical properties of Nigella seed oil. Journal of Agricultural Research. 2001;27:471–486. [Google Scholar]
- 51.Salem M.L. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int. Immunopharmacology Journal. 2005;5:1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 52.Salma Cheikh-Rouhou, Souhail Besbes, Basma Hentati, Christophe Blecker, Claude Deroanne, Hamadi Attia. Nigella sativa L.: Chemical composition and physicochemical characteristics of lipid fraction. Food Chemistry. 2007;101:673–681. [Google Scholar]
- 53.Sathish Z, Natarajan K, Mukesh, Madhavrao, Nikhad Effect of Hygrophila spinosa T. Anders on ethylene glycol induced urolithiasZis in rats. Asian Journal of Pharmaceutical and Clinical Research. 2010;3:61–63. [Google Scholar]
- 54.Sayed-Ahmed M.M, Nagi M.N. Thymoquinone supplementation prevents the development of gentamicin-induced acute renal toxicity in rats. Clin. Exp. Pharmacol. Physiol. 2007;34:399–405. doi: 10.1111/j.1440-1681.2007.04560.x. [DOI] [PubMed] [Google Scholar]
- 55.Scheid C.R, Cao L.C, Honeyman T, Jonassen J.A. How elevated oxalate can promote kidney stone disease: changes at the surface and in the cytosol of renal cells that promote crystal adherence and growth. Front. Biosci. 2004;9:797–808. doi: 10.2741/1265. [DOI] [PubMed] [Google Scholar]
- 56.Selvam R, Kalaiselvi P, Govindaraj A, Murugan V.M, Satishkumar A.S. Effect of A. lanata leaf extract and Vediuppu chunnz on the urinary risk factors of calcium oxalate urolithiasis during experimental hyperoxaluria. Pharmacol. Res. 2001;43:89–93. doi: 10.1006/phrs.2000.0745. [DOI] [PubMed] [Google Scholar]
- 57.Soundararajan P, Mahesh R, Ramesh T, Begum V.H. Effect of Aerva lanata on calcium oxalate urolithiasis in rats. Ind. J. Exp. Biol. 2006;44:981–986. [PubMed] [Google Scholar]
- 58.Sriboonlue P, Suwantrai S, Prasongwatana V. An indirect method for urinary oxalate estimation. Clinica Chimica Acta. 1998;273:59–68. doi: 10.1016/s0009-8981(98)00024-2. [DOI] [PubMed] [Google Scholar]
- 59.Takruri H.M.H, Dameh M.A.F. Study of the nutritional value of black cumin seeds (Nigella sativa L.) Journal of the Sciences of Food Agriculture. 1998;76:404–410. [Google Scholar]
- 60.Thamilselvan S, Khan S.R, Menon M. Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: Effect of antioxidants. Urol. Res. 2003;31:3–9. doi: 10.1007/s00240-002-0286-x. [DOI] [PubMed] [Google Scholar]
- 61.Velho-Pereira R.M, Barhate C.R, Kulkarni S.R, Jagtap A.G. Validated high performance thin-layer chromatographic method for the quantification of thymoquinone in Nigella sativa extracts and formulations. Phytochem. Anal. 2011;22:367–373. doi: 10.1002/pca.1289. [DOI] [PubMed] [Google Scholar]
- 62.Vihan V.S, Panwar H.S. Galactopoietic effect of Nigella sativa in clinical cases of agalactia in goats. Indian Veterinary Journal. 1987;4:347–349. [Google Scholar]
- 63.Williams H.E, Wandzilak T.R. Oxalate synthesis, transport and the hyperoxaluric syndromes. Urol. J. 1989;141:742–749. doi: 10.1016/s0022-5347(17)40999-2. [DOI] [PubMed] [Google Scholar]
- 64.Yaman I, Balikci E. Protective effects of Nigella sativa against gentamicin induced nephrotoxicity in rats. Exp. Toxicol. Pathol. 2010;62:183–190. doi: 10.1016/j.etp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 65.Zaoui A, Cherrah Y, Mahassini N, Alaoui K, Amarouch H, Hassar M. Acute and chronic toxicity of Nigella sativa fixed oil. Phytomedicine. 2002;9:69–74. doi: 10.1078/0944-7113-00084. [DOI] [PubMed] [Google Scholar]


