Abstract
Background:
TQ has been used as treatment and preventive agent for many diseases over the years. The goal of this study was to investigate the effects of TQ supplement on fractions of serum proteins.
Materials and methods:
Fourteen male Wistar-Albino rats (200-250 g weight) were used as material for two groups; (control (C) and thymoquinone (TQ) respectively. Each group contained seven rats. The control group had only corn oil, while the TQ group was dissolved in corn oil. 30 mg/kg/day were given by oral gavage for four weeks. The serum protein fractions were identified using cellulose acetate technique.
Results:
The total protein level and albumin, α-1, α-2 fractions and A/G ratio have showed no difference between groups (p>0.05). β-globulin fractions of TQ group were higher than control’s (p<0.05). In addition, it was observed that the γ-globulin levels of TQ group were lower than that of the control group’s (p<0.05).
Conclusion:
From the results, it was observed that the changes of these fractions may have originated from elevation or decline synthesis, or activities of containing proteins.
Keywords: Electrophoresis, rat, serum protein fractions, thymoquinone
Introduction
Nigella sativa is a medicinal herb used in the treatment of several diseases in Middle Eastern and Far East countries for over 2000 years. Thymoquinone (TQ) is the basic component in black sesame (Nigella sativa) seeds with various beneficial effects. In addition to antioxidant, anti-carcinogen, antibacterial, antiulcer, antifungal, antitumor, anti-allergic, anti-inflammatory, antineoplastic properties, there are several studies on the positive effects of TQ on cell metabolism, SSS, apoptosis, and immune system (Ali and Blunden, 2003; Kanter et al., 2003; Gali Muhtasib et al., 2006; Aggarwal et al., 2008; Ragheb et al., 2009). TQ has very low acute toxicity but the only effect it has resulting from prolonged use is hypoglycemia (Hosseinzadeh and Pavardeh, 2004).
There are several specialized types of proteins with different tasks performing functions within the cell. They exist in different amounts in the serum under different physiological and pathologic conditions. Serum proteins fulfill several physiological functions. Physiological, nutritional, gender, environmental, and genetic factors can affect serum proteins (Karagül et al., 2000; Onat et al., 2002). Electrophoretic analysis of serum proteins has been conducted in clinical laboratories for many years. Serum protein fractions differ quantitatively and qualitatively depending on physiological conditions such as age, temperature, and pregnancy; also, some environmental conditions such as nutrition and gender; and certain diseases such as genetic polymorphism (Karagül et al., 2000; Onat et al., 2002; Zaias et al., 2009; Apaydin and Dede, 2010; Yüksek et al., 2013; Dede et al., 2014).
The present study was done to determine whether TQ, was extensively used for its therapeutic and inhibitory effects, and to know how effective it has been on serum protein fractions.
Material and Methods
Animals
Fourteen male rats, weighing (200-250 g) and obtained from Yuzuncu Yil University, Medical Faculty Empirical Research Laboratory were used in this study. Study groups consisted one control and one test groups of 7 rats in each. Rats were kept in cages under 12 hrs of daylight/darkness schedule and 22 ± 2°C temperature interval containing freely accessible fresh feed and water.
Preparation of test groups
Control Group: Control group had seven rats. They were orally fed corn oil daily for four weeks.
TQ Group: Seven rats in this group were fed 30 mg/kg/day TQ solution dissolved in corn oil orally every day for four weeks.
Samples preparation
At the end of study, blood samples were taken from the left heart ventricles of the rats using Ketalar anesthesia passed into serum tubes with gel and serum samples separated through centrifugation for 10 minutes (3000 rpm at +4°C).
Biochemical analysis
The obtained serum samples were used for total protein and serum protein electrophoresis. The total protein concentration was analyzed using the biuret method. The serum protein fractions were obtained by Helena Lab-Titan III® Serum Protein Electrophoresis system (Helena, Bioscience Europe, UK). The determined bands after electrophoresis were analyzed with Platinum 3.0 program. The total protein concentrations were calculated after Biuret test.
Statistical analysis
The data obtained were analyzed with paired samples t-test. Statistical differences were considered when p value was less than 0.05 using SPSS 22.0 statistical software.
Results
The results for the serum samples are summarized in Tables 1 and 2
Table 1.
% g values for protein fractions
| Parameters (%) | TQ group | Control group | p |
|---|---|---|---|
| Albumin (% g) | 49.79±1.42 | 53.11±1.35 | .066 |
| α1-globulin (% g) | 7.94±0.64 | 9.17±0.89 | .406 |
| α2-globulin (% g) | 11.26±1.05 | 9.18±0.57 | .119 |
| β-globulin (% g) | 23.65±0.70 | 19.30±0.86 | .012 |
| γ-globulin (% g) | 7.36±0.38 | 9.24±0.71 | .024 |
| A/G | 1.0013±.05693 | 1.15±0.07 | .070 |
Table 2.
Preotein fraction concentrations obtained from study groups
| Parameters | TQ group | Control group |
|---|---|---|
| Total protein(gr/dl) | 5.905±0.303 | 6.370±0.676 |
| Albumin (%) | 2.940±0.189 | 3.380±0.347 |
| α1-globulin (%) | 0.469±0.038 | 0.584±0.107 |
| α2-globulin (%) | 0.668±0.043 | 0.585±0.043 |
| β-globulin (%) | 1.395±0.046 | 1.230±0.055 |
| γ-globulin (%) | 0.435±0.026 | 0.589±0.034 |
| A/G | 1.01±0.057 | 1.13±0.067 |
Although it was observed that albumin, α1-globulin levels, A/G ratio and total protein levels comparatively decreased in the TQ group and alpha 2 levels increased, the variances were not statistically significant.
It was determined that the beta globulin levels of TQ group had higher values than the control group (p < 0.05).
It was identified that gamma globulin levels decreased statistically in TQ group (p < 0.05).
Data obtained as a result of assessing the serum protein fraction percentages in total protein summarized in (Table 3). The comparison conducted for the same exhibited similar results with the concentration values.
The band images and electropherograms of serum protein fractions identified in control and TQ groups are showed in Figures 6 and 7
Figure 1.
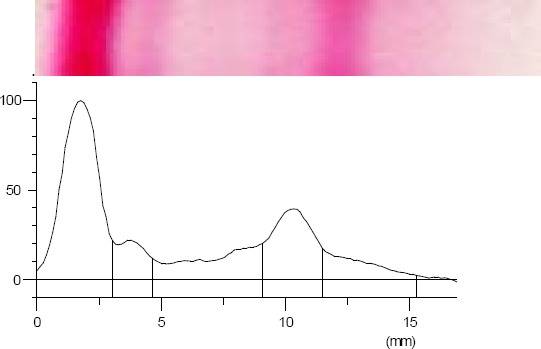
Control group band image and electropherogram
Figure 2.
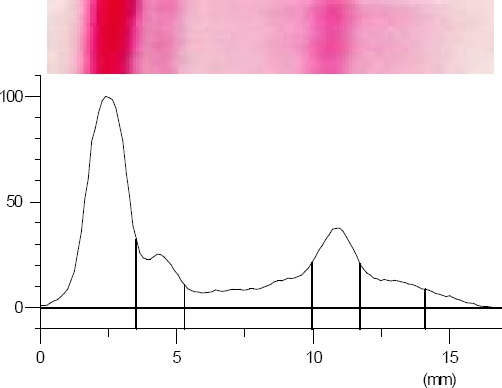
TQ group band image and electropherogram
Discussion
Clinically significant serum proteins have several functions including transport of lipids, vitamins, and metalloenzymes in circulation, and the regulation of supplementary components, extracellular activity, and the immune system. As a result of a normal serum protein electrophoresis, serum proteins are separated into 5 – 6 bands (albumin, α1, α2, β and γ-globulin) (Murray, 2003; Metzler and Metzler, 2001).
Albumin had a key role in general binding and transport mechanism. Globulins were separated as α1, 2, β and γ globulin fractions, with different functions (Kaneko et al., 1997; Metzler and Metzler, 2001; Prinsen et al., 2004). Albumin is one negative phase protein because its amount decreases during acute phase response; α1, 2, β and γ globulins are positive acute phase proteins becaiuse their amounts increases during acute phase response (Zaias et al., 2009).
Although literature review showed no earlier studies on the effects of TQ applications on serum protein fractions, there are publications that revealed the effects of its application in different doses, periods, and conditions on serum total protein and albumin levels. In daily 10 mg/kg TQ application on rats for 5 weeks, it was reported that serum total protein concentrations and albumin levels were not affected (Abdel-Wahab, 2013). Also the same amount of TQ was applied to drinking water of the rats for 2 weeks and no change in total protein levels was observed (Badary et al., 2000).
Jaswal et al. (2013) did not determine a significant change in albumin levels of rats that were applied 10, 20, and 40 mg/kg TQ dissolved in olive oil for 8 weeks (Elbarbry et al., 2012). Furthermore, in rats that were given 5 mg/kg intraperitoneal TQ for 5 weeks, it was indicated that total protein levels were not affected (El-Khouly et al., 2012).
In a study that examined the effects of TQ application on serum total proteins, it was observed that the total protein levels increased in 60 mg/kg/day applied group (p < 0.05), and did not change in 30 mg/kg/day applied group (Kurt et al., 2015). It was further observed that, although the total protein concentrations relatively decreased in 30 mg/kg/day TQ applied group, but, this result was not important (p > 0.05) in this study.
There are several physiological, toxicological, pathological, and nutritional empirical studies that examined the effects of TQ and N. sativa applications that contain plenty of TQ on serum protein levels. There was a study, which demonstrated that the reductions in serum total protein and albumin concentrations due to insecticides with organic chlorine were brought back to normal with N. sativa application (Mahmoud et al., 2002). In rats with oxidative liver damage induced by aluminum chloride, application of N. sativa oil significantly reduced albumin and total protein concentrations (Bouasla et al., 2014).
When TQ was used as a supplementary nutrient, it was determined that the total serum protein levels increased, but, albumin and globulin levels stayed at normal range (Al-Gaby, 1998). An increase in serum albumin levels of N. sativa applied rats (Al-Jishi and Abuo Hozaifa, 2003), and plasma total protein, albumin and globulin levels in rabbits (Tousson et al., 2011) were observed. It was reported that the application of different levels of N. sativa had no effects on serum protein and albumin levels in broiler chicks (Toghyani et al., 2010). TQ asserts its metabolic effects by binding to serum albumin (Lupidi et al., 2010; Yasseen et al., 2014). There are several studies conducted with different objectives, different dosage and durations of TQ applications. In a study that investigated the effects of TQ applications on serum albumin levels, it was identified that there was no statistical differences between the study and control groups (Kurt et al., 2015). In another study, there was no significant effect with the application of 10 and 20 mg/kg/day oral TQ on albumin levels (El-Barbry et al., 2012). Also in this study, in compliance with the above mentioned study results, it was observed that serum albumin levels relatively decreased in TQ applied group, however this reduction was not important for statistic purposes (p>0.05). However, it was reported that serum total protein, albumin, and albumin/globulin rates decreased due the fact that gentamycin application increased after TQ application and came close to the levels reached within the control group (Galaly et al., 2014).
Serum globulin levels are important in the diagnosis of liver diseases, together with other available tests. It was noted that serum globulin concentrations of chickens in N. sativa oil diet increased significantly (Bölükbaşı et al., 2009). In a study that determined the effects of TQ application on serum globulin levels, it was found that serum globulin levels increased significantly in the 60 mg/kg/day TQ applied group (p<0.05), however they stayed the same in 30 mg/kg/day TQ applied group (Kurt et al., 2015). It was observed in this study that TQ application did not change the total globulin level as well.
The first group of α-1 globulins are a1-antitrypsin, a1-acid glycoprotein, a1-lipoprotein (Apo-lipoprotein A), and a1-fetoprotein (AFP) (Turgut, 2000). It was observed in this study that, although a1-globulin fraction relatively decreased in TQ applied group, but this status was not statistically significant (p > 0.05), α-2 globulins are glycoproteins, macroglobulin, haptoglobulin, and ceruloplasmin (Mehmetoğlu, 2002). a2-macroglobulin, heptoglobulin, and ceruloplasmin are affected by several metabolic conditions significantly. Ceruloplasmin that was synthesized primarily in the liver was accepted as an acute phase protein that shows a moderate response to conditions such as inflammation and tissue damage; as well as an antioxidant (Güngör et al., 2004). Haptoglobulin (Hp) induction provides protection against oxidative stress and kidney damage, creates a stable complex with free plasma hemoglobin, and is a genetic polyphormic glycoprotein (Wobeto et al., 2009; Blum et al., 2010). Plasma haptoglobin increases in acute infections, trauma, and nephrotic syndrome. In individuals with active hemolysis induced haptoglubin deficiency, an independent hemoglobin band that migrates to α-2 or β regions (Karagül et al., 2000; Onat et al., 2002). Although literature review revealed no studies that investigated its effects on α-2 globulin, it was reported that the inflammation proteins, whose anti-inflammatory effects are known as belonging to this group, were affected by TQ application (Galaly et al., 2014; Cikman et al., 2015). Although it was observed that α-2 band increased somewhat in TQ applied study group in this study, these differences were not important as statistically (p > 0.05).
β-lipoproteins (apolipoprotein B), complimentary proteins (C3, C4), β2-microglobulin, hemopexin and transferrin (siderophilin) are in the β globulin group. There was no study that demonstrated the effects of TQ application on β-globulin fractions. However, the present studies’ results determined that β-globulin levels increased in TQ applied group (p < 0.05). It was considered that this high level for β-globulin was due to the effects of TQ on the proteins that are among β-globulins and certain physiologic activities and that these were a part of γ-globulins, which are also defined as antibody proteins that help in the fight against infections. The important proteins of the γ-globulin fraction are immunoglobulins (Ig G, Ig M, Ig A, Ig E ve Ig D), C1q complimentary system protein, and C-reactive protein (CRP) (Turgut, 2000; Mehmetoğlu, 2002). It was determined in this study that γ-globulin concentrations significantly decreased in TQ applied group (p < 0.05). It was also interesting to note that, in addition to the decrease in γ-globulin, beta globulin fraction increased.
There are studies that identified TQ application to affect immunoglobulin levels. In a study that implemented TQ application in conjunctivitis treatment, it was reported that, in addition to other immunity parameters, Ig E level decreased as well (Hayat et al.,. Anti-inflammatory properties of TQ on rats with asthma were proven by demonstrating its detractive effect on serum Ig E (Ammar et al., 2011). N. sativa application is defined as an initiator effect of IgG and IgM induced immunity response (Al-Suhaimi,. In a study that scrutinized the effect of TQ on imidacloprid (IC) induced immunotoxicity, phagocytic activity, chemo kinesis, chemotaxis, immunoglobulin levels and biochemical, histopathologic, and immunologic variations were observed, and antibody hemagglutination improved (Mohany et al., 2012).
This study was designed to investigate whether TQ, was widely used in various ways and for various reasons in recent times. The study also showed its effectiveness on serum protein fractions, although the total protein levels, albumin, α-1 and α-2 fractions were not significantly affected after 30 mg/kg/day dose TQ application, β-globulin fraction increased, while γ-globulin fractions decreased. It was concluded that these variations could have been due to synthesis and increase or decrease in the activity of the proteins that constitute the related fractions as a result of TQ application. It is suggested that further studies would cover expression and synthesis, and activity phases should be conducted for exact determination of the proteins that are in serum protein fractions that are affected by TQ application, and to show which phase they were affected by it.
References
- 1.Abdel Wahab WM. Protective effect of thymoquinone on sodium fluoride-induced hepatotoxicity and oxidative stress in rats. J Basic Applied Zool. 2013;66:263–270. [Google Scholar]
- 2.Aggarwal BB, Kunnumakkara AB, Harikumar KB, Tharakan ST, Sung B, An P. Potential of spice-derived phytochemicals for cancer prevention. Planta Med. 2008;74:1560–1569. doi: 10.1055/s-2008-1074578. [DOI] [PubMed] [Google Scholar]
- 3.Al-Gaby AM. Amino acid composition and biological effects of supplementing broad bean and corn proteins with Nigella sativa (black cumin) cake protein. Nahrung. 1998;42(5):290–294. doi: 10.1002/(sici)1521-3803(199810)42:05<290::aid-food290>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 4.Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003;17:299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- 5.Al-Jishi SA, Abuo Hozaifa B. Effect of Nigella sativa on blood hemostatic function in rats. J Ethnopharmacol. 2003;85:7–14. doi: 10.1016/s0378-8741(02)00356-2. [DOI] [PubMed] [Google Scholar]
- 6.Al-Suhaimi EA. Hepatoprotective and immunological functions of Nigella sativa seed oil against hypervitaminosis A in adult male rats. Int J Vitam Nutr Res. 2012;82:288–297. doi: 10.1024/0300-9831/a000121. [DOI] [PubMed] [Google Scholar]
- 7.Ammar el SM, Gameil NM, Shawky NM, Nader MA. Comparative evaluation of anti-inflammatory properties of thymoquinone and curcumin using an asthmatic murine model. Int Immunopharmacol. 2011;11(12):2232–2236. doi: 10.1016/j.intimp.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Apaydin B, Dede S. Electrophoretic profile of serum protein fractions from sheep naturally infected with Babesia ovis. Rev Méd Vét. 2010;161:57–60. [Google Scholar]
- 9.Attia AM, El-Banna SG, Nomeir FR, Abd El-Basser MI. Lindane-induced biochemical perturbations in rat serum and attenuation by omega-3 and Nigella sativa seed oil. Ind J Biochem Biophys. 2011;48:184–190. [PubMed] [Google Scholar]
- 10.Badary OA, Abdel-Naim AB, Mohamed H, Hamada FM. The influence of thymoquinone on doxorubicin-induced hyperlipidemic nephropathy in rats. Toxicology. 2000:219–226. doi: 10.1016/s0300-483x(99)00179-1. [DOI] [PubMed] [Google Scholar]
- 11.Blum S, Vardi M, Brown JB, Russell A, Milman U, Shapira C, Levy NS, Miller-Lotan R, Asleh R, Levy AP. Vitamin E reduces cardiovascular disease in individuals with diabetes mellitus and the haptoglobin 2-2 genotype. Pharmacogenomics. 2010;11:675–684. doi: 10.2217/pgs.10.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouasla I, Bouasla A, Boumendjel A, Messarah M, Abdennour C, Boulakoud MS, El Feki A. Nigella sativa oil reduces aluminium chloride-induced oxidative injury in liver and erythrocytes of rats. Biol Trace Elem Res. 2014;162:252–261. doi: 10.1007/s12011-014-0114-5. [DOI] [PubMed] [Google Scholar]
- 13.BölükbasıŞ C, Erhan MK, Ürüşan H. The effects of supplementation of Nigella Sativa oil on performance and egg fatty acid composition during the late laying period in hens. J Tekirdag Agricultur Fac. 2009;6:283–289. [Google Scholar]
- 14.Çıkman O, Taysi S, Gülşen MT, Demir E, Akan M, Diril H, Kiraz HA, Karaayvaz M, Tarakçıoğlu The radio-protective effects of caffeic acid phenethyl ester and thymoquinone in rats exposed to total head irradiation. Wien Klin Wochenschr. 2015;127:103–108. doi: 10.1007/s00508-014-0635-0. [DOI] [PubMed] [Google Scholar]
- 15.Dede S, Altuğ N, Değer Y, Özdal N, Ceylan E. Serum biochemical profile and protein fractions in cattle with Theileriosis. Revue Méd. Vét. 2014;165:137–143. [Google Scholar]
- 16.El-Barbry F, Ragheb A, Marfleet T, Shoker A. Modulation of hepatic drug metabolizing enzymes by dietary doses of thymoquinone in female New Zealand White rabbits. Phytother Res. 2012;26:1726–1730. doi: 10.1002/ptr.4628. [DOI] [PubMed] [Google Scholar]
- 17.El-Khouly D, El-Bakly WM, Awad AS, El-Mesallamy HO, El-Demerdash E. Thymoquinone blocks lung injury and fibrosis by attenuating bleomycin-induced oxidative stress and activation of nuclear factor Kappa-B in rats. Toxicology. 2012;302:106–113. doi: 10.1016/j.tox.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Galaly SR, Ahmed OM, Mahmoud AM. Thymoquinone and curcumin prevent gentamicin-induced liver injury by attenuating oxidative stress, inflammation and apoptosis. J Physiol Pharmacol. 2014;65:823–832. [PubMed] [Google Scholar]
- 19.Gali-Muhtasib H, Roessner A, Schneider-Stock R. Thymoquinone promising anticancer drug from natural sources. Int J Biochem Cell Biol. 2006;38:1249–1253. doi: 10.1016/j.biocel.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Güngör Ö, Sunar B, Özçelik F, Aktaş, Süer Gökmen S. Akut myokard infarktüsünde sialik asit düzeyleri ve seruloplazmin ile ilişkisi. Türk Biyokimya Derg. 2004;29:226–231. [Google Scholar]
- 21.Hayat K, Asim MB, Nawaz M, Li M, Zhang L, Sun N. Ameliorative effect of thymoquinone on ovalbumin-induced allergic conjunctivitis in Balb/c mice. Curr Eye Res. 2011;36:591–598. doi: 10.3109/02713683.2011.573898. [DOI] [PubMed] [Google Scholar]
- 22.Hosseinzadeh H, Parvardeh S. Anticonvulsant effects of thymoquinone, the major constituent of N sativa seeds in mice. Phytomedicine. 2004;11:56–64. doi: 10.1078/0944-7113-00376. [DOI] [PubMed] [Google Scholar]
- 23.Jaswal A, Sinha N, Bhadauria M, Shrivastava S, Shukla S. Therapeutic potential of thymoquinone against anti-tuberculosis drugs induced liver damage. Environ Toxicol Pharmacol. 2013;36:779–786. doi: 10.1016/j.etap.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Kanter M, Meral İ, Dede S, Cemek M, Özbek H, Uygan İ, Gündüz H. Effects of Nigella sativa l and Urtica dioca l on lipid peroxidation, antioxidant enzyme systems and some liver enzymes in CCl4-treated rats. J Vet Med A. 2003;50:264–268. doi: 10.1046/j.1439-0442.2003.00537.x. [DOI] [PubMed] [Google Scholar]
- 25.Kaneko JJ, Harvey JW, Bruss ML. Clinical Biochemistry of Domestic Animals. 5th edition. San Diego, California: Academic Press; 1997. [Google Scholar]
- 26.Karagül H, Fidancı UR, Altıntaş A, Sel T. Klinik Biyokimya, Medisan Yayın Seri No 40, Ankara. 2000 [Google Scholar]
- 27.Kurt E, Dede S, Ragbetli C. The investigations of total antioxidant status and biochemical serum profile in thymoquinone-treated rats. Afr J Tradit Complement Altern Med. 2015;12:68–72. [Google Scholar]
- 28.Lupidi G, Scire A, Camaioni E, Khalife KH, De Sanctis G, Tanfani F, Damiani E. Thymoquinone, a potential therapeutic agent of Nigella sativa binds to site I of human serum albümin. Phytomedicine. 2010;17:714–720. doi: 10.1016/j.phymed.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Mahmoud MR, El-Abhar HS, Saleh S. The effect of Nigella sativa oil against the liver damage induced by Schistosoma mansoni infection in mice. J Ethnopharmacol. 2002;79:1–11. doi: 10.1016/s0378-8741(01)00310-5. [DOI] [PubMed] [Google Scholar]
- 30.Mehmetoğlu İ. Klinik Biyokimya LaboratuarıEl Kitabı. Konya: İnci Ofset; 2002. [Google Scholar]
- 31.Metzler DE, Metzler CM. Biochemistry: the chemical reactions of living cells. 2nd. Ed. Academic Press; 2001. p. 58. [Google Scholar]
- 32.Mohany M, El-Feki M, Refaat I, Garraud O, Badr G. Thymoquinone ameliorates the immunological and histological changes induced by exposure to imidacloprid insecticide. J Toxicol Sci. 2012;37:1–11. doi: 10.2131/jts.37.1. [DOI] [PubMed] [Google Scholar]
- 33.Murray RK. In: Plasma Proteins and Immunglobulins, chapter 50, in Harper’s Illustrated Biochemistry. Twenty-sixth Edition. Murray RK, Granner DK, Mayes PA, Radwell VW, editors. Mc Graw Hill Co; 2003. pp. 580–582. [Google Scholar]
- 34.Onat T, Kaya E, Sözmen EY. İnsan Biyokimyası. Palme Yayıncılık; Ankara: 2002. pp. 184–218. [Google Scholar]
- 35.Prinsen B, Monique GM, Velden S. Albumin turnover: experimental approach and its application in health and renal diseases. Clin Chim Acta. 2004;347:1–14. doi: 10.1016/j.cccn.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Ragheb A, Attia A, Eldin WS, Elbarbry F, Gazarin S, Shoker A. The protective effect of thymoquinone, an anti-oxidant and anti-inflammatory agent, against renal injury: A review. Saudi J Kidney Dis Transpl. 2009;20:741–752. [PubMed] [Google Scholar]
- 37.Toghyani M, Toghyani M, Gheisari A, Ghalamkari G, Mohammadrezaei M. Growth performance, serum biochemistry and blood hematology of broiler chicks fed different levels of black seed (Nigella sativa) and pepper mint (Mentha piperita) Livestock Sci. 2010;129:173–178. [Google Scholar]
- 38.Tousson E, El-Moghazy M, El-Atrsh E. The possible effect of diets containing Nigella sativa and Thymus vulgaris on blood parameters and some organs structure in rabbit. Toxicol Ind Health. 2011;27:107–116. doi: 10.1177/0748233710381891. [DOI] [PubMed] [Google Scholar]
- 39.Turgut K. Veteriner Klinik Laboratuar Teşhis, Geliştirilmiş2. Baskı, Bahçıvanlar Basım Sanayi, Konya, s. 2000:496. [Google Scholar]
- 40.Wobeto VP, Garcia PM, Zaccariotto TR, Sonati Mde F. Haptoglobin polymorphism and diabetic nephropathy in Brazilian diabetic patients. Ann Hum Biol. 2009;36:437–441. doi: 10.1080/03014460902960263. [DOI] [PubMed] [Google Scholar]
- 41.Yasseen ZJ, Hammad JH, Altalla HA. Thermodynamic analysis of thymoquinone binding to human serum albumin. Spectrochim Acta A Mol Biomol Spectrosc. 2014;124:677–681. doi: 10.1016/j.saa.2013.12.112. [DOI] [PubMed] [Google Scholar]
- 42.Yuksek V, Dede S, Ceylan E. The electrophoretical determination of serum protein fractions in lycopene treated experimental diabetic rats. Cell Biochem Biophys. 2013;67:1283–1289. doi: 10.1007/s12013-013-9660-2. [DOI] [PubMed] [Google Scholar]
- 43.Zaias J, Mineau M, Cray C, Yoon D, Altman NH. Reference values for serum proteins of common laboratory rodent strains. J Am Assoc Lab Anim Sci. 2009;48:387–390. [PMC free article] [PubMed] [Google Scholar]


