Abstract
Background:
Aster tataricus L. f. is used as a traditional Chinese drug to relieve cough and asthma symptoms and to eliminate phlegm. However, Aster tataricus L. f. possesses toxicity, and little systematic research has been conducted on its toxic effects in the laboratory.
Methods and Materials:
The acute group was administered 75% alcohol extract of Aster tataricus L. f. in a single dose. A subchronic toxicity study was performed via daily oral administration of Aster tataricus L. f. at a dose of 0.34 g/kg body weight in SD rats. The rats were divided into six groups: a petroleum ether extract (PEA) group, an ethyl acetate extract (EEA) group, an n-butyl alcohol extract (NEA) group, a remaining lower aqueous phases (REA) group, a 75% alcohol extract (AEA) group and a control group. Quantitative measurements of cytokines were obtained by fluorescence with a laser scanner using a Cy3 equivalent dye.
Results:
The LD50 of the 75% alcohol extract of Aster tataricus L. f. was 15.74 g/kg bw. In the subchronic toxicity study, no significant differences were observed among groups in relative organ weights, urine traits, liver antioxidase levels, or cytokine levels. However, significant sporadic differences were observed in body weight gains, haematology indices, biochemistry values, and histopathology features in PEA, EEA group. In addition, sporadic changes in other groups in measures such as WBC, MCHC, CK, ALP, AST, ALT, LDH, T-BIL, LDL-C, HDL-C, and TC were observed.
Conclusion:
The toxicity study showed that Aster tataricus L. f. can produce toxic effects, mainly on the liver; much less on the heart. The LD50 was 15.74 g/kg BW in mice, and the subchronic toxicity study, used a dosage of 0.34 g/kg/d.BW, showed that the toxic components of Aster tataricus L. f. were mainly concentrated in the petroleum ether fraction, followed by the ethyl acetate fraction, the n-butyl alcohol fraction, the lower aqueous phase and the 75% ethanol extracts.
Abbreviations: PEA, petroleum ether extract of Aster tataricus L. f.; EEA, ethyl acetate extract of Aster tataricus L. f.; NEA: n-butyl alcohol extract of Aster tataricus L. f.; REA: lower aqueous phases of Aster tataricus L. f.; AEA, 75% alcohol extract of Aster tataricus L. f.; WBC, white blood cell; RBC, red blood cell, PLT, platelet; HCT, haematocrit; MCV, mean corpuscular volume; HGB, haemoglobin; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; CREA, creatinine; LDH, lactate dehydrogenase; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; T-BIL, total bilirubin; ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; TP, total protein; ALB, albumin; Glu, glucose; TC, total cholesterol; TG, triglycerides; CK, creatine kinase; GSH, Glutathione; MDA, malondialdehyde; T-SOD, total superoxide dismutase; TNF, tumour necrosis factor; IFN, interferon; MCP, monocyte chemotactic protein C.
Keywords: Aster tataricus L. f, Acute toxicity, Subchronic toxicity, Cytokines, Hepatic injury
Introduction
Aster tataricus L. f., a perennial herb of the Asteraceae (Morita et al., 1996), is widely distributed across Asia, Europe and North America. Majority of Aster species grow in low-lying wetlands, hills and low mountain meadows and marshes. There are an estimated 250 species of Aster worldwide, including 100 species that occur in China. A variety of compounds have been isolated from Aster tataricus L. f., including coumarins, flavonoids, anthraquinones (Sawai et al., 2011), sterols, peptides, terpenes (Akihisa et al., 1999), sesquiterpenes, astersaponin (Zhou et al., 2014), a triterpene and epifriedelinol (Fujioka et al., 1997; Shirota et al., 1994). A number of volatile oil constituents have been identified in Aster tataricus L. f. by gas chromatography (Tori et al., 2001).
However, in China, shionone is known as the main triterpenoid component of Aster tataricus L. f. The dried roots of this plant have been used in traditional Chinese medicine to eliminate phlegm and relieve cough (Sawai et al., 2011). Aster tataricus L. f. has been used as an expectorant, and it possesses diuretic, antibacterial, antitumour, antiviral and anti-ulcer activities (Morita et al., 1996; Shao et al., 1997; Wang et al., 1998). Yen et al. (1998) demonstrated that emodin, a component of Aster tataricus L. f., has antioxidant properties. Ng et al. (2003) showed that quercetin and kaempferol are effective antioxidants for inhibiting erythrocytic haemolysis and brain lipid peroxidation and that quercetin, kaempferol, scopoletin and emodin can inhibit the formation of superoxide free radicals. Additionally, terpene can cause cytotoxicity in tumour cells by inducing tumour cell apoptosis and DNA mutations (Zhou et al., 2014). In recent years, studies of the pharmacological activity of Aster tataricus have focused on cough relief and phlegm elimination or its anti-tumour effects, with few assessments of other pharmacological activities and no reports on liver toxicity associated with Aster. Considering its pharmacological activities and potential health benefits, and the lack of toxicological studies, there is a pressing need to clarify the toxicological profile of Aster tataricus L. f. Thus, we evaluated the LD50 and potential toxic effects of a 75% alcohol extract and four organic agentia extracts (petroleum ether extract, ethyl acetate extract, n-butyl alcohol extract, and aqueous phases) of Aster tataricus L. f. over 91 days to identify the extract with the greatest toxicity and determine the relationship between the toxicity and the duration of oral dosing. Haematology, biochemistry, urinalysis, liver biochemistry, histopathology, inflammation array, weight change and organ-to-body weight ratio were evaluated.
Materials and methods
Materials and Chemicals
Urine was examined by using a KNF-100 Automated Urine Chemistry Analyser (Uritest Inc., China). An automatic biochemical analyser (BS-420) and biochemical kits were purchased from ShenZhen Mindray Bio-medical Electronics Co., Ltd. An automatic microplate reader was provided by Molecular Devices, Inc. (USA). Antioxidant enzyme kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China), and an inflammatory cytokines kit and reagents were provided by RayBiotech, Inc. (Guangzhou, China). Ethanol, petroleum ether, ethyl acetate extract, and n-butyl alcohol were purchased from Fuyu Chemical Co., Ltd. (Tianjin, China).
Animals
Young mice weighing 18-22 g were purchased from the Animal Physiology Laboratory of Lanzhou University, and a total of 36 male and 36 female 5-week-old Sprague-Dawley (SD) rats were supplied by the production facility of the Animal Physiology Laboratory, Gansu University of Traditional Chinese Medicine (Gansu, China). The accession number is GTCM-140823. The animals were fed in a separate room with a barrier system under a controlled light-dark cycle (12-12 h, lights on 7:00-19:00), ventilation (air exchange rate of 18 cycles/h), temperature (23±2 °C) and relative humidity (55±15%) during the study. The cages and chip bedding were changed twice per week. The experiments began after the rats had acclimatized for one week. This study was performed in accordance with the rulings of Gansu Experimental Animal Center (Gansu, China) approved by the Ministry of Health, P.R. China, in accordance with NIH guidelines.
Plant Material
All the Aster tataricus root material used in this study were collected from The Yellow River Medicine Market (GanSu, China). Plant samples were identified by Professor Jifang Zheng at the Lanzhou Institute of Husbandry and Pharmaceutical Sciences of CAAS (Sample No. TONE-140512).
One thousand grams of Aster tataricus L. f. was weighed and added to water-diluted ethanol (purified water 25% v/v and ethanol 75% v/v) by three iterations of steam distillation over 2 hrs, filtered, and concentrated to remove the alcohol at 45 °C in a rotary evaporator. Next, an equal amount of ether petroleum (60-90 °C) that had been extracted 3 times was added to the 75% ethanol extract using a merged extraction and concentrated to a paste with a weight of 6.885 g (0.68% yield) to generate a petroleum ether extract of Aster tataricus L. f. (PEA) (Ye et al., 2014).
The lower aqueous phases were extracted 3 times with an equal amount of ethyl acetate using a merged extraction and concentrated to a paste with a weight of 13.529 g (1.35% yield) to generate an ethyl acetate extract of Aster tataricus L. f. (EEA). The remaining aqueous phases were extracted 3 times with equal n-butyl alcohol using a merged extraction and concentrated to a paste with a weight of 42.493 g (4.25% yield) to generate an n-butyl alcohol extract of Aster tataricus L. f. (NEA). The remaining lower aqueous phases were evaporated to a paste under reduced pressure to obtain 397.045 g (39.70% yield) to generate REA.(PEA, EEA, NEA, REA extract from AEA)
Aster tataricus L. f. (100 g) was extracted with 75% ethanol (400 mL of solvent ×3, 2 hrs per extraction) using a reflux unit, and the filtered solutions were combined and evaporated to a paste under reduced pressure at 40 °C to eliminate the alcohol, resulting in 48.002 g of alcohol extract (48.00% yield), or AEA. AEA was kept at -20 °C until use and suspended in distilled water.
Acute oral toxicity in mice
Sixty healthy Kunming mice (equal numbers of males and females, weighing 18-22 g) were randomly divided into 6 groups, with 5 males and 5 females in each group. AEA was dissolved to different concentrations in distilled water and administered to the Kunming mice via oral gavage at doses of 25.08, 20.81, 17.27, 14.34, 11.90 or 9.88 g/kg body weight per day at a dose ratio of 0.83 according to Research Methodology of Traditional Chinese Medicine Pharmacology (People’s Medical Publishing House, 2006) (Chen, 2006). All of the mice were fasted overnight to eliminate food from the gastrointestinal tract before dosing. After gavage, the mice were observed for 14 days to observe symptoms, mortality, and changes in behaviour, skin, eyes, fur and somatic motor activity. After the experiment, all of the mice were euthanized, and their vital organs were individually observed for overt pathology by necropsy. Finally, the LD50 was calculated using SPSS 17.0 software.
Subchronic oral toxicity test
Experimental design
The five extracts were ground in Tween-80, dissolved in 0.5% water carboxymethyl cellulose Na and administered to the rats at a dose of 0.34 g original drug/kg/d.BW. Tween-80 and 0.5% water carboxymethyl cellulose Na were chosen because of their inert nature and the insolubility of PEA, EEA, and NEA. The rats were divided into 6 groups (PEA group, EEA group, NEA group, REA group, AEA group, and Control group). The treatment groups received Aster tataricus L. f. extract at a dose of approximately 0.34 g/kg body weight (50/LD50), and the control group received an equal dose of 0.5% carboxymethyl cellulose Na. Both the treatment and control groups were dosed daily between 9:00 am and 12:00 am for 13 consecutive weeks.
Urine samples were collected for analysis at the end of the experiment. On the last day of the experiment, the animals were fasted overnight. Before collecting blood, the rats received a 3% sodium pentobarbital solution. Blood was collected on day 92 in 10% ethylenediaminetetraacetic acid (EDTA) tubes for haematology and in non-oxalate tubes for serum separation. The abdominal cavity was opened to dissect out and weigh the liver, lungs, kidneys, heart, spleen, thymus, testes and ovaries.
Observation of gross toxicity and Urine examination
All the animals were observed twice daily for mortality. General observations were made for clinical signs including behavioural changes, signs of gross toxicity, fur condition and somatic motor activity, and these observations were performed once daily during the subchronic toxicity study.
On day 91, each rat was placed in a metabolic cage from 2:00-4:00 pm to collect urine. Urine colour, turbidity, pH, specific gravity (USG), protein, nitrite, bilirubin, urobilinogen, ketones, WBC, vitamin C and glucose levels were analysed using a KNF-100 Automated Urine Chemistry Analyser.
Body weight and food consumption
The animals were weighed on the first day of administration, and then individual body weights and food consumption were recorded every day. The mean weekly body weight gain and food consumption were calculated for each sex and measured weekly. The rats were allowed unlimited access to food throughout the study.
Macroscopic examination and relative organ weights
The rats were euthanized by administering sodium pentobarbital at dose of 30 mg/kg BW. The abdominal cavity of each rat was opened to observe the position, shape, size, colour, and consistency of the organs. Gross lesions were examined in all of the animals in all groups. The animals were eviscerated concomitantly. The liver, kidneys, spleen, lungs, heart, thymus, ovaries and testes were weighed. A portion of the liver (approximately 0.2-0.5 g) was immediately preserved at -80 °C for liver biochemical and cytokine analyses. The absolute organ weights were converted to relative organ weights based on the organ-to-body weight ratio.
Haematology and Serum Biochemistry
After the collection of blood in anticoagulant tubes on day 92, haematology indices were detected by using a pocH-100iV haematology analyser and reagents. The indices consisted of white blood cell (WBC), red blood cell (RBC), and platelet (PLT) counts; haematocrit (HCT); mean corpuscular volume (MCV); haemoglobin (HGB); mean corpuscular haemoglobin (MCH); and mean corpuscular haemoglobin concentration (MCHC).
Serum was isolated from non-anticoagulated tubes by centrifugation (Xiang Yi L-550, Changsha, China) at 3000 r/min for 15 min. Measurements of the following were obtained: creatinine (CREA-j), lactate dehydrogenase (LDH), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total bilirubin (T-BIL), alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), total protein (TP), albumin (ALB), urea, glucose (GLU), total cholesterol (TC), triglycerides (TG), and creatine kinase (CK).
Liver biochemical analysis
The supernatant was isolated from the liver-water suspension (volume ratio liver: water=1:9). GSH, MDA, and T-SOD tests were conducted with the liver supernatant according to the instructions provided with the kits.
Histopathology
After weighing the collected organs, all of the samples were fixed in 10% buffered formalin. The histological analysis aimed to assess the tissue integrity of the organs and to observe the presence of degeneration, necrosis, apoptosis, leukocyte infiltration, congestion, blood extravasation or fibrosis.
Quantitative measurements of liver inflammation using an array
Cytokines (IL-1α, IL-β, IL-2, IL-4, IL-6, IL-10, IL-13, MCP-1, IFNγ, TNFα) were detected by fluorescence with a laser scanner using a Cy3 equivalent dye (Watanabe et al., 2005) between the control group, PEA and EEA group. Hepatic tissues were homogenized with 10 L protease inhibitors and 990 L 1X Cell Lysis Buffer and then centrifuged at 13,000 rpm for 20 min. The supernatants were used for the experiment. Cytokine standard dilutions were prepared before the test according to the kit instructions, and all of the kits and reagents were provided by RayBiotech, Inc., Guangzhou.
Statistical analysis
The LD50 values and data on weekly body weight, food consumption, relative organ weights, haematology, serum biochemistry, urinalysis and liver assessments were evaluated by one-way ANOVA using SPSS 17.0 statistical software. All of the values are expressed as the mean ± SD. P values less than 0.05 were considered significant.
Results
Acute toxicity
All of the mice were observed for mortality and changes in behavioural patterns over 14 days. The mice became drowsy and depressed after dosing, and their fur lost its gloss. (Table 1) shows the group mortality rates. The LD50 of AEA in the mice was 15.74 g/kg bw based on calculations performed using Bliss in SPSS 17.0. The mice were convulsive before they died. The organs exhibited no macroscopic signs of toxicity, except in the liver. Significant pathological changes and lesions were observed in the liver.
Table 1.
Mice mortality in the acute toxicity study.
| Groups | Doses (g/kg) | Number of mice | Mortality | Survival rate (%) |
|---|---|---|---|---|
| 1 | 25.08 | 10 | 10 | 0 |
| 2 | 20.81 | 10 | 8 | 20 |
| 3 | 17.27 | 10 | 5 | 50 |
| 4 | 14.34 | 10 | 5 | 50 |
| 5 | 11.90 | 10 | 2 | 80 |
| 6 | 9.88 | 10 | 0 | 100 |
Subchronic toxicity
Observations of gross toxicity and mortality
No deaths were observed throughout the experimental period. Compared with the solvent group, rats in the PEA, EEA, and NEA groups were drowsy and depressed, reacted slowly to outside stimulation, and displayed some fur loss beginning at 21 days after oral administration. No significant changes were observed in the remaining groups. These results suggest that the administration of PEA, EEA, and NEA in rats at levels up to 0.34 g/kg/day for 91 days have adverse effects on clinical observations.
Food consumption, body weight gain and relative organ weights
The gain in body weight was significantly higher (P < 0.05) in males of the petroleum ether extract group compared with the male controls at week 2 (no significant difference was observed in females). However, significant decreases in body weight gain relative to male controls were observed in males of the PEA group at weeks 7, 9, 11, and 12 (P < 0.05). In addition, almost all of the treatment groups lost weight sporadically from week 7 to week 12. Table 2 and Fig. 1 show the body weight gains and trends in weight gain in the rats. Food consumption is shown in Fig. 2. There were no significant differences in the weekly total food consumption between any of the extract groups and the control group throughout the experimental period. This result indicated that the Aster tataricus L. f. extracts affected rat weight gain in both sexes during the stable phase (weeks 7 to 12), especially in the groups that received the petroleum ether extract and the ethyl acetate extract of Aster tataricus L. f. No significant differences in the relative organ weights in either sex were observed between any of the extract groups and the control group (Table 3).
Table 2.
Weekly body weight gain of rat treatment groups at 91 days.
| Time | Groups | |||||
|---|---|---|---|---|---|---|
| Control group | PEA group | EEA group | NEA group | REA group | AEA group | |
| Male | ||||||
| 1 week | 48.00±7.94 | 53.00±1.41 | 50.67±9.02 | 46.50±10.61 | 53.33±7.51 | 55.50±2.12 |
| 2 weeks | 44.33±6.03 | 53.50±2.12* | 49.67±4.93 | 49.00±1.41 | 48.00±3.00 | 46.50±0.71 |
| 3 weeks | 42.33±4.16 | 42.50±3.54 | 41.67±4.62 | 40.50±3.54 | 43.33±5.86 | 51.00±2.83 |
| 4 weeks | 32.30±5.51 | 34.50±2.12 | 38.33±2.08 | 35.50±3.54 | 35.00±7.81 | 42.00±1.41* |
| 5 weeks | 25.32±5.51 | 25.00±1.41 | 28.33±5.51 | 33.50±7.78 | 34.44±2.08 | 30.00±5.66 |
| 6 weeks | 25.34±7.64 | 23.50±2.12 | 21.00±5.00 | 18.50±3.54 | 19.67±6.35 | 20.00±0.00 |
| 7 weeks | 25.67±4.93 | 14.00±2.82* | 18.00±6.08 | 15.50±0.71* | 19.33±5.04 | 17.50±3.54 |
| 8 weeks | 15.67±1.53 | 10.00±1.41 | 13.67±3.79 | 16.50±6.36 | 10.00±2.65 | 9.50±0.71 |
| 9 weeks | 17.00±5.29 | 6.50±0.71* | 14.00±3.61 | 11.50±6.36 | 20.00±6.00 | 12.00±2.83 |
| 10 weeks | 9.00±2.00 | 4.50±0.71 | 7.67±2.08 | 13.50±2.12 | 18.00±8.66* | 14.00±1.41 |
| 11 weeks | 12.33±5.03 | 20.00±4.24* | 16.33±3.51 | 16.00±0.00 | 23.67±3.06** | 19.00±1.41 |
| 12 weeks | 11.33±1.53 | 4.50±0.50* | 5.33±0.0.58* | 16.50±0.71 | 12.00±5.29 | 13.50±2.12 |
| 13 weeks | 8.00±1.02 | 9.00±1.41 | 8.00±2.00 | 5.50±0.72 | 5.65±1.52 | 6.50±0.72 |
| Female | ||||||
| 1 week | 38.50±4.95 | 37.00±2.65 | 26.50±4.95* | 38.67±6.11 | 39.50±0.71 | 33.00±1.73 |
| 2 weeks | 21.50±4.95 | 24.33±2.08 | 21.00±1.41 | 26.67±4.51 | 22.00±5.66 | 17.33±0.58 |
| 3 weeks | 17.00±0.00 | 15.00±3.00 | 13.50±3.54 | 15.67±1.15 | 19.50±2.12 | 21.33±3.21 |
| 4 weeks | 12.00±2.83 | 18.33±8.38 | 14.00±4.24 | 14.33±6.02 | 10.50±4.95 | 12.67±2.52 |
| 5 weeks | 16.00±1.41 | 15.32±2.89 | 12.50±4.95 | 17.67±2.08 | 13.00±1.41 | 11.00±1.73 |
| 6 weeks | 11.50±0.71 | 12.00±1.00 | 10.00±1.41 | 13.67±3.52 | 13.00±2.83 | 10.00±3.00 |
| 7 weeks | 7.00±1.41 | 6.33±1.53 | 3.50±0.71* | 10.33±1.53* | 10.00±1.41 | 9.67±1.53 |
| 8 weeks | 5.50±0.71 | 2.33±0.58** | 2.00±1.41** | -1.33±0.58** | -1.50±0.71** | 3.33±1.15** |
| 9 weeks | 6.50±0.71 | 9.00±3.00 | 2.50±0.71 | 10.00±3.61 | 3.50±0.71 | 5.30±1.00 |
| 10 weeks | 9.50±2.12 | 2.67±1.15** | 7.50±2.12 | 6.67±1.53 | 6.00±1.41* | 4.33±1.15** |
| 11 weeks | 1.50±0.71 | 7.33±2.31** | 1.50±0.71 | 5.00±1.00* | 3.00±1.41 | 3.67±1.15 |
| 12 weeks | 7.50±2.12 | 0.67±0.26** | 5.50±0.71* | 1.33±0.58** | 4.00±1.41** | 0.33±0.15** |
| 13 weeks | 2.50±0.80 | 3.00±1.00 | 1.75±0.35 | 1.35±0.58 | 3.50±0.72 | 3.00±1.00 |
Values are the mean ± SD for 6 rats in each group.
indicates a significant difference from controls (P < 0.05).
indicates a highly significant difference from controls (P < 0.01).
Figure 1.
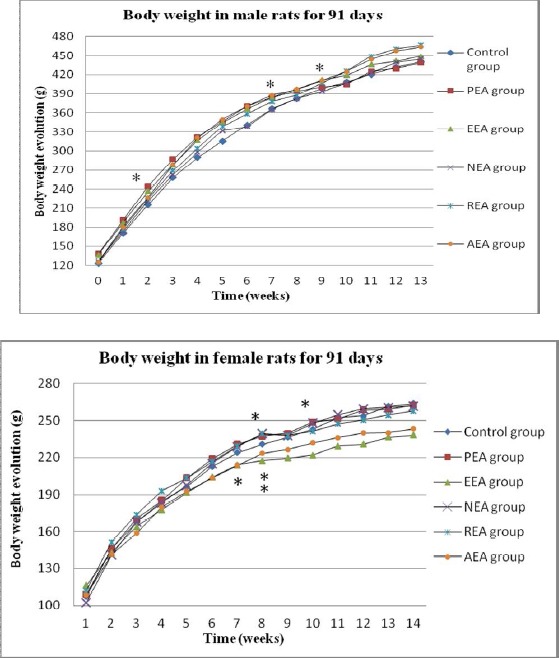
Body weight changes in rats over 91 days.
The weekly average body weights (g) of male and female rats that received Aster tataricus L. f. extracts. * = statistically significant difference in body weight gain compared with the control group.
Figure 2.
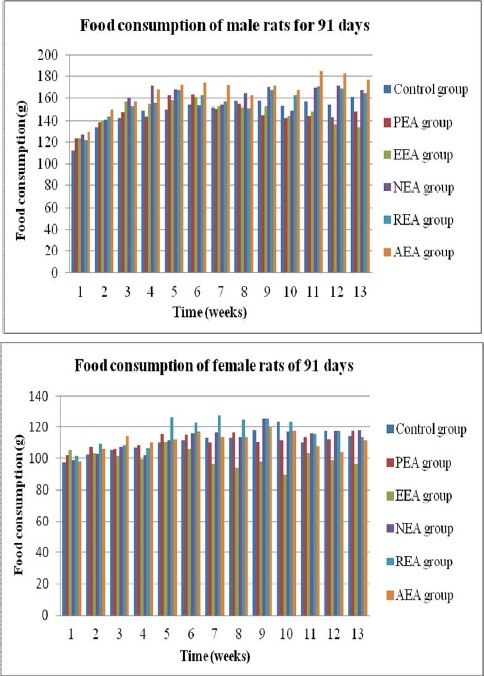
Average weekly food consumption.
Values are presented as the mean ± SD (6 rats/sex/group). * = significant difference compared with the control group at P < 0.05.
Table 3.
Effects of different Aster tataricus L. f. extracts on relative organ weights
| Parameters | Groups | |||||
|---|---|---|---|---|---|---|
| Control group | PEA group | EEA group | NEA group | REA group | AEA group | |
| Male | ||||||
| Liver | 2.91±0.06 | 2.68±0.29 | 2.64±0.28 | 2.79±0.05 | 2.77±0.05 | 2.73±0.12 |
| Heart | 0.32±0.02 | 0.36±0.04 | 0.31±0.03 | 0.35±0.01 | 0.32±0.02 | 0.33±0.04 |
| Spleen | 0.17±0.04 | 0.15±0.02 | 0.16±0.01 | 0.15±0.03 | 0.17±0.00 | 0.15±0.03 |
| Lungs | 0.44±0.02 | 0.45±0.01 | 0.43±0.06 | 0.49±0.06 | 0.41±0.00 | 0.47±0.06 |
| Kidneys | 0.65±0.08 | 0.59±0.07 | 0.61±0.00 | 0.67±0.03 | 0.60±0.02 | 0.70±0.09 |
| Thymus | 0.10±0.02 | 0.12±0.06 | 0.11±0.02 | 0.12±0.02 | 0.10±0.01 | 0.14±0.03 |
| Testes or ovaries | 0.81±0.03 | 0.87±0.03 | 0.80±0.06 | 0.77±0.04 | 0.74±0.05 | 0.79±0.02 |
| Female | ||||||
| Liver | 2.93±0.18 | 2.68±0.29 | 2.79±0.13 | 3.05±0.06 | 2.90±0.03 | 3.01±0.35 |
| Heart | 0.36±0.04 | 0.37±0.04 | 0.40±0.03 | 0.41±0.05 | 0.38±0.03 | 0.41±0.04 |
| Spleen | 0.19±0.02 | 0.21±0.03 | 0.22±0.03 | 0.22±0.03 | 0.21±0.03 | 0.20±0.01 |
| Lungs | 0.53±0.05 | 0.55±0.01 | 0.63±0.03 | 0.55±0.10 | 0.60±0.04 | 0.58±0.05 |
| Kidneys | 0.68±0.03 | 0.64±0.06 | 0.63±0.03 | 0.69±0.02 | 0.66±0.03 | 0.68±0.04 |
| Thymus | 0.16±0.04 | 0.17±0.00 | 0.15±0.07 | 0.18±0.01 | 0.14±0.02 | 0.15±0.01 |
| Testes or ovaries | 0.048±0.01 | 0.046±0.01 | 0.046±0.01 | 0.046±0.01 | 0.047±0.00 | 0.050±0.01 |
Values are the mean ± SD for 6 rats in each group. *indicates a significant difference from controls (P < 0.05).** indicates a highly significant difference from controls (P < 0.01).
Urine examination
Following urine collection, the urine was observed to be luminous yellow in colour. The quantitative analyses of urine revealed no significant differences between any of the extract-treated groups and the control group in either sex. The urine parameters examined included pH (6.5-8.0), specific gravity (1.030), nitrite, glucose, protein, bilirubin, urobilinogen (3.3 μmοΙ/L), ketones, WBC and vitamin C.
Haematology
No significant changes were observed in any haematological index except MCHC (Table 4). MCHC was decreased in females of the PEA, NEA, and REA groups relative to controls.
Table 4.
Haematological parameters of Sprague-Dawley rats treated orally with Aster tataricus L. f. extracts
| Parameters | Groups | |||||
|---|---|---|---|---|---|---|
| Control group | PEA group | EEA group | NEA group | REA group | AEA group | |
| Males | ||||||
| WBC (109/L) | 9.70±0.57 | 8.65±2.19 | 11.37±1.79 | 11.65±0.07 | 11.13±0.80 | 8.90±0.14 |
| RBC (1012/L) | 8.11±0.39 | 8.59±0.58 | 8.51±0.53 | 8.50±0.33 | 8.26±0.35 | 8.38±0.62 |
| HGB (g/L) | 175.67±9.07 | 180.50±4.95 | 180.00±6.00 | 176.00±5.66 | 175.67±3.06 | 178.50±4.95 |
| HCT | 0.46±0.02 | 0.48±0.02 | 0.49±0.03 | 0.48±0.01 | 0.48±0.01 | 0.48±0.03 |
| MCV (fL) | 57.33±1.19 | 55.15±0.07 | 55.17±1.91 | 56.55±3.04 | 57.70±1.40 | 57.10±1.13 |
| MCH (pg) | 21.67±0.15 | 20.35±0.49 | 20.33±0.57 | 20.75±1.48 | 21.27±0.67 | 21.30±0.99 |
| MCHC (g/L) | 378.00±6.55 | 370.00±9.90 | 374.67±6.66 | 366.50±6.36 | 368.67±3.21 | 373.00±9.90 |
| PLT (109/L) | 1068.67±24.01 | 1138.00±80.61 | 1183.50±61.52 | 1052.00±189.50 | 877.67±32.53 | 1056.00±34.65 |
| Females | ||||||
| WBC (109/L) | 6.83±0.22 | 7.43±0.55 | 5.75±0.21 | 9.13±2.93 | 9.15±1.48 | 8.20±0.20 |
| RBC (1012/L) | 7.76±0.24 | 8.19±0.14 | 7.81±0.20 | 8.26±0.28 | 8.04±0.76 | 8.09±0.36 |
| HGB (g/L) | 172.60±6.02 | 183.00±8.19 | 167.00±5.66 | 173.00±3.61 | 169.50±10.61 | 178.00±10.15 |
| HCT (%) | 0.45±0.01 | 0.47±0.03 | 0.45±0.01 | 0.47±0.00 | 0.47±0.03 | 0.47±0.03 |
| MCV (fL) | 58.16±0.80 | 60.40±2.45 | 57.05±0.07 | 56.70±1.47 | 58.00±1.56 | 58.20±1.18 |
| MCH (pg) | 22.24±0.11 | 22.33±1.36 | 21.40±0.14 | 21.27±0.29 | 21.10±0.71 | 22.00±0.62 |
| MCHC (g/L) | 382.60±6.88 | 369.67±7.09* | 375.00±2.83 | 369.67±5.86* | 364.50±2.12** | 378.00±13.45 |
| PLT (109/L) | 944.40±78.74 | 1021.67±139.95 | 1004.00±83.44 | 1029.00±71.63 | 945.00±4.24 | 914.33±116.14 |
Values are the mean ± SD for 6 rats in each group.
indicates a significant difference from controls (P < 0.05).
indicates a highly significant difference from controls (P < 0.01).
Serum chemistry
The results of biochemical analyses of blood are presented in Table 5. In male rats, the administration of each of the five extracts of Aster tataricus L. f. resulted in significant increases in CK, and similar trends were observed for the levels of ALT, AST, LDL-C, HDL-C, and TC in the PEA group. A significant increase in CK relative to controls was also noted in females in all of the tested groups. The plasma levels of ALT in female rats treated with PEA increased relative to the controls. Other parameters that showed relative increases were T-BIL (EEA and REA groups), LDH (PEA, EEA, NEA, and REA groups), and TC (AEA group). However, ALP was decreased in the PEA and AEA groups compared with the controls. The changes in ALT, AST, ALP, T-BIL, and CK indicate liver damage; other activities were not considered in any of the liver toxicological significance tests, although some irrelevant changes were observed.
Table 5.
Serum biochemistry of SD rats treated orally with Aster tataricus L. f. extracts
| Indices | Groups | |||||
|---|---|---|---|---|---|---|
| Control group | PEA group | EEA group | NEA group | REA group | AEA group | |
| Male | ||||||
| ALT (U/L) | 46.67±2.31 | 78.00±26.87* | 57.67±14.05 | 53.50±2.12 | 57.33±10.79 | 47.00±7.07 |
| AST (U/L) | 116.33±15.95 | 175.50±41.72* | 142.00±32.08 | 155.5±3.54 | 147.67±15.31 | 125.00±42.23 |
| ALP (U/L) | 155.67±13.43 | 149.50±2.12 | 150.00±19.52 | 124.00±16.97 | 143.67±29.54 | 132.50±19.09 |
| T-BIL (μmol/L) | 6.05±1.75 | 7.55±1.93 | 5.71±0.17 | 4.65±0.01 | 5.51±0.43 | 5.25±0.66 |
| TP (g/L) | 67.00±2.50 | 74.10±2.62 | 69.27±0.80 | 61.55±4.74 | 64.60±4.25 | 66.45±3.04 |
| ALB (g/L) | 34.17±2.59 | 37.30±2.26 | 35.77±0.46 | 33.15±1.91 | 34.10±1.65 | 34.35±0.21 |
| CK (U/L) | 654.67±54.00 | 1958.50±468.81** | 1745.00±87.73** | 1281.00±2.83** | 1982.33±112.22** | 1884.00±100.41** |
| LDL-C(mmol/L) | 0.26±0.04 | 0.42±0.03** | 0.28±0.03 | 0.28±0.04 | 0.25±0.06 | 0.25±0.05 |
| LDH (U/L) | 1146.67±381.34 | 1458.00±386.08 | 1179.67±310.78 | 1641.00±21.21 | 1258.00±416.45 | 1274.00±490.73 |
| Urea (mmol/L) | 6.57±1.19 | 5.95±2.33 | 6.10±0.26 | 7.50±0.14 | 7.83±2.48 | 6.50±1.84 |
| Creatinine (μmol/L) | 64.33±8.62 | 74.50±6.36 | 74.00±5.29 | 62.00±1.41 | 66.33±3.79 | 60.50±2.12 |
| HDL-C (mmol/L) | 1.07±0.15 | 1.61±0.24* | 1.10±0.05 | 1.12±0.17 | 1.19±0.22 | 1.02±0.36 |
| Glucose (mmol/L) | 5.72±0.83 | 5.29±1.53 | 4.74±0.34 | 6.10±0.56 | 5.33±0.59 | 5.37±1.02 |
| TC (mmol/L) | 1.60±0.30 | 2.30±0.28* | 1.63±0.15 | 1.60±0.28 | 1.67±0.32 | 1.70±0.14 |
| TG (mmol/L) | 1.05±0.13 | 1.15±0.07 | 1.30±0.49 | 0.75±0.07 | 1.20±0.18 | 1.21±0.07 |
| Female | ||||||
| ALT (U/L) | 45.50±4.95 | 58.67±2.52* | 45.50±2.12 | 50.33±5.51 | 45.50±3.54 | 47.33±11.01 |
| AST (U/L) | 131.40±32.74 | 141.00±30.12 | 156.50±24.75 | 152.67±43.59 | 140.00±36.77 | 116.33±23.29 |
| ALP (U/L) | 112.50±0.71 | 91.67±4.93** | 99.50±0.71 | 109.33±11.72 | 100.00±1.41 | 89.00±4.00** |
| T-BIL (μmol/L) | 4.97±1.31 | 5.48±0.92 | 7.12±0.14* | 4.96±0.66 | 7.53±0.79* | 5.19±1.06 |
| TP (g/L) | 72.24±7.50 | 71.47±4.48 | 72.65±1.77 | 67.53±1.46 | 65.65±3.75 | 72.50±1.23 |
| ALB (g/L) | 37.70±2.27 | 37.43±0.91 | 36.80±0.85 | 35.93±0.64 | 34.85±2.62 | 38.03±1.36 |
| CK (U/L) | 1544.50±286.48 | 2548.33±458.26** | 4490.50±441.94** | 3010.00±284.96** | 3084.50±665.39** | 2521.67±487.26* |
| LDL-C (mmol/L) | 0.21±0.06 | 0.27±0.04 | 0.26±0.01 | 0.24±0.05 | 0.27±0.01 | 0.27±0.07 |
| LDH (U/L) | 683.67±32.32 | 1206.67±212.59** | 1347.50±178.90** | 1265.67±197.00** | 1120.00±247.49* | 978.67±220.00 |
| Urea (mmol/L) | 7.22±0.43 | 7.20±1.57 | 6.65±0.64 | 6.93±2.14 | 7.95±1.34 | 8.10±1.84 |
| Creatinine (μmol/L) | 70.40±9.56 | 79.67±9.61 | 72.50±9.19 | 67.67±7.51 | 68.00±2.83 | 70.67±2.52 |
| HDL-C (mmol/L) | 1.81±0.22 | 1.66±0.42 | 1.72±0.05 | 1.65±0.17 | 1.77±0.54 | 2.28±0.19 |
| Glucose (mmol/L) | 5.85±1.08 | 5.07±0.80 | 4.76±0.08 | 6.09±0.55 | 5.56±1.06 | 5.08±0.57 |
| TC (mmol/L) | 2.24±0.36 | 2.20±0.46 | 2.40±0.14 | 2.10±0.20 | 2.40±0.57 | 2.83±0.31* |
| TG (mmol/L) | 0.94±0.39 | 1.13±0.22 | 1.06±0.17 | 0.76±0.14 | 0.84±0.23 | 0.59±0.11 |
Values are the mean ± SD for 6 rats in each group.
indicates a significant difference from controls (P < 0.05).
indicates a highly significant difference from controls (P < 0.01).
Liver biochemical analysis
Results of the liver biochemical analysis are summarized in Table 6. No significant changes were observed in glutathione (GSH), total superoxide dismutase (T-SOD), or malondialdehyde (MDA) activities in any of the groups compared with the control group.
Table 6.
Liver biochemical indices during the 91-day subchronic oral administration test
| Indices | Groups | |||||
|---|---|---|---|---|---|---|
| Solvent group | PEA group | EEA group | Groups NEA group | REA group | AEA group | |
| Male | ||||||
| GSH | 25.62±0.98 | 26.96±3.47 | 28.49±3.73 | 28.99±3.80 | 29.11±3.50 | 30.71±7.83 |
| T-SOD | 474.09±24.50 | 541.95±8.20 | 519.43±55.29 | 491.70±79.83 | 504.78±63.11 | 539.40±113.07 |
| MDA | 1.00±0.25 | 1.04±0.34 | 0.75±0.10 | 0.90±0.08 | 1.01±0.16 | 0.83±0.03 |
| Female | ||||||
| GSH | 23.77±0.39 | 24.40±4.75 | 26.62±3.20 | 25.34±3.09 | 26.48±1.19 | 27.00±1.71 |
| T-SOD | 550.76±54.73 | 563.32±21.43 | 572.90±14.50 | 512.50±36.71 | 497.35±49.92 | 528.36±62.98 |
| MDA | 1.11±0.04 | 1.17±0.05 | 1.19±0.06 | 0.96±0.06 | 1.17±0.21 | 1.10±0.03 |
Values are the mean ± SD for 6 rats in each group. *indicates a significant difference from controls (P < 0.05). ** indicates a highly significant difference from controls (P < 0.01).
Quantitative measurement of cytokines
There were no significant differences in cytokine measurements (Fig. 3). The alignment of the cytokine arrays is shown in Fig. 4A. Examples of the array blots after incubation with liver tissue samples (groups PEA and EEA) are shown in Fig. 4B. Twelve positively stained inflammation spots were clearly identified. All of the spots were slightly darker compared to those of the control rats, demonstrating that there were no significant changes in the levels of inflammatory cytokines.
Figure 3.
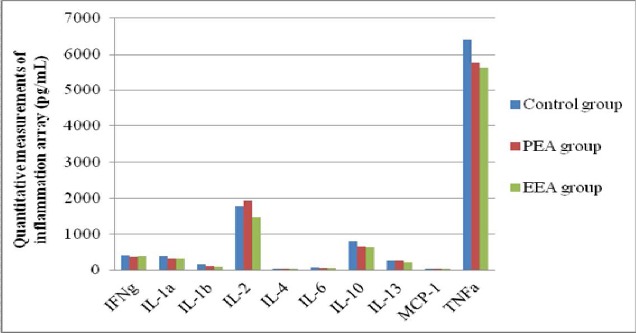
Quantitative measurements of cytokines. Values are means ± SEM for 4 rats in each of the PEA, EEA and Control groups.
*P < 0.05, **P < 0.01 (extract groups vs. controls).
Figure 4.
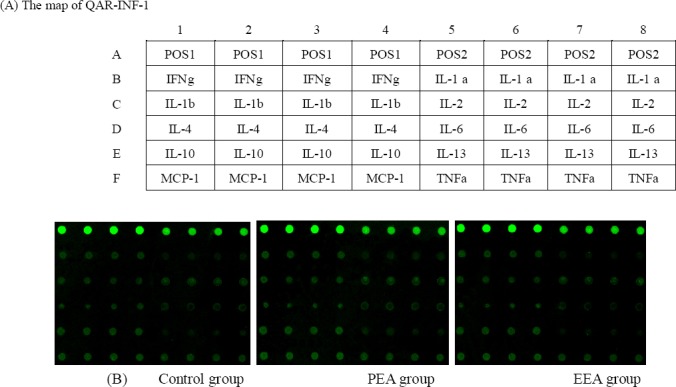
Results of the rat inflammation array. (A) The alignment of 12 cytokines in the Rat Inflammation Array. (B) Examples of Rat Inflammation Array blots probed with liver samples from the PEA and EEA groups.
Histopathology
There were no significant macroscopic changes in observable toxicity in the main organs of the extract-treated rats at 91 days. However, the histopathological examination of organs from the extract-treated groups revealed sporadic lesions, slight congestion and hepatic cord disorders in the liver in the PEA and EEA groups (Fig. 5B, 5C). In addition, signs of swelling and dissolution of the cardiac muscle fibres were observed in myocardial cells (Fig. 5H) in the PEA group; however, no significant differences were observed between any of the remaining extract-treated groups compared with the controls. No changes in the other organs, including the kidneys, spleen, lungs, thymus, ovaries or testes, were detect
Figure 5.
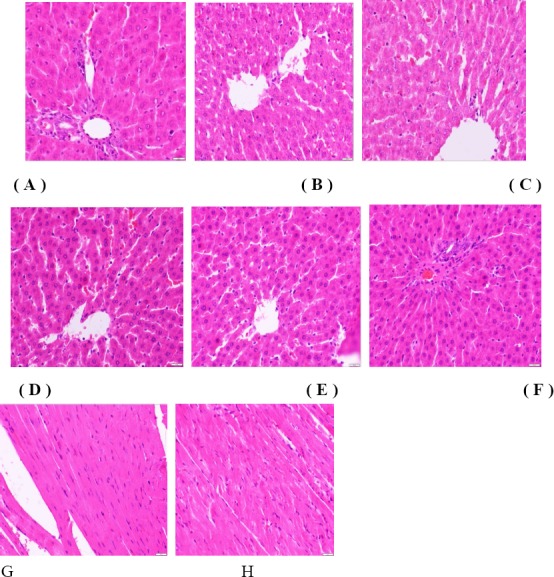
Histopathological photomicrographs obtained at 91 days.
Photomicrographs of liver tissue from the control group (A) and B,C,D,E,F for PEA, EEA, NEA, REA,ANA group. Image G shows the condition of the heart in normal rats, and H shows that of the PEA group. All of the samples were stained with haematoxylin and eosin (40x).
Discussion
In recent years, there has been an increasing emphasis on the safety and efficacy of natural plant products and the elimination of side effects. The diversity and complexity of herbal components make it necessary to establish their safety, quality and effectiveness (Lee et al., 2012; Shin et al., 2011). We found that Aster tataricus L. f. extracts were toxic to animals and resulted in death during a pharmacologic study. Considering its lack of toxicological properties, to clarify the toxicological profile of Aster tataricus L. f., an acute toxicity test was conducted. The results demonstrated the toxic effects of Aster tataricus L. f. in mice. The LD50 of the 75% ethanol extract was greater than 15.74 g/kg BW in mice, and the detection of liver lesions indicated that Aster tataricus L. f. toxicity mainly affected the liver.
The preliminary hypothesis that Aster tataricus L. f. extracts can cause liver toxicity can be evaluated by examining the nidus in the liver. The lack of significant differences in the urine traits between each of the treatment groups and controls demonstrated that kidney function was normal in the extract-treated rats. No deaths related to extract administration were noted, but clinical signs such as unkempt fur, drowsiness and reduced appetite were observed in the treatment groups during the later period of the subchronic toxicity test compared with the control group. Increases or decreases in body weight usually reflect physiological changes, such as changes in liver function or hormones. The observed differences in weight gain indicate that these five extracts of Aster tataricus L. f., especially the petroleum ether and ethyl acetate extracts, can affect weight gain of rats, potentially via the accumulation of toxic effects.
The haematopoietic system can reflect toxic effects because of its sensitivity to toxic compounds (Adeneye et al., 2006). The significant decrease in MCHC observed may be due to diminished cell absorption caused by the accumulation of toxins. Iron deficiency can cause a decrease in MCHC values (Patel et al., 2008). Plasma ALT and AST levels are associated with changes in the permeability of the hepatocyte membrane or hepatic damage because soluble cytosolic enzymes are released into the blood when the permeability of the hepatocellular membrane is altered by either hepatocytic membrane injury or metabolic disturbances (Hor et al., 2011; Ihsan et al., 2010). Haematological analyses indicate that these phenomena generally result in the elevation of these enzymes in the blood. This type of release typically occurs from the apical side of the hepatocyte plasma membrane facing the canaliculi (Ramaiah, 2007). Cholestasis increases total serum bilirubin levels. Therefore, the significant increase in bilirubin observed in female rats confirmed the presence of disturbances in liver function because hyperbilirubinaemia caused by drug inhibition results in bilirubin transport into hepatocytes or the inhibition of bilirubin conjugation in the liver (Zucker et al., 2001). Lactate dehydrogenase (LDH) levels increased in the treated group, and this effect was very clear in females. Lactate dehydrogenase (LDH) leakage has been occasionally encountered in the literature as a potential marker of hepatocellular toxicity. Alkaline phosphatase (ALP) is considered to be a cholestatic induction enzyme of hepatobiliary origin and shows minimal activity in normal hepatic tissue. ALP is generally used to detect impaired bile flow (cholestasis), but serum ALP activity typically increases rapidly in rats following a meal due to its high sensitivity, and thus, it cannot be reliably employed to detect cholestasis (Amacher, 2002). Considering the fasting employed during the last night of the test and the sensitivity of ALP to fasting, the decrease in ALP observed during the test period (a significant difference in female rats in the PEA and AEA groups) is not a good toxicity-related parameter. Increases in CK, LDH, and AST are considered to be indicators of myocardial damage. Nevertheless, the measurement of AST, CK, and LDH activities involves poor sensitivity and specificity in myocardial tissue, and therefore, the use of these measurements in clinical practice is limited. Currently, troponins are used as a replacement (Kulthinee et al., 2010; O’Brien, 2008; Walker, 2006). Hence, the observed increases in CK and LDH might only be a reference for myocardial damage, with greater cardiac toxicity observed in female compared to male rats. Serum creatine (Crea) and urea levels are used to assess renal toxicity (Kuroiwa et al., 2006), but no abnormities were observed in these activities in the present study. The observed increases in LDL-C, HDL-C and cholesterol in males in the PEA group suggest that petroleum ether extract of Aster tataricus L. f. affects lipid and carbohydrate metabolism in rats. HDL is known to be a strong inverse predictor of cardiovascular disease (Liju et al., 2013). Although some sporadic differences were observed, these differences were not considered to be related to toxicity because the observed changes were within the normal reference laboratory range (Petterino et al., 2006). Serum chemistry results showed that extracts of Aster tataricus L. f., especially petroleum ether extract, affect liver function and have some effect on the heart.
The production of excessive free radicals in liver tissue creates tissue injury, resulting in decreases in the levels of SOD and GSH. MDA is an important indicator of biological membrane system damage because it is a product of lipid peroxidation, and its content can reflect the degree of lipid peroxidation in the body and indirectly reflect the degree of cell injury. However, there were no significant changes in the three antioxidases. Furthermore, the results of the cytokine assessment revealed little effect of the petroleum ether extract and the ethyl acetate extract on inflammation, although these results may have been caused by inter-individual differences in the rats. The lack of obvious differences in these parameters indicated that liver toxicity was not severe at the dose of 0.34 g/kg/dBW. The histopathological results from the selected organs (heart, liver, lungs, spleen, thymus, testis, ovaries and kidneys) revealed normal architecture except for the liver in both sexes of group PEA and EEA. Therefore, these lesions can be considered to be spontaneous and/or incidental in nature but relevant to Aster tataricus L. f. treatment, especially in animals that receive petroleum ether extract or ethyl acetate extract of Aster tataricus L. f. (Lu et al., 2014).
The administration of low oral doses of Aster tataricus L. f. extracts to rats can cause mild liver injury, whereas the administration of high doses can cause acute liver toxicity or death. The results of our tests revealed that the petroleum ether and ethyl acetate extracts of Aster tataricus L. f. could cause liver toxicity after 91 days at a daily oral dose of 0.34 g/kg bw. The main components in petroleum ether and ethyl acetate extracts are shionone, friedelin, epifriedelinol, chrysophanol, emodin, quercetin, and luteolin (Ye, 2007). The pharmacologically active ingredients are also concentrated in these two polar segments; for example, shionone is the main active ingredient that eliminates phlegm and relieves cough (Qiny et al., 1984). An experimental study by Yanhua Lu et al. (1999) showed that petroleum ether and ethyl acetate extracts could eliminate phlegm. Emodin and quercetin have anti-inflammatory and bacteriostasis effects. Considering the toxicity of Aster tataricus L. f., it is important to consider this toxicity in clinical settings. Our future research will be focused on identifying drugs or other measures that can potentially inhibit the toxic effects of Aster tataricus L. f. to allow full benefit from its pharmacological effects.
Conclusion
Despite sporadic differences, the toxicity-related changes observed in this study were significant. The results of this study showed the toxic effects primarily affected the liver, with slight effects on the heart. The acute oral toxicity experiment showed that Aster tataricus L. f. is capable of toxic effects and resulted in an LD50 of 15.74 g/kg BW in mice, The subchronic experiment, conducted at a dose of 0.34 g/kg/d.BW, demonstrated that the toxic components of Aster tataricus L. f. were mainly concentrated in the petroleum ether fraction, followed by the ethyl acetate fraction, the n-butyl alcohol fraction, the lower aqueous phase and the 75% ethanol extract.
Acknowledgments
This work was financially supported by the Special Fund for Agro-Scientific Research in the Public Interest (No. 201303040-18).
Footnotes
Conflict of Interest: The authors of this paper declare no conflicts of interest.
References
- 1.Adeneye A.A, Ajagbonna O.P, Adeleke T.I, Bello S.O. Preliminary toxicity and phytochemical studies of the stem bark aqueous extract of Musanga cecropioides in rats. Journal of Ethnopharmacology. 2006;105(3):374–379. doi: 10.1016/j.jep.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 2.Akihisa T, Kimura Y, Tai T, Arai K. Astertarone B, a hydroxy-triterpenoid ketone from the roots of Aster tataricus L. Chemical & Pharmaceutical Bulletin. 1999;47(8):1161–1163. [Google Scholar]
- 3.Amacher D.E. A toxicologist’s guide to biomarkers of hepatic response. Human & Experimental Toxicology. 2002;21(5):253–262. doi: 10.1191/0960327102ht247oa. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q. People’s Medical Publishing House. 2.nd. Beijing: China; 2006. Research Methodology of Traditional Chinese Medicine Pharmacology; pp. 107–110. [Google Scholar]
- 5.Fujioka T, Sakurai A, Mihashi K, Kashiwada Y, Chen I. S, Lee K.H. Antitumor agents. 169. Dysoxylum cumingianum. 5. Cumingianosides P and Q, new cytotoxic triterpene glucosides with an apotirucallane-type skeleton from Dysoxylum cumingianum. Chemical & Pharmaceutical Bulletin. 1997;45(1):202–206. doi: 10.1248/cpb.45.202. [DOI] [PubMed] [Google Scholar]
- 6.Hor S.Y, Ahmad M, Farsi E, Lim C.P, Asmawi M.Z, Yam M.F. Acute and subchronic oral toxicity of Coriolus versicolor standardized water extract in Sprague-Dawley rats. Journal Of Ethnopharmacology. 2011;137(3):1067–1076. doi: 10.1016/j.jep.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Ihsan A, Wang X, Huang X.J, Liu Y, Liu Q, Zhou W, Yuan Z.H. Acute and subchronic toxicological evaluation of Mequindox in Wistar rats. Regulatory Toxicology And Pharmacology. 2010;57(2-3):307–314. doi: 10.1016/j.yrtph.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Kulthinee S, Wyss J.M, Jirakulsomchok D, Roysommuti S. High sugar intake exacerbates cardiac reperfusion injury in perinatal taurine depleted adult rats. Journal of Biomedical Science. 2010;17(Suppl 1):S22. doi: 10.1186/1423-0127-17-S1-S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuroiwa K, Shibutani M, Inoue K, Lee K.Y, Woo G.H, Hirose M. Subchronic toxicity study of water pepper extract in F344 rats. Food and Chemical Toxicology. 2006;44(8):1236–1244. doi: 10.1016/j.fct.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Lee M.Y, Shin I.S, Seo C.S, Kim J.H, Han S.R, Shin H.K. Subchronic oral toxicity studies of the traditional herbal formula Bangpungtongseong-san in Crl: CD (SD) rats. Journal of Ethnopharmacology. 2012;144(3):720–725. doi: 10.1016/j.jep.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Liju V.B, Jeena K, Kuttan R. Acute and subchronic toxicity as well as mutagenic evaluation of essential oil from turmeric (Curcuma longa L) Food and Chemical Toxicology. 2013;53:52–61. doi: 10.1016/j.fct.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 12.Lu L, Fan Y, Yao W, Xie W, Guo J, Yan Y, Yang F, Xu L. Safety assessment of the fermented Phylloporia ribis (Lonicera japonica Thunb.) mycelia by oral acute toxicity study in mice and 90-day feeding study in rats. Food and Chemical Toxicology. 2014;69:18–24. doi: 10.1016/j.fct.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 13.Lu Y.H, Dai Y, Wang Z.T, Xu L.S. Effeets of pH and Azone on Transdermal Absorption o f Chrysophanic Acid. Chinese Traditional and Herbal Drugs. 1999;30(5):360–362. [Google Scholar]
- 14.Morita H, Nagashima S, Uchiumi Y, Kuroki O, Takeya K, Itokawa H. Cyclic peptides from higher plants. XXVIII. Antitumor activity and hepatic microsomal biotransformation of cyclic pentapeptides, astins, from Aster tataricus. Chemical & Pharmaceutical Bulletin. 1996;44(5):1026–1032. doi: 10.1248/cpb.44.1026. [DOI] [PubMed] [Google Scholar]
- 15.Ng T.B, Liu F, Lu Y, Cheng C.H, Wang Z. Antioxidant activity of compounds from the medicinal herb Aster tataricus Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2003;136(2):109–115. doi: 10.1016/s1532-0456(03)00170-4. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien P.J. Cardiac troponin is the most effective translational safety biomarker for myocardial injury in cardiotoxicity. Toxicology. 2008;245(3):206–218. doi: 10.1016/j.tox.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Patel C, Dadhaniya P, Hingorani L, Soni M.G. Safety assessment of pomegranate fruit extract: acute and subchronic toxicity studies. Food and Chemical Toxicology. 2008;46(8):2728–2735. doi: 10.1016/j.fct.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 18.Petterino C, Argentino-Storino A. Clinical chemistry and haematology historical data in control Sprague-Dawley rats from pre-clinical toxicity studies. Experimental and Toxicologic Pathology. 2006;57(3):213–219. doi: 10.1016/j.etp.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Qiny Q, Xu S.M. Active extraction on expectorant action of Aster tataricus. Chemical & Pharmaceutical Bulletin. 1984;19(11):698–699. [Google Scholar]
- 20.Ramaiah S.K. A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food and Chemical Toxicology. 2007;45(9):1551–1557. doi: 10.1016/j.fct.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Sawai S, Uchiyama H, Mizuno S, Aoki T, Akashi T, Ayabe S, Takahashi T. Molecular characterization of an oxidosqualene cyclase that yields shionone, a unique tetracyclic triterpene ketone of Aster tataricus. FEBS Letters. 2011;585(7):1031–1036. doi: 10.1016/j.febslet.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 22.Shao Y, Ho C.T, Chin C.K, Poobrasert O, Yang S.W, Cordell G.A. Asterlingulatosides C and D, cytotoxic triterpenoid saponins from Aster lingulatus. Journal of Natural Product. 1997;60(7):743–746. doi: 10.1021/np970080t. [DOI] [PubMed] [Google Scholar]
- 23.Shin I.S, Yu Y.B, Seo C.S, Ha H.K, Lee M.Y, Huang D.S, Kim J.H, Shin H.K. Subchronic toxicity of Sipjeondaebo-tang (SDT) in Sprague-Dawley rats. Regulatory Toxicology and Pharmacology. 2011;59(3):375–384. doi: 10.1016/j.yrtph.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Shirota O, Morita H, Takeya K, Itokawa H. Cytotoxic aromatic triterpenes from Maytenus ilicifolia and Maytenus chuchuhuasca. Journal of Natural Product. 1994;57(12):1675–1681. doi: 10.1021/np50114a009. [DOI] [PubMed] [Google Scholar]
- 25.Tori M, Murata J, Nakashima K, Sono M. The structure of linoleic acid ester of trans-lachnophyllol isolated from Aster tataricus. Spectroscopy: An International Journal. 2001;15(2):119–123. [Google Scholar]
- 26.Walker D.B. Serum chemical biomarkers of cardiac injury for nonclinical safety testing. Toxicologic Pathology. 2006;34(1):94–104. doi: 10.1080/01926230500519816. [DOI] [PubMed] [Google Scholar]
- 27.Wang C.Z, Yu D.Q. Triterpenoid saponins from Aster auriculatus. Planta Medica. 1998;64(1):50–53. doi: 10.1055/s-2006-957365. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe M, Guo W, Zou S.P, Sugiyo S, Dubner R. Antibody array analysis of peripheral and blood cytokine levels in rats after masseter inflammation. Neuroscience Letters. 2005;382(1-2):128–133. doi: 10.1016/j.neulet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Ye B.G, Feng Y, Wang S. Scientific evaluation of the acute toxicity and 13-week subchronic toxicity of Rheum emodi rhizome extracts in Sprague Dawley rats. Food And Chemical Toxicology. 2014;66:278–285. doi: 10.1016/j.fct.2014.01.051. [DOI] [PubMed] [Google Scholar]
- 30.Ye J. Study on the Constituents and Their Primary Antitumor Effect in Aster tataricus & Semiaquilegia adoxoides. Tianjin. PR. China: Tianjin University; 2007. [Google Scholar]
- 31.Yen G, Chen H, Duh P. Extraction and identification of an antioxidative component from Jue Ming Zi (Cassia toral.) Journal Of Agricultural And Food Chemistry. 1998;46(3):820–824. [Google Scholar]
- 32.Zhou W.B, Zeng G.Z, Xu H.M, He W.J, Zhang Y.M, Tan N.H. Astershionones A-F, six new anti-HBV shionane-type triterpenes from Aster tataricus. Fitoterapia. 2014;93:98–104. doi: 10.1016/j.fitote.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 33.Zucker S.D, Qin X, Rouster S.D, Yu F, Green R.M, Keshavan P, Feinberg J, Sherman K.E. Mechanism of indinavir-induced hyperbilirubinemia. Mechanism of indinavir-induced hyperbilirubinemia. Proc. Natl. Acad. Sci. U. S. A. 2001;98(22):12671–12676. doi: 10.1073/pnas.231140698. [DOI] [PMC free article] [PubMed] [Google Scholar]


