Abstract
Objective:
This Study observed the relevant brain areas activated by acupuncture at the Taichong acupoint (LR3) and analyzed the functional connectivity among brain areas using resting state functional magnetic resonance imaging (fMRI) to explore the acupoint specificity of the Taichong acupoint.
Methods:
A total of 45 healthy subjects were randomly divided into the Taichong (LR3) group, sham acupuncture group and sham acupoint group. Subjects received resting state fMRI before acupuncture, after true (sham) acupuncture in each group. Analysis of changes in connectivity among the brain areas was performed using the brain functional connectivity method.
Results:
The right cerebrum temporal lobe was selected as the seed point to analyze the functional connectivity. It had a functional connectivity with right cerebrum superior frontal gyrus, limbic lobe cingulate gyrus and left cerebrum inferior temporal gyrus (BA 37), inferior parietal lobule compared by before vs. after acupuncture at LR3, and right cerebrum sub-lobar insula and left cerebrum middle frontal gyrus, medial frontal gyrus compared by true vs. sham acupuncture at LR3, and right cerebrum occipital lobe cuneus, occipital lobe sub-gyral, parietal lobe precuneus and left cerebellum anterior lobe culmen by acupuncture at LR3 vs. sham acupoint.
Conclusion:
Acupuncture at LR3 mainly specifically activated the brain functional network that participates in visual function, associative function, and emotion cognition, which are similar to the features on LR3 in tradition Chinese medicine. These brain areas constituted a neural network structure with specific functions that had specific reference values for the interpretation of the acupoint specificity of the Taichong acupoint.
Keywords: acupuncture, Taichong(LR3), functional magnetic resonance imaging (fMRI), functional connectivity
Introduction
Acupoint specificity has important significance for studying the basic theory of acupuncture. Acupoint specificity refers to, after appropriate stimulation of an acupoint, the corresponding reaction produced by the body having a specificity effect compared with the reaction induced by the stimulation of sham acupoints or other acupoints (Lai, et al., 2007, Zhang, et al., 2015).
An increasing number of studies on acupoint specificity have used the technology of brain functional connectivity. Through the measurement of the temporal correlation of blood oxygen level-dependent (BOLD) low-frequency signals, this technology investigates whether there is connectivity among brain areas and the strength of this connectivity, which is a dynamic mode of dependence. A high temporal consistency among brain areas indicates the presence of brain functional connectivity, and these areas together constitute a closely related neural network structure (Cordes D, et al., 2000). The commonly used method is to select a brain area as the reference region and to perform comparative analysis of a time series between other voxels in the whole brain and the reference brain area. If the low-frequency fluctuation (LFF) of the blood oxygen level shows a high temporal correlation, then it is considered that these brain areas and reference region constitute a brain network structure with specific functions (Greicius MD, et al., 2003). In recent years, an increasing number of studies on functional connectivity have been reported. Under physiological conditions, some (Ren Y, et al., 2010; Zhong C, et al., 2012; You Y, et al., 2013) have compared the differences in brain functional connectivity using specific acupoints, sham acupoints, or other acupoints, some studies (Jiang Y, et al., 2012,2013; Zyloney CE, et al., 2010) have compared the influence of different acupuncture modalities, including sham acupuncture on brain functional connectivity, and proposed different mechanisms for different acupuncture modalities, and some studies (Zhang G, et al., 2013) have compared the post-acupuncture effect on brain functional connectivity. Under pathological conditions, most studies (Chen H, et al., 2013; Chen J, et al., 2014; Liang P, et al., 2014) have compared the differences in the influences on the brain network between acupuncture patients and healthy individuals to try to explain the mechanism underlying the treatment of related diseases by acupuncture. However, a comparison of specific brain functional connectivity among true acupuncture at specific points, sham acupuncture, and acupuncture at sham acupoints has not been reported. In 1997, NIH (Acupuncture, 1997) proposed sham acupuncture or placebo acupuncture as control groups to study the clinical effect of acupuncture in random clinic trail (RCT) methods. The results of recently studies (Berman BM, et al., 2004; Linde KJ, et al., 2006; Andersen D, et al., 2010) demonstrated the effective of acupuncture, but the difference between acupuncture and sham acupuncture is not determined. The placebo acupuncture is including sham acupuncture in true acupoint and true acupuncture in sham acupoint. So, we want to analyze the difference among the three states from the perspective of acupuncture mechanism.
LR3 is one of the important acupoints for studying the acupoint specificity in clinical settings. Our group’s previous studies used the combination of the amplitude of low-frequency fluctuation (ALFF) and regional homogeneity (ReHo) to analyze changes in brain functions using true versus sham acupuncture (Wu C, et al., 2014). The present study aimed to use randomized and before-after study designs to observe and compare the changes in relevant brain areas before and after acupuncture at LR3, after true and sham acupuncture, and after acupuncture at sham acupoints. In addition, the functional connectivity method was adopted to further investigate the influences of acupuncture on corresponding regions in the brain from the perspective of the neural network to discuss whether specific acupoints had specific areas in the brain and the functional connectivity among different brain areas, thus to validate the acupoint specificity of LR3.
Data and Methods
General Data
90 healthy subjects were randomly selected from universities and colleges in Guangzhou city, China. The inclusion criteria: (1) age between 21 and 28 years; right handedness; (2) regular diet with minimal liquor, tobacco, tea, and coffee consumption; normal sleeping patterns (before 12 a.m.); moderate-sized body mass index of 18.5–23.9 (Chinese); no history of nervous system disease; (3) no pain (including dysmenorrheal) or insomnia within 1 month before the test; (4) no metallic substances in the body, such as stents; (5) no noise exposure and hypothermia; no fear of confined spaces; and (6) no acupuncture procedure within 1 month before the test. The subjects were informed about the experiment and voluntarily signed informed consent in advance. There were only 45 subjects completed our study. This experiment was approved by the Chinese Ethics Review Committee (ChiECRCT-2012011) and was registered at the Chinese Clinical Trial Registry (ChiCTR-TRC-12002427).According to the complete randomized design, the 45 subjects were distributed into three groups, with each group consisting of 15 people. There were no statistically significant differences between the groups in terms of age, height, and weight (P > 0.05; Table 1).
Table 1.
Basic information of the volunteers (χ̅ ± 5)
| Group | LR3 Group (n=15) | Sham acupuncture Group (n=15) | Sham acupoint Group (n=15) | χ2 | P |
|---|---|---|---|---|---|
| Age in years | 21.80±0.56 | 21.80±0.86 | 21. 87± 1.18 | 0.048 | 0.976 |
| Height in cm | 168.60±6.81 | 167.60±8.58 | 170.07±4.52 | 0.397 | 0.820 |
| Weight in kg | 55.40±8.34 | 56.40±9.18 | 61.20±7.84 | 3.465 | 0.177 |
Methods
Trials and Processing Methods
The LR3 group subjects underwent true acupuncture at LR3 acupoint, while the sham acupuncture group underwent sham acupuncture at LR3 acupoint. The subjects in sham acupoint group underwent true acupuncture at the sham point. Subjects were asked to pass urine and stool prior to treatment. The volunteers’ eyes were masked with eyeshades, and earplugs were simultaneously worn so that their audiovisual system could not be stimulated. The flowchart is as followed (Figure 1).
Figure 1.
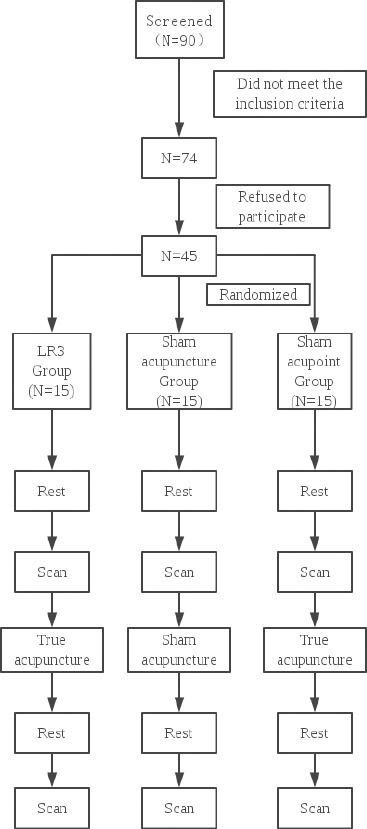
Flowchart
2.2.2 Acupuncture Methods
2.2.2.1 Acupoints Localization
LR3 : On the dorsum of the foot, in the depression anterior to the junction of the first and second metatarsal bones (Chinese National Standards GB/T12346) (Figure 2).
Figure 2.
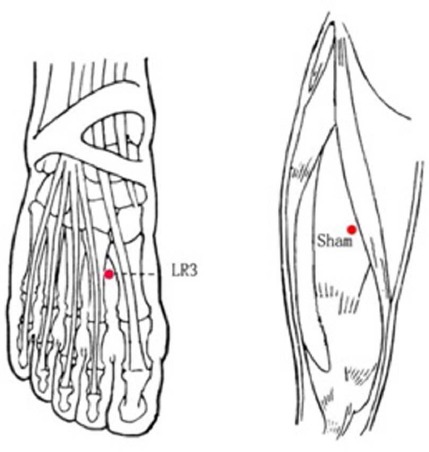
The location of the acupoints
Sham point: On the midpoint of the line connecting the anterior superior iliac spine and lateral border of the patella, 2 cm inside.
Acupuncture Operation
The physician’s hands and volunteers’ skin around the acupoints were sterilized with alcohol before needling. DONGBANG needles (0.30 × (25–15 mm); DONGBANG AcuPrime, Exeter, UK) were used in this study. The acuponits are stimulated bilaterally.
① Sham acupuncture at the LR3 acupoint: In accordance with a previously published method of sham acupuncture (Huang Y, et al., 2012), the auxiliary part of the tube was applied to the skin, with a sham needle placed in the tube over the acupoint. The sham needle was then tapped to make its tip touch the skin without puncturing it and was maintained in place for 30 min.
② True acupuncture at the LR3 acupoint: The tube needling technique was used; the needle handle was tapped softly using a forefinger. The puncturing depth was controlled. After removal of the tube, the needle was vertically punctured at 15±2 mm. After developing needle sensation, twirling at an angle of 90–180° and a frequency of 60–90 times/min and lifting and thrusting at a range of 0.3–0.5 cm and a frequency of 60–90 times/min were conducted. After manipulating the needle for 1 min, the needle was held in place for 30 min. During the 30 min, the physician repeated this manipulation for 1 min every 10 min.
③ True acupuncture at the sham point: The same procedure was followed as that of true acupuncture at the LR3 acupoint.
Resting-state fMRI Scan
The subjects were awake, with normal respiration, and lay supine on an examination bed. The head was placed in a foam headrest for maximum restriction of passive and active movements of the head. The subjects were instructed to avoid any systematic mental activity, and visual and audio stimulations were minimized with earplugs and eyeshades. Scanning was initiated once the subjects were familiarized with the circumstances.
Experiments were performed using a GE 3.0T MRI scanner with an 8-channel head coil. The MRI data (resting-state BOLD sequence) were collected at before needling and 15 min after withdrawing the needle. The scanning parameters are the same as those in our previous study (Wu C, et al., 2014). (Figure 3)
Figure 3.
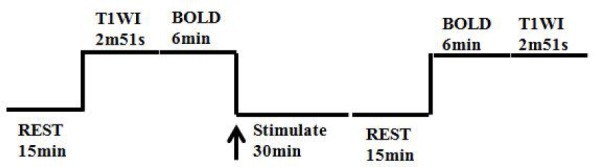
The scan flowchart
Image Processing and Analytical Methods
Preprocessing was carried out using Data Processing Assistant for Resting-State fMRI (DPARSF) (Yan & Zang, 2010, http://www.restfMRI.net), which is based on Statistical Parametric Mapping (SPM8) (http://www.fil.ion.ucl.ac.uk/spm) and the Resting-State fMRI Data Analysis Toolkit (REST, Song et al., 2011. http://www.restfMRI.net) (Yan CC, et al., 2010; Song XW, et al., 2011). This includes DICOM format conversion, removal of 10 time points before image scanning, time correction, correction of the head movement, space standardization and space smoothing. After preprocessing, all 45 subjects were included in the statistical analysis. ReHo Analysis
The parameters are the same as those in our previous study (Wu C, et al., 2014). ALFF analysis: using REST1.8 software, linear tendency of the data after preprocessing (space smoothing was completed) was removed by linear regression. Time and curve were convolved using Hamming bandpass filtering. ALFF was obtained (0.01–0.08 Hz). ALFF of each subject was computed, so ALFF maps were obtained. ALFF value was divided by the mean of the whole brain, and standardized ALFF was obtained. ROI Identification
Regarding our ALFF results, comparing these three groups, the highest peak scores were in the right cerebrum temporal lobe [36, -45, 9]. In the present study, we followed the work of Greicius et al (Grecius MD, et al., 2003) to select our region of interest (ROI). We chose the above three regions as our seed points, which is the center of a sphere with a radius of 1 mm. fMRI Analyses
We used the REST1.8 software and ROIs mentioned above to analyze functional connectivity.
Statistical Analyses
Data were analyzed using REST1.8 software. In the statistical analysis, t-test was used to explore the differences between before and after acupuncture in LR3, true and sham acupuncture in LR3, between acupuncture in LR3 and sham acupoint. Rest1.8 software Viewer was employed to identify the precise anatomical position in the brain with statistical significance on the corresponding MNI coordinate (AlphaSim correction P < 0.05, continuous voxel > 85). The results are presented as images visualized with the BrainNet Viewer (Xia et al., 2013, http://www.nitrc.org/projects/bnv/).
Result
The changes in functional connectivity before and after acupuncture in LR3
We selected the right temporal lobe as the ROI to compare the changes in functional connectivity before and after acupuncture in LR3: right cerebrum superior frontal gyrus, limbic lobe cingulate gyrus and left cerebrum inferior temporal gyrus (BA 37), inferior parietal lobule. (Table.2, Figure 4)
Table 2.
The changes in functional connectivity before and after acupuncture in LR3
| Cluster | Regions | HS | BA | Cluster Size (voxels) | Peak intensity | Peak MNI coordinate | ||
|---|---|---|---|---|---|---|---|---|
| 1 | Superior Frontal Gyrus | R | - | 275 | -4.3844 | 18 | 42 | -18 |
| 2 | Limbic Lobe Cingulate Gyrus | R | - | 124 | -4.0361 | 6 | 21 | 33 |
| 3 | Inferior Temporal Gyrus | L | 37 | 1278 | -5.7516 | -51 | -39 | -21 |
| 4 | Inferior Parietal Lobule | L | - | 99 | -3.7757 | -42 | -39 | 27 |
HS: hemispheres BA: Brodmanns area. The same below.
Figure 4.
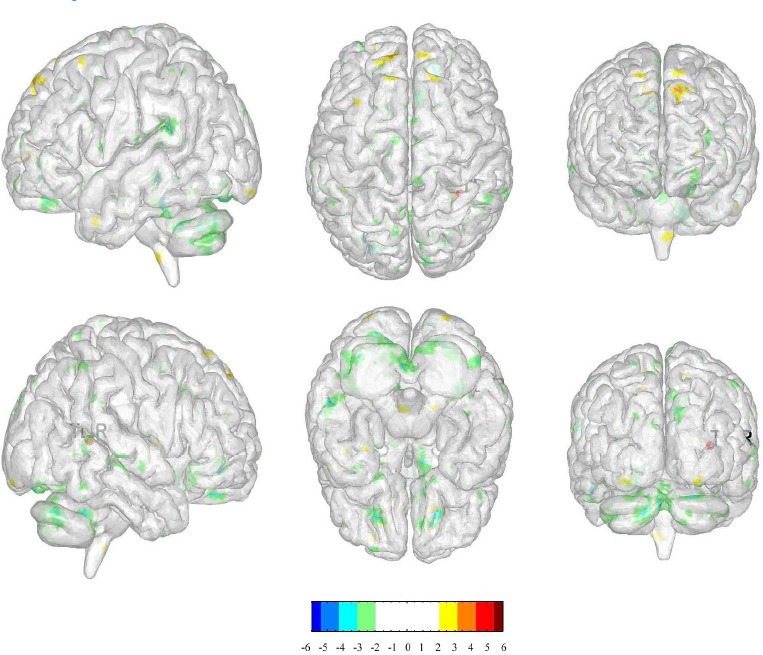
The changes in functional connectivity before and after acupuncture in LR3
(The positive numbers (colors) represent activation, negative number s (colors) represent de activation.)
The changes in functional connectivity between true and sham acupuncture in LR3
We selected the right temporal lobe as the ROI to compare the changes in functional connectivity between true and sham acupuncture in LR3: right cerebrum sub-lobar insula and left cerebrum middle frontal gyrus, medial frontal gyrus. (Table.3, Figure.5)
Table 3.
The changes in functional connectivity between true and sham acupuncture in LR3
| Cluster | Regions | HS | BA | Cluster Size (voxels) | Peak intensity | Peak MNI coordinate | ||
|---|---|---|---|---|---|---|---|---|
| 1 | Sub-lobar Insula | R | - | 146 | -3.3825 | 42 | -21 | 18 |
| 2 | Middle Frontal Gyrus | L | - | 95 | -3.9104 | -21 | 36 | 42 |
| 3 | Medial Frontal Gyrus | L | - | 110 | -3.2984 | -15 | 51 | 6 |
Figure 5.
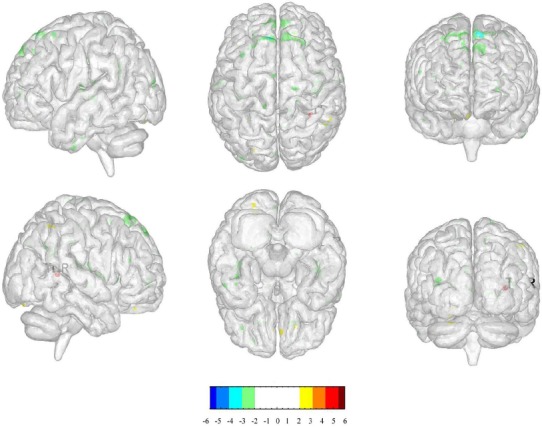
The changes in functional connectivity between true and sham acupuncture in LR3
(The positive numbers (colors) represent activation, negative number s (colors) represent de activation.)
3.3 The changes in functional connectivity between acupuncture in LR3 and sham acupoint
We selected the right temporal lobe as the ROI to compare the changes in functional connectivity between acupuncture in LR3 and sham acupoint: right cerebrum occipital lobe cuneus, occipital lobe sub-gyral, parietal lobe precuneus and left cerebellum anterior lobe culmen. (Table.4, Figure.6)
Table 4.
The changes in functional connectivity between acupuncture in LR3 and sham acupoint
| Cluster | Regions | HS | BA | Cluster Size (voxels) | Peak intensity | Peak MNI coordinate | ||
|---|---|---|---|---|---|---|---|---|
| 1 | Occipital Lobe Cuneus | R | - | 95 | 3.5757 | 18 | -84 | 3 |
| 2 | Occipital Lobe Sub-Gyral | R | - | 139 | 4.3823 | 30 | -90 | -6 |
| 3 | Parietal Lobe Precuneus | R | - | 110 | 4.6498 | 18 | -54 | 42 |
| 4 | Cerebellum Anterior Lobe Culmen | L | - | 1443 | 4.3066 | -3 | -57 | -24 |
Figure 6.
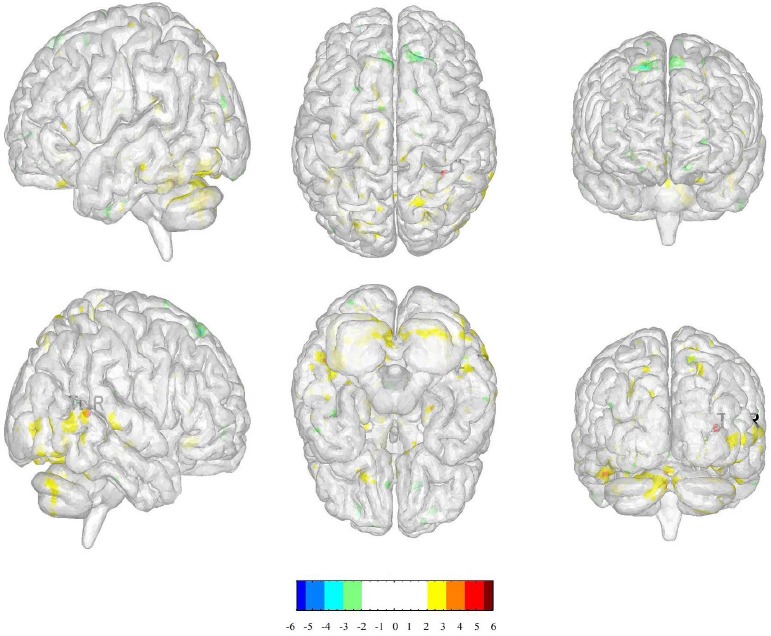
The changes in functional connectivity between acupuncture in LR3 and sham acupoint
(The positive numbers (colors) represent activation, negative number s (colors) represent de activation)
Discussion
We compared the differences of ALFF after acupuncture at LR3, sham acupuncture at LR3 and sham acupoint acupuncture, and found the highest peak score was in the right temporal lobe. Thus, we chose this lobe as the seed point, which is the center of a sphere with a radius of 1 mm, to compare the indexes in different conditions. Both sham acupoint and LR3 belong to the lower limbs. LR3 belongs to liver meridian, while sham acupoint belongs to non-meridian. The sensory function and motor function of LR3 area are controlled by the femoral nerve, which are issued by the L2-4 spinal nerves. Meanwhile, the sensory function and motor function of sham acupoint area are controlled by the lateral popliteal nerve, which are issued by the L4-5, S1-2 spinal nerves. In order to avoid the influence of innervations from the same neurons, we selected the sham acupoint from different areas.
In these comparisons, we found that after acupuncturing at LR 3, the seed point could have a special functional connectivity to superior frontal gyrus, middle frontal gyrus, medial frontal gyrus, occipital lobe, inferior temporal gyrus, inferior parietal lobule, cingulate gyrus, insula, cuneus, precuneus and cerebellum anterior lobe, most of which are associated with function of emotion and vision.
Frontal lobe has always been playing an important part of brain function in emotion. Yang (Yang W, et al., 2016) found that compared to healthy participants, depressed patients had more activities in superior frontal gyrus, inferior frontal gyrus, superior parietal lobule and inferior temporal gyrus, which could demonstrate that these patients recruited had more frontal and parietal inhibitory control resources. Dai (Dai XJ, et al., 2014) had reported that the ReHo of superior frontal gyrus in left insomnia female patients were decreased. The abnormal spontaneous activity areas provided important information on the neural mechanisms underlying emotion and sleep-quality impairment. Li (Li G, et al., 2015) used negative emotional pictures to stimulate depressed patients who had experienced SLEs and found that their medial frontal gyrus were activated. Townsend JD (Townsend JD, et al., 2013) compared bipolar disorder patients to healthy participants and found deactivation in medial frontal gyrus of the patients. With the help of rfMRI, Liu (Liu Q, et al., 2015) found lower functional connectivity in middle frontal gyrus of Premenstrual syndrome than that in healthy volunteers. These previous studies have indicated those specific frontal gyri are closely related to the emotion.
In addition, there are some other brain regions are related to emotion. Engelen T (Engelen T, et al., 2015) used transcranial magnetic stimulation (TMS) to stimulate inferior parietal lobule (IPL) and found it specifically enhanced fearful body processing, which could demonstrate that IPL plays a causal role in processing of fearful bodies. Potvin S (Potvin S, et al., 2015) found that neutral images prompted hyper-activations in the cingulate gyrus in schizophrenia patients. A research (Kwan CL, et al., 2000) showed that stabbing pain could activate some parts of the anterior cingulated. Liu (Liu Q, et al., 2015) also showed that there were significantly positive correlations between the stress perception scores and functional connectivity in the middle frontal gyrus and cuneus. The Beck Depression Inventory scores in the Premenstrual syndrome group were correlated negatively with the functional connectivity in the middle frontal gyrus and precuneus while correlated positively with the functional connectivity in the medial temporal gyrus. McPherson MJ (McPherson MJ, et al., 2016) discovered that emotional intent directly modulated functional connectivity of limbic and paralimbic areas such as the amygdala and insula in jazz pianists. Ebisch SJ (Ebisch SJ, et al., 2015) found that individual differences in emotional susceptibility were associated with differential anterior insula in responses to primary sensory (flavor) stimuli.
Besides these brain regions related to emotions, inferior temporal gyrus (BA 37) (Brodmann K, 1909) and occipital lobe are also found related to visual process. In previous studies, Siedentopf (Siedentopf CM, et al., 2002) suggested that acupuncture at acupoints on the foot activated the visual cortex and treated vision-related disease. Simultaneously, fMRI studies (Wu C, et al., 2014; Yan B, et al., 2005) confirmed that acupuncture at LR3 specifically activated BA19, which was also participant in visual process.
This study had several limitations. First, the selection of the sham acupoint has no unified standard, and the selection of sham acupoints at different locations might have different influences on the study results. Secondly, the selection of ROIs also has a certain influence on the study results. The selection of seed points and radius in this study also requires validation. Finally, we mainly performed specificity studies of LR3 in healthy individuals and it is not known whether similar results can be found in patients. Therefore, the treatment guidance in a clinical setting is uncertain.
In summary, we found that acupuncture at LR3 mainly specifically activated the brain functional network of participates in visual function, associative function, and emotion cognition, which are similar to the features on LR3 in tradition Chinese medicine. In our study, we not only confirmed the function of LR3 from brain functional connectivity, but also compared sham acupuncture and sham acupoint states to conclude the specificity of LR3.
Funding: This study was supported by the National Key Basic Research and Development Project (973 Program), grant no. 2012CB518504; the Guangdong Province College student’s science and technology innovation special funds “Climbing Program”.
Acknowledgements
We are very grateful to the healthy volunteers and staff from the MRI Center of the First Affiliated Hospital of Guangzhou University of Chinese Medicine in China.
Footnotes
Conflict of interest statement None declared.
References
- 1.Acupuncture. NIH Consens Statement Online. 1997;15(5):1. [PubMed] [Google Scholar]
- 2.Andersen D, Lossl K, Nyboe Andersen A, Fürbringer J, Bach H, Simonsen J, Larsen EC. Acupuncture on the day of embryo transfer: a randomized controlled trial of 635 patients. Reprod Biomed Online. 2010;21(3):366–72. doi: 10.1016/j.rbmo.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 3.Berman BM, Lao L, Langenberg P, Lee WL, Gilpin AM, Hochberg MC. Effectiveness of acupuncture as adjunctive therapy in osteoarthritis of the knee: a randomized, controlled trial. Ann Intern Med. 2004;141(12):901–10. doi: 10.7326/0003-4819-141-12-200412210-00006. [DOI] [PubMed] [Google Scholar]
- 4.Brodmann K. Vergleichende Lokalisationslehre der GroBhirnrinde. Leipzig: Johann Ambrosius Barth; 1909. [Google Scholar]
- 5.Chen H, Dai J, Zhang X, Wang K, Huang S, Cao Q, Wang H, Liang Y, Shi C, Li M, Ha T, Ai L, Li S, Ma J, Wei W, You Y, Liu Z, Tian J, Bai L. Hypothalamus-related resting brain network underlying short-term acupuncture treatment in primary hypertension[J] Evid Based Complement Alternat Med. 2013 doi: 10.1155/2013/808971. 808971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Wang J, Huang Y, Lai X, Tang C, Yang J, Wu J, Zeng T, Qu S. Modulatory effect of acupuncture at Waiguan (TE5) on the functional connectivity of the central nervous system of patients with ischemic stroke in the left basal ganglia [J] PLoS One. 2014;9(6):e96777. doi: 10.1371/journal.pone.0096777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cordes D, Haughton V M, Arfanakis K, Wendt GJ, Turski PA, Motitz CH, Quigley MA, Meyerand ME. Mapping functionally related regions of brain with functional connectivity MR imaging[J] AJNR Am J Neuroradiol. 2000;21(9):1636–44. [PMC free article] [PubMed] [Google Scholar]
- 8.Dai XJ, Peng DC, Gong HH, Wan AL, Nie X, Li HJ, Wang YX. Altered intrinsic regional brain spontaneous activity and subjective sleep quality in patients with chronic primary insomnia: a resting-state fMRI study. Neuropsychiatr Dis Treat. 2014;10:2163–75. doi: 10.2147/NDT.S69681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebisch SJ, Bello A, Spitoni GF, Perrucci MG, Gallese V, Committeri G, Pastorelli C, Pizzamiglio L. Emotional susceptibility trait modulates insula responses and functional connectivity in flavor processing. Front Behav Neurosci. 2015;9:297. doi: 10.3389/fnbeh.2015.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelen T, de Graaf TA, Sack AT, de Gelder B. A causal role for inferior parietal lobule in emotion body perception. Cortex. 2015;73:195–202. doi: 10.1016/j.cortex.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis [J] Proc Natl Acad Sci USA. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greicius MD, Krasnow B, Boyett-Anderson JM, Eliez S, Schatzberg AF, Reiss AL, Menon V. Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study [J] Hippocampus. 2003;13(1):164–74. doi: 10.1002/hipo.10064. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y, Tang C, Wang S, Lu Y, Shen W, Yang J, Chen J, Lin R, Cui S, Xiao H, Qu S, Lai X, Shan B. Acupuncture regulates the glucose metabolism in cerebral functional regions in chronic stage ischemic stroke patients-a PET-CT cerebral functional imaging study [J] BMC Neuroscience. 2012;13:75. doi: 10.1186/1471-2202-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang Y, Hao Y, Zhang Y, Liu J, Wang X, Han J, Fang J, Zhang J, Cui C. Thirty minute transcutaneous electric acupoint stimulation modulates resting state brain activities: a perfusion and BOLD fMRI study [J] Brain Res. 2012;1457:13–25. doi: 10.1016/j.brainres.2012.03.063. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y, Wang H, Liu Z, Dong Y, Xiang X, Xiang X, Bai L, Tian J, Wu L, Han J, Cui C. Manipulation of and sustained effects on the human brain induced by different modalities of acupuncture: an fMRI study [J] PLoS One. 2013;8(6):e66815. doi: 10.1371/journal.pone.0066815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwan CL, Crawley AP, Mikulis DJ, Davis KD. An fMRI study of the anterior cingulate cortex and surrounding medial wall activations evoked by noxious cutaneous heat and cold stimuli. Pain. 2000;85(3):359–74. doi: 10.1016/S0304-3959(99)00287-0. [DOI] [PubMed] [Google Scholar]
- 17.Lai X, Huang Y. A cerebral functional definition on the specificity of acupoints, needling sensation and association of acupoints based on the “acupoints-brain relation hypothesis” [J] Chinese Acupuncture & Moxibustion. 2007;27(10):777–780. [PubMed] [Google Scholar]
- 18.Li G, Ma X, Bian H, Sun X, Zhai N, Yao M, Qu H, Ji S, Tian H, Zhuo C. A pilot fMRI study of the effect of stressful factors on the onset of depression in female patients. Brain Imaging Behav. 2015;10(1):195–202. doi: 10.1007/s11682-015-9382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang P, Wang Z, Qian T, Li K. Acupuncture Stimulation of Taichong (Liv3) and Hegu (LI4) Modulates the Default Mode Network Activity in Alzheimer’s Disease [J] Am J Alzheimers Dis Other Demen. 2014;29(8):739–48. doi: 10.1177/1533317514536600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linde K1, Weidenhammer W, Streng A, Hoppe A, Melchart D. Acupuncture for osteoarthritic pain: an observational study in routine care. Rheumatology (Oxford) 2006;45(2):222–7. doi: 10.1093/rheumatology/kei252. [DOI] [PubMed] [Google Scholar]
- 21.Liu Q, Li R, Zhou R, Li J, Gu Q. Abnormal Resting-State Connectivity at Functional MRI in Women with Premenstrual Syndrome. PLoS One. 2015;10(9):e0136029. doi: 10.1371/journal.pone.0136029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McPherson MJ, Barrett FS, Lopez-Gonzalez M, Jiradejvong P, Limb CJ. Emotional Intent Modulates The Neural Substrates Of Creativity: An fMRI Study of Emotionally Targeted Improvisation in Jazz Musicians. Sci Rep. 2016;6:18460. doi: 10.1038/srep18460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potvin S, Tikàsz A, Lungu O, Dumais A, Stip E, Mendrek A. Emotion processing in treatment-resistant schizophrenia patients treated with clozapine: An fMRI study. Schizophr Res. 2015;168(1-2):377–80. doi: 10.1016/j.schres.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 24.Ren Y, Bai L, Feng Y, Tian J, Li K. Investigation of acupoint specificity by functional connectivity analysis based on graph theory[J] Neurosci Lett. 2010;482(2):95–100. doi: 10.1016/j.neulet.2010.06.091. [DOI] [PubMed] [Google Scholar]
- 25.Siedentopf CM, Golaszewski SM, Mottaghy FM, Ruff CC, Felber S, Schiager A. Functional magnetic resonance imaging detects activation of the visual association cortex during laser acupuncture of the foot in humans [J] Neurosci Lett. 2002;327(1):53–56. doi: 10.1016/s0304-3940(02)00383-x. [DOI] [PubMed] [Google Scholar]
- 26.Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF. REST: A toolkit for resting-state functional magnetic resonance imaging data processing[J] PLoS ONE. 2011;6(9) doi: 10.1371/journal.pone.0025031. Article ID e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Townsend JD, Torrisi SJ, Lieberman MD, Sugar CA, Bookheimer SY, Altshuler LL. Frontal-amygdala connectivity alterations during emotion downregulation in bipolar I disorder. Biol Psychiatry. 2013;73(2):127–35. doi: 10.1016/j.biopsych.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu C, Qu S, Zhang J, Chen J, Zhang S, Li Z, Chen J, Ouyang H, Huang Y, Tang C. Correlation between the Effects of Acupuncture at Taichong (LR3) and Functional Brain Areas: A Resting-State Functional Magnetic Resonance Imaging Study Using True versus Sham Acupuncture[J] Evid Based Complement Alternat Med: 729091. 2014 doi: 10.1155/2014/729091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan B, Li K, Xu J, Wang W, Li K, Liu H, Shan B, Tang X. Acupoint-specific fMRI patterns in human brain[J] Neurosci Lett. 2005;383(3):236–240. doi: 10.1016/j.neulet.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Yan CC, Zang YF. DPARSF: A MATLAB toolbox for Pipeline Data Analysis of Resting-State fMRI. [J] Frontiers in Systems Neuroscience. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang W, Chen Q, Liu P, Cheng H, Cui Q, Wei D, Zhang Q, Qiu J. Abnormal brain activation during directed forgetting of negative memory in depressed patients. J Affect Disord. 2016;190:880–8. doi: 10.1016/j.jad.2015.05.034. [DOI] [PubMed] [Google Scholar]
- 32.You Y, Bai L, Dai R, Cheng H, Liu Z, Wei W, Tian J. Altered hub configurations within default mode network following acupuncture at ST36: a multimodal investigation combining fMRI and MEG [J] PLoS One. 2013;8(5):e64509. doi: 10.1371/journal.pone.0064509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang G, Qu S, Zheng Y, Chen J, Deng G, Yang C, Huang Y. Key regions of the cerebral network are altered after electroacupuncture at the Baihui (GV20) and Yintang acupoints in healthy volunteers: an analysis based on resting fcMRI[J] Acupunct Med. 2013;31(4):383–8. doi: 10.1136/acupmed-2012-010301. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Q, Li A, Yue J, Zhang F, Sun Z, Li X. Using functional magnetic resonance imaging to explore the possible mechanism of the action of acupuncture at Dazhong (KI 4) on the functional cerebral regions of healthy volunteers[J] Intern Med J 2015. 2015;45(6):669–671. doi: 10.1111/imj.12767. [DOI] [PubMed] [Google Scholar]
- 35.Zhong C, Bai L, Dai R, Xue T, Wang H, Feng Y, Liu Z, You Y, Chen S, Tianya J. Modulatory effects of acupuncture on resting-state networks: a functional MRI study combining independent component analysis and multivariate Granger causality analysis[J] J Magn Reson Imaging. 2012;2012;35(3):572–81. doi: 10.1002/jmri.22887. [DOI] [PubMed] [Google Scholar]
- 36.Zyloney CE, Jensen K, Polich G, Loiotile RE, Cheetham A, LaViolette PS, Tu P, Kaptchuk TJ, Gollub RL, Kong J. Imaging the functional connectivity of the Periaqueductal Gray during genuine and sham electroacupuncture treatment [J] Mol Pain. 2010;6:80. doi: 10.1186/1744-8069-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]


