Abstract
Background:
Cognitive disorders associated with aging have been successfully managed by African traditional medical practitioners using various plants. This study evaluated the cognitive enhancing potentials of Morinda lucida (L) Rubiaceae and Peltophorum pterocarpum (DC) ex. K Heyne in scopolamine induced amnesic animals.
Materials and Methods:
The anti-amnesic activity of the ethyl acetate extracts of Morinda lucida and Peltophorum pterocarpum at doses of 4 mg/kg, 6 mg/kg and 8 mg/kg were assessed in scopolamine induced amnesic mice using Morris water maze test model. Effect of the extracts on the histology of the hippocampus was also evaluated.
Results:
The ethyl acetate extract of Morinda lucida and Peltophorum pterocarpum ameliorated scopolamine induced memory deficit in the animals under study. There was no effect of the extract on the histology of the hippocampus. However, there was an increase in the density of cells in the hippocampus of treated group as compared to the untreated.
Conclusion:
Morinda lucida and Peltophorum pterocarpum showed considerable enhancement of cognition in scopolamine induced amnesic mice.
Keywords: Morinda lucida, Peltophorum pterocarpum, Morris water maze, Scopolamine, Hippocampus
Introduction
There is usually a decline in memory and the rate of information processing as a person grows older (Buckner, 2004., Lesne et al., 2006). Like the body’s muscles, bones and other vital organs, the brain also feels the effect of aging (Sack et al., 2009). Through years of constant use and biological wear and tear, the brain gradually loses some of its sharpness in processing information and in relaying the multitude of signals that are essential for day to day functioning (Sack et al., 2009). However, age related memory loss does not indicate diminished intelligence or ability to learn (Friedman et al., 2006). The brain may sometimes need more time to recall information from memory or to learn new information. Therefore, simple forgetfulness is not a disease.
A decline in cognitive functions has however been linked with several conditions such as neurodegenerative diseases like Alzheimer’s disease (AD) (Lesne et al., 2006) and as such is being clinically treated. Due to several limitations such as toxicity, short half-life and high cost (Giacobini, 2004) associated with some of these clinically available therapies, researchers are still working at getting better and cheaper alternatives especially from natural sources.
Memory and learning impairment is the most characteristic manifestation of dementia (Lee et al., 2009) and this can be chemically induced in experimental animal with the injection of scopolamine, a cholinergic antagonist which interferes with acetylcholine (ACh) transmission in the central nervous system (Misane and Ogren 2003). Moreover, scopolamine induced amnesic animal model have been used widely in screening for compounds with therapeutic potential as anti-dementia (Lee et al., 2009, Rubaj et al., 2003). Also, Morris water maze test is widely accepted for the assessment of spatial memory in experimental animal model (Lee et al., 2009, Moris, 1984, Kin et al., 2003).
In Nigeria, ethno-medicine memory loss is taken seriously particularly by the youths who seek memory enhancing remedies to increase their ability to pass examinations. The old people also take such remedies as memory enhancers and anti-aging to alleviate suffering associated with old age (Elufioye et al., 2013). Peltophorum pterocarpum (DC) ex. K Heyne and Morinda lucida (L) Rubiaceae are commonly found in recipes used as memory enhancer or anti-aging in Nigeria ethno-medicine. The leaves of these plants have been previously reported to inhibit cholinesterase (Elufioye et al., 2010., Sridharamurthy et al., 2012) an enzyme that causes a decline of ACh in the brain by breaking it down. Acetylcholine is critical for an adequately functioning memory and it is the subject of the major researches for treatment of memory defects like those found in Alzheimer’s disease. Any mutual health issue that involves memory or lack of it is directly related to acetylcholine (Gais and Born, 2004). A major attempt to the treatment of Alzheimer’s disease has involved attempts to augment the cholinergic function of the brain (Auld et al., 2002).
This study is, therefore, aimed at evaluating the in vivo cognitive enhancing potential of extracts Peltophorum pterocarpum and Morinda lucida in scopolamine induced animal model using Morris water maze test.
Materials and Methods
Plants Collection and extraction
Leaves of M. lucida and P. peltophorum were collected at the University of Ibadan in October 2014. The plants were authenticated at the Department of Pharmacognosy herbarium University of Ibadan with DPHUI NO; 1451 for Morinda lucida and DPHUI NO; 1453 for Peltophorum pterocarpum. Approximately 1 g of fresh leaves of P. peltophorum and M. lucida were air dried for two weeks before oven drying at 40°C for 2 hours, powdered and stored until use. 500 g of the powdered sample was macerated with ethyl acetate for 48 hrs, filtered and concentrated in vacuo.
Cognitive study
Animals
Thirty-five albino mice were purchased from Institute of Advance Medical Research and Training (IMRAT), College of medicine, University of Ibadan.
Administration of doses for the various groups
The mice were weighed, labeled and grouped into seven groups of 5 animals each after which all animals were pre-injected intraperitoneally with 3 mg/kg scopolamine. Groups 1-3 were administered 0.2 ml equivalent doses of 4 mg/kg, 6 mg/kg and 8 mg/kg of the extract of Morinda lucida while groups 4-6 were given same doses of Peltophorum pterocarpum extract and group 7 was given 0.2 ml of distilled water (negative control) for 3 consecutive days.
Morris Water Maze Test Procedure
This test is usually done to assess the spatial memory function. It was performed according to the method of Morris, 1984 with slight modifications according to Kim et al., 2003 and Lee et al., 2009.
The water maze is a circular pool (90 cm in diameter and 45 cm in height) filled with water mixed with 157 mL of evaporated milk to a height of 30 cm. The pool was divided into four equal quadrants and a platform submerged below water level in one of the quadrants at 1 cm such that it was not visible from the surface.
On day 1 of the experiment, the animals were trained to swim for 60 sec. in the absence of the platform. Subsequently, the animals were given two swimming trial sessions each day with the platform in place for four days. Their locations from start position to the platform was monitored, the escape latencies of each animal were measured with a stop watch and an average calculated for each trial session. Each mouse was allowed a period of 10 sec. on the platform after locating it. However, any animal which could not locate the platform after 120 sec. was also placed on the platform for 10 sec and then removed from the pool.
The time interval of 30 min was observed between daily trials. While the point of entry into the pool for the animals and the location of the platform remained unchanged between daily trials, it was changed on a daily basis. Changes in the escape latency from day-to-day stands for long term or reference memory, while changes from trial 1 to trial 2 on the same day represent working or short-term memory.
Five mice were used per treatment and control group. Amnesia was induced in all animals by intra-peritoneal injection of 3 mg/kg scopolamine dissolved in water/DMSO. All animals were assessed for spatial memory 24 hours after the administration of scopolamine to establish amnesia. Treatment with different doses (0.2 mL of 4 mg/kg, 6 mg/kg and 8mg/kg) of M. lucida and P. peltophorum extracts commenced after establishing amnesia in the animals. The control group was given 0.5 mL/kg distilled water.
Histopathology
All the animals were sacrificed by cervical dislocation at the end of the experiments with their brains removed and preserved in phosphate formalin buffer for histopathological studies. Slides of the forebrain and hippocampus were prepared and observed under light microscope. Photomicrographs were taken, the number of cells per area in the CA1 region of the hippocampus was estimated and density of each slide was calculated as follows:
All area values were multiplied by a constant value of 0.088899 Density = Number of cells Areax 0.088899

Results and Discussion
Impairment of memory and learning, the most characteristic manifestation of cognitive dysfunction can be induced chemically in people (Broks et al., 1988) as well as in experimental animals by scopolamine, a cholinergic antagonist known to interfere with acetylcholine transmission in the central nervous system (Misane and Ogren 2003). Herbal and traditional medicine which has been a major aspect of socio cultural heritage in Africa for hundred years (Okigbo and Mmeka, 2006) has successfully managed memory impairment with the use of memory enhancing and anti-aging herbs.
The effect of Morinda lucida and Peltophorum pterocarpum (two plants commonly included in recipes for memory enhancing) on scopolamine-induced memory deficit was evaluated using Morris water maze test. Morris water maze evaluates spatial, working and reference memory (Lee et al., 2009).
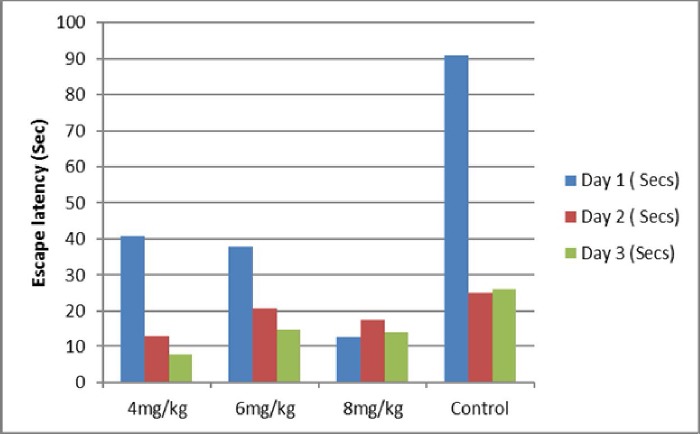
In this study, the escape latency time in animals induced by scopolamine was significantly reduced by ethyl acetate extracts of both Morinda lucida and Peltophorum pterocarpum when compared with the control group that received distilled water, as shown in figures 1 and 2.
Figure 1.
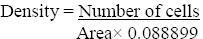
Effect of Morinda lucida on scopolamine induced memory impairment in Morris water maze test.
Figure 2.
Effect of Peltophorum pterocarpum on scopolamine induced memory impairment mice in Morris Water maze test.
It was observed that Morinda lucida and Peltophorum pterocarpum improved the memory of the animals in a dose dependent manner. Animals administered with 4 mg/kg, 6 mg/kg and 8 mg/kg responded to the ethyl acetate extracts as they exhibited a reduction in escape latency times from Day 1, Day 2 and Day 3. The escape latencies as depicted in table 1 and table 2 showed a significant decrease to the ethyl acetate extracts of Morinda lucida and Peltophorum pterocarpum while the scopolamine treated group exhibited a characteristic swimming behavior consisting of circling around the pool and longer escape latency indicating impairment of their spatial memory.
Table 1.
Effects of M. lucida extract on scopolamine induces memory impairment in mice showing mean escape latency
| Doses | Mean escape latency (+ SEM) |
|---|---|
| 4 mg/kg | 25.11±3.89 |
| 6 mg/kg | 23.83±9.09 |
| 8 mg/kg | 23.33±4.33 |
| Scopolamine | 47.33± 21.85 |
Table 2.
Effects of P. pterocarpum extract on scopolamine induces memory impairment in mice showing mean escape latency
| Doses | Mean escape latency (+ SEM) |
|---|---|
| 4 mg/kg | 20.37±8.4 |
| 6 mg/kg | 24.30±6.95 |
| 8 mg/kg | 14.60±1.55 |
| Scopolamine | 47.33± 21.85 |
The extracts showed dose-dependent cognitive enhancing activity with Peltophorum pterocarpum having shorter escape latency time of 14.60 + 1.55 at 8 mg/kg and thus appears to have better cognitive enhancing ability when compared with Morinda lucida with escape latency of 23.33 + 4.33 at the same dose.
The cognitive enhancing activity observed in this study can be due to previously reported cholinesterase inhibitory property of these plants (Elufioye et al., 2010) since acetyl cholinesterase inhibitors are known to counteract scopolamine induced amnesia (Lee et al., 2009). This is probably because the effects of scopolamine in learning and memory are also mediated by cholinergic neurons in the ventral forebrain, particularly in those arising from the medial septal nuclei or the diagonal band of Broca and projecting to the hippocampus (Bejar et al., 1999).
In the histopathology study, there was no significant change in the histology of the brain. However, it was observed that there was a reduction in density of cells in the hippocampus of the control mice pretreated with scopolamine who received only distilled water. However, there was an increase in the number and density of cells in the animals tested with extracts of Morinda lucida and Peltophorum pterocarpum (tables 3 and 4). The resultant increase may signify cognitive enhancement.
Table 3.
Result of the Histopathology studies on the Hippocampus of mice treated with Morinda lucida extract showing the cell count per area counted and density
| Doses (mg/kg) | Cells counted per area | Area (Square meter) | Density |
|---|---|---|---|
| 4mg/kg | 75 | 662400.00 | 0.001238 |
| 6mg/kg | 33 | 294100.00 | 0.001262 |
| 8mg/kg | 25 | 2029500.00 | 0.0001386 |
| Scopolamine (3mg/kg) | 35 | 456875.00 | 0.000086813 |
Table 4.
Result of the Histopathology studies on the Hippocampus of mice treated with Peltophorum pterocarpum extract showing the cell count per area counted and density.
| Doses (mg/kg) | Cells counted per unit area | Area (Squmetre) | Density |
|---|---|---|---|
| 4mg/kg | 61 | 402500.00 | 0.001705 |
| 6mg/kg | 33 | 297118.80 | 0.001249 |
| 8mg/kg | 45 | 463137.50 | 0.001093 |
| Scopolamine (3mg/kg) | 35 | 456875.00 | 0.000086813 |
Figure 3.
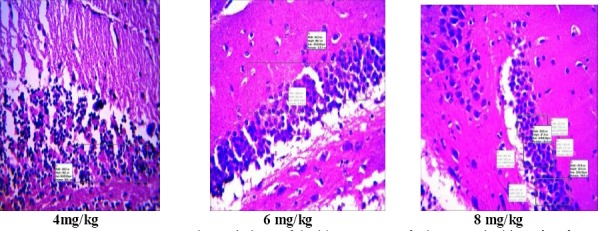
Histopathology of the hippocampus of mice treated with M. lucida
Figure 4.
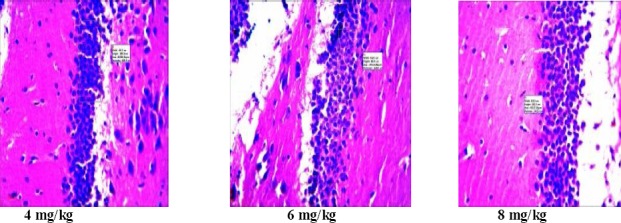
Histopathology of the hippocampus of mice treated with P. pterocarpum
Figure 5.
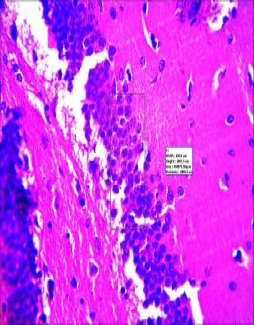
Histopathology of the hippocampus of mice pretreated with 3 mg/kg Scopolamine Morinda lucida and Peltophorum pterocarpum at doses of 4 mg/kg, 6 mg/kg and 8 mg/kg exhibited significant gradual improvement in memory and cognition.
Conclusion
The ethyl acetate extracts of Morinda lucida and Peltophorum pterocarpum possess significant cognitive-enhancing activity based on the results of the Morris water maze and histopathology studies of the hippocampus, and may thus be effective in the treatment of cognitive disorders.
References
- 1.Auld D. S, Kornecook T. J, Bastianetto S, Quirion R. Alzheimer’s disease and the basal forebrain cholinergic system: relation to βamyloid peptides, cognition and treatment strategies. Progress in neurobiology. 2002;68(3):209–245. doi: 10.1016/s0301-0082(02)00079-5. [DOI] [PubMed] [Google Scholar]
- 2.Bejar C, Wang R. H, Weinstock M. Effect of rivastigmine on scopolamine-induced memory impairment in rats. Europrean Journal of Pharmacology. 1999;383:231–240. doi: 10.1016/s0014-2999(99)00643-3. [DOI] [PubMed] [Google Scholar]
- 3.Broks P, Preston G. C, Traub M, Poppleton P, Ward C, Stahl S. M. Modelling dementia: effects of scopolamine on memory and attention. Neuropsychologia. 1988;26(5):685–700. doi: 10.1016/0028-3932(88)90004-8. [DOI] [PubMed] [Google Scholar]
- 4.Buckner R. L. Memory and executive function in aging and AD: Multiple factors that cause decline and reverse factors than compensate. Neuron. 2004;44(1):195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Elufioye T. O, Obuotor E. F, Agbedahunsi J. M, Adesanya S. A. Cholinesterase inhibitory activity of Morinda lucida benth. Journal of Pharmacognosy and phytotherapy. 2013;5:77–82. [Google Scholar]
- 6.Elufioye T. O, Obuotor E. M, Sennuga A. T, Agbedahunsi J. M, Adesanya S. A. Acetyl cholinesterase and butyryl cholinesterase inhibitory activity of some selected Nigerian medicinal plants. Brazilian Journal of Pharmacognosy. 2010;20(4):472–477. [Google Scholar]
- 7.Friedman N. P, Miyake A, Corley R. P, Young S. E, Defries J. C, Hewitt J. K. Not all executive functions are related to intelligence. Psychological science. 2006;17(2):172–179. doi: 10.1111/j.1467-9280.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- 8.Gais S, Born J. Low acetylcholine during slow-wave sleep is critical for declarative memory consolidation. Proceedings of the National Academy of Sciences of the United states of America. 2004;101(7):2140–2144. doi: 10.1073/pnas.0305404101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giacobini E. Cholinesterase inhibitors: new roles and therapeutic alternatives. Pharmacological research. 2004;50(4):433–440. doi: 10.1016/j.phrs.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Kim S. R, Kang S. Y, Lee K. Y, Kim S. H, Markelonis G. J, Oh T. H, Kim Y. C. Anti-amnesic activity of E-P- methoxy cinnamic acid from Scrophularia buergeriana. Cog. Brain Res. 2003;17:454–461. doi: 10.1016/s0926-6410(03)00161-7. [DOI] [PubMed] [Google Scholar]
- 11.Lee K. Y, Sung H. S, Kim H. S, Jang P. Y, Oh H. T, Kim C.Y. Cognitive enhancing activity of Loganin isolated from Cornus officinalis in scopolamine -induced amnesic mice. Arch. Pharm. Res. 2009;32(5):677–683. doi: 10.1007/s12272-009-1505-6. [DOI] [PubMed] [Google Scholar]
- 12.Lesne S, Koh M. T, Kotilinek L, Kayed R, Glabe C. G, Yang A, Gallagher M, Ashe K. A. Specific amyloid protein assembly in the brain impairs memory. Nature. 2006;16(440(7082)):352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 13.Misane I, Ogren S. O. Selective 5-HT(IA) antagonist WAY 100635 and NAD-299 attenuate the impairment of passive avoidance caused by scopolamine in rats. Neuropsycho Pharmacology. 2003;28:253–264. doi: 10.1038/sj.npp.1300024. [DOI] [PubMed] [Google Scholar]
- 14.Morris R. G. Development of a water maze procedure for studying spatial learning in the rat. J. Neurosci. Meth. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 15.Okigbo R. N, Mmeka E. C. An appraisal of phytomedicine in Africa. KMITL Science and technology Journal. 2006;6(2):83–94. [Google Scholar]
- 16.Rubaj A, Zgodzinski W, Sickluck-Dziuba M. The influence of adenosine A3 receptor agonist IB-MECA on scopolamine and MK-810 induced memory impairment. Behav. Brain. Res. 2003;141:11–17. doi: 10.1016/s0166-4328(02)00314-5. [DOI] [PubMed] [Google Scholar]
- 17.Sack I, Beierbach B, Wuerfel J, Klatt D, Hamhaber U, Papazoglou S, Martus P, Braun J. The impact of aging and gender on brain viscoelasticity. Neuroimage. 2009;46(3):652–657. doi: 10.1016/j.neuroimage.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 18.Sridharamurthy N. B, Ashok B, Yogananda R. Evaluation of antioxidant and acetyylcholinesterase inhibitory activity of Peltophorum pterocarpum in scopolamine treated rats. International Journal of drug development and research. 2012;4(3):115–127. [Google Scholar]


